Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Earlier use of high-dose cytarabine during induction II therapy improves EFS for ML-DS patients.
MRD assessment by flow cytometry after induction I is a new prognostic variable for ML-DS.
Abstract
Patients with myeloid leukemia of Down syndrome (ML-DS) have favorable event-free survival (EFS), but experience significant treatment-related morbidity and mortality. ML-DS blast cells ex vivo have increased sensitivity to cytarabine (araC) and daunorubicin, suggesting that optimizing drug dosing may improve outcomes while reducing toxicity. The Children’s Oncology Group (COG) AAML0431 trial consisted of 4 cycles of induction and 2 cycles of intensification therapy based on the treatment schema of the previous COG A2971 trial with several modifications. High-dose araC (HD-araC) was used in the second induction cycle instead of the intensification cycle, and 1 of 4 daunorubicin-containing induction cycles was eliminated. For 204 eligible patients, 5-year EFS was 89.9% and overall survival (OS) was 93.0%. The 5-year OS for 17 patients with refractory/relapsed leukemia was 34.3%. We determined the clinical significance of minimal residual disease (MRD) levels as measured by flow cytometry on day 28 of induction I. MRD measurements, available for 146 of the 204 patients, were highly predictive of treatment outcome; 5-year disease-free survival for MRD-negative patients (n = 125) was 92.7% vs 76.2% for MRD-positive patients (n = 21) (log-rank P = .011). Our results indicated that earlier use of HD-araC led to better EFS and OS in AAML0431 than in past COG studies. A 25% reduction in the cumulative daunorubicin dose did not impact outcome. MRD, identified as a new prognostic factor for ML-DS patients, can be used for risk stratification in future clinical trials. This trial was registered at www.clinicaltrials.gov as #NCT00369317.
Introduction
Children with Down syndrome (DS) are at a higher risk of developing both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) compared with children without DS.1 AML in DS children is associated with several unique features. First, there is a high prevalence of the acute megakaryocytic leukemia (AMKL) phenotype.2 Second, the precursor condition, transient myeloproliferative disorder (TMD; also referred to as transient abnormal hematopoiesis), occurs in up to 10% of neonates with DS, which progresses to AML in ∼20% to 30% of patients.3 Third, somatic mutations in the gene for the X-linked transcription factor GATA1 occur in almost all DS patients with TMD and/or AMKL.3-6 Before being diagnosed with AML, DS patients may develop myelodysplasia, which is characterized by progressive anemia and thrombocytopenia, with dysplastic erythroid cells and megakaryocytes in the bone marrow.2 This myelodysplastic phase frequently precedes the development of AML, and both the myelodysplastic syndrome (MDS) and AML are now collectively referred to as myeloid leukemia of DS (ML-DS) according to the World Health Organization (WHO) 2008 classification.
The Pediatric Oncology Group (POG) 8498 trial reported high cure rates for ML-DS patients.7 Subsequent studies confirmed that children with ML-DS represented a very favorable prognostic subgroup of AML.8-12 Increasing the dose intensity in ML-DS patients to that given for non-DS children with AML resulted in excessive deaths in children with DS primarily due to infections and cardiac dysfunction.13-15 ML-DS patients with either refractory or relapsed disease have a very poor prognosis even after receiving stem cell transplants (SCTs).16-18 Hence, identifying the optimal treatment intensity for ML-DS patients remains a challenge, balancing the need to use curative therapy while minimizing treatment-associated morbidity and mortality.
