Abstract
Hyperglycemia is the major risk factor for microvascular complications in patients with type 2 diabetes (T2D). However, cardiovascular disease (CVD) is the principal cause of death, and lowering HbA1c has only a modest effect on reducing CVD risk and mortality. The recently published LEADER and SUSTAIN-6 trials demonstrate that, in T2D patients with high CVD risk, the glucagon-like peptide 1 receptor agonists liraglutide and semaglutide reduce the primary major adverse cardiac events (MACE) end point (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke) by 13% and 24%, respectively. The EMPA-REG OUTCOME, IRIS (subjects without diabetes), and PROactive (second principal end point) studies also demonstrated a significant reduction in cardiovascular events in T2D patients treated with empagliflozin and pioglitazone. However, the benefit of these four antidiabetes agents (liraglutide, semaglutide, empagliflozin, and pioglitazone) on the three individual MACE end points differed, suggesting that different underlying mechanisms were responsible for the reduction in cardiovascular events. Since liraglutide, semaglutide, pioglitazone, and empagliflozin similarly lower the plasma glucose concentration but appear to reduce CVD risk by different mechanisms, there emerges the intriguing possibility that, if used in combination, the effects of these antidiabetes agents may be additive or even multiplicative with regard to cardiovascular benefit.
Individuals with type 2 diabetes (T2D) have a twofold increased risk for cardiovascular disease (CVD) (myocardial infarction, stroke, peripheral vascular disease), and CVD is the principal cause of death in T2D patients (1). Clinical trials (2–5) consistently have demonstrated that lowering HbA1c in T2D patients has no (2,3) or only a modest (4,5) effect on reducing cardiovascular (CV) risk. In contrast, correction of traditional CVD risk factors, e.g., blood pressure and cholesterol, markedly reduces CVD risk and mortality in patients with T2D. The recently published LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) (6) and SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes) (7) trials provide evidence that glucagon-like peptide 1 receptor agonists (GLP-1 RAs) (liraglutide and semaglutide) reduce CVD risk beyond their glucose-lowering effect and improvement in other CVD risk factors in T2D patients with established CVD. Together with EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) (8), IRIS (Insulin Resistance Intervention After Stroke Trial) (9), and PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) (10)—which showed reduction in major adverse cardiac events (MACE) end points of 14%, 24%, and 16% (main secondary end point), respectively—these studies make it clear that we are entering a new era in T2D management, where the newer antidiabetes medications, in addition to lowering plasma glucose, also exert a CV protective effect that is independent of reduction in plasma glucose concentration and traditional CVD risk factors.
CV RISK IN T2D
The major benefit of reducing plasma glucose levels in T2D is prevention of long-term microvascular complications and, to lesser extent, macrovascular complications. Individuals with T2D have two- to threefold greater risk of CV events compared with subjects without diabetes, and CVD is responsible for ∼80% of the mortality in T2D (1). Hyperglycemia is a weak risk factor for CVD (5,11), and interventions (2–4) focused on reducing plasma glucose have failed to significantly reduce CV risk and mortality, particularly in secondary prevention trials. Moreover, in the United Kingdom Prospective Diabetes Study (UKPDS) (11) and Veterans Affairs Diabetes Trial (VADT) (12), it took 10 years to observe the CV benefit associated with improved glycemic control. Most individuals with T2D manifest moderate to severe insulin resistance, which is associated with multiple CV risk factors (obesity, dyslipidemia, hypertension, endothelial dysfunction, procoagulant state). This cluster of CV/metabolic disturbances is known as insulin resistance (metabolic) syndrome and is a principal factor responsible for increased CV risk in T2D (13,14). A multifactorial intervention that improves CV risk factors has been shown to reduce CV events and mortality in T2D (15). Further, the molecular mechanisms responsible for insulin resistance directly contribute to pathogenesis of atherosclerosis, independent of associated metabolic abnormalities (13,14). Thus, obese individuals without diabetes but with insulin resistance syndrome manifest a similarly increased risk for CVD compared with T2D patients, supporting the concept that hyperglycemia is not the major risk factor for CVD (16). Consequently, it is not surprising that lowering blood pressure and improving the lipid profile lead to greater reduction in CVD risk than lowering plasma glucose in T2D. Consistent with this, antidiabetes agents like insulin (17), sulfonylureas (18,19), and dipeptidyl peptidase 4 (DPP-4) inhibitors (20–22), which lower plasma glucose without affecting insulin resistance or other metabolic abnormalities associated with insulin resistance syndrome, do not lower CVD risk and mortality in T2D. Conversely, pioglitazone, which improves insulin sensitivity and multiple components of insulin resistance syndrome, i.e., blood pressure, lipids, and endothelial dysfunction (23), exerts a favorable effect on CVD risk in T2D, independent of its glucose-lowering action (9,10). In PROactive (9) pioglitazone lowered MACE (CV death, nonfatal myocardial infarction [MI], nonfatal stroke), which was the main secondary end point, by 16% (P = 0.027), and in IRIS (9) pioglitazone reduced the incidence of recurrent stroke and MI by 24% in insulin-resistant individuals without diabetes who had suffered a recent transient ischemic attack or stroke.
