Abstract
During mammary gland involution, the epithelial mesenchymal transition (EMT) process plays an important role in tissue remodelling and in the termination of milk production. Transforming growth factor β (TGFβ) has been known as a central inducer to EMT and contributor to the mammary gland involution. However, the whole mechanism has accomplished the EMT process in mammary gland is still unclear. Here, we show that arachidonic acid, one of the major products in milk, is new player to control the EMT together with TGFβ during mammary gland involution. Firstly, we observed decrease in CDH1 (epithelial marker gene) expression and increases in VIM and TWIST1 (mesenchymal marker genes), TGFB1, and PLCG2 (arachidonic acid synthesis gene) at involution. In epithelial cells culture experiments, depletion of lactogenic hormones to mimic the involution induced TGFβ1 and PLCG2 expressions. Treatment with arachidonic acid in epithelial cells increased VIM and TWIST1 expressions without decrease of CDH1 expression, while TGFβ1 decreased CDH1 and increased VIM and TWIST1; more importantly, TGFβ1 induced the expression of PLCG2, but arachidonic acid did not induce the expression of TGFB1. Finally, arachidonic acid accelerated the TGFβ1 increasing VIM and TWIST1 expressions, meanwhile arachidonic acid synthase inhibitor partially blocked the TGFβ1 increasing VIM and TWIST1 expressions. In conclusion, TGFβ1 stimulates arachidonic acid synthesis and the arachidonic acid has a function to postulate the EMT process together with TGFβ1 during mammary gland involution.
Keywords: Arachidonic acid, Epithelial-mesenchymal transition, Involution, Mammary gland, TGFβ1
The mammary gland is a unique organ, providing nutrition and immune protection for a newborn child. Significant structural and functional alternations occur during mammary gland development [1]. During pregnancy, steroid hormones and prolactin stimulate epithelial cell proliferation and differentiation to generate ductal branching and alveolar development, which support milk production during the following lactation period [2]. Lactation is controlled by a sophisticated regulation mechanism taking into account the conflicting requirements to minimize energy cost and maximize the chances for offspring survival. The subtle balance between mother and offspring could influence the lactogenesis and, eventually, mammary gland involution [1]. During involution, the mammary gland undergoes a highly coordinated tissue remodelling process to return to a primitive state in non-pregnancy.
The epithelial mesenchymal transition (EMT) is an important tissue remodelling process that allows polarised epithelial cells to lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal cells [3, 4]. Epithelial cells express high levels of E-cadherin, whereas mesenchymal cells express high levels of N-cadherin, Snail, and Vimentin. Loss of E-cadherin is considered a fundamental event in EMT. There are two types of transcription regulators for E-cadherin expression: Snail1/2 (also known as Slug) and ZEB1/2 can directly bind to the E-cadherin promoter and repress its transcription, whereas factors such as Twist1 can repress E-cadherin indirectly [5, 6]. Therefore, decreased expression of E-cadherin and/or increased expression levels of Vimentin, Snail1/2, ZEB1/2, or Twist1 are molecular markers for EMT.
Transforming growth factor β (TGFβ) is a secreted protein, which regulates cell proliferation, cell differentiation, apoptosis, and other cellular functions [7,8,9,10]. There are three mammalian TGFβ isoforms, including TGFβ1, TGFβ2, and TGFβ3. TGFβs play an important local role in mammary gland development [11, 12]. TGFβ expression levels are relatively high during pregnancy but are greatly reduced during lactation because TGFβs inhibit the synthesis of milk protein. During involution, the expression of TGFβs is restored to that during pregnancy. TGFβ has been known as an inducer and enhancer of EMT and may regulate mammary gland involution though the EMT process [13, 14].
It is believed that inflammation could promote EMT during organ fibrosis and wound healing [15]. The inflammation modulator arachidonic acid and its metabolites have been found in mammary glands and other tissues [16, 17]. Our previous study showed that the arachidonic acid metabolizing enzyme COX-2 has contribution to the mammary gland involution, which indicates the possible function of arachidonic acid or its metabolites in mammary gland involution [18]. Generally, the synthesis of arachidonic acid in cells is depended on two enzymes mediating pathways including phospholipase A2 (PLA2) and phospholipase C (PLC) [19]. The role of arachidonic acid from milk in the mammary gland has been studied over the past decades. While arachidonic acid acts as a milk secretion promoter [20], its contribution to the EMT process during mammary gland involution has not been studied.
The aim of this study was to identify the relationship between TGFβ1 and arachidonic acid in the EMT process during mammary gland involution. We analysed the expression profiles of EMT marker genes, arachidonic acid synthesis genes, and TGFβ1 mRNA during mammary gland development. In addition, we investigated the effects of TGFβ1 and arachidonic acid on expression of EMT marker genes in mammary epithelial cells in vitro.
