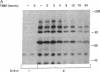
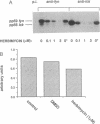
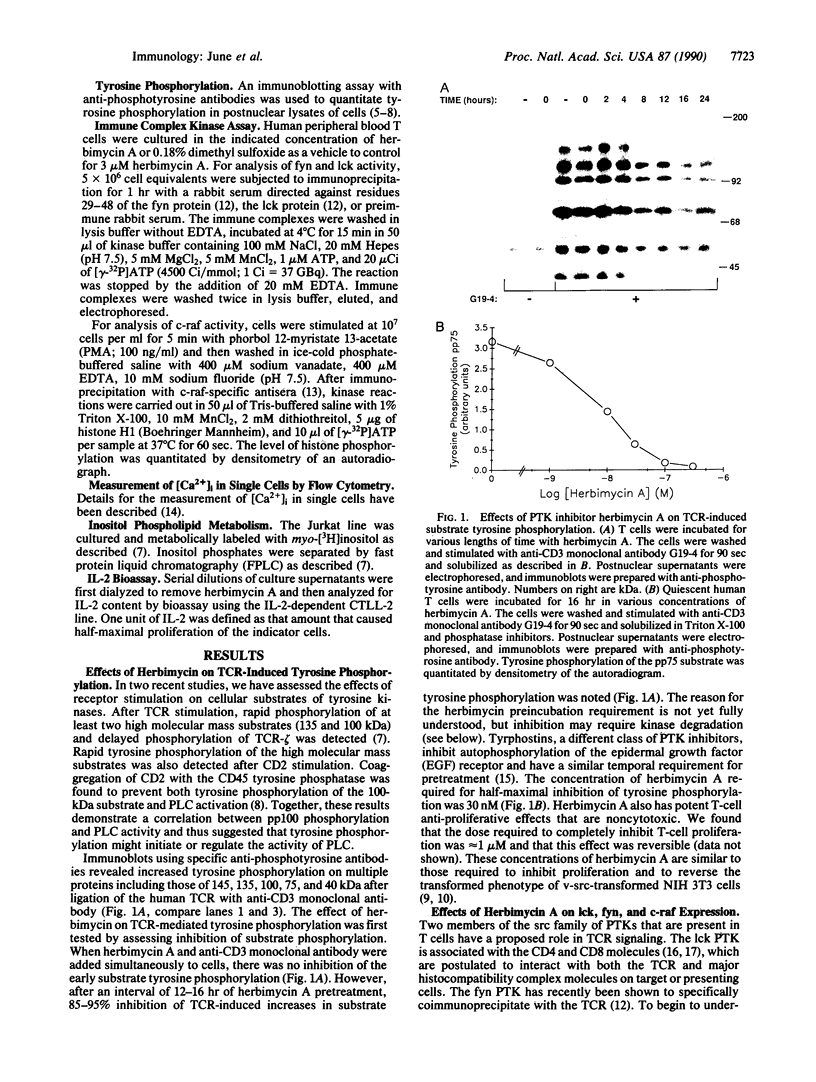
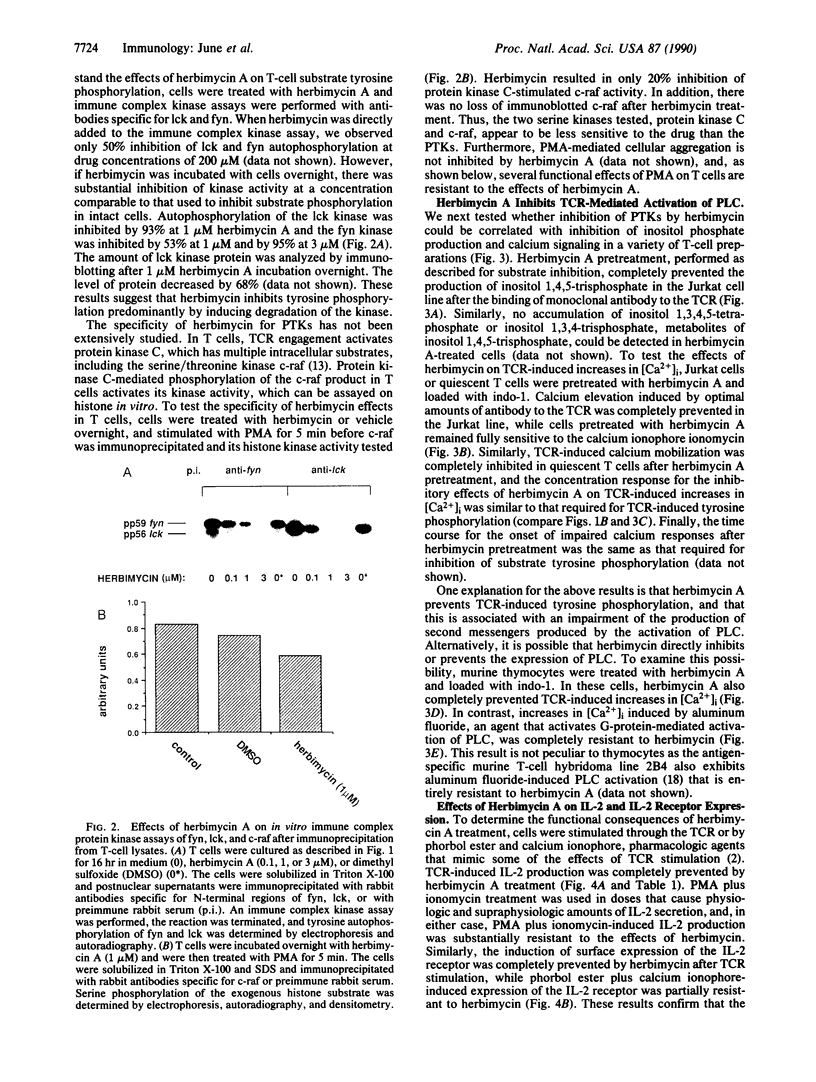
Abstract
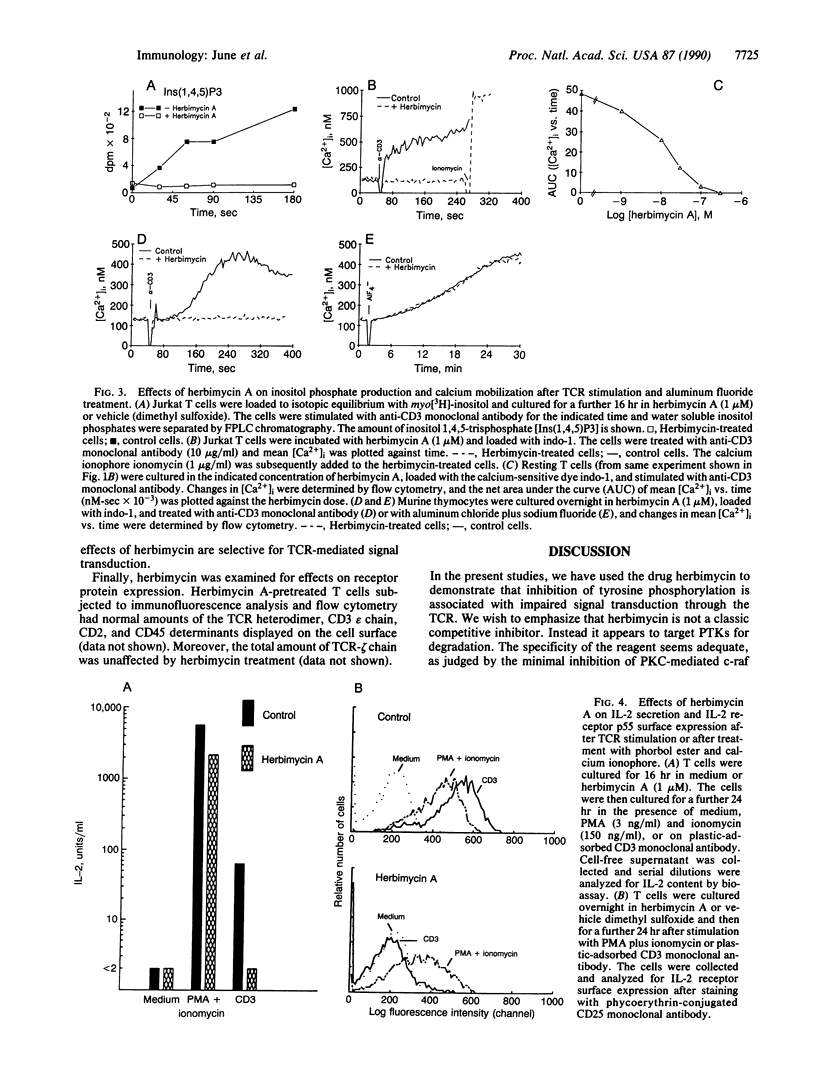
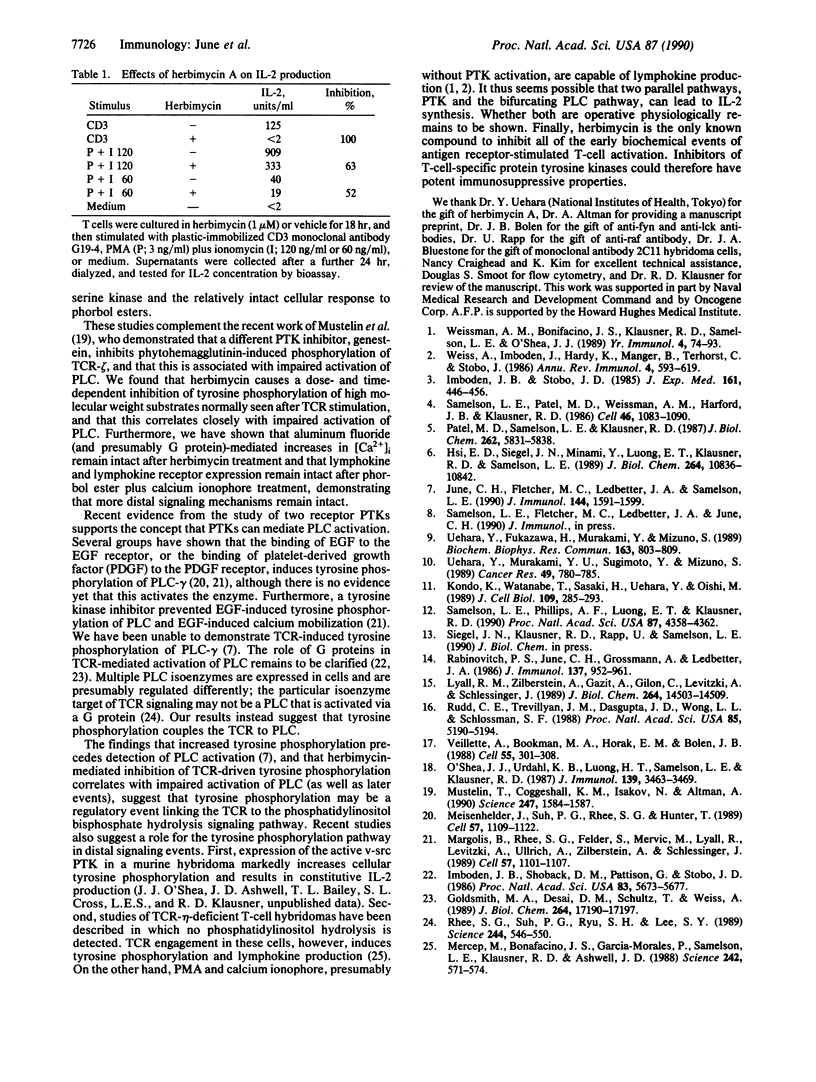
The binding of antigen to the multicomponent T-cell receptor (TCR) activates several signal transduction pathways via coupling mechanisms that are poorly understood. One event that follows antigen receptor engagement is the activation of inositol phospholipid-specific phospholipase C (PLC). TCR activation by antigen, lectins, or anti-TCR monoclonal antibody has also been shown to cause increases in tyrosine phosphorylation of TCR-zeta and other substrates, suggesting stimulation of protein tyrosine kinase (PTK) activity. A critical question is whether these two pathways, PLC and PTK, are independently activated or whether one initiates and/or regulates the other. In the former case, PLC activation could be coupled to the TCR via a GTP-binding protein (G protein). We have reported, however, that tyrosine phosphorylation of intracellular substrates