Abstract
Objective
One of the rate-limiting barriers within the field of vascular tissue engineering is the lengthy fabrication time associated with expanding appropriate cell types in culture. One particularly attractive cell type for this purpose is the adipose-derived mesenchymal stem cell (AD-MSC), which is abundant and easily harvested from liposuction procedures. However, even this cell type has its drawbacks including the required culture period for expansion which could pose risks of cellular transformation or contamination. Eliminating culture entirely would be ideal to avoid these concerns. In this study we utilized the raw population of cells obtained after digestion of human liposuction aspirates – known as the stromal vascular fraction (SVF) – as an abundant, culture-free cell source for tissue engineered vascular grafts (TEVG).
Methods
SVF cells and donor-paired cultured AD-MSCs were first assessed for their abilities to differentiate into vascular smooth muscle cells (SMCs) after angiotensin II stimulation and to secrete factors (e.g. conditioned media) that promote SMC migration. Next, both cell types were incorporated into TEVG scaffolds, implanted as an aortic graft in a Lewis rat model, and assessed for their patency and composition.
Results
In general, cells from human SVF were able to perform the same functions as AD-MSCs isolated from the same donor via culture expansion. Specifically, cells within the SVF performed two important functions, namely, they were able to differentiate into SMCs (SVF calponin expression: 16.4% ± 7.7 vs. AD-MSC: 19.9% ± 1.7) and could secrete pro-migratory factors (SVF migration rate relative to control: 3.1 ± 0.3 vs. AD-MSC: 2.5 ± 0.5). Additionally, SVF was also capable of being seeded within biodegradable, elastomeric, porous scaffolds that, when implanted in vivo for 8 weeks, generated patent TEVGs (SVF: 83% patency vs. AD-MSC: 100% patency) populated with primary vascular components (e.g. SMCs, endothelial cells, collagen, and elastin).
Conclusion
Human adipose tissue can be utilized as a culture-free cell source to create TEVGs, laying the groundwork for the rapid production of cell-seeded grafts.
Keywords: Culture-free, mesenchymal stem cell, tissue engineered blood vessel, fabrication time
1. INTRODUCTION
Tissue engineers have developed small diameter (<6 mm) vascular grafts which possess both reduced intimal hyperplasia and thrombosis compared to current clinical standards, representing excellent progress towards clinical application1-5. However, despite the significant degree of pre-clinical testing that cell-based tissue engineered vascular grafts (TEVGs) have undergone3, few approaches have reached clinical trials6-8. This is likely attributed to a number of practical rate-limiting barriers still present which hinder the clinical translation of TEVGs, such as appropriate testing of patient-specific cells (e.g. clinically appropriate demographics)9, lack of feasible and consistent modes of manufacture to appropriate sizes, the need for culture expansion of cells to a suitable number, and lengthy fabrication time10. While the first two of these concerns have been validated in recent years3, 9, the necessity of in vitro culture and lengthy fabrication times which are employed by many TEVG designs3 are still major concerns. Specific concerns for clinical application include excess waiting time for the patient, significant costs (such as cell culture reagents and personnel), and potential cellular transformation or contamination.
In order to successfully translate cell-based TEVGs to the clinic, it is important to identify a tissue source which can provide a high number of quality cells in the lowest amount of time. One of the most attractive tissues for this purpose is fat, which can be obtained in abundance from liposuction procedures and provides human adipose-derived mesenchymal stem cells (AD-MSCs). However, even AD-MSCs require time for vitro expansion into necessary numbers11. Alternatively, the digested liposuction population of cells used to obtain AD-MSCs, known as the stromal vascular fraction (SVF), could be utilized as it is progenitor rich, containing AD-MSCs, pericytes, endothelial progenitor cells, and endothelial cells12. As all of these cell types have been successfully used in vascular tissue engineering3, SVF could hold significant promise as a cell source. Additionally, typical liposuction volumes are sufficient to obtain large enough quantities of cells for construction of a graft of clinically relevant dimensions without additional in vitro expansion13, 14. Utilizing SVF to seed scaffolds for vascular tissue engineering could therefore be ideal for the clinical translation of TEVGs, relieve many regulatory and financial concerns, and make a significant step forward in the fabrication of stem cell-based TEVGs.
