Abstract
Francisella tularensis is an intracellular pathogen that causes the fatal zoonotic disease tularaemia. Critical for its pathogenesis is the ability of the phagocytosed bacteria to escape into the cell cytosol. For this, the bacteria use a non-canonical type VI secretion system (T6SS) encoded on the Francisella pathogenicity island (FPI). Here we show that in F. novicida T6SS assembly initiates at the bacterial poles both in vitro and within infected macrophages. T6SS dynamics and function depends on the general purpose ClpB unfoldase, which specifically colocalizes with contracted sheaths and is required for their disassembly. T6SS assembly depends on iglF, iglG, iglI and iglJ, whereas pdpC, pdpD, pdpE and anmK are dispensable. Importantly, strains lacking pdpC and pdpD are unable to escape from phagosome, activate AIM2 inflammasome or cause disease in mice. This suggests that PdpC and PdpD are T6SS effectors involved in phagosome rupture.
The pathogenicity of Francisella species largely depends on their escape from phagosomes in macrophages, mediated by a type VI secretion system (T6SS). Here, the authors show dynamics of T6SS assembly and disassembly and identify the genes essential for phagosome escape and pathogenicity in mice.
Francisella tularensis is a Gram-negative bacterium that causes the zoonotic disease tularaemia in human and animal host. The severity of tularaemia varies depending on the route of infection and the type of strain. The Francisella tularensis subsp. tularensis is the most virulent strain and aerosol transmission of a few bacteria can cause lethal pneumonia in humans1. Given the low infectious dose and the severity of the infection, subsp. tularensis has been classified as Tier 1 select agent. The related strain Francisella tularensis subsp. novicida (F. novicida) has in contrast low virulence in humans, but is highly virulent in mice and thus often used as a laboratory model for tularaemia2. The pathogenicity of both Francisella species is linked to their ability to replicate in the cytosol of phagocytes, such as macrophages or dendritic cells. After phagocytosis, the bacteria shortly reside within a membrane-bound phagosome, but subsequently disrupt the phagosomal membrane and escape into the host cell cytosol, where they replicate3.
While phagosomal escape is essential for Francisella intracellular replication and virulence in vivo, it also allows the host to mount anti-microbial and innate immune defenses. Among these are the production of type I interferons (type I IFNs) via the cGAS-STING-IRF3 axis, the production of antimicrobial guanylate-binding proteins (GBPs) and the activation of the AIM2 (absent in melanoma 2) inflammasome, which controls the release of mature IL-1β and IL-18 as well as the induction of host cell death through pyroptosis4,5,6,7,8,9,10,11. Interferon production and inflammasome activation require the recognition of bacterial DNA in the cytosol, and have been linked to the lysis of cytosolic Francisella. Mice deficient in these responses fail to control bacterial replication, resulting in a fatal disease4,5,6,8,9. Francisella virulence and the escape from the phagosomal compartment requires a gene cluster referred to as the Francisella Pathogenicity Island (FPI)12. Two nearly identical copies of the FPI are found in subspecies tularensis, holarctica and mediasiatica. The F. novicida genome contains only a single FPI copy13, but features a related island called ‘Francisella novicida Island (FNI)’14,15. The FPI has been suggested to encode a non-canonical type VI secretion system (T6SS)16,17, which based on gene content and phylogeny is proposed to represent a unique T6SS subtype (T6SSii)18.
T6SS is a nanomachine capable of delivery of effector proteins across target cell membranes of both bacterial and eukaryotic cells and thus is often required for bacterial competition and pathogenesis19,20,21,22,23. One of the hallmarks of this system is its highly dynamic assembly that can be visualized by live-cell fluorescence microscopy24,25. Assembly of T6SS starts by formation of a membrane complex formed of TssJ, TssL and TssM26. This is followed by assembly of a baseplate complex from TssE, TssF, TssG, TssK and also VgrG, PAAR spike as well as TssA in some organisms27,28,29,30,31. Baseplate complex then initiates assembly of a long Hcp tube and TssB, TssC (or VipA, VipB) sheath wrapped around the tube32. Both spike and Hcp tube can associate with effectors and are delivered together into target cells upon rapid sheath contraction33,34,35,36,37,38,39.
Even though the F. novicida sheath is structurally similar to the sheath of canonical T6SS of V. cholerae40,41, it is unclear to what extent the canonical T6SS assembly mechanisms apply to Francisella. The reason is that Francisella T6SS is highly divergent and clear homologues of several core components are missing, such as TssE, TssF and TssG. In addition, many components such as TssK, VgrG, Hcp and PAAR have only low primary sequence homology to the canonical T6SS components. For example, IglG was recently shown to be structurally similar to PAAR proteins, which are required for T6SS function15,29. On the other hand, the FPI cluster contains many genes of unknown function, such as iglF, iglI, iglJ, pdpA, pdpC, pdpE, pdpD and anmK. PdpA, PdpC and PdpD were identified by mass-spectrometry as secreted by Francisella T6SS and PdpC/PdpD were proposed to be effectors required for phagosomal escape, intracellular growth and virulence42,43,44,45,46,47,48. Interestingly, the FPI cluster lacks a homologue of an unfoldase ClpV, which is present in all canonical T6SS clusters and recycles contracted sheaths14,24,49,50. Overall, the non-canonical gene composition suggests a unique mode of action of the Francisella T6SS.
Here we show that F. novicida T6SS sheath cycles between assembly, contraction and disassembly. Interestingly, the vast majority of T6SS sheath assemblies initiate close or at the cell pole. We show that ClpB colocalizes with contracted sheaths and is required for sheath disassembly, however, is dispensable for sheath assembly and contraction. T6SS dynamics and function depends on iglF, iglG, iglI and iglJ, while pdpC and pdpD are specifically required for phagosomal escape and virulence in a mouse model of tularaemia, but also for the engagement of the host innate immune response.
Results
Francisella T6SS is dynamic and assembles on the cell pole
Francisella T6SS has a non-canonical gene composition and lacks ClpV suggesting unique mode of action (Fig. 1). To understand Francisella T6SS assembly and function, we searched for conditions that would allow us to image subcellular localization of TssB homologue IglA. We have serendipitously discovered that F. novicida iglA-sfGFP grown to an exponential phase in BHI media induced expression of IglA-sfGFP upon prolonged incubation on an agarose pad under a glass coverslip. Importantly, the increase in expression correlated with an increase in number of IglA-sfGFP structures detected in the bacteria (Fig. 2a,b). Time-lapse imaging at a rate of 20 frames per minute showed that IglA-sfGFP structures extended across the bacteria within 30 and 120 s with assembly speeds between 5 and 15 nm s−1. After full assembly, the IglA-sfGFP structures immediately contracted to approximately half of their original length and became brighter (Fig. 2c; Supplementary Fig. 1a; Supplementary Movies 1 and 4). After contraction, the sheath structures were disassembled during the next ∼2–3 min (Fig. 2c; Supplementary Fig. 1a). The average fluorescence intensity of the bacteria before and after one cycle of assembly, contraction and disassembly was similar, suggesting that IglA-sfGFP remained stable and folded during this cycle (Supplementary Fig. 1b). Importantly, no IglA-sfGFP structures were detected in the bacteria lacking the TssM homologue encoded by pdpB (Fig. 1), suggesting that assembly of IglA-sfGFP structures is dependent on the function of the whole T6SS (Fig. 2d; Supplementary Movie 2). The dynamics of IglA-sfGFP localization is similar to that of VipA-sfGFP in V. cholerae and is consistent with the fact that IglA and IglB form a structure closely resembling V. cholerae T6SS sheath25,40,41.
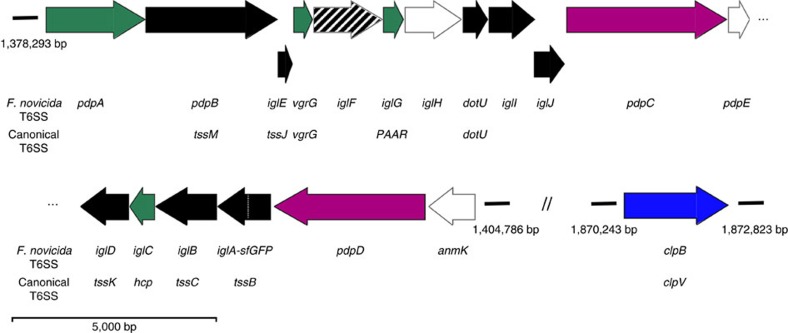
Figure 1. A schematic overview of Francisella T6SS genes.