LEADER AND SUSTAIN-6
LEADER (6) and SUSTAIN-6 (7) examined the effect of once-daily liraglutide and once-weekly semaglutide on CV risk (Supplementary Table 1). In LEADER (6), 9,340 T2D patients at high CVD risk (82% with prior CV event) were randomized to liraglutide, 1.8 mg/day, or placebo for a mean of 3.8 years. Investigators were blinded to the study intervention and instructed to maintain HbA1c <7.0% with any antidiabetes medication except a GLP-1 RA or DPP-4 inhibitor. The primary outcome was 3-point MACE. Liraglutide-treated patients experienced a 13% reduction in MACE, which was driven by a 22% reduction in CV mortality (P = 0.007). Nonfatal MI was decreased by 12% (P = 0.11), while nonfatal stroke was reduced by 11% (P = 0.30).
In SUSTAIN-6 (phase 3 trial) (7), 3,297 T2D patients at high risk for CVD were randomized to semaglutide, 0.5 and 1 mg/week, or placebo and followed for 104 weeks. This study was designed to demonstrate noninferiority, which accounts for the smaller number of subjects. The primary outcome was 3-point MACE. Most participants (83%) had established CVD and the remaining 17% were >60 years of age with multiple CV risk factors that were well controlled. Investigators were instructed to lower HbA1c to <7.0% according to local guidelines without using incretin-based therapies. Subjects receiving semaglutide experienced greater HbA1c reduction than those on placebo (1.4% vs. 0.4%). This difference (1.0%) in HbA1c was considerably greater than in LEADER (0.4%). Weight loss (4 kg) and systolic blood pressure reduction (3 mmHg) were twice as great in SUSTAIN-6 versus LEADER. In SUSTAIN-6, the primary outcome (3-point MACE) was reduced by 26%, and that decrease was driven primarily by a 39% reduction in nonfatal stroke (P = 0.04) and a 26% reduction in nonfatal MI (P = 0.12); CV mortality was not decreased (hazard ratio [HR] = 0.98). Of note, the placebo group had significantly more revascularization procedures than the semaglutide group, which could have reduced future deaths; it may be that the similar overall CV death rate between the two groups resulted from the increased number of revascularization procedures in the control group.
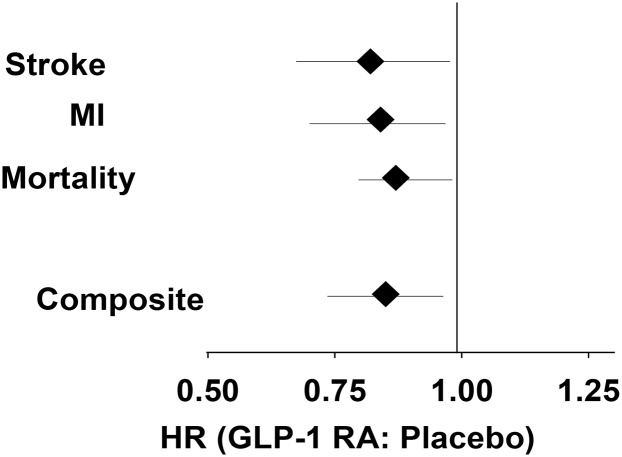
Two aspects of LEADER and SUSTAIN-6 deserve emphasis: 1) patients at higher CVD risk benefited more from GLP-1 RA treatment, and 2) the benefit of liraglutide and semaglutide was evident on top of optimal control of traditional CV risk factors. In a meta-analysis of the two long-acting GLP-1 RAs (Fig. 1), 3-point MACE was decreased by 15%, with similar and significant benefit for all three components: nonfatal stroke, nonfatal MI, and mortality by 18%, 16%, and 13%, respectively.
Figure 1.
Effect of long-acting GLP-1 RAs on CVD outcome. Data are combined from LEADER (6) and SUSTAIN (7).
However, there are some concerns about the generalizability of findings in LEADER. In the 23% of participants without a prior CV event, there was no reduction in MACE (HR = 1.20, P not significant). There was more sulfonylurea and insulin use in the placebo group. In SUSTAIN-6, the number of participants was one-third of that in LEADER, the follow-up was short (two years), the decrement in HbA1c (1.0%) was much greater in the treatment group than the placebo group (although hyperglycemia is not considered to be a major risk factor for CVD), and the incidence of serious eye complications (vitreous hemorrhage, blindness, and photocoagulation) was significantly increased.