In this study, the use of human SVF was validated for vascular tissue engineering using both in vitro and in vivo studies. This initial small-scale study showed that SVF can be used to produce rapidly fabricated TEVGs with robust in vivo performance functionally similar to TEVGs composed of culture expanded AD-MSCs generated from the same human donors.
2. METHODS
2.1 Isolation of Human SVF and AD-MSCs
Adipose-derived cell fractions were isolated from non-diabetic human patients using previously described methods12, 15. Informed consent was not required due to the University of Pittsburgh IRB determination that the study involved solely deidentified waste from the liposuction procedure. Under this type of protocol determination only the presented information (age, gender, BMI, and diabetic status) could be provided (Table I). Liposuction yields were on the order of several kg, from which 200 mL was removed and dissociated via mincing and digestion with collagenase. The resulting solution was filtered to remove debris, centrifuged to eliminate buoyant adipocytes, resuspended in an erythrocyte lysis buffer to eliminate red blood cells, and centrifuged to obtain a final cell pellet. A typical cell yield from this procedure was 30-40 million cells. These cells will be referred to in this manuscript as SVF. For this study, a cultured AD-MSC control was established from each SVF donor by passage expanding a portion of the SVF four times9, 15. Using this procedure, SVF was available for graft fabrication within a matter of hours whereas AD-MSCs took upwards of 4 weeks. Cell culture was performed in 75-cm2 tissue culture flasks (Corning) under defined culture media9, 15 and was replenished every 2-3 days. To passage expand cells, Trypsin-EDTA (#25200-056; Gibco) was utilized. In addition, for in vitro experimentation SMCs purchased from ATCC were cultured as previously described15 using their own defined media.
Table 1.
AD-MSC Human Donor Information.
| Human Donors Used for Cell Seeding Experiments | |||
|---|---|---|---|
| ID Number | Gender | Age | BMI |
| 1 | F | 38 | 33 |
| 2 | F | 33 | 28 |
| 3 | F | 43 | 26 |
| Human Donors Used for Differentiation, Migration, and Implantation Experiments | |||
|---|---|---|---|
| ID Number | Gender | Age | BMI |
| 4 | F | 44 | 23 |
| 5 | F | 26 | 33 |
| 6 | F | 40 | 26 |
| 7 | F | 38 | 35 |
2.2 Smooth Muscle Cell Differentiation and Migration Assays
Two relevant functions of stem cells in the context of TEVG development are differentiation into vascular SMC3, 15-18 and secretion of factors that can promote SMC migration2, 15, 19-21. To induce an SMC-like differentiation of SVF, a previously described method15 was utilized where cells were stimulated with angiotensin II (1 μM in culture media) for 4 days. As this type of analysis was previously presented in a more robust manner when characterizing multiple populations of AD-MSCs15 this data is presented in Supplemental Figure 1. In this figure, only staining of calponin expression is presented as markers of terminal differentiation (e.g. myosin heavy chain, smoothelin) do not yet appear with 4 days of angiotensin II treatment, and the early marker smooth muscle alpha actin is ubiquitously expressed in AD-MSCs prior to differentiation. Donor-paired cultured AD-MSCs were utilized as a positive control for differentiation and non-stimulated SVF were used as a negative control. Differentiation was assessed by calponin expression and a morphological shift to a typical SMC spindle-like shape. Quantification of this morphological shift was obtained by measuring each cell's shape factor (Shape factor = 4*pi*area/perimeter2, ~0 = ellipsoid, ~1 = circular) wherein differentiation is reflected by conversion from a round (nearer to 1) to spindle-like (nearer to 0) shape.
Stimulation of SMC migration also was performed utilizing previous protocols15. Briefly, SMCs were plated near confluence in a 24-well plate and a scratch wound was made via a single stroke of a pipette tip per well. During migration SMCs were stimulated with conditioned media from either SVF, donor-matched AD-MSCs, or non-conditioned media. All cells were maintained under culture conditions (37°C, 5% CO2) for the duration of the experiment. The extent of migration was monitored every 2 hours and was reported as the average rate of scratch wound closure over the first 8 hours. Three donor lines were used in these studies (Table I).