Materials and Methods
Animals
ICR mice were purchased from SLC (Japan SLC, Shizuoka, Japan) and maintained at 23 ± 2°C under a 14-h light schedule (lights on from 0500 to 1900 h). Food and tap water was provided ad libitum. The day of the vaginal plug was designated as day 0 of pregnancy (P0). The day of parturition was designated as day 0 of lactation (L0). The pups were rearranged randomly and the equal pups number presented with different mother. Day 10 of lactation, corresponding to the day on which the pups were separated from their mother, was designated as day 0 of forced weaning (W0). Mammary glands were collected from the mice at day 14 of pregnancy (P14); at day 7 of lactation (L7); at days 1 and 2 after forced weaning (involution, I1 and I2); and at days 1, 2, and 3 after putting the pups back with their mother (recovery, R1, R2 and R3) at day I1. Three animals in each stage were used for the experiment. The mammary glands were harvested from the same animals throughout the experimental period. Harvested glands were frozen immediately and stored at –80°C for use in immunofluorescence and real-time quantitative PCR (qRT-PCR) experiments. All experiments with mice were performed according to the guidelines of the Institutional Animal Care and Use Committee of Tokyo University of Agriculture and Technology.
Cell culture
Eph4 cells were originally isolated from the mammary tissue of a mid-pregnant Balb/c mouse and routinely maintained in growth media consisting of Dulbecco’s Modified Eagle Medium with high glucose (DMEM), 10% foetal bovine serum (FBS), penicillin (50 U/ml), streptomycin (50 µg/ml), amphotericin B (0.125 μg/ml), and insulin (5 µg/ml) [21]. To induce differentiation, confluent cells were incubated for three days with daily replacement of DMEM medium containing lactogenic hormones mix: dexamethasone (1 µM) (Sigma-Aldrich, MO, USA), insulin (5 µg/ml) (Sigma-Aldrich), and prolactin (9 µg/ml) (Sigma-Aldrich). To induce involution, the medium from the differentiated cells was changed to DMEM medium without dexamethasone and prolactin, and cells were incubated for further one day. To investigate the effect of TGFβ1 and arachidonic acid, confluent cells were cultured with TGFβ1 (5 ng/ml) or arachidonic acid (50 μM) or arachidonic acid synthase inhibitor (varespladib, 10 μM) for one day.
Immunofluorescence
Frozen sections (6 μm) of mammary gland tissue were fixed in 4% paraformaldehyde for 10 min, permeabilized, and blocked with 5% bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS for 30 min. Sections were incubated with anti E-cadherin (24E10, Cell Signaling Technology, MA, USA) or anti-vimentin (D21H3, Cell Signaling Technology) antibody 4°C for overnight and with anti-rabbit IgG Alexa Fluor 568 conjugate (Invitrogen, CA, USA) for 1 h at room temperature. These sections were then mounted with ProLong Gold Antifade reagent and DAPI (Invitrogen). Images were captured using a BX-51 immunofluorescence microscope (Olympus, Tokyo, Japan).
Quantitative real-time PCR analysis
Total RNA was extracted from mammary gland tissue or cultured Eph4 cells by using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Complementary DNA was synthesised using the PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). The oligonucleotide primers for qRT-PCR analysis were designed using the Primer3 program (Table 1). PCR reactions were conducted in a 10-μl volume with Ex Taq Hot Start Version containing SYBR-Green I (Takara Bio) and performed using the chromo4 Real-Time PCR System (Bio-Rad, CA, USA) under the following conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, and a dissociation protocol. The expression level of each target mRNA was normalized to the reference gene γ-tubulin using the 2-ΔΔCt method.
Table 1. Oligonucleotide primers used for quantitative real time PCR.
| Gene name | Forward | Reverse |
| CDH1 | CAAGGACAGCCTTCTTTTCG | TGGACTTCAGCGTCACTTTG |
| CSN2 | AGAGGGATGTGCTCCAGGCTA | TAAGGAGGGGCATCTGTTTG |
| TWIST1 | CCCCACTTTTTGAGGAAGAA | CAGTTTGATCCCAGCGTTTT |
| VIM | ATGCTTCTCTGGCACGTCTT | CAGTTTGATCCCAGCGTTTT |
| TGFΒ1 | TGCGCTTGCAGAGATTAAAA | GCTGAATCGAAAGCCCTGTA |
| PLA2G4A | GCCTCTCTTCACGTGTCTCC | ACCCATCAAGAAATGCAAGG |
| PLCG2 | GGAGCTGAAGACCATCTTGC | CCTAGGATGAACACGGAGGA |
| TUBG1 | GCTGACCAGTGCACGGT | AAACCTGGGGGGCTGGGT |
Statistical analysis
Statistical comparisons were made using Student’s t-test or one-way ANOVA followed by Tukey’s multiple range tests by using Prism 5 software (GraphPad, CA, USA). A P-value of < 0.05 was considered statistically significant.