Assignments for gene functions are based on previous studies cited in the main text and our observations: Black—structural components; Green—secreted structural components; Purple—secreted effectors; Blue—unfoldase; White—no clear evidence for function; Shaded—required for efficient assembly. The Francisella FPI (pdpA–anmK) nomenclature and the canonical T6SS nomenclature for the F. novicida genes is shown. Genes are drawn in scale.
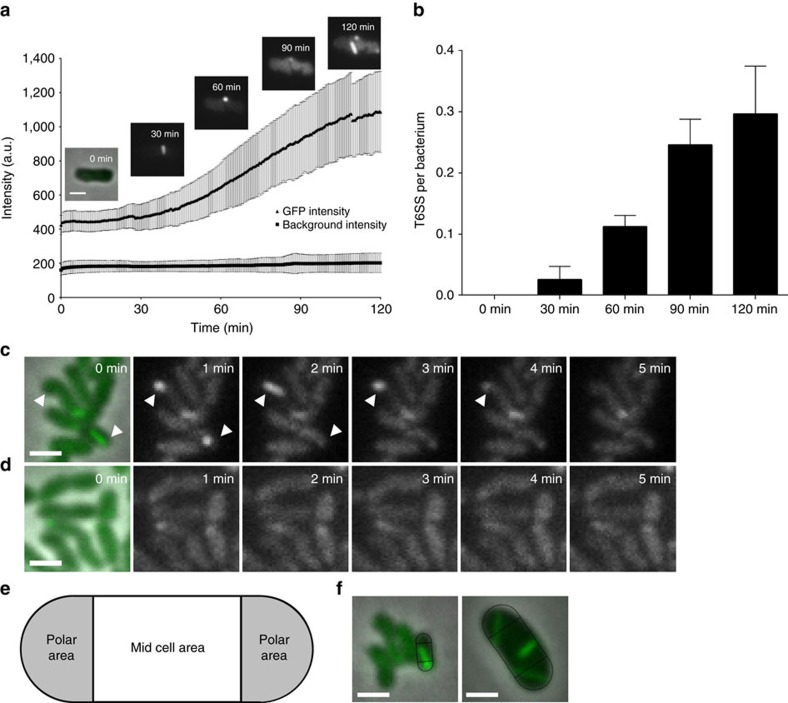
Figure 2. Increase of GFP intensity correlates with increased number of dynamic T6SS per bacterium.
(a) GFP signal intensities of F. novicida U112 iglA-sfGFP and fluorescence background were measured every minute for three regions of interest containing 1–30 bacteria. Two independent experiments were carried out. GFP intensity increase in a single F. novicida U112 iglA-sfGFP bacterium is shown at different time points. First image is a merge of phase contrast and GFP channels, following images represent GFP channel only. (b) Number of bacteria and T6SS structures were counted at time points between 0 and 120 min in three regions of interest containing 36–191 bacteria. Two independent experiments were carried out. Error bars represent s.d. (c,d) IglA-sfGFP localization in F. novicida U112 iglA-sfGFP wild type (c) and ΔpdpB (d). Arrowheads indicate T6SS sheath assembly and contraction. First image is a merge of phase contrast and GFP channels, following images represent GFP channel only. (e) Model for quantification of T6SS assembly position. Pole area was determined as 50% of total surface area equally distributed to both poles. (f) Model from e applied to F. novicida U112 iglA-sfGFP and V. cholerae 2740-80 vipA-msfGFP. Merge of phase contrast and GFP channels is shown. For a,c,d and f 3.3 × 3.3 μm fields of view are shown. Scale bar, 1 μm.
Interestingly, we also noticed that IglA-sfGFP sheaths were preferentially assembled from the bacterial pole and thus often formed structures as long as the bacterial length. To quantify the preference for subcellular localization, we divided the bacterial perimeter equally to a polar region and a mid-cell region (Fig. 2e) and counted assemblies initiated in these two equally large regions. Out of 851 assemblies, 821 assemblies (96.5%) were initiated in the polar region. As a control, we performed the same analysis for V. cholerae and show that only 53.8% (425 from 790) assemblies were initiated in the polar region (Fig. 2f) as expected for assemblies without preferred localization24,25,51. Taken together, we show that F. novicida assembles a dynamic T6SS sheath on the cell poles and that the sheath cycles through assembly, contraction and disassembly similarly to what was previously described for other canonical T6SSs.
ClpB is required for disassembly of contracted sheaths
The fact that contracted sheaths were quickly disassembled without apparent degradation of IglA-sfGFP suggested that F. novicida recycles contracted sheaths using a mechanism similar to the canonical ClpV-mediated sheath disassembly. The closest homologue of V. cholerae ClpV in F. novicida genome is ClpB (FTN_1743) (36% sequence identity). Interestingly, clpB was previously shown to be required for survival of various stresses52 but also essential for intracellular replication and virulence of F. novicida53,54.
Here we show that F. novicida lacking clpB mainly contained bright IglA-sfGFP foci (Fig. 3a). Time-lapse imaging showed that the F. novicida ΔclpB occasionally assembled new sheaths with kinetics similar to that of the parental strain but after contraction, the sheaths were never disassembled and remained intact in the bacteria (Fig. 3a,b; Supplementary Movies 1 and 4). Such assembly was still dependent on functional T6SS, as no sheath extensions and contractions were detected in F. novicida ΔclpB/pdpB. However, some bright, non-dynamic IglA-sfGFP foci were detected in the absence of both clpB and pdpB (Supplementary Fig. 2a; Supplementary Movie 2). This indicates that activity of ClpB is required for recycling of contracted sheaths, however, in case of a defect in ClpB function, some non-dynamic IglA-sfGFP foci may form also in the absence of a fully functional T6SS.
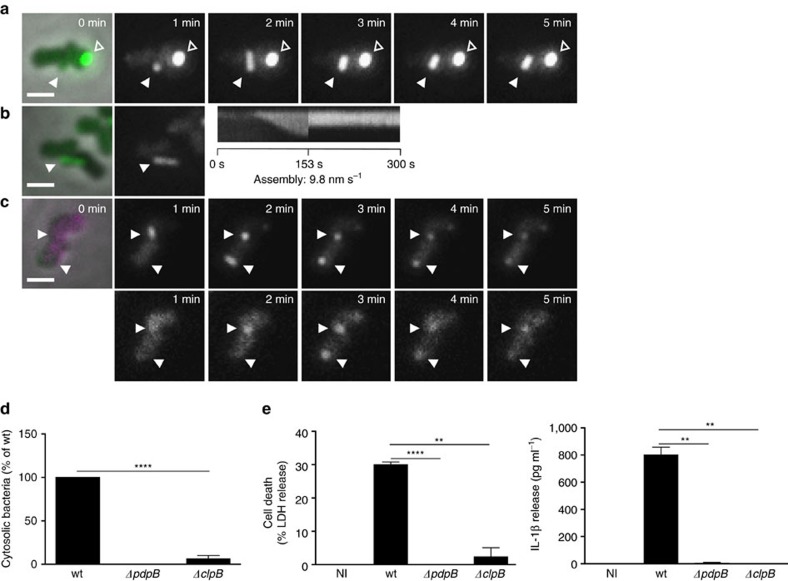
Figure 3. Phagosomal rupture and AIM2 inflammasome activation is dependent on disassembly of T6SS sheaths by ClpB.
(a) T6SS dynamics in F. novicida U112 iglA-sfGFP ΔclpB. Arrowheads indicate T6SS sheath assembly, contraction and location of sheath after contraction. Empty arrowheads indicate non-dynamic IglA-sfGFP foci. First image is a merge of phase contrast and GFP channels, following images represent GFP channel only. (b) Kymogram of F. novicida U112 iglA-sfGFP ΔclpB over 5 min (3 s per pixel). First image is a merge of phase contrast and GFP channels, following images represent GFP channel only. (c) Colocalization of ClpB-mCherry2 with IglA-sfGFP (arrows) in F. novicida U112 iglA-sfGFP clpB-mCherry2. First image is a merge of phase contrast, GFP and mCherry channels, following images represent GFP channel (upper panel) and mCherry channel (lower panel). (d) Quantification of cytosolic bacteria in unprimed wild-type BMDMs 4 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB or ΔclpB (normalized to wild type). (e) Release of LDH and mature IL-1β from primed wild-type BMDMs 10 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB or ΔclpB (NI—noninfected control). (a–c) 3.3 × 3.3 μm fields of view are shown. Scale bars, 1 μm. (d,e) Data are pooled from three independent experiments (d) (mean and s.d. are shown) or representatives of three independent experiments (e) (mean and s.d. of triplicate wells are shown). **P<0.01 and ****P<0.0001 (two-tailed unpaired t-test with Welch’s correction).