2.3 Scaffold Fabrication
Small (1.3 mm ID, 10 mm length) bilayered, tubular, biodegradable, elastomeric scaffolds consistent with the size of the rat abdominal aorta were constructed from poly(ester urethane)urea (PEUU) as previously described22. Briefly, a porous inner layer was created via thermally induced phase separation, which was then coated with an external mechanical sheath via electrospinning. This scaffold has already shown efficacy in the TEVG context using multiple cell types1, 4, 9, 23.
2.4 Cell Seeding
A rotational vacuum seeding device (RVSD) technology was used to seed PEUU scaffolds as previously described1, 4, 23, 24. Briefly, scaffolds were mounted within the device, infused lumenally with a cell suspension, and exposed to both rotation (15 rpm) and vacuum (−127 mmHg). A total of 3 million cells (either SVF or donor-matched AD-MSCs) were incorporated within each scaffold utilizing this process. Post-seeding, the resulting constructs were incubated in static media for 4 hours to allow for cell adhesion after which they were subjected to a dynamic culture (spinner flask, 15 rpm) for 48 hours which primed them for either seeding analysis or implantation. For analysis of uniform seeding, constructs were fixed (4% paraformaldehyde; 30 min), cut into three axial portions, and frozen. Scaffolds were then sectioned and stained with DAPI to analyze the cell distribution. Images were acquired at 10x using NIS Elements software and were analyzed using custom scripts built within ImageJ (NIH, Bethesda, Maryland)9 wherein complete cross sectional images were generated, virtually segmented into radial or circumferential pieces, and cell densities were compared with respect to radial, circumferential, or longitudinal pieces.
2.5 In Vivo Implantation and Explant
Seeded scaffolds were implanted as end-to-end abdominal aortic grafts using our Lewis rat model1, 4, 9, 23. All 4 patients noted in Table I under “Human Donors Used for Differentiation, Migration, and Implantation Experiments” were used as the source of cells to produce, with some replicates, n=6 SVF and n=5 AD-MSC grafts for implantation. Rats were anesthetized and the infrarenal abdominal aorta was exposed. Microclamps were applied to the aorta prior to transection upon which a seeded scaffold was sutured with 10-0 prolene. After securing the graft, the clamps were released and the patency verified by observation of the distal pulse pressure. The animals were then closed with 3-0 polyglactin sutures and maintained on an anti-coagulant (dipyridamole, aspirin) schedule for 4 weeks as previously described9. After 8 weeks, the rats were euthanized and an angiogram was performed to assess TEVG patency. All procedures were performed under an approved Institutional Animal Care and Use Committee protocol. TEVGs were then explanted, fixed, and frozen for histologic analysis.
2.6 Immunofluorescence and Histologic Evaluation
Frozen TEVGs were sectioned using a cryo-microtome at an 8 μm thickness prior to being mounted on gelatin coated slides and stained using standard indirect immunofluorescent chemistry. Primary antibodies utilized were as follows: von Willebrand Factor (vWF) [1:250; US Biological #V2700-07], smooth muscle alpha-actin (SMA) [1:1000; Sigma #A5228], calponin [1:250; Abcam #ab46794], and elastin [1:100; EPC #RA75]. The following secondary antibodies were utilized: [Rockland #611-1202 (1:1000)], [Invitrogen #A10521 (1:1000)], [Sigma #C2821 (1:300)]. Explants were also stained with H&E. All images were taken using a 20x objective with an epifluorescent microscope utilizing NIS Elements software. Analysis was restricted to the newly developed luminal tissue within TEVGs (termed as “neotissue”).
2.7 Statistics
All statistical analyses were performed in Minitab (version 16) software. Data was first assessed for assumptions of a normal distribution and homogeneity of variance. Following this, a student's t-test was performed with significance being defined at p < 0.05. Data was expressed as mean ± standard deviation.