Results
EMT gene expression during mammary gland development
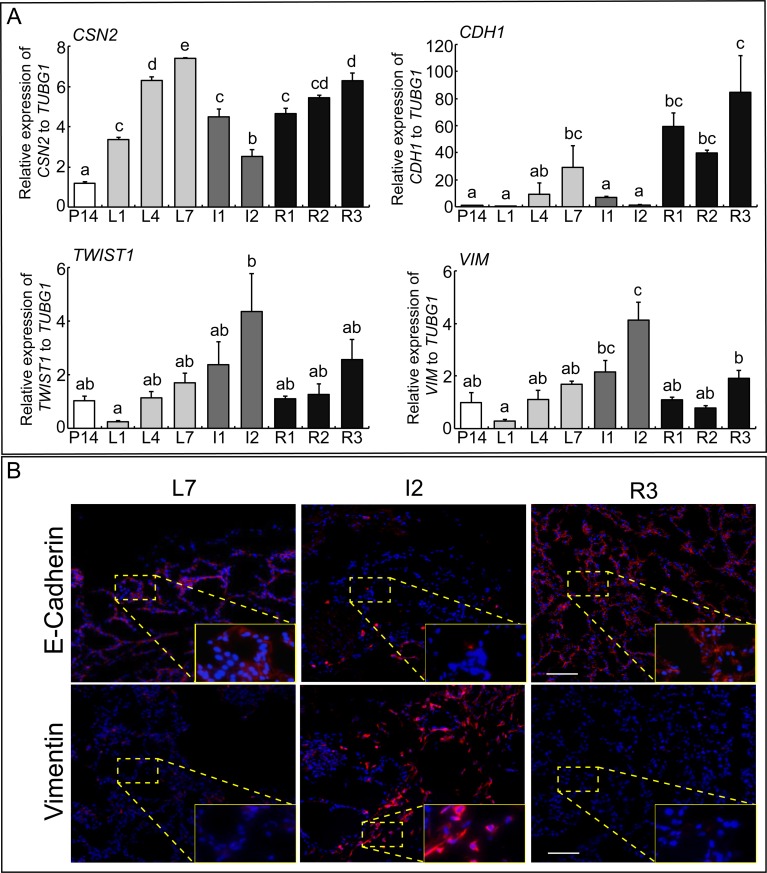
The expression levels of β-Casein (CSN2), E-cadherin (CDH1), Twist1 (TWIST1), and Vimentin (VIM) in the mammary gland during pregnancy, lactation, upon pup removal, and reintroduction of the pups are shown in Fig. 1A. Expression of the gene for the milk protein β-Casein was gradually increased during lactation and reached its peak expression at L7. During involution, when pups were removed from their mother, the CSN2 expression decreased, but the expression levels recovered when the pups were reintroduced. The expression pattern of the epithelial marker gene CDH1 was similar to that of CSN2, showing high expression levels during lactation (L4 and L7) and the recovery period (R1, R2, and R3), but low levels in the involution period (I1 and I2). In contrast, the mesenchymal marker genes TWIST1 and VIM, showed constant expression levels throughout the pregnancy and lactation period, a significantly increased expression at day 2 of involution (I2), and sharply decreased expression during the recovery period (Fig. 1A, lower panel). To confirm the manifestation of EMT in the mammary gland, immunofluorescence experiments were conducted for E-cadherin and Vimentin. Similar to the mRNA expression results, high E-cadherin and low Vimentin protein levels were observed in the mammary gland during the lactation and recovery period, while low E-cadherin and high Vimentin levels were detected during involution (Fig. 1B). These results indicate that the EMT is a critical event in mammary gland involution.
Fig. 1.
EMT genes expression during mammary gland development. (A) Real-time PCR of CSN2, CDH1, TWIST1 and VIM mRNAs in mouse mammary gland at day 14 of pregnancy (P14), day 1, 7 and 10 of lactation (L1, L7 and L10), day 1 and 2 of involution (I1 and I2), and day 1, 2 and 3 of recovery period (R1, R2 and R3). Bars represent means ± SEM for four independent experiments. Different letters indicate significant differences (P < 0.05). (B) Immunostained for E-cadherin and Vimentin in the mouse mammary gland at L7, I2 and R3. E-cadherin and Vimentin are shown in red; DAPI is shown in blue. Scale bar, 50 μm.