The unique sensitivity of ML-DS blasts to chemotherapy can be used to maximize the efficacy of therapy, while reducing the risk of toxicity in ML-DS patients. Blasts from children with ML-DS have increased ex vivo sensitivity to cytarabine (araC) and generate significantly higher levels of the intracellular metabolite, araC triphosphate, compared with blast cells from children without DS.19-22 Thus, more effective use of high-dose araC (HD-araC)-containing treatment cycles could improve treatment outcomes for DS patients. The increased ex vivo sensitivity of ML-DS blast cells to daunorubicin20,23 suggested that daunorubicin dosing could be reduced to minimize cardiac toxicity in children with DS,9 which arises in part due to overexpression of the chromosome 21–localized gene, carbonyl reductase, which catalyzes the reduction of anthracyclines to cardiotoxic alcohol metabolites.24
The Children’s Oncology Group (COG) trial AAML0431, the largest trial to date for ML-DS, was designed to answer 2 outstanding questions in the management of patients with ML-DS. First, could the use of HD-araC earlier in the second induction cycle (rather than the intensification cycle of A2971) improve disease-free survival (DFS)? Second, could a 25% lower cumulative dose of daunorubicin reduce the risk of adverse cardiac events without compromising outcomes?8,12 We determined whether minimal residual disease (MRD) levels, previously identified as an important prognostic factor for non-DS AML patients, could identify risk groups in patients with ML-DS.25,26
Materials and methods
The COG phase 3 AAML0431 trial, “The Treatment of Down Syndrome Children with Acute Myeloid Leukemia and Myelodysplastic Syndrome Under the Age of 4 Years,” used the treatment schema of the A2971 trial with 3 major modifications: (1) HD-araC with asparaginase was administered in induction II instead of intensification, thus replacing 1 of 4 cycles of continuous-infusion araC and daunorubicin and reducing the cumulative daunorubicin dose by 25%; (2) 2 intensification cycles with moderate-dose araC and etoposide were administered instead of HD-araC; and (3) the number of prophylactic intrathecal doses was reduced from 7 to 2 (Table 1; supplemental Figure 1, available on the Blood Web site).
Table 1.
Patient characteristics and outcome for eligible patients on AAML0431
| Characteristic | All patients, n = 204 | AML, n = 144 | MDS, n = 60 | AML vs MDS | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P | |
| Sex | |||||||
| Male | 99 | 48.5 | 73 | 50.7 | 26 | 43.3 | .338 |
| Female | 105 | 51.5 | 71 | 49.3 | 34 | 56.7 | |
| Race | |||||||
| Asian | 12 | 6.6 | 9 | 7.0 | 3 | 5.6 | 1.000 |
| Native Hawaiian or other Pacific Islander | 2 | 1.1 | 2 | 1.6 | 0 | 0.0 | 1.000 |
| Black or African American | 28 | 15.4 | 21 | 16.4 | 7 | 13.0 | .556 |
| White | 140 | 76.9 | 96 | 75.0 | 44 | 81.5 | .343 |
| Unknown | 22 | 16 | 6 | ||||
| Ethnicity | |||||||
| Hispanic | 48 | 9.6 | 32 | 22.7 | 16 | 26.7 | .546 |
| Not Hispanic | 153 | 90.4 | 109 | 77.3 | 44 | 73.3 | |
| Unknown | 3 | 3 | 0 | ||||
| History of TMD | |||||||
| No | 141 | 69.1 | 103 | 71.5 | 38 | 63.3 | .248 |
| Yes | 63 | 30.9 | 41 | 28.5 | 22 | 36.7 | |
| Received prior treatment of TMD | |||||||
| No | 198 | 97.1 | 141 | 97.9 | 57 | 95.0 | .362 |
| Yes | 6 | 2.9 | 3 | 2.1 | 3 | 5.0 | |
| FAB | |||||||
| M0 | 3 | 1.5 | 3 | 2.1 | 0 | 0.0 | * |
| M1 | 5 | 2.5 | 5 | 3.5 | 0 | 0.0 | * |
| M2 | 7 | 3.4 | 7 | 4.9 | 0 | 0.0 | * |
| M5 | 1 | 0.5 | 1 | 0.7 | 0 | 0.0 | * |
| M6 | 3 | 1.5 | 3 | 2.1 | 0 | 0.0 | * |
| M7 | 85 | 41.7 | 85 | 59.0 | 0 | 0.0 | * |
| AML not further classified | 40 | 19.6 | 40 | 27.8 | 0 | 0.0 | * |
| MDS refractory anemia | 3 | 1.5 | 0 | 0.0 | 3 | 5.0 | * |
| MDS RA with excess blasts | 18 | 8.8 | 0 | 0.0 | 18 | 30.0 | * |
| MDS RAEB in transformation | 6 | 2.9 | 0 | 0.0 | 6 | 10.0 | * |
| MDS not further classified | 33 | 16.2 | 0 | 0.0 | 33 | 55.0 | * |