3. RESULTS
3.1 Human SVF differentiated into SMC-like cells and secreted factors to promote SMC migration
To initially investigate the relevance of SVF for TEVG applications, the abilities of SVF to differentiate into SMC and to induce SMC migration were analyzed. Both of these stem cell functions are important for achieving maturation of a TEVG into a native-like vessel15. Both donor-matched AD-MSCs – a cell population previously validated in this manner15 – and SVF underwent differentiation into SMC like-cells and secreted factors to promote SMC migration. For SMC differentiation this was shown by induced expression of the SMC marker calponin (Supplemental Figure 1A) and a morphologic change to a more spindle-like cell shape (e.g. a decreased shape factor) (Supplemental Figure 1B). It is important to note that even though the broad percentage of differentiated cells was low, discernable donor differences can be detected with this assay15. The ability of both AD-MSCs and SVF to promote migration of SMCs was evident by the significant increase in migration rate observed in SMCs when stimulated with conditioned media from either cell type (Supplemental Figure 1C). Taken together these results verify that SVF has functions present in AD-MSCs necessary for TEVG maturation and thus can be utilized in the context of vascular tissue engineering.
3.2 Human SVF was able to be seeded within tissue engineering scaffolds and generated robust, patent vessels in vivo
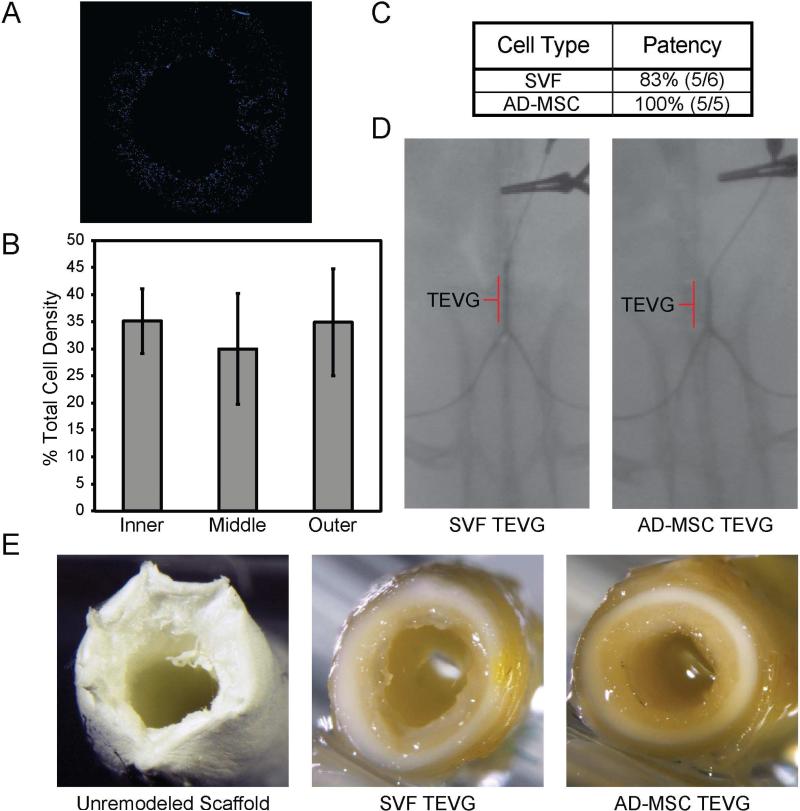
To verify that SVF could be seeded within our porous tubular scaffolds and as a point of quality control, the extent to which SVF was incorporated within our scaffolds using our RVSD was quantified. SVF was evenly seeded throughout the scaffolds in cross section (Figure 1A) and equivalent cell densities were observed across the radial, circumferential, and longitudinal directions (Figure 1B, radial shown). This is in line with previously published work analyzing AD-MSC seeded scaffolds9. SVF seeded scaffolds were then implanted in a Lewis rat model where after 8 weeks in vivo they remained patent, as confirmed by angiography, similar to their AD-MSC counterpart grafts (Figure 1C,D). No dilation was observed for any implanted vessel. On gross observation, TEVGs generated from either SVF or AD-MSCs showed an evident tissue-like appearance (Figure 1E). Together these results show that both SVF and AD-MSCs are capable of generating patent, tissue-like TEVGs.