To test directly the role of ClpB in disassembly of the contracted sheaths, we introduced clpB-mCherry2 fusion to the native locus on the chromosome of the iglA-sfGFP or wild-type strain. Fusing mCherry2 to ClpB had no influence on the ability of F. novicida to survive heat shock indicating that such fusion is fully functional (Supplementary Fig. 2d). ClpB-mCherry2 subcellular localization cycled between uniform cytosolic and punctate localization and this dynamics was dependent on the presence of pdpB (Supplementary Fig. 2b,c, Supplementary Movie 3). When IglA-sfGFP and ClpB-mCherry2 were imaged simultaneously, ClpB spots colocalized specifically with the contracted sheaths (Fig. 3c; Supplementary Movies 3 and 5).
F. novicida uses the T6SS to escape from phagosome of cells like macrophages and consistently IglA-sfGFP spots could be detected in intracellular bacteria, implying the assembly of T6SS sheaths40. To test whether sheath assembly is dynamic under physiological conditions during infection, we infected primary murine bone marrow-derived macrophages (BMDMs) from wild-type C57BL/6 mice for 1 h with exponentially grown F. novicida. After washing away non-phagocytosed bacteria, the infected cells were fixed, stained with phalloidin and anti-F. novicida LPS antibody and analysed by super resolution structured illumination microscopy (SIM) to determine the relative localization of actin, bacteria and T6SS sheaths (Fig. 4a,b). This analysis confirmed that F. novicida reside inside the macrophage and assemble T6SS sheaths.
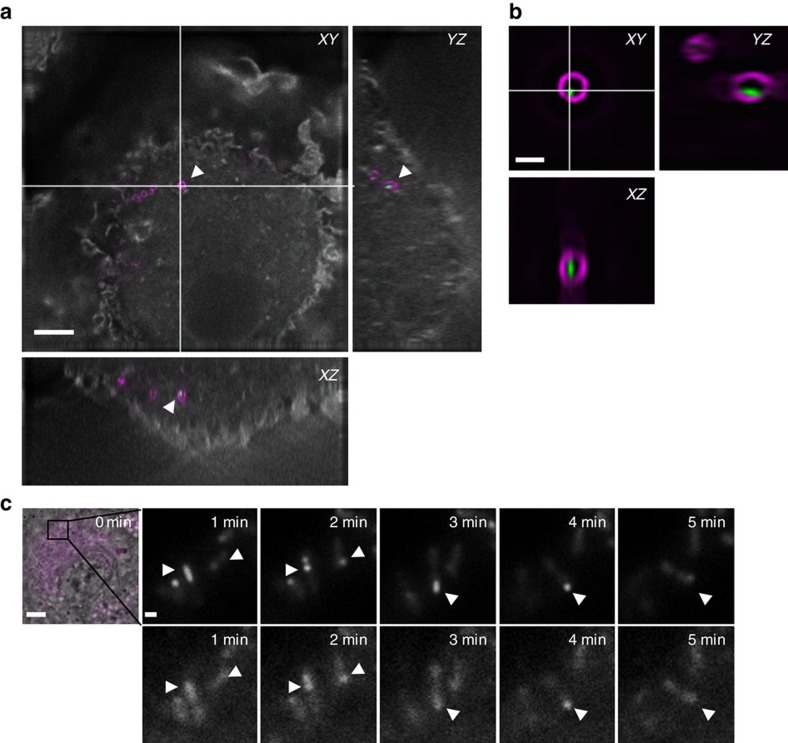
Figure 4. T6SS dynamics in bone marrow-derived macrophages (BMDMs).
(a) Merged wide field image and orthogonal view of BMDMs infected for 1 h with F. novicida U112 iglA-sfGFP clpB-mCherry2; in grey: actin staining, in magenta: LPS staining, in green: IglA-sfGFP. 41 × 41 μm field of view, scale bar, 5 μm. (b) Close up and orthogonal SIM view of bacterium highlighted with arrowheads in a; magenta: LPS staining, green: IglA-sfGFP. 5.1 × 5.1 μm field of view, scale bar, 1 μm. (c) Time-lapse images of unprimed wild-type BMDMs infected with F. novicida U112 iglA-sfGFP clpB-mCherry2 for 1 h. First image consists of merged phase contrast, GFP and mCherry channels. 30 × 30 μm field of view is shown. Scale bar, 5 μm. Close ups consist of GFP channel (upper panel) and mCherry channel (lower panel). Close ups show 5 × 5 μm. Scale bar, 1 μm. Arrowheads indicate T6SS sheath assembly, contraction and location of sheath after contraction.
Next, we imaged IglA-sfGFP and ClpB-mCherry2 dynamics within F. novicida in live macrophages and observed that the sheaths cycled through assembly, contraction and disassembly. Importantly, ClpB-mCherry2 dynamically localized into spots that colocalized with the contracted sheaths, suggesting that ClpB is responsible for disassembly of the contracted sheaths also within phagosomes of infected macrophages (Fig. 4c; Supplementary Movie 6). In total, we analysed 30 sheath assembly, contraction and disassembly events inside live macrophages and all of the assemblies originated from the cell pole (Fig. 4c; Supplementary Movie 6). Together, these data suggest that sheath dynamics and subcellular localization observed during imaging of F. novicida on agarose pads is similar to that of the sheath in the bacteria residing inside of live macrophages.
To determine the importance of ClpB for F. novicida pathogenesis, we infected BMDMs with F. novicida wild-type, ΔpdpB and ΔclpB and determined the percentage of phagosomal and cytosolic bacteria using a phagosome-protection assay based on selective permeabilization of the plasma membrane with digitonin9. F. novicida ΔclpB had a significant defect in phagosomal escape at 4 h post infection, similarly to bacteria lacking the essential structural component PdpB (Fig. 3d; Supplementary Fig. 3a). Consistent with reduced cytosolic localization, we observed significantly reduced levels of pyroptosis induction and cytokine release in LPS-primed BMDMs infected for 10 h with F. novicida ΔpdpB and ΔclpB, while the wild-type strain elicited strong immune responses (Fig. 3e). Finally, we evaluated the role of ClpB in vivo in a mouse model of tularaemia. We infected age- and sex-matched wild-type C57BL/6 mice subcutaneously with 104 colony-forming units (CFUs) of F. novicida wild-type, ΔpdpB and ΔclpB and measured the bacterial burden at 2 days post infection. Mice infected with F. novicida ΔclpB displayed significantly reduced bacterial counts in the liver and spleen as compared to the mice infected with F. novicida wild type, and in many cases no bacteria could be recovered, similarly to what was observed with F. novicida ΔpdpB (Supplementary Fig. 3b). Overall these results indicate that ClpB acts as an unfoldase for the FPI-encoded T6SS sheath, and that its activity is essential for T6SS dynamics and consequently F. novicida virulence.
Differential requirement of FPI genes for sheath dynamics
Almost all FPI genes were shown to be required for intracellular replication probably due to a lack of phagosomal escape, however, many genes of the FPI cluster have no known homologues or were not characterized in detail14. Importantly, both structural components of T6SS as well as putative effectors secreted by T6SS are in principle essential for overall T6SS function, however, effectors may be to a certain degree dispensable for T6SS assembly. To provide an insight into which FPI genes are required for assembly of T6SS and which may potentially encode secreted effectors, we generated in-frame deletions of genes for which we were unable to predict function based on homology to known canonical T6SS components (Fig. 1). IglA-sfGFP subcellular localization was then imaged in those strains under the same conditions as used before for the parental strain.