| Cytogenetic classification | |||||||
| Normal (trisomy 21 only) | 52 | 28.0 | 37 | 28.2 | 15 | 27.3 | .893 |
| Monosomy 7 | 3 | 1.6 | 2 | 1.5 | 1 | 1.8 | 1.000 |
| Del(7q) only | 5 | 2.7 | 3 | 2.3 | 2 | 3.6 | .633 |
| Monosomy 5/del(5q) | 3 | 1.6 | 3 | 2.3 | 0 | 0.0 | .556 |
| +8 | 49 | 26.3 | 35 | 26.7 | 14 | 25.5 | .858 |
| Multiple trisomy 21 | 25 | 13.4 | 22 | 16.8 | 3 | 5.5 | .039† |
| Trisomy 21 with other abnormalities | 49 | 26.3 | 29 | 22.1 | 20 | 36.4 | .044† |
| Unknown | 18 | 13 | 5 | ||||
| Age from diagnosis, (median, range), y | 1.59 | (0.38-3.78) | 1.7 | (0.38-3.75) | 1.42 | (0.53-3.78) | .036† |
| WBC, (median, range), ×109/L | 5.75 | (1.6-118.3) | 6.5 | (1.6-118.3) | 4.9 | (1.6-28.6) | <.001† |
| Platelets, (median, range), ×109/L | 34.5 | (2.0-1280) | 34 | (2-1280) | 36.5 | (3-176) | .716 |
| Peripheral blasts, (median, range), % | 3.55 | (0-89) | 7 | (0-89) | 0 | (0-31) | <.001† |
| Outcome, from study entry | |||||||
| 5-y EFS | 89.9 | 95% CI: 84.8-93.4 | 88.6 | 95% CI: 82.0-92.8 | 93.2 | 95% CI: 82.9-97.4 | .329 |
| 5-y OS | 93 | 95% CI: 88.5-95.8 | 92.2 | 95% CI: 86.4-95.6 | 94.9 | 95% CI: 85.1-98.3 | .492 |
WBC, white blood cell.
Not compared.
Bold P values represent statistical significance <.05.
Patients
Eligibility criteria were as follows: (1) confirmed diagnosis of DS or DS mosaicism, (2) diagnosis of AML according to the French-American-British (FAB) classification, excluding promyelocytic leukemia, and (3) age <4 years at diagnosis. Patients with a diagnosis of MDS (with <30% blasts) were eligible for the trial. Patients older than 90 days old at diagnosis of AML or MDS with a history of TMD (which may or may not have required chemotherapy intervention) were eligible if they (1) had ≥30% blasts in the bone marrow, regardless of the time since resolution or (2) were >8 weeks since TMD resolution with ≥5% blasts in the bone marrow in association with myelodysplastic changes. The same eligibility criteria were used in the prior COG A2971 trial.12 Children who had previously received chemotherapy or radiation therapy or any antileukemic therapy were not eligible for this protocol, with the exception of intrathecal araC given at diagnosis, and prior therapy for TMD. Adequate cardiac function (shortening fraction of ≥27% by echocardiogram) and pulmonary function (pulse oximetry >94% on room air) were required.
The trial was approved by the central institutional review board of the National Cancer Institute and institutional review boards of each enrolling center. Patients and their families provided informed consent or assent as appropriate. The trial was conducted in accordance with the Declaration of Helsinki.
Treatment and monitoring
The treatment consisted of 4 cycles of induction therapy and 2 cycles of intensification therapy. Prophylactic intrathecal araC was administered at diagnosis/start of induction I and at induction III. Induction cycles I, III, and IV consisted of continuous-infusion araC 6.7 mg/kg per day for 4 days (96 hours), continuous-infusion daunorubicin 0.67 mg/kg per 24 hours for 4 days (96 hours), and oral 6-thioguanine 1.65 mg/kg twice daily for 4 days. Induction cycle II consisted of araC 100 mg/kg administered as a 3-hour infusion every 12 hours for 4 doses on days 1 and 2 and repeated on days 8 and 9 (total 8 doses) with Escherichia coli asparaginase (200 U/kg) being administered intramuscularly 3 hours following the last dose of araC on days 2 and 9. Intensification cycles I and II consisted of continuous-infusion araC 3.3 mg/kg per 24 hours for 7 days (168 hours) and etoposide 4.2 mg/kg per dose administered as a 1-hour infusion for 3 days. For patients over the age of 36 months, chemotherapy dosages were based on mg/m2 using the conversion factor (mg/kg × 30 = mg/m2). The total cumulative chemotherapy doses were as follows: araC, 27 800 mg/m2; daunorubicin, 240 mg/m2, and etoposide, 750 mg/m2.