Figure 1. Human SVF can be seeded uniformly within porous tubular scaffolds and generate patent vessels to a similar extent as AD-MSCs.
(A) SVF (n=3) was obtained from human patients and seeded directly into PEUU scaffolds where they seeded uniformly (blue, DAPI stain) throughout the thickness of the scaffold wall. A representative image is shown. (B) This uniformity was quantitatively confirmed by comparing radial distribution densities of cells across three radial regions, which each contained ~33% of the total cells. This is in line with our previous work quantitatively showing uniformity of AD-MSC seeded scaffolds13. Data is presented as mean ± SD with a statistically significant difference defined at P < .05. (C) Both SVF and donor-matched AD-MSCs were capable of generating patent TEVGs based on patency rates. (D) Seeded scaffolds with either SVF or donor-matched AD-MSCs were implanted in a Lewis rat model wherein they maintained angiographic patency through an 8 week timepoint. The TEVG location is indicated. (E) All explanted TEVGs displayed a clear tissue-like gross appearance, compared to the unremodeled PEUU scaffold.
3.3 SVF develop vascular-like tissue within TEVGs that is rich in primary vascular components
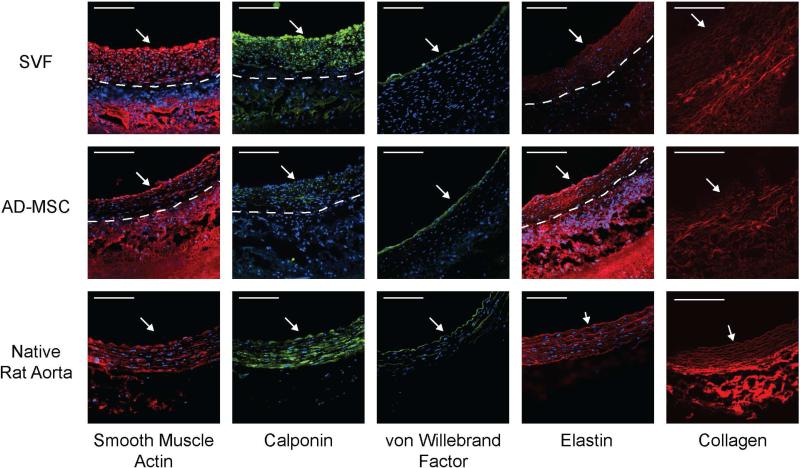
To analyze the composition of the newly developed tissue within the TEVGs, the primary cellular (SMC, endothelial cells), and extracellular (elastin, collagen) vascular components were detected using multiple imaging modalities. Immunofluorescence (Figure 2) revealed that the cellular components of all explanted TEVGs – either SVF or AD-MSCs – primarily consisted of SMCs (SMA and calponin labeling) with a luminal lining of endothelial cells (vWF labeling). Immunofluorescence also confirmed the presence of elastin in both SVF and AD-MSC-based TEVGs, however, it had not yet formed into fully-developed elastic lamellae. Finally, to detect the presence of the extracellular matrix component collagen within explanted TEVGs multiphoton microscopy was employed. This imaging modality revealed prominent, circumferentially aligned collagen fibers within all TEVGs explants (Figure 2). Taken together, these results show that when SVF is utilized as the cell source for TEVGs a vascular-like composition emerges similar to that observed with donor-matched AD-MSCs.
Figure 2. Human SVF-based TEVGs developed into a vascular-like tissue containing the primary cellular and matrix constituents of a small diameter artery, similar to that observed with AD-MSC-based TEVGs.
TEVGs, using either SVF or donor-matched AD-MSCs displayed significant remodeling. TEVGs stained positively for SMCs (smooth muscle actin and calponin), endothelial cells (von Willebrand Factor), and elastin. Multiphoton imaging demonstrated the presence of collagen. White arrow indicates lumen. The dashed line represents the boundary between newly-developed vascular-like tissue (neotissue) and the PEUU scaffold. For comparison, native artery images are included from13.