TGFβ1 and arachidonic acid synthase gene expression during involution period
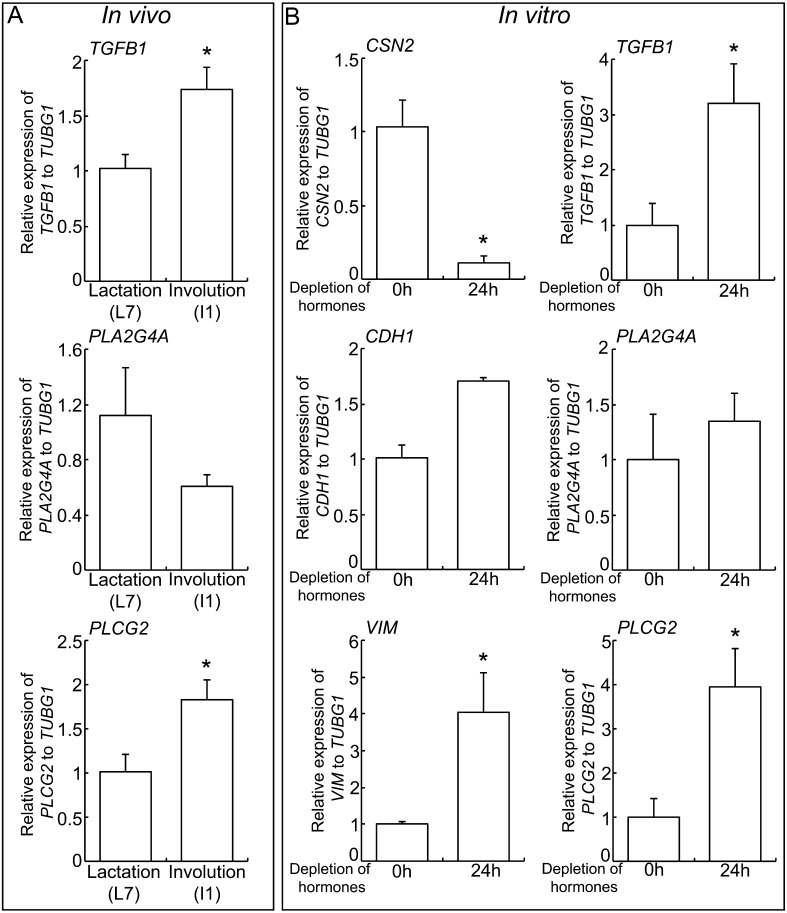
To seek the reason for the changes in expression of EMT genes during involution, TGFβ1 and arachidonic acid synthase genes expressions were evaluated by in vivo and in vitro experiments. The expressions of TGFB1 and the arachidonic acid synthase PLCG2, but not PLA2G4A, were increased in the mammary gland tissues during involution period compared to lactation period (Fig. 2A). To investigate a state change from lactation to involution in vitro, Eph4, mammary gland epithelial cells, were pre-treated with lactogenic hormones mix, insulin, prolactin and dexamethasone, for 3 days to induce cell differentiation observed at lactation, and then further incubated in lactogenic hormones-depleted medium (kept insulin for cell survival) for 1 day to mimic involution [18]. Depletion of prolactin and dexamethasone decreased CSN2 gene expression and increased VIM gene expression even not affected CDH1 gene expression (Fig. 2B). In this involution model, a significant increase of TGFB1 and PLCG2 gene expressions were observed, consistent with the in vivo results.
Fig. 2.
TGFβ1 and arachidonic acid synthesis genes expression in mammary gland involution. (A) Real-time PCR of TGFB1, PLA2G4A and PLCG2 in mouse mammary gland at L7 and I1. Bars represent means ± SEM for four independent experiments. Asterisk indicates significant differences (P < 0.05). (B) Real-time PCR of CSN2, CDH1, VIM, TGFB1, PLA2G4A and PLCG2 in Eph4 cells incubated in lactogenic hormones-depleted medium for 1 day. Bars represent means ± SEM for four independent experiments. Asterisk indicates significant differences (P < 0.05).
