Abstract
Skeletal muscle is adapting to the needs of the body by changes of various gene expression that control mitochondrial biogenesis, angiogenesis, and the composition of muscle fiber types. Recently, it was revealed that PGC-1α, which is an auxiliary transcription factor, plays a key role in the aforementioned adaptation phenomena. It means that various signal transduction systems within muscle directly affect the expression and activation of PGC-1α and also PGC-1s activates various programs for muscle adaptation. Therefore, this review assessed PGC-1α to understand the reaction and adaptation phenomena of muscle against the biological stimulus such as exercise and came to the conclusion that PGC-1α and PGC-1β significantly affect skeletal muscle in various ways, and also have an affect on the increase of exercise capacity, inducing of angiogenesis and the prevention of muscle atrophy and degeneration.
Keywords: exercise, mitochondrial biogenesis, PGC-1α, skeletal muscle
1. Introduction
Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) is an auxiliary transcription activating factor (transcriptional coactivator), and it controls the genes related to energy metabolism. PGC-1α also controls mitochondrial biogenesis and its functions1 and it has complicated interaction with transcription factors, using the interaction with nuclear hormone receptor peroxisome proliferator-activated receptor-r (PPAR-r)-γ, and it controls interactions or activity level of cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) and nuclear respiratory factors (NRFs). Also, PGC-1α directly connects exogenous physiological stimulus and mitochondrial biogenesis and controls them, and it is a main factor of deciding the type of muscle fiber.
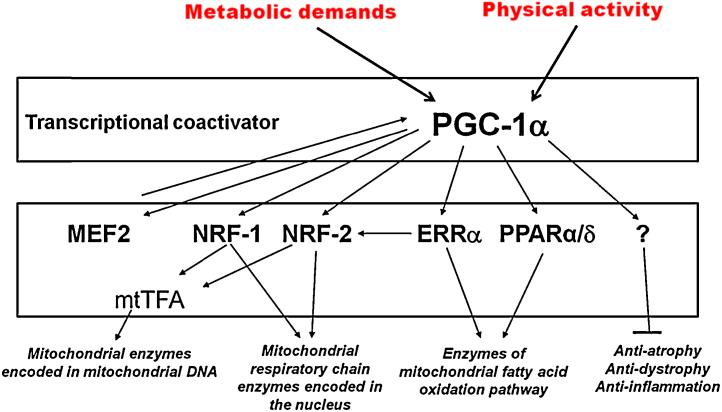
PGC-1α is structurally composed of the N-terminal region (aa1-200), the middle region (aa200-400), and C-terminal region (aa400-797).2 The N-terminal region includes transactivation domain (TAD) and two auxiliary activation factors—steroid receptor coactivator-1 (SRC-1) and CREB-binding protein (CBP)/p300 are combined.1 The lower area of TAD, where leucine is abundant, not only controls interaction with nuclear receptors activated by the ligand but also controls interaction with various transcription factors such as Nuclear respiratory factor 1(NRF1), myocyte enhancer factor-2C (MEF2C), and forkhead box protein O1 (FOXO1).3, 4, 5 Its middle region of TAD is where p160 myb binding protein (p160MBP) is combined and it plays the role of limiting PGC-1α.6 PGC-1α’s C-terminal region contains RNA recognition motifs7 and it controls protein stability.8 The role of PGC-1α in muscle plasticity is illustrated in Fig. 1.
Fig. 1.
Schematic of the role of PGC-1α in muscle plasticity.
ERRα, Estrogen related receptor alpha; MEF2, myocyte enhancer factor-2; NRF, nuclear respiratory factor; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PPARα/δ, Peroxisome proliferator-activated receptor.