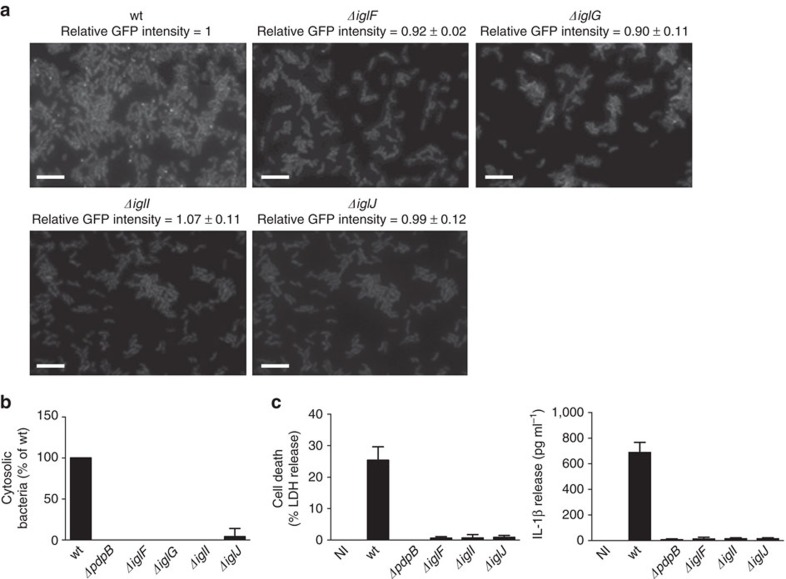
In ΔiglF and ΔiglG strains, we detected on average 1 dynamic sheath assembly per 400 and 500 cells, respectively, in 5 min (Supplementary Movie 2). This suggests that IglF and IglG may be required for efficient initiation of T6SS assembly. On the other hand, iglI and iglJ are essential for sheath assembly as no sheath assemblies were detected in more than 1,000 cells in 5 min even though IglA-sfGFP was expressed to the same level as in the parental strain (Fig. 5a; Supplementary Movie 2). Consistent with the defect in T6SS assembly, we found that ΔiglF, ΔiglG, ΔiglI and ΔiglJ strains were unable to escape into the cytosol of the infected macrophages, and consequently failed to activate cytosolic innate immune signalling (Fig. 5b,c). We cannot completely rule out the possibility that the observed phenotypes of mutants are due to polar effects on expression of other T6SS genes. However, defect in intracellular growth was previously successfully complemented for iglF, iglG and iglI genes55.
Figure 5. Identification of genes required for assembly and function of F. novicida T6SS.
(a) IglA-sfGFP localization in F. novicida U112 iglA-sfGFP wild type, ΔiglF, ΔiglG, ΔiglI and ΔiglJ. The GFP channel is shown. The numbers above the images represent the ratio of average GFP intensity of mutants compared to the parental strain with s.d. The average GFP intensities were quantified in three independent experiments. Thirty bacteria were analysed per experiment; 39 × 26 μm fields of view are shown. Scale bars, 5 μm. (b) Quantification of cytosolic bacteria in unprimed wild-type BMDMs 4 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB, ΔiglF, ΔiglG, ΔiglI or ΔiglJ (normalized to wild type). (c) Release of LDH and IL-1β from primed wild-type BMDMs 10 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB, ΔiglF, ΔiglI or ΔiglJ (NI—noninfected control). (b,c) Data are pooled from three independent experiments (b) (mean and s.d. are shown) or are representatives of three independent experiments (c) (mean and s.d. of triplicate wells are shown).
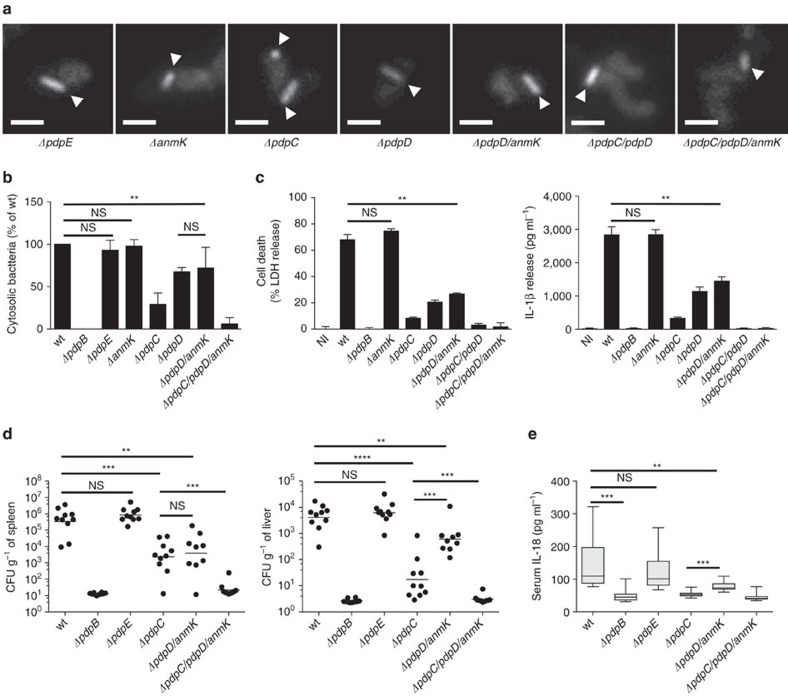
Single deletion of pdpE, pdpC, pdpD and anmK or deletion of both pdpD and anmK (ΔpdpD/anmK) or pdpC and pdpD (ΔpdpC/pdpD) had no significant influence on sheath dynamics or localization (Fig. 6a; Supplementary Movie 1). Only deletion of all three genes pdpC, pdpD and anmK in the same strain decreased frequency of sheath assembly by 30% from an average of one structure per three cells to about one structure per five cells (Supplementary Fig. 4a). Nevertheless, sheath assemblies in ΔpdpC/pdpD/anmK still preferentially localized to the cell pole, assembled with a similar speed and cycled through extension, contraction and disassembly like in the parental strain (Supplementary Fig. 4b,c; Supplementary Movie 1). Importantly, ΔpdpE and ΔpdpC/pdpD/anmK assembled sheaths with dynamics undistinguishable from the parental strain within infected macrophages (Supplementary Fig. 5e,f). In conclusion, our analysis allowed us to identify FPI genes (iglF, iglG, iglI and iglJ) essential for T6SS assembly and a distinct set of FPI genes (pdpE, pdpC, pdpD and anmK) that are dispensable for T6SS assembly.
Figure 6. Contribution of uncharacterized FPI genes to T6SS function.
(a) T6SS sheath assembly (arrowheads) in F. novicida U112 iglA-sfGFP ΔpdpE, ΔanmK, ΔpdpC, ΔpdpD, ΔpdpD/anmK, ΔpdpC/pdpD and ΔpdpC/pdpD/anmK. GFP channel and 3.3 × 3.3 μm fields of view are shown. Scale bars, 1 μm. (b) Quantification of cytosolic bacteria in unprimed wild-type BMDMs 4 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB, ΔpdpE, ΔanmK, ΔpdpC, ΔpdpD, ΔpdpD/anmK or ΔpdpC/pdpD/anmK (normalized to wild type). (c) Release of LDH and IL-1β from primed wild-type BMDMs 10 h after infection with F. novicida U112 iglA-sfGFP wild type, ΔpdpB, ΔanmK, ΔpdpC, ΔpdpD, ΔpdpD/anmK, ΔpdpC/pdpD or ΔpdpC/pdpD/anmK (NI—noninfected control). (d,e) Bacterial burden (as colony-forming units (CFUs) per gram tissue) in the spleen and liver (d) and serum IL-18 levels (e) of wild-type C57BL/6JRj mice infected subcutaneously for 2 days with 1 × 104 F. novicida U112 iglA-sfGFP wild type, ΔpdpB, ΔpdpE, ΔpdpC, ΔpdpD/anmK and ΔpdpC/pdpD/anmK. Small horizontal lines indicate the mean. Each symbol represents an individual mouse (n=5 per experiment). Graphs show pooled data from two independent biological replicates with n=5 per experiment (n=10 total per group); small horizontal lines indicate the mean. (b–e) Data are pooled from three independent experiments (b) (mean and s.d. are shown) or two independent experiments (d,e) or are representatives of three independent experiments (c) (mean and s.d. of triplicate wells are shown). **P<0.01, ***P<0.001 and ****P<0.0001 (two-tailed unpaired t-test with Welch’s correction (b,c) or Mann–Whitney test (d,e)).
PdpC and PdpD are required for phagosomal escape
To test whether pdpE, pdpC, pdpD and anmK genes are required for the escape of F. novicida from phagosome, we infected BMDMs with F. novicida ΔpdpE, ΔanmK, ΔpdpC, ΔpdpD, ΔpdpD/anmK or ΔpdpC/pdpD/anmK and determined the percentage of phagosomal and cytosolic bacteria compared to wild-type and ΔpdpB bacteria as outlined above (Supplementary Fig. 3a). Interestingly, we found that deletion of pdpC resulted in a very strong defect in phagosomal escape in comparison to wild-type bacteria, although the reduction was smaller than with bacteria lacking the structural component PdpB (Fig. 6b). F. novicida ΔpdpD and ΔpdpD/anmK also showed a defect in phagosomal escape, which was however less severe than the phenotype of a pdpC or pdpB deletion. No significant difference in phagosomal escape was observed between ΔpdpD and ΔpdpD/anmK strains, indicating that AnmK plays no role in phagosomal escape, consistent with the finding that phagosomal escape of the ΔanmK strain was indistinguishable from the wild-type strain (Fig. 6b). To determine whether the effect of a pdpC and pdpD deletion was additive, we generated a strain lacking pdpC, pdpD and also anmK. Interestingly, bacteria lacking pdpC/pdpD/anmK were unable to escape from the phagosomal compartment similarly to the ΔpdpB strain. In contrast, deletion of pdpE had no significant effect on phagosomal escape (Fig. 6b).