PIOGLITAZONE AND CVD
In PROactive, 5,238 patients with T2D with established CVD (population similar to EMPA-REG OUTCOME) were randomized to pioglitazone or placebo plus standard of care for CV risk factors and glycemic control (10). The 3-point MACE, the “main secondary end point,” was significantly reduced (HR = 0.84, P = 0.027). The primary end point (3-point MACE plus coronary and leg revascularization) did not reach statistical significance (HR = 0.90, P = 0.09); however, it is now well recognized that peripheral vascular disease is refractory to antihypertensive, lipid-lowering, and glucose-lowering therapy (24,25). Further, by preserving people from death, MI, and stroke, pioglitazone would make more people available for leg revascularization (26).
In IRIS (9), 3,876 insulin resistant (HOMA-IR >3.0) nondiabetic individuals with a recent (within 6 months) ischemic stroke or transient ischemic attack were randomized to pioglitazone or placebo for 4.8 years. Subjects receiving pioglitazone experienced a 24% reduction in fatal/nonfatal stroke plus MI (HR 0.76, P = 0.007); mortality was reduced slightly (by 7%) but not significantly.
Meta-analysis was performed to examine the combined effect of the treatments compared with the placebo-treated control group. Outcomes were expressed as risk ratios and combined risk difference (with 95% CI) and were calculated using a fixed effect model. To examine the appropriateness of the model, Cochran's Q was calculated to measure inconsistency between studies and I2 was calculated to describe the percentage of variation. CMA statistical package was used for the analysis. Differences were considered significant at P < 0.05 (Fig. 2 and Supplementary Table 1).
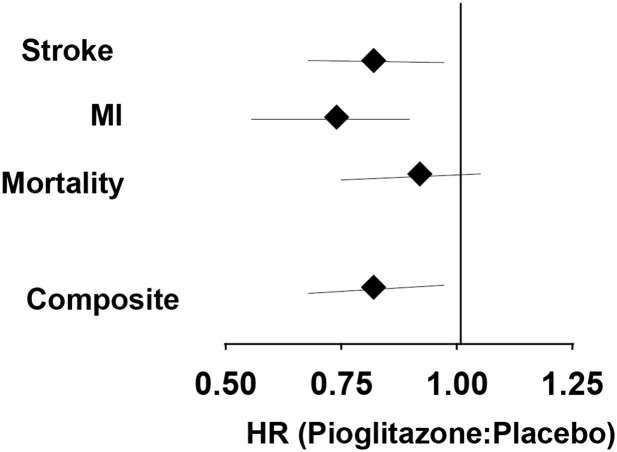
Figure 2.
Effect of pioglitazone on CVD outcomes. Data are combined from PROactive (10) and IRIS (9).
In a meta-analysis of PROactive plus IRIS (Fig. 2), pioglitazone reduced 3-point MACE by 18%, with the main effect being driven by an 18% reduction in stroke and a 26% reduction in MI, while total mortality decreased, but not significantly, by 8%.
EMPA-REG OUTCOME STUDY
In EMPA-REG OUTCOME, empagliflozin caused a 14% reduction (P = 0.04 for superiority) in 3-point MACE in 7,020 patients with T2D with established CVD over 3.1 years (Supplementary Table 1). Several outcomes were surprising and different from LEADER, SUSTAIN-6, PROactive, and IRIS. First, the primary outcome was driven by a robust 38% reduction in CV mortality. Second, there was a striking disconnect between the three MACE components (Fig. 3): 1) for nonfatal MI, HR (0.87) decreased slightly but not significantly (P = 0.22); 2) for nonfatal stroke, HR (1.24) increased slightly but not significantly (P = 0.22); 3) for CV death, HR (0.62) decreased markedly and significantly by 38% (P = 0.001). Third, unlike in LEADER, SUSTAIN-6, and PROactive, separation between the empagliflozin and placebo curves occurred very early, such that reduction in the primary outcome was evident at 3 months after starting treatment.
Figure 3.
Effect of empagliflozin on CVD outcomes in the EMPA-REG OUTCOME trial (8).
POTENTIAL MECHANISMS TO EXPLAIN CV BENEFIT
Glucose Control
Although investigators were instructed to maintain HbA1c <7.0% in all trials, subjects treated with the active drug experienced significantly lower HbA1c than patients receiving placebo (0.3% in EMPA-REG OUTCOME, 1.0% in SUSTAIN-6, 0.4% in LEADER, 0.5% in PROactive). However, it is unlikely that such HbA1c differences over 2–4 years can explain the difference in primary outcome (MACE). First, hyperglycemia is a weak risk factor for CV disease. Intensive glycemic control failed to decrease CV events in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (2), Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) (3), and VADT (4) studies in T2D patients with long-standing diabetes duration, and in UKPDS (5,11) and VADT (12) it took 10 years to demonstrate a small (∼10%), though significant, reduction in CV events in newly diagnosed individuals. The beneficial CV effects of empagliflozin, liraglutide, and pioglitazone were evident after 3, 18, and 24 months, respectively. More conclusively, IRIS participants were normoglycemic, making it unlikely that improved glucose control was a contributory factor to the reduction in MI and stroke. The HbA1c difference between semaglutide and placebo in SUSTAIN-6 was clinically meaningful (1.0%), but similar HbA1c reductions in ACCORD and ADVANCE had no benefit on MACE. Of note, in both LEADER and SUSTAIN-6 the majority of treatment intensification in the placebo group was done with insulin and sulfonylureas, which have been associated with increased atherosclerotic CV events in some studies (14,18,19), although no randomized control trials have demonstrated such an adverse effect.