2. Function of mitochondria and PGC-1α
Skeletal muscle comprises the biggest portion of total body mass and is the most active part, especially when there is an increase in physical activity; it increases mitochondria's oxidative function and thus maintains and controls the body's overall energy balance. To activate mitochondria's function in skeletal muscle, it is important to activate several signal transduction mechanisms including Ca2+-regulated CaMKIV-calcineurin/NFAT and MEF2 axis, adrenergic/cholinergic signaling and AMP-activated protein kinase (AMPK). Such signal/transcription mechanisms are activating PGC-1α and it was reported that the mouse, which had an overexpression of PGC-1α in the skeletal muscle with gene manipulation, had an increased amount of mitochondria and increased transition of muscle fiber into slow muscle fiber, which has a higher oxidizing power.9
On the contrary, different mouse from previous paragraph, which had removed PGC-1α in skeletal muscle, had a lack of mitochondrial protein expression and amyotrophy and with such results, we think PGC-1α not only controls mitochondrial biogenesis but also controls gene expression.10 Studies using animals and cells reported evidence of the role of PGC-1α on mitochondrial protein expression,11, 12 Glucose transporter 4 (GLUT4),13 Pyruvate dehydrogenase kinase 4 (PDK4),14 and angiogenesis within skeletal muscle.15 Nevertheless, there is not enough validation on whether PGC-1α is actually playing the role of inducing exercise-induced adaptation phenomenon or which area of skeletal muscle adaptation phenomenon will be affected by the absence of functional PGC-1α. Leick et al16 reported that although the level of expression of metabolic enzymes was reduced during a rest period for the PGC-1α-knock out (KO) mouse, hexokinase II, aminolevulinate synthase 1, and cytochrome oxidase (COX) I protein expressions were increased after endurance exercise. From such results, Leick et al16 came to the conclusion that PGC-1α is not an essential factor for exercise or training-induced adaptive gene response. Also, Adhihetty et al17 reported that there was no reduction of endurance exercise capacity when a PGC-1α-KO mouse was taking a rest, even though mitochondrial respiratory function was decreased. However, it was reported that the PGC-1α-KO mouse showed overactivity as a result of the damage of the central nervous system with abnormal circadian rhythm and AMPK activation within skeletal muscle was shown even during a rest.18, 19 Therefore, it is necessary to reduce potential polluting factors as much as possible, caused by PGC-1α-KO of the entire body because of the specific destruction of genes within skeletal muscle.
Recently, the mouse was identified (PGC-1α-MKO), which had specific PGC-1α-KO within skeletal muscle.11 The PGC-1α-MKO mouse showed the decrease of activity and maximal exercise capacity, the damage of muscle function and the reduction of oxidative metabolism capacity. Although IIb-to-IIa fiber type transition within skeletal muscle during the voluntary activity and exercise was normal for the PGC-1α-MKO mouse, there was weakening of the expression of endurance exercise-induced mitochondria enzymes (cytochrome c, COX IV) and the proliferation of platelet endothelial cell adhesion molecule-1-positive endothelial cells.20 With such results, we think it was confirmed that PGC-1α plays an important role in mitochondrial biogenesis and angiogenesis caused by endurance exercise.
3. Types of PGC-1α
PGC-1α has various biological functions within diverse tissues including muscle, and most of their activities are related to oxidative metabolism. PGC-1α and PGC-1β are highly expressed in oxidative tissues such as heart, kidney, and muscle.21 If PGC-1α and PGC-1β are expressed, mitochondrial biogenesis is induced and cellular respiration is increased. For PGC-1α−/− and PGC-1β−/− animals, energy metabolism of skeletal and heart muscle was abnormal22, 23 and the PGC-1α−/− mouse had a higher risk of heart attack because of stress.24 If the expression within skeletal muscle was done by gene manipulation, both PGC-1α and PGC-1β experience a significant increase in mitochondrial biogenesis.15, 24 Gene-manipulated muscle showed an increase of oxidizing power, fatigue-resisting ability, and endurance exercise capacity.25