Next, we tested the role of pdpE, pdpC, pdpD and anmK in cytosolic innate immune detection of F. novicida. Consistent with the reduced level of cytosolic localization, we found that F. novicida ΔpdpC and ΔpdpC/pdpD/anmK induced significantly lower levels of type I IFN production in unprimed BMDMs infected for 10 h at an MOI of 100 (Supplementary Fig. 5c). The triple mutant ΔpdpC/pdpD/anmK had the most severe phenotype and only elicited IFN levels in the range of the ΔpdpB strain (Supplementary Fig. 5c).
Since type I IFNs control the activation of the AIM2 inflammasome during F. novicida infection5, we examined the level of inflammasome activation in LPS-primed infected macrophages at different time points (Fig. 6c; Supplementary Fig. 5a). While infection with F. novicida lacking pdpC or pdpD resulted in significantly reduced levels of inflammasome activation, only the deletion of both pdpC and pdpD completely abrogated cell death induction and cytokine production in infected macrophages, which was consistent with the reduced levels of cytosolic localization and type I IFN induction in macrophages infected with mutants lacking both proteins (Fig. 6b; Supplementary Fig. 5c). Cell death induction and cytokine production in infected macrophages was unchanged between cells infected with wild-type and ΔanmK bacteria indicating that AnmK is not involved in modulating inflammasome activation (Fig. 6c). Consistently, cell death and cytokine production was comparable between cells infected with F. novicida ΔpdpC/pdpD and ΔpdpC/pdpD/anmK or F. novicida ΔpdpD and ΔpdpD/anmK. Importantly, the observed changes in inflammasome activation were independent of macrophage priming, since unprimed macrophages infected with wild-type or mutant F. novicida responded similarly (Supplementary Fig. 5b). Deletion of pdpE had no significant effect on the level of type I IFN induction, pyroptosis and cytokine release (Supplementary Fig. 5a–c).
Previous work has implicated the FPI in intracellular replication55, therefore, we also examined intracellular replication of wild-type or mutant F. novicida. We monitored growth over 24 h of infection in BMDMs lacking the inflammasome adaptor protein ASC as they fail to trigger pyroptosis in response to bacterial infection8. F. novicida wild-type and ΔpdpE replicated over the course of the infection (Supplementary Fig. 5d), while bacteria that lacked a dynamic T6SS (ΔpdpB or ΔclpB) or bacteria that had a dynamic T6SS, but were deficient in phagosomal escape (ΔpdpC or ΔpdpC/pdpD/anmK), were cleared over the course of the infection. Consistent with reduced phagosomal escape, pdpD/anmK-deficient bacteria also displayed a reduced rate of replication compared to wild-type bacteria, however, the difference was not significant.
Finally, we examined the role of potential T6SS effectors in vivo. Age- and sex-matched wild-type C57BL/6 mice were infected subcutaneously with 104 CFUs of F. novicida wild-type or strains deficient for the putative effectors, and the bacterial burden in the liver and spleen as well as serum IL-18 levels were assessed at 2 days post infection (Fig. 6d,e). The bacterial burden closely correlated with phagosomal escape, in that a partial reduction in virulence could be observed in ΔpdpC and ΔpdpD/anmK-infected mice. Deletion of pdpC alone had a stronger effect than deletion of pdpD/anmK although this difference was only significant in the liver. Deleting all three potential effectors, ΔpdpC/pdpD/anmK, rendered the bacteria largely avirulent, similarly to the deletion of the T6SS structural component pdpB. Consistent with the reduced levels of inflammasome activation in vitro (Fig. 6c; Supplementary Fig. 5a), we found that deletion of pdpB, pdpC, pdpD/anmK or pdpC/pdpD/anmK resulted in significantly lower levels of serum IL-18. A deficiency in pdpE appeared to have no effect on virulence or host response, since infection with F. novicida ΔpdpE resulted in bacterial burden and cytokine levels that were comparable to infections with F. novicida wild type (Fig. 6d,e). In summary, these results confirm previous studies indicating that PdpC and PdpD are T6SS-secreted effectors. Moreover, we show that PdpC and PdpD are dispensable for T6SS dynamics and specifically facilitate the escape of F. novicida from the phagosome into the host cell cytosol and therefore are essential for Francisella virulence.
Discussion
We show here that Francisella T6SS sheath is under certain conditions highly dynamic and ClpB is necessary for sheath disassembly. Since ClpB-mCherry2 specifically colocalizes with the contracted sheaths, our data suggest that ClpB is directly involved in Francisella sheath disassembly similarly to ClpV in canonical T6SS (refs 24, 49, 50, 56, 57). Interestingly, Francisella ClpB was also shown to alter the immune response in vivo58 and to be required for heat shock survival52. However, we show here that T6SS activity is dispensable for heat shock survival (Supplementary Fig. 2d). This suggests that, in contrast to canonical T6SS where ClpV is apparently solely dedicated to sheath disassembly, Francisella ClpB has a dual role. This raises the question how ClpB recognizes different substrates and whether a specific adaptor protein is required to recognize contracted sheaths similarly to adaptor proteins that recognize substrates for AAA+-mediated unfolding59,60,61,62. We show that ClpB is important for F. novicida virulence, which is consistent with what was shown previously52,53,54,58. Since all virulence related phenotypes of clpB-negative strain correlated with the phenotypes of the other strains with impaired T6SS dynamics (Fig. 3; Supplementary Figs 2 and 3), we propose that in vivo ClpB is mainly important for T6SS sheath disassembly. However, refolding of substrates unrelated to T6SS may be required to survive certain stresses, which Francisella encounter during pathogenesis.
Detailed analysis of subcellular localization of dynamic T6SS sheath shows that Francisella T6SS assembles on the bacterial cell poles both in vitro as well as during infection of macrophages (Figs 2e,f and 3c; Supplementary Fig. 5e,f). Interestingly, similarly to what we show here for Francisella, ClpV-5 from T6SS-5 of Burkholderia thailandensis was found to preferentially localize to spots on bacterial poles63. Those spots were however less dynamic than ClpB spots in Francisella and thus it remains to be directly tested if assembly of T6SS-5 of B. thailandensis indeed initiates at the poles. Interestingly, both T6SS-5 of B. thailandensis and Francisella T6SS are required for manipulation of the eukaryotic cells after bacterial internalization. However, unlike in Francisella, T6SS-5 of B. thailandensis is only required for formation of multinucleated giant cells after bacteria escape from endosomes using T3SS63,64,65,66,67. Since T6SS sheaths almost always assemble as long as the bacteria, one possible advantage of the polar localization could be that the sheaths assembled from the pole in rod shaped bacteria would be generally longer than the sheaths assembled from the side of the cells. Given that the T6SS sheaths only contract to about half of their extended size25, longer sheaths may increase the distance to which T6SS can deliver effectors. Interestingly, restricted subcellular localization was shown to decrease T6SS efficiency in inter-bacterial competitions despite increased overall activity51. However, since F. novicida is completely surrounded by phagosome membrane, restricted directionality of T6SS assembly should have no consequences for delivery of effectors to the host cell. In addition, polar localization of T6SS may increase chances of puncturing phagosomal membranes, as those may be physically closer to the bacterial poles when bacteria are in a tight membrane compartment. As it was shown previously for inter-bacterial interactions, proper aiming of the T6SS apparatus at the target bacteria increases efficiency of substrate translocation37,51.
The primary function of the Francisella T6SS is to promote the escape of Francisella from the phagosome. We show that phagosome escape depends entirely on PdpC and PdpD, which are dispensable for T6SS assembly and dynamics (Fig. 6a,b; Supplementary Movie 1), suggesting that these proteins function as effectors necessary for phagosomal escape. It is also possible that PdpD and PdpC are required for activity or secretion of yet uncharacterized T6SS effectors to promote phagosomal escape, however previous work by Eshraghi et al.42 has shown that F. novicida PdpC and PdpD are released by the T6SS in an in vitro secretion assay, supporting the hypothesis that these proteins function as secreted effectors in the target cell. Moreover, F. tularensis and F. holarctica lacking pdpC are unable to escape from the phagosome, induce cytotoxicity and replicate intracellularly, and they are avirulent in a mouse model of tularaemia43,44,46,47,48. These observations support our conclusions that PdpC contribute to Francisella virulence, independent of the Francisella tularensis subspecies. Whereas pdpC is conserved in all subspecies of Francisella tularensis, pdpD is differentially encoded45. Therefore, PdpD might have subspecies-specific virulence related functions.