Effect of TGFβ1 on mammary gland epithelial cells
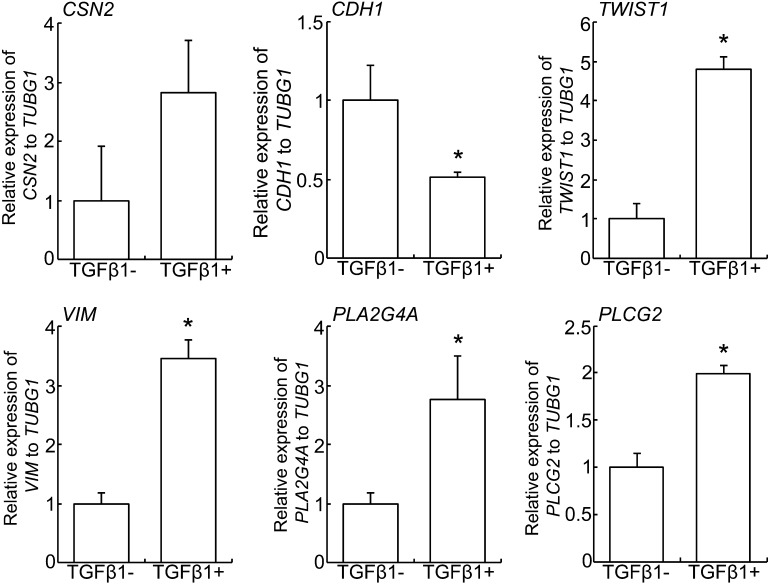
Eph4 cells were treated with recombinant human TGFβ1 for 1 day prior to gene expressions analysis (Fig. 3). The expression of both TWIST1 and VIM was significantly increased in TGFβ1-treated Eph4 cells, meanwhile the expression of CDH1 was decreased. In addition, the expressions of PLA2G4Aand PLCG2, encode the consisting proteins of PLA2 and PLC respectively, were significantly increased in TGFβ1-treated Eph4 cells. There was no significant difference in the expression of CSN2.
Fig. 3.
Effect of TGFβ1 on EMT and arachidonic acid synthesis genes expression. Real-time PCR of CSN2, CDH1, TWIST1, VIM, PLA2G4A and PLCG2 in Eph4 cells treated with or without 5 ng/ml human recombinant TGFβ1 for 1 day. Bars represent means ± SEM for four independent experiments. Asterisk indicates significant difference (P < 0.05).
Effect of arachidonic acid on mammary gland epithelial cells
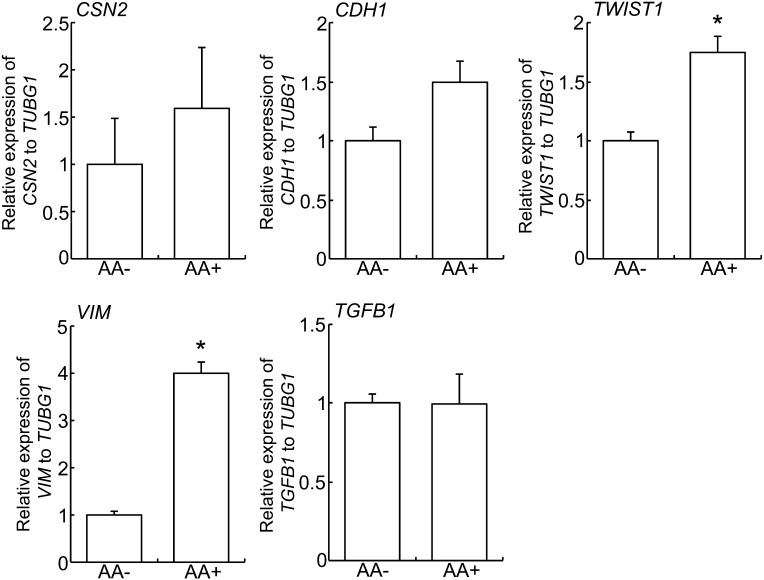
The results of the gene expression analysis in arachidonic acid-treated Eph4 cells are shown in Fig. 4. Treatment of arachidonic acid induced high expression levels of TWIST1 and VIM, but did not affect CDH1 gene expression. There were no significant differences in the expression of CSN2 and TGFB1.
Fig. 4.
Effect of arachidonic acid on EMT and TGFβ1 genes expression. Real-time PCR of CSN2, CDH1, TWIST1, VIM and TGFB1 in Eph4 cells treated with or without 50 μM arachidonic acid for 1 day. Bars represent means ± SEM for four independent experiments. Asterisk indicates significant difference (P < 0.05). AA, arachidonic acid.