PGC-1α’s methods of increasing mitochondrial biogenesis are being revealed with various studies and it activates nuclear-encoded genes related to mitochondrial biogenesis through auxiliary activation of transcription factors such as NRF-1, NRF-2, and ERRα. Such factors activate or interact with regulatory regions of various mitochondria genes encoded within nucleus. Fatty acid oxidation genes are controlled by coactivation of nuclear receptor PPAR-α. Also, PGC-1α increases the expression of Tfam, factors B1 and B2. Such factors are the proliferation and transcription control factors of the mitochondrial genome. From a study on the gene manipulation of mice, defective mitochondria was clearly shown after eliminating PGC-1α or PGC-1β in various tissues.26, 27 However, mitochondria's functions were maintained in such mice.28, 29
4. Type of muscle fiber and PGC-1α
Type I, which is skeletal muscle's slow muscle fiber, and type IIa, which is fast muscle fiber, have many mitochondria and relatively high oxidizing power. Type IIb fiber has low density of mitochondria and high relevant capacity. It is well know that PGC-1α reconfigures the fiber formation of skeletal muscle and in general, glycolytic type IIb fiber is reduced compared to oxidative type I and type IIa fibers. PGC-1α within skeletal muscle is easily expressed from both short-term exercise and endurance training.30, 31, 32 Until recently, PGC-1α’s biological functions on the structure and functions of skeletal muscle was largely revealed thanks to the use of gene-manipulated mouse model. The mouse with over-expression of skeletal muscle-specific PGC-1α had a transition rate of 20% and 10% in plantaris muscle, respectively, for type IIa and type I fibers of fast-twitch type IIb muscle fibers; in addition, genes related to mitochondria oxidative metabolism were activated. Also, resistance to fatigue was increased after providing electrical stimulus on the separated muscle from MCKPGC-1transgenic mouse.10
Recently, Mortensen et al33 reported that after overexpressing PGC-1α in skeletal muscle fiber of mice, messenger RNA (mRNA) increase of myosin heavy chain (MHC) isoform (MHCIb) related to the oxidation of slow muscle and mRNA decrease of MHC isoforms (MHCIIx and MHCIIb) related to fast muscle fibers were observed.33 Mice lacking PGC-1α had a decreased number of mitochondria; thus, respiratory capacity within slow muscle fiber was reduced and therefore a decrease of endurance exercise capacity and resistance to fatigue occurred.22
5. PGC-1α’s activation mechanism during exercise
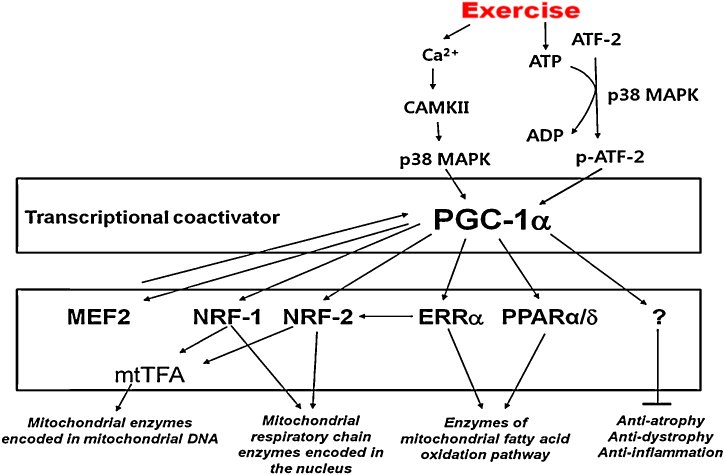
Signaling pathways are illustrated in Fig. 2. Higher signal, which activates PGC-1α, is not clearly revealed yet but several mechanisms were suggested—calcineurin A, CaMK, p38 MAPK, and AMPK pathways.34, 35 Although the importance of such kinases’ controlling ability of PGC-1α is not yet clearly known, many studies reported that calcineurin and CaMK pathways play an important role in the expression of PGC-1α. Increased neuromuscular input and muscle contraction from exercise express various transcription factors such as MEF2 and CREB36 and it was reported that such phenomenon was induced by calcineurin and CaMK.35 An increase of MEF2 expression increases the bonding of MEF2 onto the promoter region of PGC-1α genes, and that will increase the expression of PGC-1α. PGC-1α is directly combined to MEF2 and works as an assistant factor in the transcription activity of genes either included in the characteristics of slow muscle fiber or related to mitochondrial oxidative metabolism.36 Also, according to the recent study of Garcia-Roves et al,37 calcineurin is not required for the increase of exercise-induced PGC-1α and the protein expression within mitochondria. Further studies are necessary to clearly define the control mechanism of PGC-1α in calcineurin and calcium signaling pathways.
Fig. 2.
Signaling pathways involved in exercise-induced PGC-1α regulation in skeletal muscle.
ADP, adenosine diphosphate; ATF2, activating transcription factor; ATP, adenosine triphosphate; CAMKII, calcium/calmodulin-dependent protein kinase; ERRα, Estrogen related receptor alpha; MAPK, mitogen activated protein kinase; MEF2, myocyte enhancer factor-2C; NRF, nuclear respiratory factor; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PPARα/δ, Peroxisome proliferator-activated receptor.