PdpC and PdpD share no homology with known effectors or pore forming toxins, such as Listeriolysin O, type C phospholipases or phenol-soluble modulins, that allow other cytosolic bacteria (Listeria monocytogenes, Shigella flexneri, Burkholderia thailandensis or Staphylococcus aureus) to escape from the phagosome, and thus might represent a novel class of effectors with membranolytic function68,69. The exact mechanism of how these effectors destabilize the phagosomal membrane and if this results in the recruitment of galectin-8, a marker of ruptured vacuoles that recruits antimicrobial autophagy70, remains to be analysed. The Francisella O-antigen allows the bacteria to avoid ubiquitination and uptake into LC3-positive compartments71, but whether Francisella can actively inhibit or escape autophagy by injected effectors, as reported for Listeria and Shigella is unknown72.
PdpE and AnmK, which are dispensable for T6SS assembly and phagosomal escape (Fig. 6a,b), might be effectors whose function is required once the bacteria enter the cytosol. However, their contribution to overall bacterial replication and virulence in vivo is minor (Fig. 6d,e; Supplementary Fig. 5d). In addition, OpiA and OpiB, encoded outside of the FPI cluster, were recently identified as T6SS secreted proteins, however, their contribution to intracellular replication is also minimal in comparison to the effects of a pdpC or pdpD deletion42. It is possible that these effectors have tissue-specific functions, or that they are required for Francisella replication in amoeba or within arthropod hosts73,74.
Live-cell imaging of T6SS sheath dynamics suggests that IglF, IglG, IglI and IglJ are putative structural components required for T6SS assembly in Francisella (Fig. 5a; Supplementary Movie 2). These proteins could be homologues of components of canonical T6SS baseplate, which are difficult to identify using homology modelling14,75 (Fig. 1). However, it is also conceivable that some of these proteins may be secreted effectors or be required for effector secretion, because deletion of certain effectors decreases T6SS function in V. cholerae37,76. Nonetheless, our finding that the dynamics of Francisella T6SS is possible to image in vitro will help to dissect the assembly of this non-canonical T6SS and to differentiate between structural components and translocated substrates. Further analysis of the structural components will reveal principles of T6SS evolution and defining the molecular mechanisms by which Francisella effectors modulate host cell signalling will significantly contribute to our understanding of Francisella virulence.
Methods
Bacterial strains and growth conditions
Francisella tularensis subsp. novicida strain U112 (hereafter F. novicida) and the derivative strains were grown at 37 °C with aeration in brain heart infusion (BHI) medium supplemented with 0.2% L-cysteine (Sigma) and appropriate antibiotics. Antibiotic concentrations used were 100 μg ml−1 ampicillin (AppliChem) or 15 μg ml−1 kanamycin (AppliChem). A detailed strain list can be found in Supplementary Table 1. For infection with F. novicida, BHI medium was inoculated with bacteria from BHI agar plate (supplemented with 0.2% L-cysteine (Sigma) and appropriate antibiotics) and were grown overnight at 37 °C with aeration.
Bacterial mutagenesis
All in-frame deletions were generated by homologous recombination using the suicide vector pDMK3 as previously described77. A list of plasmids, primers as well as remaining peptides encoded by deleted genes can be found in Supplementary Table 2. To obtain single colonies after recombination, bacteria were grown overnight at 37 °C on Mueller-Hinton agar (MHA) supplemented with 0.1% D-glucose (Millipore), 0.1% FCS (BioConcept), 100 μg ml−1 ampicillin (AppliChem) and 0.1% L-cysteine (Sigma) (hereafter MHA plate). Cloning product sequences were verified and chromosomal mutations were tested by PCR using primers located outside of the replaced region. Sites of homologous recombination of the chromosomal mutations were verified by sequencing.
Heat shock survival assay
Heat shock survival assay was adapted from ref. 52. In brief, bacteria were grown overnight as described above, diluted 1:40 in BHI medium and grown for 3 h at 37 °C with aeration. Then bacteria were diluted 1:10 in 250 μl BHI in a 1.5 ml tube and incubated in a water bath at 50 °C for 0, 15 or 30 min. At each time point the bacteria were transferred on ice and serial dilutions were plated on MHA plates. The next day, CFUs were counted and the concentration of surviving bacteria was calculated.
Fluorescence microscopy
Procedures and settings to detect a fluorescence signal in F. novicida were employed as previously described37,41. All imaging was carried out at 37 °C and humidity was regulated to 95% using a T-unit (Oko-lab). The exposure time was set to 150 ms for all channels. For bacterial imaging on agarose pads, F. novicida strains from BHI plate were washed once with BHI, diluted 1:40 in BHI medium and grown at 37 °C with aeration for 3–4 h. Bacteria from 1 ml culture were re-suspended in 50–100 μl phosphate-buffered saline (PBS), spotted on a pad of 1% agarose in PBS, covered with a cover glass (Roth) and either imaged directly or incubated at 37 °C for 1 h before imaging. Images were collected every 3 s for T6SS assembly speed quantification and every 30 s for assessment of T6SS dynamics. For imaging of infected macrophages, BMDMs were seeded onto cover glass (VWR) in 24-well plates at a density of 1.5 × 105 cells per well and infected with F. novicida at a multiplicity of infection of 100 in 1 ml OptiMEM (Life Technologies) as described below. Thirty minutes post infection, the BMDMs were washed three times with OptiMEM and the cover glass was mounted on a pad of 1% agarose in PBS BMDMs facing down. Images were collected every 30 s for assessment of T6SS dynamics.
Image analysis
Fiji78 was used for all image analysis and manipulations as described previously37,51. The ‘Time Series Analyzer V3.0’ plugin was used for quantification of GFP signal intensity. For comparison of GFP signal intensities of mutants and wild type, only bacteria without assembled T6SS structures were considered. For quantification of T6SS activity in different mutants from 5 min time-lapse movies the ‘temporal colour code’ function was used. For kymograms and T6SS assembly speed quantification the ‘reslice’ function was used. For determination of subcellular localization of T6SS assembly the surface area of bacteria was divided into an equally sized polar and mid cell area. The surface area was calculated based on the model of a capsule using the manually measured length and width of the bacteria (see formulas below). T6SS assemblies initiating in one of the two pole areas were considered as T6SS assemblies at pole.
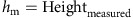 |
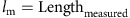 |
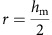 |
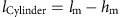 |
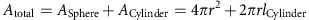 |
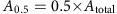 |
For determination of subcellular localization of T6SS assembly, images of V. cholerae 2740-80 were reanalysed from ref. 37. Contrast on compared sets of images was adjusted equally. All imaging experiments were performed with at least two biological replicates.
Structured illumination microscopy
BMDMs were seeded onto cover glass (VWR) in 24-well plates at a density of 1.25 × 105 cells per well and infected with F. novicida at a multiplicity of infection of 100 for 1 h as described below. BMDMs were washed three times with PBS and fixed for 10 min at 37 °C with 4% paraformaldehyde (Electron Microscopy Science). Cover glass was incubated with chicken anti-F. novicida (1:2,000; a gift from D.M. Monack, Stanford University) for 1 h at room temperature, then was washed three times with PBS, incubated with goat anti-chicken coupled to Alexa 568 (1:500; Life Technologies) and DY-647-Phalloidin (1:500; Dyomics) for another 45 min at room temperature, washed three times with PBS and was mounted on glass slides with Vectashield (Vector labs). 3D-SM was performed on a microscope system DeltaVision OMX-Blaze version 4 (Applied Precision, Issaquah, WA). Images were acquired using a Plan Apo N 60 × 1.42 numerical aperture oil immersion objective lens (Olympus) and four liquid-cooled sCMOS cameras (pco Edge, full frame 2,560 × 2,160; Photometrics). Optical z-sections were separated by 0.125 μm. The laser lines 488 and 568 were used for 3D-SIM acquisition. Exposure times were typically between 10 and 140 ms, and the power of each laser was adjusted to achieve optimal intensities of between 5,000 and 8,000 counts in a raw image of 15-bit dynamic range at the lowest laser power possible to minimize photobleaching. Phalloidin Alexa-647 was acquired using the widefield mode of the system. Raw 3D-SIM images were processed and reconstructed using the DeltaVision OMX SoftWoRx software package (Applied Precision).