Interaction between TGFβ1 and arachidonic acid on mammary gland epithelial cells
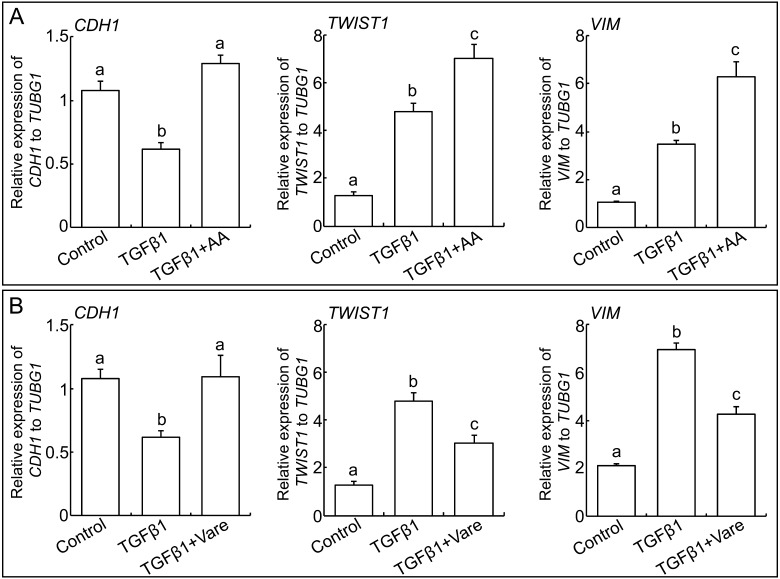
Eph4 cells were treated with TGFβ1 alone, TGFβ1 with arachidonic acid or TGFβ1 with arachidonic acid synthase inhibitor, and the expressions of TWIST1, VIM and CDH1 were analysed by real-time PCR (Fig. 5). Same as in Fig. 3, TGFβ1 stimulated EMT by decreasing CDH1 and increasing TWIST1 and VIM genes expression. Co-treatment with arachidonic acid and TGFβ1 showed higher increase of TWIST1 and VIM genes expression than TGFβ1 alone, but inconsist increase of the expression of CDH1 was observed (Fig. 5A). On the other hand, co-treatment with arachidonic acid synthase inhibitor and TGFβ1 increased CDH1 and decreased TWIST1 and VIM compared to treatment of TGFβ1 alone, indicating that arachidonic acid have a role for induction of EMT by TGFβ1 (Fig. 5B).
Fig. 5.
Interaction between TGFβ1 and arachidonic acid on EMT genes expression. Real-time PCR of CDH1, TWIST1 and VIM in Eph4 cells treated with TGFβ1 alone, TGFβ1 with 50 μM arachidonic acid (A) or TGFβ1 with 10 μM Varespladib (B) for 1 day. Bars represent means ± SEM for four independent experiments. The different letters indicate significant difference (P < 0.05). AA, arachidonic acid. Vare, Varespladib.
Discussion
It has been proposed that mammary gland involution, a rapid and extensive period of tissue remodelling, is accompanied by EMT. In this study, we demonstrated that TGFβ1 and arachidonic acid synthase expression is increased during the involution period in mammary glands and that this increase is observed in cell culture experiments by depletion of lactogenic hormones, prolactin and dexamethasone. Furthermore, TGFβ1 and arachidonic acid could enhance mesenchymal marker gene expression; most importantly, TGFβ1 also increased arachidonic acid synthase genes expression. These results indicate that TGFβ1 and arachidonic acid are both involved in the EMT process during mammary gland involution and that there is possible interaction between TGFβ1 and arachidonic acid signalling (Fig. 6).
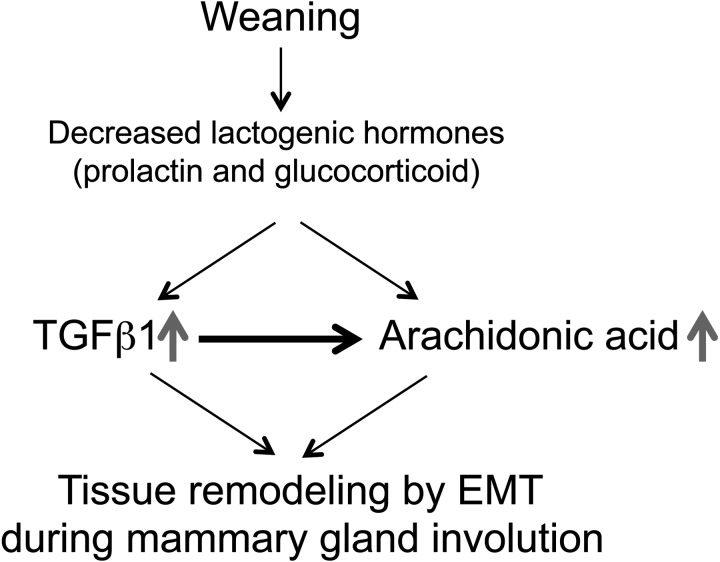
Fig. 6.
A schematic representation of the crosstalk between TGFβ1 and arachidonic acid during mammary gland involution. At weaning, a reduction of sucking stimuli decreases lactogenic hormones, prolactin and glucocorticoid, concentration in mother and increase TGFβ1 and arachidonic acid genes expression. TGFβ1 increase of arachidonic acid production in the mammary gland. The interaction between TGFβ1 and arachidonic acid is an important for successful tissue remodelling during mammary gland involution.