Expedited reporting was required for all unexpected grade 4 (Common Terminology Criteria for Adverse Events) and all grade 5 events. Routine reporting was required for all grade 3 or higher nonhematological toxicities and all grades of QTc prolongation and ventricular systolic dysfunction.
Disease response criteria
The recommended time points for assessing response in bone marrow aspirates (BMAs) were on day 14 and day 28 of induction I. Patients who had ≥20% blasts in BMAs on day 14 of induction I were to receive induction II regardless of peripheral blood count recovery. For all other patients, BMAs were assessed on day 28 to determine remission status. For patients achieving a complete remission (CR), induction II was recommended when the absolute neutrophil count (ANC) was ≥1000/µL and when platelets were ≥100 × 109/L. Subsequent BMAs were assessed on day 28 of induction IV and at the end of intensification II. For patients with a partial response (PR) or refractory disease (RD), regardless of cellularity after day 28 BMA, BMA assessment was to be repeated on day 14 and day 28 of induction II. Patients with a PR or RD after induction IV were taken off protocol therapy. Each subsequent cycle of therapy was recommended to be administered when the ANC was ≥1000/µL and platelets were ≥100 × 109/L. Hospitalization after each chemotherapy cycle during periods of neutropenia was at the discretion of each treating institution.
MRD analysis
MRD was an optional biology study performed at the above BMA time points in a single reference center at the St. Jude Children’s Research Hospital (Memphis, TN). Samples were shipped from the referring centers and processed within 24 to 48 hours of collection using methods similar to those used in the previously reported AML-02 trial25 (described in supplemental Methods).
Cytogenetics and mutational analysis
Cytogenetic analysis was performed at individual institutions based on standard G-banding procedures, the final karyotype, and any relevant fluorescence-in-situ hybridization images reviewed centrally by S.R. and B.H. (described in supplemental Methods). Somatic GATA1 mutations were analyzed as previously described.6
Statistical methods
Data from AAML0431 were current as of June 30, 2016, with a median follow-up of 5.6 years (range, 0-8.7 years) (described in supplemental Methods).
Results
Patient characteristics
Between March 2007 and December 2011, 205 children (106 girls, 99 boys) with DS or DS mosaicism were enrolled on the AAML0431 trial. After review, 1 patient with ALL was deemed ineligible prior to starting therapy. Table 1 presents patient characteristics and clinical data including sex, race, ethnicity, history of TMD, institutional FAB classification, cytogenetic classification, age at diagnosis, and presenting complete blood counts for the 204 eligible patients. Congenital heart defects were present in 90 (44%) patients. No patient had central nervous system (CNS) involvement with leukemia at diagnosis.
Cytogenetics
Cytogenetic findings were reviewed centrally and considered acceptable for 186 of 204 (91%) patients (Table 1). None of the patients harbored the recurring balanced rearrangements seen in non-DS AML such as t(8;21), inv(16), or 11q23 translocations. There were 116 acquired structural chromosomal rearrangements (ie, non–germ line). Of these, 15 (13%) were balanced structural abnormalities (eg, translocations or inversions) and 111 (87%) were unbalanced structural rearrangements (eg, derivative chromosomes resulting in loss and/or gain of chromosomal material). The most frequent structural abnormalities were gain of material from the long arm of chromosome 1 (1q) (n = 27; 23% of structural abnormalities), loss of material from the short arm and/or long arm of chromosome 7 (7p and/or 7q) (n = 23; 20%), and gain of material from the long arm of chromosome 11 (11q) (n = 10; 9%). The 4 most common numerical abnormalities involved trisomy 8 (n = 49), gain of a fourth copy of chromosome 21 (n = 32), trisomy 11 (n = 11), and trisomy 19 (n = 10). Monosomy 7 occurred in 3 patients (1.6%) and 52 patients had a normal karyotype other than for constitutional trisomy 21.