Prevention of Atherosclerosis
Reduction in CV events in LEADER, SUSTAIN-6, IRIS, and PROactive started at 1–2 years after initiation of therapy and widened thereafter. This time course is reminiscent of interventions that slow atherosclerosis, e.g., statins and blood pressure–lowering therapy. Similar to results with statins and antihypertensive medications, MI and stroke were reduced in SUSTAIN-6, LEADER, and PROactive, although the magnitude of reduction varied (Figs. 1 and 2); the effect on mortality varied most among the three studies. Pioglitazone improves multiple CV risk factors (blood pressure, triglycerides, plasminogen activator inhibitor 1, endothelial dysfunction, insulin resistance, visceral fat) (23) and has documented antiatherogenic effects in preclinical and clinical studies (9,10,23,27,28). Thus, the beneficial effect of pioglitazone in PROactive and IRIS is likely the result of this thiazolidinedione’s antiatherogenic effect.
GLP-1 RAs also improve many CVD risk factors (obesity, hypertension, dyslipidemia, inflammation, visceral fat) in T2D patients. However, the magnitude of improvement in CV risk factors in SUSTAIN-6 and LEADER was modest (2.3 kg weight loss and 1.2 mmHg decrease in systolic blood pressure in LEADER; 3.6–4.9 kg weight loss and 3.4–5.4 mmHg decrease in systolic blood pressure in SUSTAIN-6). LDL cholesterol was not reported in either study. It is unlikely that these changes in CV risk factors can explain the 13% (LEADER) and 26% (SUSTAIN-6) reduction in primary outcome. In ADVANCE, a greater reduction in systolic blood pressure (5.6/2.2 mmHg) was associated with a nonsignificant 8% reduction in MI, stroke, and CV death. Similarly, it is unlikely that weight loss was the principal factor responsible for reduction in the primary outcome. In Look AHEAD: Action for Health in Diabetes, a mean weight loss of 4 kg in patients with T2D (twice that in liraglutide-treated patients in LEADER), as well as modest reductions in HbA1c, blood pressure, and triglycerides, was associated with a nonsignificant (5%) reduction in MI, stroke, and CV death.
Insulin Sensitization
Insulin resistance is a core defect in T2D and is associated with multiple metabolic and CV risk factors, which collectively are known as insulin resistance (metabolic) syndrome (14,16). Furthermore, the molecular etiology of insulin resistance promotes the development of atherosclerosis (14). It follows that interventions which improve insulin sensitivity might reduce CV events in T2D patients. Although neither insulin resistance nor surrogate markers of insulin resistance were measured in any of the CV outcome trials except IRIS, all active interventions (GLP-1 RAs, sodium–glucose cotransporter 2 inhibitors [SGLT2i], thiazolidinediones) are known to improve insulin sensitivity in T2D. Pioglitazone is a powerful insulin sensitizer in skeletal muscle, liver, and adipocytes (23), and in IRIS (9) HOMA-IR decreased by 24% (P < 0.001). GLP-1 RAs do not have a direct insulin-sensitizing effect, but they promote weight loss, which is associated with enhanced insulin action. Lastly, treatment with dapagliflozin for as little as 2 weeks modestly increases insulin-mediated glucose disposal secondary to reversal of glucotoxicity (29). Thus, improved insulin sensitivity could have contributed to the reduction in MACE in LEADER, SUSTAIN-6, EMPA-REG OUTCOME, PROactive, and IRIS.
Direct Action on CV System
GLP-1 receptors are expressed in the myocardium and vasculature (30), and GLP-1 and GLP-1 RAs can directly affect CV function via multiple mechanisms: 1) direct action on the myocardium; 2) direct effect on blood vessels to increase nitric oxide production, cause vasodilation, and increase blood flow; 3) direct effect on atherosclerotic plaque formation; and 4) change in autonomic nervous system balance (31–33). With respect to the latter, GLP-1 RAs have been shown to stimulate the parasympathetic nervous system (34). This could explain the increase in heart rate, as well as the cardioprotective effect, seen with this class of antidiabetes drugs. Each of these GLP-1 actions potentially could have contributed to decreased CV events in SUSTAIN-6 and LEADER. If GLP-1 RAs increase coronary blood flow, especially in patients with existing heart disease, this effect could reduce ischemia, infarct size, and risk of arrhythmias. A recent study in subjects with normal glucose tolerance demonstrated that GLP-1 infusion augments blood flow in small vessels in skeletal muscle and heart (35), and increased blood flow in small coronary vessels after myocardial ischemia has been shown to predict increased survival and reduced infarct size after MI (36).