Cell culture and infection
Primary wild-type BMDMs from C57BL/6JRj mice (Janvier) were differentiated in DMEM (Sigma) with 20% M-CSF (supernatants of L929 mouse fibroblasts), 10% v/v FCS, 10 mM HEPES, nonessential amino acids and penicillin (100 IU ml−1)/streptomycin (100 μg ml−1) (all BioConcept). One day before infection, BMDMs were seeded into 24- or 96-well plates (Eppendorf) at a density of 1.5 × 105 or 5 × 104 cells per well in DMEM (Sigma) with 10% M-CSF (supernatants of L929 mouse fibroblasts), 10% v/v FCS, 10 mM HEPES and nonessential amino acids (all BioConcept). Where required, BMDMs were pre-stimulated overnight with LPS (from Escherichia coli strain O111:B4 (InvivoGen; tlr-3pelps)). F. novicida were grown overnight at 37 °C with aeration as described above. The bacteria were added to the BMDMs at a multiplicity of infection of 100 or the indicated value. The plates were centrifuged for 5 min at 500g to ensure similar adhesion of the bacteria to the cells and were incubated for 120 min at 37 °C. Next, the medium was replaced with fresh medium containing 10 μg ml−1 gentamicin (BioConcept) to kill extracellular bacteria, then plates were incubated at 37 °C for the indicated length of time.
Cytokine and LDH release measurement
IL-1β and IL-18 were measured by enzyme-linked immunosorbent assay (eBioscience). Lactate dehydrogenase (LDH) was measured with an LDH Cytotoxicity Detection Kit (Takara). To correct for spontaneous cell lysis and to normalize the values, the percentage of LDH release was calculated as follows:
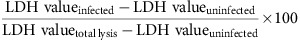 |
Phagosome protection assay
The amount of cytoplasmic and vacuolar bacteria was measured as previously described79. In brief, BMDMs were seeded into 24-well plates at a density of 1.5 × 105 cells per well and F. novicida were grown for 4 h at 37 °C with aeration as described above. BMDMs were infected with F. novicida at a multiplicity of infection of 100 for 4 h as outlined above. BMDMs were washed three times with KHM buffer (110 mM potassium acetate, 20 mM Hepes, 2 mM MgCl2) and incubated for 1 min with 75 μg ml−1 digitonin (Sigma) followed by differential staining of cytoplasmic and total bacteria. Antibodies used for staining were chicken anti-F. novicida (1:2,000; a gift from D.M. Monack, Stanford University) and goat anti-chicken coupled to Alexa 647 (cytoplasmic bacteria) or Alexa 488 (total bacteria) (1:500; both from Life Technologies). Stained bacteria were analysed on a FACS-Canto-II. Percentage of cytosolic bacteria were normalized to wild-type F. novicida as follows:
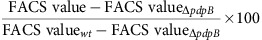 |
Intracellular bacterial growth assay
BMDMs were seeded into 24-well plates at a density of 1.5 × 105 cells per well and infected with F. novicida at a multiplicity of infection of 1 as described above. After 2 and 24 h of infection, the BMDMs were washed three times with PBS and lysed with 0.1% Triton-X 100 (Promega) for 10 min at 37 °C. The bacteria were stained for 10 min with chicken anti-F. novicida (1:2,000; a gift from D.M. Monack, Stanford University), washed once with PBS and stained for 10 min with goat anti-chicken coupled to Alexa 647 and Alexa 488 (1:500 each; both from Life Technologies). A volume of 20 μl 123count eBeads (eBioscience) was added to each sample. The samples were analysed on a FACS-Canto-II by counting the number of bacteria per 5,000 beads. The CFU ratio was calculated by dividing the number of bacteria at 24 h (output) with the number of bacteria at 2 h (input).
Type I interferon measurement
One day before infection, ISRE-L929 reporter cells (a gift from D.M. Monack, Stanford University) were seeded into black 96-well plates with micro-clear bottom (Greiner) at a density of 1 × 105 cells per well in DMEM (Sigma) with 10% v/v FCS and penicillin (100 IU ml−1)/streptomycin (100 μg ml−1) (both BioConcept). BMDMs were seeded into 96-well plates at a density of 5 × 104 cells per well and infected with F. novicida at a multiplicity of infection of 100 as described above. After 10 h of infection, type I IFN production was measured with the Bright-Glo Luciferase Assay System (Promega) as previously described80.
Animal infection
All animal experiments were approved (licence 2535-26742, Kantonales Veterinäramt Basel-Stadt) and were performed according to local guidelines (Tierschutz-Verordnung, Basel-Stadt) and the Swiss animal protection law (Tierschutz-Gesetz). Female 10 weeks old wt C57BL/6JRj mice (Janvier) were infected subcutaneously with 104 CFUs of indicated stationary-phase F. novicida strain in 50 μl PBS. Mice were killed 48 h post infection. Bacterial load of spleen and liver was analysed by plating the bacteria on MHA plates. The plates were incubated for 24 h at 37 °C. IL-18 levels in the blood were measured by enzyme-linked immunosorbent assay (eBioscience). No randomization or ‘blinding’ of researchers to sample identity was used.
Statistical analysis
Statistical data analysis was done using Prism 6.0h (GraphPad Software, Inc.). To evaluate the difference between two groups (T6SS per cell, T6SS assembly speed, subcellular localization of T6SS, bacterial survival, cell death, cytokine release, phagosomal escape, bacterial growth and IFN production) the unpaired two-tailed t-test with Welch’s correction was used. Animal experiments were evaluated with a two-tailed Mann–Whitney test. P values are given in the figure legends.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.
Additional information
How to cite this article: Brodmann, M. et al. Francisella requires dynamic type VI secretion system and ClpB to deliver effectors for phagosomal escape. Nat. Commun. 8, 15853 doi: 10.1038/ncomms15853 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank M.A. Horwitz (UCLA) for providing the strain F. novicida U112 iglA-sfGFP, D.M. Monack (Stanford University) for the conjugation plasmid, the anti-Francisella primary antibody and the ISRE-L929 cells, A. Harms and C. Dehio (Biozentrum, University of Basel) for the E. coli conjugation strain and the Imaging Core Facility and FACS Core Facility (Biozentrum, University of Basel) for technical support. We thank P.D. Ringel for his help with the analysis of subcellular localization of T6SS assembly. The work was supported by SNSF Starting Grant BSSGI0_155778 (to M.Ba.), SNSF grant 31003A_159525 (to M.Ba.), SNSF grant PP00P3_139120/1 (to P.B.) and the University of Basel. M.Br. was supported by the Biozentrum Basel International PhD Program ‘Fellowships for Excellence’.
Footnotes
The authors declare no competing financial interests.
Author contributions M.Br., R.F.D., P.B. and M.Ba. designed experiments, analysed and interpreted the results. M.Br. and R.F.D. generated strains and acquired all data. All authors wrote and approved the manuscript.