Toxicity
The highest number of adverse events occurred during induction II, in which HD-araC was administered. This cycle accounted for 27.1% of the total adverse events reported and 66% of all adverse events classified as grade 3 or greater; 7.9% of adverse events were classified as grade 4. Induction II was also associated with the longest median time to ANC recovery (>1000/µL) with a median of 37 days and a maximum length of 67 days (Table 2). Furthermore, the percentage of patients hospitalized in the intensive care unit was highest in inductions I and II (6.9% and 7%, respectively). Febrile neutropenia (grade ≥3) was the most common adverse event and was most frequent in induction II (29.7%) and induction I (27%). Sterile-site bacterial infection (grade ≥3) occurred in 22.6% and 19.1% of patients in induction II and induction I, respectively (Table 2). Supplemental Table 2 summarizes the sterile-site bacterial infections documented during induction II in which viridans group Streptococcus was the most common organism. There were no life-threatening cardiac toxicities and only 7 cardiac adverse events were classified as ≥grade 3 (sinus tachycardia, 3; pericardial effusion, 1, which persisted in 2 cycles for 1 patient, prolonged QT-1) or grade 4 (pericardial effusion, 1; cardiac arrest, 1). The only other adverse event occurring in at least 5% of patients for any of the 6 cycles was mucositis, which was reported in 7% of patients for induction II.
Table 2.
Adverse events per cycle for eligible AAML0431 patients
| Cycle | n | No. of patients with ANC recovery prior to starting next cycle | Days to ANC recovery, median (range) | ICU admission, % of patients | Febrile neutropenia grade ≥3, % of patients | Sterile site bacterial infection grade ≥3, % of patients |