Multiple studies in animals and humans (37) have demonstrated a cardioprotective effect of GLP-1 and GLP-1 RAs on myocardial function after ischemic injury. These benefits include decreased infarct size, increased coronary blood flow, improved left ventricular (LV) function, decreased LV filling pressure, and increased survival. Although the cellular/molecular mechanisms of these GLP-1 actions are not fully understood, similar effects of liraglutide and semaglutide on the heart in LEADER and SUSTAIN-6 could have contributed to the decrease in CV events and death. Studies in animals have demonstrated that the postischemic beneficial effects of GLP-1 on the heart are preserved in animals lacking the GLP-1 receptor, suggesting a GLP-1 receptor–independent mechanism (38). In humans, beneficial effects of GLP-1 RAs on LV function and filling pressure have been observed with exenatide (37), liraglutide (39), and native GLP-1 (40).
Lastly, GLP-1 RAs can reduce CV events by slowing the atherosclerotic process. Studies in experimental animals have demonstrated that liraglutide accelerates endothelial recovery after injury (41), retards atheroma formation in apolipoprotein E knockout mice (42), exerts anti-inflammatory effects on the vasculature, and inhibits reactive oxygen species formation and platelet aggregation (43). Collectively, these actions of GLP-1 RAs could slow the atherosclerotic process.
Hemodynamic Action of Empagliflozin to Reduce CVD
The beneficial effect of empagliflozin on CV events differs from that of pioglitazone and GLP-1 RAs with respect to both the time course and the individual components benefited (i.e., MI vs. stroke vs. mortality). The beneficial effect of empagliflozin on MACE was driven by a robust reduction in CV mortality, was rapid in onset, and was associated with a marked decrease in hospitalization for heart failure. Empagliflozin did not significantly reduce the risk of MI, while stroke risk increased slightly. As previously reviewed (44), the impressive reductions in mortality and heart failure most likely are explained by the rapid and simultaneous reductions in blood pressure (afterload reduction), intravascular volume (preload reduction), and arterial stiffness—i.e., hemodynamic effects—and not by slowing of the atherosclerotic process. Consistent with this, a recent preliminary study demonstrated that empagliflozin treatment for 3 months reduced LV mass index and improved diastolic dysfunction (45). Other factors suggested to account for the beneficial CV effects in EMPA-REG OUTCOME have been reviewed and include increased circulating ketone levels, reduced uric acid, and increased angiotensin (1–7) and angiotensin type 2 receptor activity, among others (44). All of these proposed mechanisms focus on nonatherosclerotic processes.
IS THERE ADDITIVE CARDIOVASCULAR BENEFIT FROM COMBINATION THERAPY WITH MULTIPLE AGENTS?
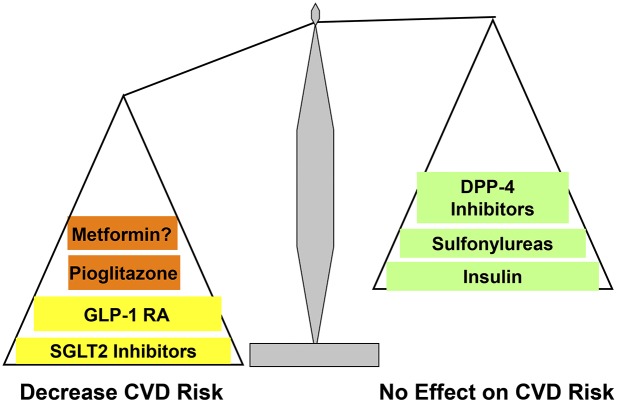
Because the beneficial CV effects of empagliflozin most likely are hemodynamically mediated, while those of GLP-1 RAs and pioglitazone represent a direct action on the vasculature (plus improved CV risk factors) to retard atherogenesis, it is plausible that combination therapy with empagliflozin plus pioglitazone and/or a GLP-1 RA will exert an additive, even synergistic, CV benefit (Fig. 4). Empagliflozin profoundly reduced CV mortality, whereas pioglitazone and GLP-1 RAs primarily reduced the risk of nonfatal MI and nonfatal stroke, so addition of empagliflozin to pioglitazone or a GLP-1 RA may produce a robust reduction in all three MACE components. Well-designed large, randomized, placebo-controlled studies should be performed to examine whether combination therapy with an SGLT2i, GLP-1 RA, and/or pioglitazone can produce an additive effect to further reduce CV events compared with monotherapy with these agents.
Figure 4.
Cardiovascular risk profile of antidiabetes medications.