The relationship between a mother and her pups could affect milk production levels in the mammary gland [1]. During lactation, pups demand milk and suck at the mother’s nipple, resulting in neural signals that stimulate oxytocin and prolactin secretion from the pituitary gland that increase milk production [22, 23]. Glucocorticoid hormone from adrenal gland postulates the milk production by stimulating prolactin receptor expression in mammary gland epithelial cells [24]. After weaning the fall in these lactogenic hormones and/or milk stasis lead to involution. In this study, EMT genes and CSN2 expressions were affected by forced weaning, involving the removal of suckling pups from the mother during lactation. In addition, the in vitro results showed that depletion of prolactin and dexamethasone in cultured mammary epithelial cells influenced EMT genes expression. These observations suggest that prolactin (and dexamethasone) plays an essential role in regulating EMT genes expression during mammary gland involution, which is consistent with previous reports claiming that prolactin may act as EMT suppressor via decrease TGFβ1 and EMT gene markers expression in breast cancer cells [25, 26]. Following the reduction or depletion of hormones stimulation, TGFβ1 plays a key role in the regulation of the EMT process during mammary gland involution, because increased TGFβ1 gene expression was observed after forced weaning in vivo and depletion of prolactin and dexamethasone in vitro. TGFβ1 has been shown to biochemically induce EMT for several types of epithelial cells, including normal mammary gland epithelial NMuMG cells, MCF7 human mammary gland tumour cells, and mouse mammary carcinoma cells [27,28,29,30]. The mechanism underling the TGFβ signalling pathway involved in the mammary gland involution has been widely investigated [31], however, the interactions between TGFβ and milk composition were remained to be investigated.
The milk compositions include water, carbohydrates, lipids, proteins and minerals, and the bio-active lipid in the milk may exerts molecular functions in mammary gland [32, 33]. The role of arachidonic acid in the mammary gland has been previously investigated with regard to milk secretion [20]. In addition, it has been reported that arachidonic acid can promote the EMT process in normal and cancer mammary epithelial cells [34, 35]. In the present study, arachidonic acid treatment induced VIM and TWIST1 gene expression in Eph4 cells. These observations indicate that increased levels of arachidonic acid and TGFβ are important for the EMT process during mammary gland involution. Interestingly, TGFβ1 could induce the expression of arachidonic acid synthase PLA2G4A and PLCG2, although arachidonic acid could not induce the expression of TGFβ1 in Eph4 cells. The similar phenomenon was also observed in other cell lines [36, 37]. TGFβ1 activates the PLA2 in cultured rat costochondral chondrocytes [36]. TGFβ1 also activates phospholipase D activity and increases the production of arachidonic acid precursor, diacyl-glycerol (DAG), in lung and kidney epithelial cells [37]. Those results indicate that TGFβ1 signalling pathway may stimulate the arachidonic acid synthesis during the involution period. Furthermore, the co-treatment of TGFβ1 and arachidonic acid could induce the significant higher expressions of mesenchymal markers including VIM and TWIST1 than TGFβ1 alone treatment. Most importantly, the co-treatment with arachidonic acid synthase inhibitor and TGFβ1 suppressed the increase of VIM and TWIST1 expressions by TGFβ1 alone. Taken together, the stimulation of arachidonic acid synthesis by TGFβ1 is important for the accomplishment of EMT process during mammary gland involution period.
In conclusion, the results of this study demonstrate that decreased lactogenic hormones, particularly prolactin and glucocorticoid, initiate EMT process by stimulation of TGFβ1 and/or arachidonic acid synthesis, in addition TGFβ1 itself also stimulates arachidonic acid synthesis. The arachidonic acid and TGFβ1 might accelerate the EMT process by regulating CDH1, VIM and TWIST1 genes expression (Fig. 6). Further studies are required to clarify exact molecular mechanisms underlying the interaction between TGFβ1 and arachidonic acid in the EMT process during mammary gland involution.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (to KN, 24780268).