4. DISCUSSION
The results of this study show that SVF can perform the same functions as paired AD-MSCs derived from the same donor for use in a TEVG. First, SVF was capable of two stem cell-based bioactivities thought to be of possible importance in vascular tissue engineering: differentiation into vascular SMCs3, 15-18 and secretion of pro-migratory factors2, 15, 19-21 (Supplemental Figure 1). Second, SVF was shown to be incorporated within porous tubular, biodegradable, elastomeric scaffolds and generate patent TEVGs (Figure 1) with vascular-like tissue after implantation (Figure 2). The results of this study indicate the functional efficacy of a freshly-isolated non-cultured adipose-derived cell source in the context of vascular tissue engineering, favorably comparable to published work with cultured AD-MSCs9. Ultimately, the use of this cell source reduces rate-limiting barriers that could hinder the clinical translation of TEVGs. Indeed, SVF has previously been shown to perform the same functions as AD-MSCs in other applications25, 26 such as Crohn's disease and myocardial infarction which has supported the use of SVF in multiple clinical trials27-29.
While non-cultured bone marrow cells have already been employed as a non-cultured cell source for vascular tissue engineering30 and can generate an equally sizable amount of cells30 as adipose tissue13, 14 (on the order of millions per mL), the use of an adipose-derived cell source confers unique benefits. In addition to the aesthetic attractiveness of harvesting adipose tissue to obtain SVF, performing a liposuction on a patient is associated with a reduction in cardiovascular disease risk factors31. This could offer resistance against recurrent vascular disease. On a cellular level, freshly isolated adipose cells (i.e. SVF) contain a higher percentage of cells that can provide anti-thrombogenic effects, compared to bone marrow, which could affect graft patency. These include cells such as MSCs (30% adipose12, 27 vs 0.001% bone marrow19, 27), endothelial cells (3% adipose12 vs 0.1% bone marrow19), and endothelial precursor cells (14% adipose12 vs. 2% bone marrow19). However, this statement remains somewhat conjecture as a side-by-side comparison of SVF vs. fresh bone marrow has yet to be performed in vascular tissue engineering.
One of the major challenges currently present in stem cell-based vascular engineering is fabrication time. To reach clinical trials, tissue engineered products face a number of hurdles32 including extensive pre-clinical testing, complicated and uncertain regulatory pathways, the necessity to improve product practicality, and funding shortages. These are amplified by the landscape of the current market where substantial reimbursement for cell-based technologies does not yet exist10. Additionally, regulations and recommendations set forth by the Food and Drug Administration (FDA)33, 34 encourage the use of fresh cells as well as approaches that minimize time between isolation and use of a patient's cells for clinical trials. Indeed one of the few literature reports of a tissue engineered vascular graft clinical trial utilized a non-cultured cell population (bone marrow mono-nuclear cells)6, 8. The FDA also encourages controllable methods33, 35 of manufacture which can be accomplished with cell processing devices. Utilizing our automated bulk cell seeding device in conjunction with SVF makes a significant practical step in the design and fabrication of stem cell-based TEVGs. However, manual methods and xenogenic enzymes are still required to isolate these cells11, 12, 15. Automated technologies36 are currently being tested to alleviate these concerns and technologies which can isolate SVF without enzymes are also being considered37. It is foreseeable that with the combination of these technologies, SVF, and our cell seeding methods, a TEVG could be fabricated at bed-side and within the same surgical procedure.
While this study acts as a small-scale feasibility investigation to the use of culture-free adipose-derived cells (e.g. SVF) to enhance improve upon TEVG fabrication methods, there are still many unaddressed areas that require further investigation. Specifically, multiple questions remain unanswered such as 1) what are the phenotypic differences between SVF and AD-MSCs?, 2) how do these differences contribute to the overall maturation of TEVGs in vivo?, and 3) what are the long term outcomes of these grafts (e.g. at time points greater than 8 weeks)? Each of these areas are of current interest for future study, for example the pro-elastogenic effect of AD-MSC is relevant to another vascular context, abdominal aortic aneurysm38. Additionally, while this proof-of-concept study was performed with immuno-tolerant rats implanted with human cells, large scale studies will need to be performed to fully understand how this graft will function in models with more similar biology and geometry to that of humans.