precedes detection of PLC activation and intracellular calcium elevation, suggesting that inositol phospholipid turnover in T cells is initiated by a PTK pathway. In this study, we test this hypothesis by treating T cells with the drug herbimycin A. We demonstrate that this agent inhibits substrate tyrosine phosphorylation, TCR-mediated inositol phospholipid hydrolysis, and calcium elevation. In contrast, under these conditions G-protein-mediated PLC activity, as tested by addition of aluminum fluoride, remains intact. Furthermore, whereas herbimycin treatment prevents TCR-mediated interleukin 2 production and interleukin 2 receptor expression, phorbol ester-induced effects are substantially resistant to herbimycin. The drug thus appears to abrogate TCR-mediated signaling without affecting distal signaling mechanisms.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldsmith M. A., Desai D. M., Schultz T., Weiss A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J Biol Chem. 1989 Oct 15;264(29):17190–17197. [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Kondo K., Watanabe T., Sasaki H., Uehara Y., Oishi M. Induction of in vitro differentiation of mouse embryonal carcinoma (F9) and erythroleukemia (MEL) cells by herbimycin A, an inhibitor of protein phosphorylation. J Cell Biol. 1989 Jul;109(1):285–293. doi: 10.1083/jcb.109.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall R. M., Zilberstein A., Gazit A., Gilon C., Levitzki A., Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989 Aug 25;264(24):14503–14509. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Patel M. D., Samelson L. E., Klausner R. D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J Biol Chem. 1987 Apr 25;262(12):5831–5838. [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Sugimoto Y., Mizuno S. Mechanism of reversion of Rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced kinase activity and increased turnover of p60v-src1. Cancer Res. 1989 Feb 15;49(4):780–785. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Bonifacino J. S., Klausner R. D., Samelson L. E., O'Shea J. J. T cell antigen receptor: structure, assembly and function. Year Immunol. 1989;4:74–93. [PubMed] [Google Scholar]