References
- 1.Knight CH, Peaker M, Wilde CJ. Local control of mammary development and function. Rev Reprod 1998; 3: 104–112. [DOI] [PubMed] [Google Scholar]
- 2.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip. Rev Dev Biol 2012; 1: 533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112: 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–428. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829. [DOI] [PubMed] [Google Scholar]
- 7.Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 2008; 1782: 197–228. [DOI] [PubMed] [Google Scholar]
- 8.Huang SS, Huang JS. TGF-β control of cell proliferation. J Cell Biochem 2005; 96: 447–462. [DOI] [PubMed] [Google Scholar]
- 9.Moses HL, Serra R. Regulation of differentiation by TGF-β. Curr Opin Genet Dev 1996; 6: 581–586. [DOI] [PubMed] [Google Scholar]
- 10.Schuster N, Krieglstein K. Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res 2002; 307: 1–14. [DOI] [PubMed] [Google Scholar]
- 11.Flanders KC, Wakefield LM. Transforming growth factor-(beta)s and mammary gland involution; functional roles and implications for cancer progression. J Mammary Gland Biol Neoplasia 2009; 14: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakefield LM, Piek E, Böttinger EP. TGF-β signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 2001; 6: 67–82. [DOI] [PubMed] [Google Scholar]
- 13.Serra R, Crowley MR. TGF-β in mammary gland development and breast cancer. Breast Dis 2003; 18: 61–73. [DOI] [PubMed] [Google Scholar]
- 14.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA 2001; 98: 6686–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009; 1: 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollock CB, Yin Y, Yuan H, Zeng X, King S, Li X, Kopelovich L, Albanese C, Glazer RI. PPARδ activation acts cooperatively with 3-phosphoinositide-dependent protein kinase-1 to enhance mammary tumorigenesis. PLoS ONE 2011; 6: e16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelsson B. Arachidonic acid metabolism: role in inflammation. Z Rheumatol 1991; 50(Suppl 1): 3–6. [PubMed] [Google Scholar]
- 18.Tanaka T, Haneda S, Imakawa K, Sakai S, Nagaoka K. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 2009; 77: 181–187. [DOI] [PubMed] [Google Scholar]
- 19.Belton O, Fitzgerald DJ. Cyclooxygenase isoforms and atherosclerosis. Expert Rev Mol Med 2003; 5: 1–18. [DOI] [PubMed] [Google Scholar]
- 20.Ollivier-Bousquet M. Effect of arachidonic acid on the secretion of milk caseins in vitro. C R Seances Acad Sci III 1982; 294: 669–672 (in French). [PubMed] [Google Scholar]
- 21.Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol 1989; 108: 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruckmaier RM, Blum JW. Simultaneous recording of oxytocin release, milk ejection and milk flow during milking of dairy cows with and without prestimulation. J Dairy Res 1996; 63: 201–208. [DOI] [PubMed] [Google Scholar]
- 23.Leng G, Caquineau C, Sabatier N. Regulation of oxytocin secretion. Vitam Horm 2005; 71: 27–58. [DOI] [PubMed] [Google Scholar]
- 24.Mizoguchi Y, Yamaguchi H, Aoki F, Enami J, Sakai S. Corticosterone is required for the prolactin receptor gene expression in the late pregnant mouse mammary gland. Mol Cell Endocrinol 1997; 132: 177–183. [DOI] [PubMed] [Google Scholar]
- 25.Sultan AS, Brim H, Sherif ZA. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through reversal of epithelial-mesenchymal transition. Cancer Sci 2008; 99: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philips N, McFadden K. Inhibition of transforming growth factor-beta and matrix metalloproteinases by estrogen and prolactin in breast cancer cells. Cancer Lett 2004; 206: 63–68. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Yan Q, Wang J, Liu S, Yang X. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by AP1-dependent EpCAM expression in MCF-7 cells. J Cell Physiol 2015; 230: 775–782. [DOI] [PubMed] [Google Scholar]
- 28.McEarchern JA, Kobie JJ, Mack V, Wu RS, Meade-Tollin L, Arteaga CL, Dumont N, Besselsen D, Seftor E, Hendrix MJ, Katsanis E, Akporiaye ET. Invasion and metastasis of a mammary tumor involves TGF-β signaling. Int J Cancer 2001; 91: 76–82. [DOI] [PubMed] [Google Scholar]
- 29.Romagnoli M, Belguise K, Yu Z, Wang X, Landesman-Bollag E, Seldin DC, Chalbos D, Barillé-Nion S, Jézéquel P, Seldin ML, Sonenshein GE. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res 2012; 72: 6268–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie L, Law BK, Aakre ME, Edgerton M, Shyr Y, Bhowmick NA, Moses HL. Transforming growth factor beta-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res 2003; 5: R187–R198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol 2011; 3: a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira PC. Milk nutritional composition and its role in human health. Nutrition 2014; 30: 619–627. [DOI] [PubMed] [Google Scholar]
- 33.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Orozco R, Navarro-Tito N, Soto-Guzman A, Castro-Sanchez L, Perez Salazar E. Arachidonic acid promotes epithelial-to-mesenchymal-like transition in mammary epithelial cells MCF10A. Eur J Cell Biol 2010; 89: 476–488. [DOI] [PubMed] [Google Scholar]
- 35.Navarro-Tito N, Robledo T, Salazar EP. Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp Cell Res 2008; 314: 3340–3355. [DOI] [PubMed] [Google Scholar]
- 36.Sylvia VL, Schwartz Z, Dean DD, Boyan BD. Transforming growth factor-beta1 regulation of resting zone chondrocytes is mediated by two separate but interacting pathways. Biochim Biophys Acta 2000; 1496: 311–324. [DOI] [PubMed] [Google Scholar]
- 37.Zhou BH, Chen JS, Chai MQ, Zhao S, Liang J, Chen HH, Song JG. Activation of phospholipase D activity in transforming growth factor-beta-induced cell growth inhibition. Cell Res 2000; 10: 139–149. [DOI] [PubMed] [Google Scholar]