References
- Oyston P. C. F., Sjostedt A. & Titball R. W. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978 (2004). [DOI] [PubMed] [Google Scholar]
- Kingry L. C. & Petersen J. M. Comparative review of Francisella tularensis and Francisella novicida. Front. Cell Infect. Microbiol. 4, 35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A. & Celli J. The Francisella intracellular life cycle: toward molecular mechanisms of intracellular survival and proliferation. Front. Microbiol. 1, 138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T., Brotcke A., Weiss D. S., Thompson L. J. & Monack D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Weiss D. S., Dixit V. M. & Monack D. M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam V. A. K. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storek K. M., Gertsvolf N. A., Ohlson M. B. & Monack D. M. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 194, 3236–3245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E. et al. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 186, 6430–6436 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E. & Schmerk C. The Francisella pathogenicity island. Ann. NY Acad. Sci. 1105, 122–137 (2007). [DOI] [PubMed] [Google Scholar]
- Bröms J. E., Sjöstedt A. & Lavander M. The role of the Francisella Tularensis pathogenicity island in type VI secretion, intracellular survival, and modulation of host cell signaling. Front. Microbiol. 1, 136 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigard M. et al. Francisella tularensis IglG belongs to a novel family of PAAR-like T6SS proteins and harbors a unique N-terminal extension required for virulence. PLoS Pathog. 12, e1005821 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L. E., Bailey C. M. & Pallen M. J. Type VI secretion: a beginner’s guide. Curr. Opin. Microbiol. 11, 3–8 (2008). [DOI] [PubMed] [Google Scholar]
- de Bruin O. M., Ludu J. S. & Nano F. E. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 7, 1 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B. et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16, 227–236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoforado Diniz J., Liu Y.-C. & Coulthurst S. J. Molecular weaponry: diverse effectors delivered by the type VI secretion system. Cell. Microbiol. 17, 1742–1751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E., Cambillau C., Cascales E. & Journet L. VgrG, Tae, Tle, and beyond: the versatile arsenal of type VI secretion effectors. Trends Microbiol. 22, 498–507 (2014). [DOI] [PubMed] [Google Scholar]
- Hachani A., Wood T. E. & Filloux A. Type VI secretion and anti-host effectors. Curr. Opin. Microbiol. 29, 81–93 (2016). [DOI] [PubMed] [Google Scholar]
- Ho B. T., Dong T. G. & Mekalanos J. J. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15, 9–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., Peterson S. B. & Mougous J. D. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 12, 137–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M. & Mekalanos J. J. Type 6 secretion dynamics within and between bacterial cells. Science 337, 815 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M., Pilhofer M., Henderson G. P., Jensen G. J. & Mekalanos J. J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483, 182–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E. et al. Biogenesis and structure of a type VI secretion membrane core complex. Nature 523, 555–560 (2015). [DOI] [PubMed] [Google Scholar]
- Brunet Y. R., Zoued A., Boyer F., Douzi B. & Cascales E. The type VI secretion TssEFGK-VgrG phage-like baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as assembly platform for tail tube/sheath polymerization. PLoS Genet. 11, e1005545 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planamente S. et al. TssA forms a gp6-like ring attached to the type VI secretion sheath. EMBO J. 35, 1613–1627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider M. M. et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500, 350–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoued A. et al. TssK is a trimeric cytoplasmic protein interacting with components of both phage-like and membrane anchoring complexes of the type VI secretion system. J. Biol. Chem. 288, 27031–27041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoued A. et al. Architecture and assembly of the type VI secretion system. Biochim. Biophys. Acta 1843, 1664–1673 (2014). [DOI] [PubMed] [Google Scholar]
- Brunet Y. R., Henin J., Celia H. & Cascales E. Type VI secretion and bacteriophage tail tubes share a common assembly pathway. EMBO Rep. 15, 315–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugnatti N. et al. A phospholipase A1 antibacterial type VI secretion effector interacts directly with the C-terminal domain of the VgrG spike protein for delivery. Mol. Microbiol. 99, 1099–1118 (2016). [DOI] [PubMed] [Google Scholar]
- Hachani A., Allsopp L. P., Oduko Y. & Filloux A. The VgrG proteins are ‘à la carte’ delivery systems for bacterial type VI effectors. J. Biol. Chem. 289, 17872–17884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. et al. Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc. Natl Acad. Sci. USA 112, 9106–9111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. M. et al. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 51, 584–593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettiger A. & Basler M. Type VI secretion system substrates are transferred and reused among sister cells. Cell 167, 99–110.e12 (2016). [DOI] [PubMed] [Google Scholar]
- Whitney J. C. et al. Genetically distinct pathways guide effector export through the type VI secretion system. Mol. Microbiol. 92, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. C. et al. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163, 607–619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Ge P., Lee B.-Y., Horwitz M. A. & Zhou Z. H. Atomic structure of T6SS reveals interlaced array essential to function. Cell 160, 940–951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashev M. et al. Structure of the type VI secretion system contractile sheath. Cell 160, 952–962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi A. et al. Secreted effectors encoded within and outside of the francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe 20, 573–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M., Eneslätt K., Bröms J. E. & Sjöstedt A. Importance of PdpC, IglC, IglI, and IglG for modulation of a host cell death pathway induced by Francisella tularensis. Infect. Immun. 81, 2076–2084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M., Bröms J. E., Meyer L., Golovliov I. & Sjöstedt A. The Francisella tularensis LVS ΔpdpC mutant exhibits a unique phenotype during intracellular infection. BMC Microbiol. 13, 20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludu J. S. et al. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J. Bacteriol. 190, 4584–4595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanic M., Marecic V., Lindgren M., Sjöstedt A. & Santic M. Phenotypic characterization of the Francisella tularensis ΔpdpC and ΔiglG mutants. Microbes Infect. 18, 768–776 (2016). [DOI] [PubMed] [Google Scholar]
- Uda A. et al. Role of pathogenicity determinant protein C (PdpC) in determining the virulence of the Francisella tularensis subspecies tularensis SCHU. PLoS ONE 9, e89075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. E., Lindemann S. R., Rasmussen J. A., Jones B. D. & Allen L.-A. H. Disruption of Francisella tularensis Schu S4 iglI, iglJ, and pdpC genes results in attenuation for growth in human macrophages and in vivo virulence in mice and reveals a unique phenotype for pdpC. Infect. Immun. 81, 850–861 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönemann G., Pietrosiuk A., Diemand A., Zentgraf H. & Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 28, 315–325 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster A. et al. Coevolution of the ATPase ClpV, the sheath proteins TssB and TssC, and the accessory protein TagJ/HsiE1 distinguishes type VI secretion classes. J. Biol. Chem. 289, 33032–33043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M., Ho B. T. & Mekalanos J. J. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152, 884–894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom K. L. et al. The heat-shock protein ClpB of Francisella tularensis is involved in stress tolerance and is required for multiplication in target organs of infected mice. Mol. Microbiol. 67, 1384–1401 (2008). [DOI] [PubMed] [Google Scholar]
- Ahlund M. K., Rydén P., Sjöstedt A. & Stöven S. Directed screen of Francisella novicida virulence determinants using Drosophila melanogaster. Infect. Immun. 78, 3118–3128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. G., Cowley S. C., Cheung K. K. M. & Nano F. E. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol. Lett. 215, 53–56 (2002). [DOI] [PubMed] [Google Scholar]
- de Bruin O. M. et al. The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology 157, 3483–3491 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerc A. J. et al. Visualization of the serratia type VI secretion system reveals unprovoked attacks and dynamic assembly. Cell Rep. 12, 2131–2142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein N. et al. ClpV recycles VipA/VipB tubules and prevents non-productive tubule formation to ensure efficient type VI protein secretion. Mol. Microbiol. 87, 1013–1028 (2013). [DOI] [PubMed] [Google Scholar]
- Barrigan L. M. et al. Infection with Francisella tularensis LVS clpB leads to an altered yet protective immune response. Infect. Immun. 81, 2028–2042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J., Molière N., Dougan D. A. & Turgay K. Adapting the machine: adaptor proteins for Hsp100/Clp and AAA+ proteases. Nat. Rev. Microbiol. 7, 589–599 (2009). [DOI] [PubMed] [Google Scholar]
- Mogk A. et al. Broad yet high substrate specificity: the challenge of AAA+ proteins. J. Struct. Biol. 146, 90–98 (2004). [DOI] [PubMed] [Google Scholar]
- Sauer R. T. & Baker T. A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 (2011). [DOI] [PubMed] [Google Scholar]
- Sauer R. T. et al. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 119, 9–18 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S. et al. VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect. Immun. 82, 1445–1452 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. T. et al. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc. Natl Acad. Sci. USA 108, 12095–12100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov E. E., Brett P. J. & DeShazer D. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu. Rev. Microbiol. 64, 495–517 (2010). [DOI] [PubMed] [Google Scholar]
- Schwarz S. et al. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6, e1001068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toesca I. J., French C. T. & Miller J. F. The type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect. Immun. 82, 1436–1444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosz M. et al. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell. Microbiol. 16, 451–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K. & Stephens R. S. Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 6, 99–110 (2008). [DOI] [PubMed] [Google Scholar]
- Thurston T. L. M., Wandel M. P., von Muhlinen N., Foeglein A. & Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case E. D. R. et al. The Francisella O-antigen mediates survival in the macrophage cytosol via autophagy avoidance. Cell. Microbiol. 16, 862–877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Saitoh T. & Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimana C. & Kwaik Y. A. Francisella-arthropod vector interaction and its role in patho-adaptation to infect mammals. Front. Microbiol. 2, 34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santic M. et al. Intra-vacuolar proliferation of F. novicida within H. vermiformis. Front. Microbiol. 2, 78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer F., Fichant G., Berthod J., Vandenbrouck Y. & Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10, 104–104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T. G., Ho B. T., Yoder-Himes D. R. & Mekalanos J. J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl Acad. Sci. USA 110, 2623–2628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H. et al. Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect. Immun. 75, 1303–1309 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014). [DOI] [PubMed] [Google Scholar]
- Peng K., Broz P., Jones J., Joubert L.-M. & Monack D. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell. Microbiol. 13, 1586–1600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.