|---|---|---|---|---|---|---|
| Induction I | 204 | 89 | 30 (5-54) | 6.9 | 27 | 19.1 |
| Induction II | 199 | 94 | 37 (1-67) | 7.0 | 29.7 | 22.6 |
| Induction III | 195 | 115 | 28 (3-47) | 1.5 | 6.2 | 11.3 |
| Induction IV | 195 | 113 | 28 (3-47) | 2.1 | 5.6 | 8.7 |
| Intensification I | 192 | 89 | 33 (3-54) | 4.2 | 11.5 | 12.5 |
| Intensification II | 186 | 117 | 32 (7-63) | 1.1 | 12.4 | 8.1 |
ICU, intensive care unit.
Outcome
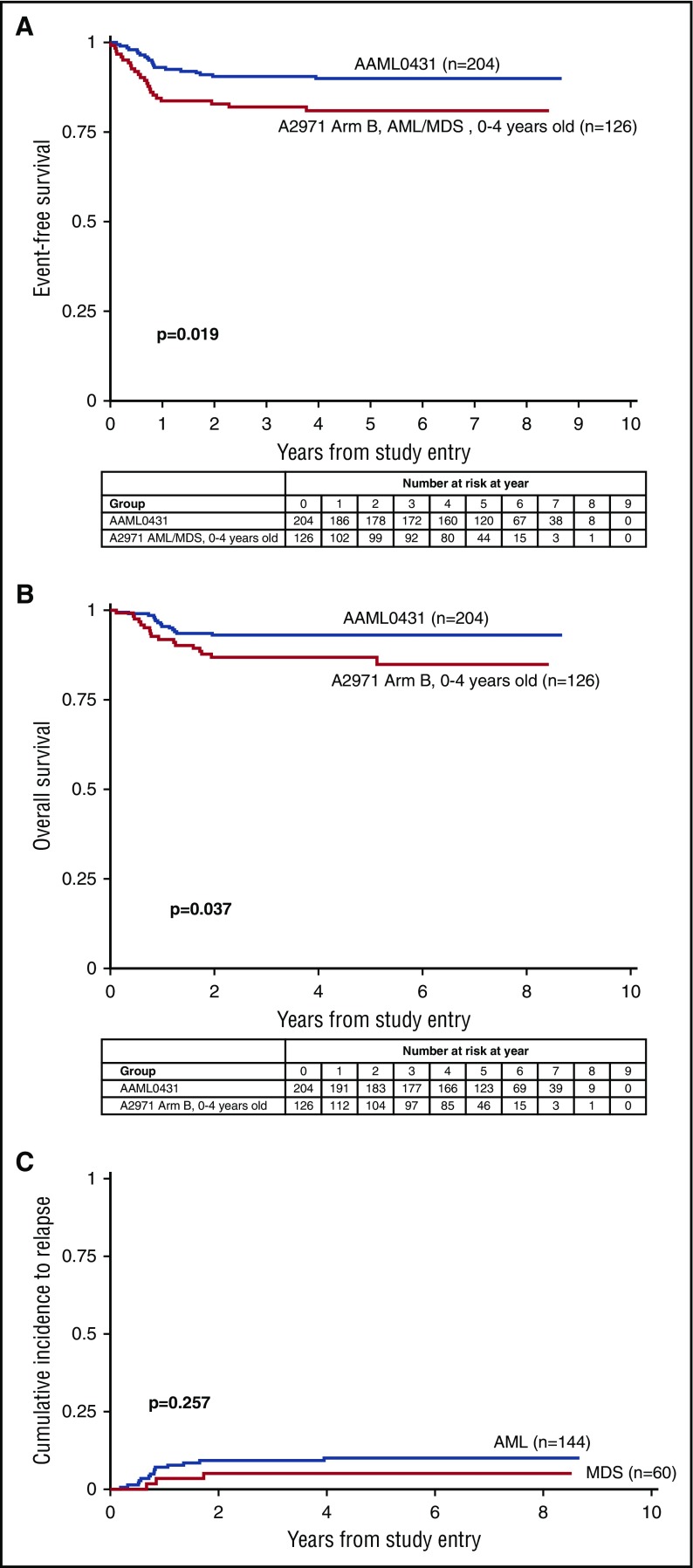
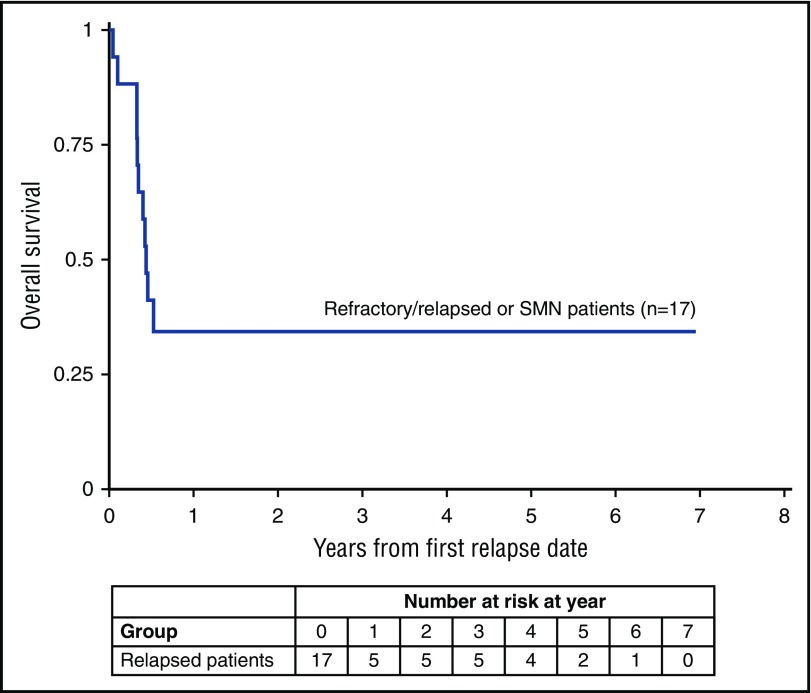
For all 204 eligible patients, the 5-year event-free survival (EFS) was 89.9% (95% confidence interval [CI], 84.8%-93.4%) and 5-year overall survival (OS) was 93.0% (95% CI, 88.5%-95.8%) (Table 1). For patients classified as having AML, EFS was 88.6% (95% CI, 82.0%-92.8%) and OS was 92.2% (95% CI, 86.4%-95.6%). For patients classified as having MDS, 5-year EFS was 93.2% (95% CI, 82.9%-97.4%) and OS was 94.9% (95% CI, 85.1%-98.3%) (Figure 1A-B). For patients with a history of TMD, the 5-year EFS was 88.5% (95% CI, 77.3%-94.3%) and OS was 91.8% (95% CI, 81.5%-96.5%). The 5-year cumulative incidence of relapse for patients with AML was 10.0% (95% CI, 5.7%-15.7%) and for MDS was 5.1% (95% CI, 1.3%-12.9%) (Figure 1C). Treatment failures occurred in 20 patients: 1 induction failure, 14 relapses, 2 secondary malignancies (SMNs), and 3 nonrelapse deaths (CONSORT diagram in Figure 2). The 3 nonrelapse deaths in patients were caused by human metapneumovirus 16 months off therapy, pneumonia during prolonged pancytopenia after induction I, and hepatic failure and pneumonia after intensification I. The 5-year OS for patients with refractory/relapsed leukemia and SMN (n = 17) was 34.3% (95% CI, 13.5%-56.5%) (Figure 3).
Figure 1.