Endurance exercise activates PGC-1α genes in skeletal muscle of humans and activated PGC-1α is stimulating mitochondrial biogenesis with two methods.38 First, PGC-1α’s activation becomes rapid in the early stage and after that, a long term increase follows based on the increased expression of PGC-1α. The phenomenon of an early activity increase was proven with various evidences and gene transcription and expression of first mitochondrial protein is increasing in a similar speed or faster than PGC-1α expression caused by exercise stimuli. Second NRF-1 and NRF-2 will combine with their response promoters prior to PGC-1α expression. Third, most of PGC-1α will be found in the cytoplasm of skeletal muscle during a rest period but it will move into the nucleus during exercise. To summarize, exercise rapidly activates PGC-1α prior to the increase of PGC-1α expression and thus increases mitochondrial biogenesis.39
Recently, two types of protease [p38 mitogen activated protein kinase (p38 MAPK), AMPK] were found that activate PGC-1α during exercise.40, 41 Sarcolemma T-tubule's nerve is stimulated during muscle contraction, and calcium/calmodulin-dependent protein kinase (CAMKII) is activated when calcium ion concentration in cytoplasm is increased by the secretion of calcium from the sarcoplasmic reticulum caused by muscle fiber contraction; CAMKII is p38 MAPK's higher signaling molecule and plays the role of connecting between muscle activity and phosphorylation of PGC-1α.39 Another important signaling molecule is AMP, which is generated by the hydration of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) because of muscle contraction. On the one hand, when AMP levels are increased in cells, AMPK is activated and thus mitochondrial biogenesis42 and phosphorylation of PGC-1α are increased.41 On the other hand, p38 MAPK is a signaling molecule that plays the role of maintaining and increasing mitochondrial biogenesis after the initial stage of endurance exercise. We can see that p38 MAPK not only activates PGC-1α with exercise stimuli but also increases the expression of PGC-1α because there is an increase of PGC-1α promoter activity if p38 MAPK mechanism was stimulated, and also, there is no change in activity when p38 MAPK inhibitor was used.34 Activating transcription factor 2 (ATF2) and myocyte enhancer factor 2 (MEF2) are PGC-1α’s higher transcription factors and they are activated by phosphorylation of p38 MAPK.43
Summarizing all the previous studies, mitochondrial biogenesis is increased by PGC-1α activation prior to the expression of PGC-1α in the early stage of endurance exercise, and long-term endurance exercise activates PGC-1α transcription factor by stimulating p38 MAPK and thus increases PGC-1α expression and therefore increases mitochondrial biogenesis. Although various evidence data were reported, a clear conclusion remains elusive and thus we believe continuous studies will be necessary.
Table 1 details exercise-induced PGC-1α regulation in skeletal muscle.
Table 1.
Exercise-induced PGC-1α regulation in skeletal muscle of rodents and humans.
| Exercise type | Exercise program | Subjects | Effects (in skeletal muscle) | References |
|---|---|---|---|---|
| Running exercise | 4 wk | C57BL/6 mice | ↑PGC-1α ↓miR-696 |
Aoi et al45 |
| Wheel running | 1, 2, 4, 6, 8 wk | Female ICR mice | ↑PGC-1α, ↑GLUT4, ↑mitochondrial proteins: soleus, plantaris muscle ↔PGC-1α, ↑GLUT4, ↑mitochondrial proteins: tibialis anterior |
Ikeda et al46 |
| Ladder climbing | High intensity 3 d/wk 8 wk |
Middle-aged male rat | ↑PGC-1α ↑AMPK ↑Mitochondria biogenesis |
Kim et al47 |
| Treadmill running | 0.8 or 1.2 km/h 50 min/d 5 d/wk 8 wk |
Middle-aged male rats | ↑SIRT-1 ↑AMPK ↑PGC-1α ↑Metabolic enzymes |
Oliveira et al48 |
| Aerobic exercise | High intensity | Trained versus untrained man | PGC-1α: trained = untrained TFAM: trained > untrained TFB2M: trained > untrained |
Popov et al49 |
| Cycle ergometer | 85% HRmax 30 min/d 5 d/wk 6 wk |
Healthy human | ↑PGC-1α ↑Lipogenesis |
Summermatter et al50 |
| Swimming exercise | Low-intensity 6 h/d 1 d |
4–5-week-old male Sprague-Dawley rats | ↑PGC-1α ↑PGC-1 mRNA expression ↑AMPK |
Terada et al51 |
| Treadmill running versus swimming |
Run (13 m/min, 3 h × 2 sessions, 45 min rest) Swim (3 h × 2 sessions, 45 min rest) 1 day |
5–6-week-old male rats | ↑PGC-1α - Running: only in soleus muscle - Swimming: only in epitrochlearis |
Terada et al52 |
AMPK, AMP-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; miR, microRNA; GLUT4, glucose transporter 4; SIRT-1, Silent Information Regulator 1; TFAM, Mitochondrial transcription factor A; TFB2M, Transcription factor B2 mitochondrial.