5. CONCLUSION
A non-cultured human cell source (i.e. SVF) can be isolated from adipose tissue and utilized in vascular tissue engineering similarly to donor-matched culture-expanded AD-MSCs. These cells are capable of essential regenerative functions, can be effectively bulk-seeded using a controllable device, and can generate TEVGs with a vascular-like composition. This study makes a significant practical step in the design and fabrication of stem cell-based TEVGs.
Supplementary Material
CLINICAL RELEVANCE.
Cells sourced from patient fat after procedures such as liposuction could ultimately be seeded into a tubular scaffold as the basis of a vascular graft for small diameter coronary and peripheral artery bypass procedures. This study serves as a preclinical evaluation of adipose-derived cells, seeded into a tubular scaffold, in the context of a rat interpositional implantation model.
ACKNOWLEDGEMENTS
This work was supported by the American Heart Association (AHA #12PRE12050163 to JTK) and National Institutes of Health (R21 #EB016138 and R21 #HL130784 to DAV, T32 HL094295 to JTK). We would also like to acknowledge the technical assistance of Alexander Josowitz.
Non-Standard Abbreviations and Acronyms
- AD-MSC
Adipose-Derived Mesenchymal Stem Cell
- PEUU
Poly(ester urethane)urea
- RVSD
Rotational Vacuum Seeding Device
- SMA
Smooth Muscle alpha-Actin
- SMC
Smooth Muscle Cell
- SVF
Stromal Vascular Fraction
- TEVG
Tissue Engineered Vascular Graft
- vWF
von Willebrand Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
No competing financial interests exist.
REFERENCES
- 1.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31(32):8235–44. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. Faseb J. 2011;25(12):4253–63. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials. 2012;33(12):3388–400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Nieponice A, Soletti L, Guan JJ, Hong Y, Gharaibeh B, Maul TM, et al. In Vivo Assessment of a Tissue-Engineered Vascular Graft Combining a Biodegradable Elastomeric Scaffold and Muscle-Derived Stem Cells in a Rat Model. Tissue Eng Pt A. 2010;16(4):1215–23. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson J, Dahl S, Prichard H, Manson R, Gage S, Kypson A, et al. VS5 human tissue-engineered grafts for hemodialysis: development, preclinical data, and early investigational human implant experience. Journal of Vascular Surgery. 2014;59(6):32S–3S. [Google Scholar]
- 7.L'Heureux N, McAllister TN, de la Fuente LM. Tissue-Engineered Blood Vessel for Adult Arterial Revascularization. New England Journal of Medicine. 2007;357(14):1451–3. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 8.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. Journal of Thoracic and Cardiovascular Surgery. 2010;139(2):431–U233. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 9.Krawiec JT, Liao HT, Josowitz AD, Weinbaum JS, D’Amore A, Rubin JP, et al. In Vivo Functional Evaluation of Tissue Engineered Vascular Grafts Fabricated Using Human Adipose-Derived Stem Cells from High Cardiovascular Risk Populations. Tissue Eng Pt A. 2016;22(9-10):765–75. doi: 10.1089/ten.tea.2015.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L'Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology Insight: the evolution of tissue-engineered vascular grafts - from research to clinical practice. Nat Clin Pract Card. 2007;4(7):389–95. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 11.Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. JoVE (Journal of Visualized Experiments) 2013;(79):e50585–e. doi: 10.3791/50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keck M, Kober J, Riedl O, Kitzinger HB, Wolf S, Stulnig TM, et al. Power assisted liposuction to obtain adipose-derived stem cells: Impact on viability and differentiation to adipocytes in comparison to manual aspiration. Journal of Plastic, Reconstructive and Aesthetic Surgery. 2014;67(1):e1–e8. doi: 10.1016/j.bjps.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Aust L, Devlin B, Foster SJ, Halvorsen YDC, Hicok K, du Laney T, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 15.Krawiec JT, Weinbaum JS, St. Croix CM, Phillippi JA, Watkins SC, Rubin JP, et al. A Cautionary Tale for Autologous Vascular Tissue Engineering: Impact of Human Demographics on the Ability of Adipose-Derived Mesenchymal Stem Cells to Recruit and Differentiate into Smooth Muscle Cells. Tissue Eng Pt A. 2014;21(3-4):426–37. doi: 10.1089/ten.tea.2014.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Yin S, Cen L, Liu QH, Liu W, Cao YL, et al. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta 1 and bone morphogenetic protein-4. Tissue Eng Pt A. 2010;16(4):1201–13. doi: 10.1089/ten.TEA.2009.0303. [DOI] [PubMed] [Google Scholar]