Combination therapy with an SGLT2i or GLP-1 RA with pioglitazone has other potential benefits. Pioglitazone improves diastolic dysfunction, enhances myocardial insulin sensitivity and reduces myocardial fat content (46), and decreases blood pressure (23). However, these CV benefits can be offset by the drug’s sodium retentive effect on the kidney. Because SGLT2i (47) and, to lesser extent, GLP-1 RAs (31) exert a natriuretic effect, the renal salt retentive effect of pioglitazone will be negated. On the other hand, it is possible that the renal sodium retentive effect of pioglitazone could reduce some of the hemodynamic benefits produced by the volume-depleting effect of the SGLT2i. Since the side effects (fluid retention and weight gain) of pioglitazone are dose related (48), we do not recommend doses in excess of 30 mg/day. Pioglitazone also promotes fat weight gain by stimulation of hypothalamic appetite centers (49). However, the fat weight gain primarily represents a cosmetic concern because the greater the weight gain, the more the decrement in HbA1c and the greater the improvements in β-cell function and insulin sensitivity (50,51). Furthermore, pioglitazone reduces CV events (9,10,28). The weight gain can be negated by combining pioglitazone with an SGLTi or GLP-1 RA or both (52). Combination therapy with an SGLT2i and GLP-1 RA is especially effective in reducing body weight (53). The only drug shown to conclusively reduce liver fat and reverse biopsy-proven nonalcoholic steatohepatitis (NASH) is pioglitazone (54). Both GLP-1 RAs (55) and SGLT2i (56) reduce visceral (hepatic) fat, making combination therapy with any two or three of these drugs an attractive option for preventing/treating NASH and nonalcoholic fatty liver disease (NAFLD), and we recommend that such a study be carried out. In EMPA-REG OUTCOME, empagliflozin reduced the composite end point of renal disease by 39% (57). Although less well appreciated, thiazolidinediones also prevent diabetic nephropathy in diabetic animal models (58), and liraglutide in LEADER significantly reduced the composite renal outcome, although this was primarily due to its effect to decrease proteinuria (6). Thus, combination therapy with any of these three classes of antidiabetes medications may provide an additive renal protective effect. Pioglitazone is a potent insulin-sensitizing agent (23) and markedly enhances and preserves β-cell function (50,51,59). SGLT2i cause a modest improvement in insulin sensitivity (by 33%) and major improvement in β-cell function (by 217%) (29,60). GLP-1 RAs exert a powerful effect to increase β-cell function (52,61–63) and indirectly improve insulin sensitivity by promoting weight loss (62). Thus, SGLT2i, GLP-1 RAs, and pioglitazone represent a triad of antidiabetes medications that, when used in combination, may provide additive effects in preventing CV complications, promoting weight loss, preserving renal function, preventing NASH/NAFLD, and improving β-cell function and insulin sensitivity. Patients treated with GLP-1 RAs plus pioglitazone should have an eye exam before initiating combination therapy with these two agents because of an increased incidence of eye complications in SUSTAIN-6 and rare cases of macular edema with pioglitazone use.
Although the increase in stroke in EMPA-REG OUTCOME did not achieve statistical significance, it nevertheless presents a worry. The mechanisms responsible for any such increased stroke risk, despite decreased blood pressure, are unclear. Since both pioglitazone (9,10) and GLP-1 RAs (6,7) significantly reduce the incidence of stroke in T2D patients, it is possible that addition of a GLP-1 RA or pioglitazone to empagliflozin will prevent any increase in stroke risk. Although results of the Dapagliflozin Effect on Cardiovascular Events (DECLARE) study (due in 2019) and the CANagliflozin cardioVascular Assessment Study (CANVAS)/Study of the Effects of Canagliflozin on Renal Endpoints in Adult Subjects With Type 2 Diabetes Mellitus (CANVAS-R) (due in 2017) are not available, a recent meta-analysis (64) suggests that all three SGLT2i will exert similar effects on CV end points and congestive heart failure.
DO THE CARDIOPROTECTIVE EFFECTS OF GLP-1 RAs AND SGLT2i REPRESENT A CLASS EFFECT?
It is not possible at this time to determine whether the CV risk reduction with liraglutide (LEADER) and semaglutide (SUSTAIN-6) is a class effect or a specific effect inherent to each individual agent. It also is difficult to determine whether other SGLT2i will exert a similar benefit on CV mortality as empagliflozin, although published data with dapagliflozin and canagliflozin suggest that this will be the case (64). Ongoing CV outcome studies with other GLP-1 RAs (exenatide, dulaglutide) and other SGLT2i (dapagliflozin, canagliflozin) will provide an answer to this question. Previous CV outcome studies reported a neutral effect on MACE of other antidiabetes agents that act via the incretin axis (lixisenatide, alogliptin, saxagliptin, and sitagliptin) (20–22). However, the ability of DPP-4 inhibitors to raise circulating GLP-1 levels is modest, whereas GLP-1 RAs achieve much higher plasma GLP-1 levels (>100 pmol/L) than DPP-4 inhibitors (∼30 pmol/L). Lixisenatide has a short half-life (∼4 h). Thus, patients are uncovered during most of the day, and this could explain its lack of CV benefit. Consistent with this, the reductions in body weight and blood pressure were smaller in ELIXA (Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 [Lixisenatide]) compared with LEADER (0.6 vs. 2.4 kg and 0.8 vs. 1.4 mmHg). Studies in experimental animals have demonstrated that some of the cardioprotective effect afforded by GLP-1 is independent of the GLP-1 receptor (38). It is possible, therefore, that the cardioprotective action of GLP-1 RAs is structure dependent, and lixisenatide has only ∼50% sequence homology with native GLP-1. Lastly, the study design and patient population in ELIXA differed significantly from LEADER. Patients in ELIXA were recruited because they had acute coronary syndrome and the primary end point was the composite of CV death, MI, stroke, and unstable angina (MACE-4).