6. Conclusion
PGC-1α’s expression and activation mechanism are variously reported but the control mechanism of a response process in skeletal muscle caused by outside stimuli is still unclear. It is also unclear how PGC-1α is induced when oxygen and nutrient are lacking and what mechanism is inducing PGC-1α during exercise, and those are still problems that need to be solved. Additional studies are necessary on what method PGC-1α is using to combine such complicated signals.
We think the control after the transcription of PGC-1s also plays an important role. PGC-1α’s role in endurance training-induced angiogenesis and mitochondrial biogenesis and muscle fiber type transition needs to be clearly defined. Changes in skeletal muscle caused by PGC-1α affect other tissues, and studies are actively ongoing on the question of the effects and what organs are involved in this mutual communication, and some of anti-inflammatory functions of PGC-1α are also being revealed. However, the most significant finding is that PGC-1α and PGC-1β affect skeletal muscle in various aspects; the effect on the increase of exercise capacity, inducing angiogenesis, and the prevention of muscle atrophy and degeneration is obvious.
As a result, understanding biological roles of PGC-1s on skeletal muscle adaptation will help in the future treatment of disease in skeletal muscle by PGC-1s induction using medications; efforts from various angles will be necessary.
Conflicts of interest
I certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Puigserver P., Adelmant G., Wu Z., Fan M., Xu J., O’Malley B. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 2.Chang J.C., Kou S.J., Lin W.T., Liu C.S. Regulatory role of mitochondria in oxidative stress and atherosclerosis. World J Cardiol. 2010;2:150–159. doi: 10.4330/wjc.v2.i6.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 4.Vega R.B., Huss J.M., Kelly D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puigserver P., Spiegelman B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 6.Fan M., Rhee J., St-Pierre J., Handschin C., Puigserver P., Lin J. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18:278–289. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monsalve M., Wu Z., Adelmant G., Puigserver P., Fan M., Spiegelman B.M. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol Cell. 2000;6:307–316. doi: 10.1016/s1097-2765(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 8.Sano M., Tokudome S., Shimizu N., Yoshikawa N., Ogawa C., Shirakawa K. Intramolecular control of protein stability, subnuclear compartmentalization, and coactivator function of peroxisome proliferator-activated receptor gamma coactivator 1alpha. J Biol Chem. 2007;282:25970–25980. doi: 10.1074/jbc.M703634200. [DOI] [PubMed] [Google Scholar]
- 9.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 10.Handschin C., Chin S., Li P., Liu F., Maratos-Flier E., Lebrasseur N.K. BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 11.Handschin C1, Rhee J., Lin J., Tarr P.T., Spiegelman B.M. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman JJ1, Barger P.M., Kovacs A., Saffitz J.E., Medeiros D.M., Kelly D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michael L.F., Wu Z., Cheatham R.B., Puigserver P., Adelmant G., Lehman J.J. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wende A.R., Schaeffer P.J., Parker G.J., Zechner C., Han D.H., Chen M.M. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 15.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leick L., Wojtaszewski J.F., Johansen S.T., Kiilerich K., Comes G., Hellsten Y. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 17.Adhihetty P.J., Uguccioni G., Leick L., Hidalgo J., Pilegaard H., Hood D.A. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol. 2009;297:C217–C225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- 18.Lin J., Wu P.H., Tarr P.T., Lindenberg K.S., St-Pierre J., Zhang C.Y. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Liu C., Li S., Liu T., Borjigin J., Lin J.D. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 20.Geng T., Li P., Okutsu M., Yin X., Kwek J., Zhang M. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C572–C579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 22.Leone T.C., Lehman J.J., Finck B.N., Schaeffer P.J., Wende A.R., Boudina S. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Arany Z., Lebrasseur N., Morris C., Smith E., Yang W., Ma Y. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Calvo J.A., Daniels T.G., Wang X., Paul A., Lin J., Spiegelman B.M. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 26.Aronson D., Boppart M.D., Dufresne S.D., Fielding R.A., Goodyear L.J. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res Commun. 1998;251:106–110. doi: 10.1006/bbrc.1998.9435. [DOI] [PubMed] [Google Scholar]