- 18.Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. Journal of Surgical Research. 2011;168(2):306–14. doi: 10.1016/j.jss.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4669–74. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashi CK, Zhu YQ, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):11915–20. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibino N, Villalona G, Pietris N, Duncan DR, Schoffner A, Roh JD, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. Faseb J. 2011;25(8):2731–9. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR, et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomaterialia. 2010;6(1):110–22. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W, Nieponice A, Hong Y, Wagner W, Vorp D. Rapid Engineered Small Diameter Vascular Grafts from Smooth Muscle Cells. Cardiovascular Engineering and Technology. 2011:1–11. [Google Scholar]
- 24.Soletti L, Nieponice A, Guan J, Stankus JJ, Wagner WR, Vorp DA. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27(28):4863–70. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Bai X, Yan Y, Song Y-H, Seidensticker M, Rabinovich B, Metzele R, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European Heart Journal. 2010;31(4):489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De-La-Quintana P, Trebol J, et al. Treatment of enterocutaneous fistula in Crohn's disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. International Journal of Colorectal Disease. 2009;24(1):27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
- 27.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and development. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 28.Riordan NH, Ichim TE, Min W-P, Wang H, Solano F, Lara F, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7(29):10.1186. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World journal of stem cells. 2011;3(4):25. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. Journal of Thoracic and Cardiovascular Surgery. 2005;129(6):1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 31.Benatti FB, Lira FS, Oyama LM. Strategies for reducing body fat mass: effects of liposuction and exercise on cardiovascular risk factors and adiposity. Diabetes, metabolic syndrome and obesity: targets and therapy. 2011;4:141. doi: 10.2147/DMSO.S12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prestwich GD, Bhatia S, Breuer CK, Dahl S, Mason C, McFarland R, et al. What is the greatest regulatory challenge in the translation of biomaterials to the clinic. Sci Transl Med. 2012;4:160cm14. doi: 10.1126/scitranslmed.3004915. [DOI] [PubMed] [Google Scholar]
- 33.Lee MH, Arcidiacono JA, Bilek AM, Wille JJ, Hamill CA, Wonnacott KM, et al. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Engineering Part B: Reviews. 2009;16(1):41–54. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 34.(FDA) UFaDA. Cellular & Gene Therapy Guidances. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guida nces/CellularandGeneTherapy/2015 [May 2015]; Available from: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/
- 35.(FDA) UFaDA Early Development Considerations for Innovative Combination Products. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126050.htm2015 [May 2015]; Available from: http://www.fda.gov/RegulatoryInformation/Guidances/ucm126050.htm.
- 36.Williams SK, Kosnik PE, Kleinert LB, Vossman EM, Lye KD, Shine MH. Adipose Stromal Vascular Fraction Cells Isolated Using an Automated Point of Care System Improve the Patency of Expanded Polytetrafluoroethylene Vascular Grafts. Tissue Eng Pt A. 2013;19(11-12):1295–302. doi: 10.1089/ten.TEA.2012.0318. [DOI] [PubMed] [Google Scholar]
- 37.Gimble JM, Wu X. Non-Enzymatic Method for Isolating Human Adipose-Derived Stromal Stem Cells. Google Patents. 2012 doi: 10.1016/j.jcyt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Blose KJ, Ennis TL, Arif B, Weinbaum JS, Curci JA, Vorp DA. Periadventitial adipose-derived stem cell treatment halts elastase-induced abdominal aortic aneurysm progression. Regen Med. 2014;9(6):733–41. doi: 10.2217/rme.14.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.