GENERALIZABILITY OF CV BENEFIT
Participants in LEADER, SUSTAIN-6, EMPA-REG OUTCOME, and PROactive had T2D and >80% had a previous CV event. It is not possible to determine whether T2D patients without established CVD and who are earlier in the natural history of their disease will similarly benefit from treatment with GLP-1 RAs, SGLT2i, or pioglitazone. It can be argued that, because pioglitazone and GLP-1 RAs slow atherosclerosis and because T2D patients are at high risk for atherosclerotic complications, these agents are likely to reduce CV events in individuals with T2D without, as well as with, established CVD. Previous studies have demonstrated that pioglitazone improves surrogate (anatomical) markers of CVD (carotid intima-media thickness and coronary atheroma volume) in T2D patients without established CVD (27,28). However, it also could be argued that since the reduction in CV events produced by GLP-1 RAs, pioglitazone, and empagliflozin was seen in patients with a prior CV event, the antidiabetes medications would not be effective or would be less effective in T2D patients without evidence of CVD. Therefore, at this stage, if considering only cardiovascular risk, evidence-based medicine dictates that pioglitazone, semaglutide, liraglutide, and/or empagliflozin should be considered to reduce CVD risk in T2D patients with established CVD, as in the published CV outcome trials. An exception is pioglitazone, which significantly reduced the incidence of stroke/MI in insulin-resistant individuals without diabetes.
IMPLICATIONS FOR DIABETES CARE
The primary goals of T2D management are to 1) improve glycemic control to prevent microvascular complications and 2) normalize CVD risk factors to reduce CV events and CV mortality. Hyperglycemia is the principal risk factor for microvascular complications, and a decrease in HbA1c, by whatever means, reduces the risk of eye, kidney, and nerve complications. Review of CV outcome trials in this Perspective demonstrates that liraglutide, semaglutide, pioglitazone, and empagliflozin reduce macrovascular events by 14–26%, independent of their glucose-lowering effect and improvement in traditional CV risk factors (Fig. 4). Although not established in T2D individuals with lower CV risk, we propose that long-acting GLP-1 RAs (liraglutide and, if approved by the U.S. Food and Drug Administration, semaglutide), SGLT2i (empagliflozin until the results of CANVAS and DECLARE become available), and pioglitazone should be given preferential consideration, along with metformin, over other agents that similarly lower HbA1c but have not been shown to reduce CV risk. Although metformin is recommended as first-line therapy by the American Diabetes Association and was reported to reduce CV events in UKPDS (5,11), the number of subjects (n = 342) was small and would not meet the standards of current CV outcome trials. On the other hand, it could be argued that the ability to demonstrate a difference with such a small sample size speaks to the significance of the results.
Supplementary Material
Article Information
Funding. This work was supported by a grant to R.A.D. from the Foundation for the National Institutes of Health (NIH-DK-24092-42). R.A.D.’s salary is supported in part by the South Texas Veterans Health Care System, Audie Murphy Division.
Duality of Interest. R.A.D. is on the advisory boards of AstraZeneca, Novo Nordisk, Janssen, Intarcia, and Boehringer Ingelheim; receives research support from Bristol-Myers Squibb, Boehringer Ingelheim, Takeda, Janssen, and AstraZeneca; and is on the speakers’ bureaus of Novo Nordisk and AstraZeneca. S.D.P. has served on the scientific board and received honoraria for consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co., GlaxoSmithKline, Merck & Co., Novartis Pharmaceuticals, Novo Nordisk, Sanofi, Servier, and Takeda Pharmaceuticals. R.C. has received research grants and has been an advisor for Amgen, Boehringer Ingelheim, Sanofi, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, and Eli Lilly and Co. R.E.J.R. has received speaker fees, consultancy fees, and educational sponsorships from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2736/-/DC1.
See accompanying article, p. 821.