- 27.Chavez J.A., Holland W.L., Bär J., Sandhoff K., Summers S.A. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 28.Sonoda J., Mehl I.R., Chong L.W., Nofsinger R.R., Evans R.M. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lelliott C.J., Medina-Gomez G., Petrovic N., Kis A., Feldmann H.M., Bjursell M. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 31.Goto M., Terada S., Kato M., Katoh M., Yokozeki T., Tabata I. cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun. 2000;274:350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 32.Norrbom J., Sundberg C.J., Ameln H., Kraus W.E., Jansson E., Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen O.H., Frandsen L., Schjerling P., Nishimura E., Grunnet N. PGC-1alpha and PGC-1beta have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am J Physiol Endocrinol Metab. 2006;291:E807–E816. doi: 10.1152/ajpendo.00591.2005. [DOI] [PubMed] [Google Scholar]
- 34.Akimoto T., Pohnert S.C., Li P., Zhang M., Gumbs C., Rosenberg P.B. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 35.Wu H., Kanatous S.B., Thurmond F.A., Gallardo T., Isotani E., Bassel-Duby R. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 36.Czubryt M.P., McAnally J., Fishman G.I., Olson E.N. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Roves P.M., Huss J., Holloszy J.O. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1172–E1179. doi: 10.1152/ajpendo.00633.2005. [DOI] [PubMed] [Google Scholar]
- 38.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright D.C., Han D.H., Garcia-Roves P.M., Geiger P.C., Jones T.E., Holloszy J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 40.Puigserver P., Rhee J., Lin J., Wu Z., Yoon J.C., Zhang C.Y. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 41.Jäger S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winder W.W., Holmes B.F., Rubink D.S., Jensen E.B., Chen M., Holloszy J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.H., Ko J.H., Kim P.S., Kim K.J. Mechanism for exercise induced PGC-1α transcription in skeletal muscle. Exercise Science. 2013;22:203–211. [Google Scholar]
- 45.Aoi W., Naito Y., Mizushima K., Takanami Y., Kawai Y., Ichikawa H. The microRNA miR-696 regulates PGC-1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. 2010;298:E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda S., Kawamoto H., Kasaoka K., Hitomi Y., Kizaki T., Sankai Y. Muscle type-specific response of PGC-1 alpha and oxidative enzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol (Oxf) 2006;188:217–223. doi: 10.1111/j.1748-1716.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 47.Jung S.R., Ahn N.Y., Kim S.H., Kim K.J. The effects of ladder climbing exercise training on PCG-1α expression and mitochondrial biogenesis of skeletal muscle in young and middle-aged rats. Exerc Sci. 2014;23:339–345. [In Korean, English abstract] [Google Scholar]
- 48.Oliveira N.R., Marques S.O., Luciano T.F., Pauli J.R., Moura L.P., Caperuto E. Treadmill training increases SIRT-1 and PGC-1 α protein levels and AMPK phosphorylation in quadriceps of middle-aged rats in an intensity-dependent manner. Mediators Inflamm. 2014;2014:987017. doi: 10.1155/2014/987017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Popov D.B., Zinovkin R.A., Karger E.M., Tarasova O.S., Vinogradova O.L. The effects of aerobic exercise upon genes expression in skeletal muscle of trained and untrained men. Fiziol Cheloveka. 2013;39:92–98. doi: 10.7868/s0131164613020124. [DOI] [PubMed] [Google Scholar]
- 50.Summermatter S., Baum O., Santos G., Hoppeler H., Handschin C. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J Biol Chem. 2010;285:32793–32800. doi: 10.1074/jbc.M110.145995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terada S., Goto M., Kato M., Kawanaka K., Shimokawa T., Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 52.Terada S., Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab. 2004;286:E208–E216. doi: 10.1152/ajpendo.00051.2003. [DOI] [PubMed] [Google Scholar]