References
- 1.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S14–S21 [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 9.Kernan WN, Viscoli CM, Furie KL, et al.; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dormandy JA, Charbonnel B, Eckland DJA, et al.; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 12.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 16.Obunai K, Jani S, Dangas GD. Cardiovascular morbidity and mortality of the metabolic syndrome. Med Clin North Am 2007;91:1169–1184 [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 18.Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. Can Med Assoc J 2006;174:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pladevall M, Riera-Guardia N, Margulis AV, Varas-Lorenzo C, Calingaert B, Perez-Gutthann S. Cardiovascular risk associated with the use of glitazones, metformin and sufonylureas: meta-analysis of published observational studies. BMC Cardiovasc Disord 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 21.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 22.White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 23.Eldor R, DeFronzo RA, Abdul-Ghani M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013;36(Suppl. 2):S162–S174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–3341 [DOI] [PubMed] [Google Scholar]
- 25.Dormandy JA, Betteridge DJ, Schernthaner G, Pirags V, Norgren L; PROactive investigators . Impact of peripheral arterial disease in patients with diabetes—results from PROactive (PROactive 11). Atherosclerosis 2009;202:272–281 [DOI] [PubMed] [Google Scholar]
- 26.Ryder RE. Pioglitazone has a dubious bladder cancer risk but an undoubted cardiovascular benefit. Diabet Med 2015;32:305–313 [DOI] [PubMed] [Google Scholar]
- 27.Nissen SE, Nicholls SJ, Wolski K, et al.; PERISCOPE Investigators . Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561–1573 [DOI] [PubMed] [Google Scholar]
- 28.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180–1188 [DOI] [PubMed] [Google Scholar]
- 29.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 31.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 2016;24:15–30 [DOI] [PubMed] [Google Scholar]
- 32.Nathanson D, Frick M, Ullman B, Nyström T. Exenatide infusion decreases atrial natriuretic peptide levels by reducing cardiac filling pressures in type 2 diabetes patients with decompensated congestive heart failure. Diabetol Metab Syndr 2016;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Liu J, Huang Y. Protective effects of glucagon-like peptide 1 on endothelial function in hypertension. J Cardiovasc Pharmacol 2015;65:399–405 [DOI] [PubMed] [Google Scholar]
- 34.Kumarathurai P, Anholm C, Larsen BS, et al. Effects of liraglutide on heart rate and heart rate variability: a randomized, double-blind, placebo-controlled crossover study. Diabetes Care 2017;40:117–124 [DOI] [PubMed] [Google Scholar]
- 35.Smits MM, Muskiet MH, Tonneijck L, et al. GLP-1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler Thromb Vasc Biol 2015;35:1538–1543 [DOI] [PubMed] [Google Scholar]
- 36.Chen JW, Wang YL, Li HW. Elevated admission microalbuminuria predicts poor myocardial blood flow and 6-month mortality in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Clin Cardiol 2012;35:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lønborg J, Vejlstrup N, Kelbæk H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–1499 [DOI] [PubMed] [Google Scholar]
- 38.Ussher JR, Baggio LL, Campbell JE, et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab 2014;3:507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arturi F, Succurro E, Miceli S, et al. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. Endocrine. 9 November 2016 [Epub ahead of print]. DOI: 10.1007/s12020-016-1166-4 [DOI] [PubMed] [Google Scholar]
- 40.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004;109:962–965 [DOI] [PubMed] [Google Scholar]
- 41.Eriksson L, Saxelin R, Röhl S, et al. Glucagon-like peptide-1 receptor activation does not affect re-endothelialization but reduces intimal hyperplasia via direct effects on smooth muscle cells in a nondiabetic model of arterial injury. J Vasc Res 2015;52:41–52 [DOI] [PubMed] [Google Scholar]
- 42.Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diab Vasc Dis Res 2013;10:353–360 [DOI] [PubMed] [Google Scholar]
- 43.Hirano T, Mori Y. Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals. J Diabetes Investig 2016;7(Suppl. 1):80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016;39:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma S, Garg A, Yan AT, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care 2016;39:e212–e213 [DOI] [PubMed] [Google Scholar]
- 46.Clarke GD, Molina-Wilkins M, Martinez S, et al. Improved left ventricular diastolic function (LVDF) following pioglitazone therapy is strongly related to increased myocardial insulin sensitivity. Diabetes 2014;63(Suppl. 1):A298 [Google Scholar]
- 47.Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol 2015;309:F889–F900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarruf DA, Yu F, Nguyen HT, et al. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 2009;150:707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000;23:1605–1611 [DOI] [PubMed] [Google Scholar]
- 50.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 51.Bajaj M, Baig R, Suraamornkul S, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015;17:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–1016 [DOI] [PubMed] [Google Scholar]
- 54.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307 [DOI] [PubMed] [Google Scholar]
- 55.Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:23–31 [DOI] [PubMed] [Google Scholar]
- 56.Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031 [DOI] [PubMed] [Google Scholar]
- 57.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 58.McCarthy KJ, Routh RE, Shaw W, Walsh K, Welbourne TC, Johnson JH. Troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Kidney Int 2000;58:2341–2350 [DOI] [PubMed] [Google Scholar]
- 59.DeFronzo RA, Tripathy D, Schwenke DC, et al.; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 60.Merovci A, Mari A, Solis C, et al. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab 2015;100:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003;52:1786–1791 [DOI] [PubMed] [Google Scholar]
- 62.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapitza C, Dahl K, Jacobsen J, Axelsen M, Flint A. The effects of semaglutide on β-cell function in subjects with type 2 diabetes. Diabetes 2016;65(Suppl. 1):A262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu JH, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016;4:411–419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.