Event-free survival, overall survival, and cumulative incidence to relapse of patients treated on AAML0431 and A2971 ARM B. (A) EFS for n = 204 eligible patients on AAML0431 and n = 126 eligible patients 0 to 4 year olds with AML/MDS on A2971. (B) OS for n = 204 eligible patients on AAML0431 and n = 126 eligible patients 0 to 4 year olds on A2971, arm B. (C) Cumulative incidence of relapse for patients classified with AML (n = 144) and MDS (n = 60).
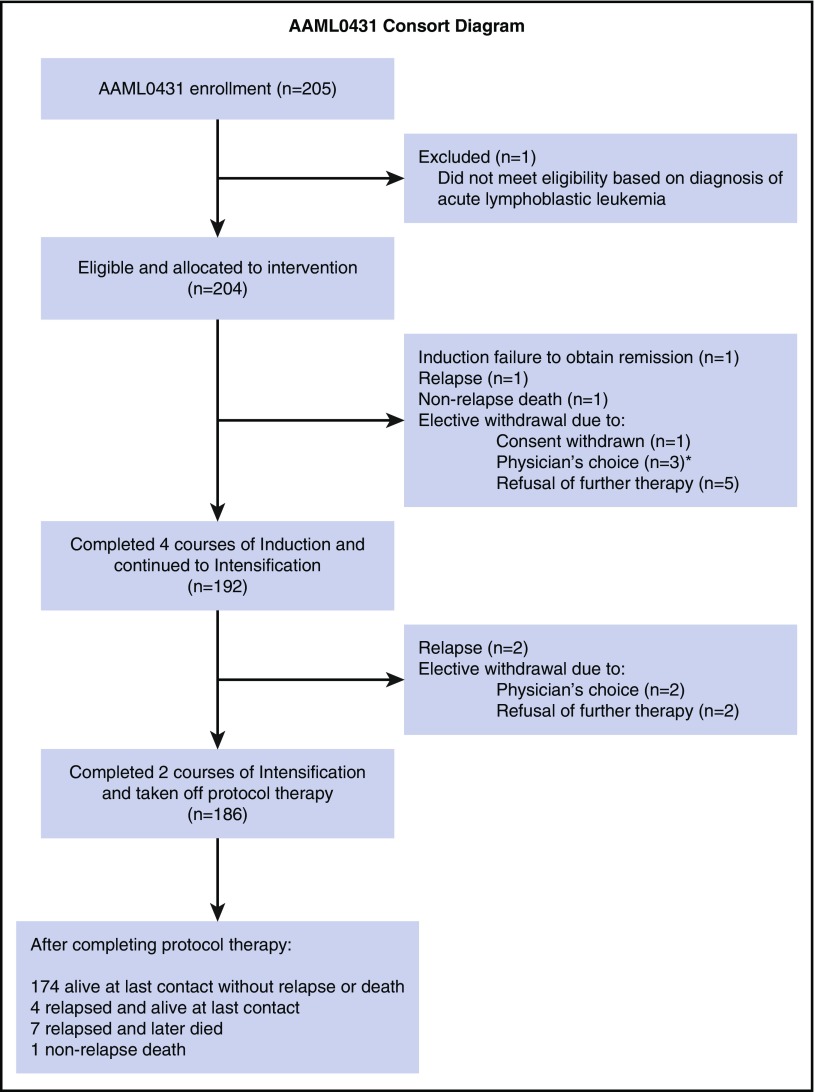
Figure 2.
Consolidated Standards for Reporting of Trials (CONSORT) diagram. *One patient had a nonrelapse death occurring 2 months postwithdrawal.
Figure 3.
OS for patients with refractory/relapsed leukemia or SMN.
A total of 13 patients were electively taken off protocol therapy on the request of the treating physician (n = 5) or parents (n = 8) (4 in induction I; 3 in induction II; 2 in induction IV; and 4 in intensification I) (CONSORT diagram in Figure 2). Of them, 1 patient taken off therapy after induction II relapsed and died, and 1 patient had a nonrelapse death after therapy was stopped because of prolonged pancytopenia after induction I (described in the preceding paragraph). The other 11 patients remain alive.
B-precursor ALL developed as a SMN in 2 patients 14 months and 3.5 years off therapy. One patient is currently alive in remission after completing ALL therapy. The second patient with a history of TMD was positive for the ETV6-RUNX1 fusion and is currently receiving ALL therapy.
MRD
As an optional biology study, MRD data from day 28 after induction I was available for 146 of the 204 patients (71.6%). For the remaining 58 patients, samples were not submitted (n = 57) or had inadequate cellularity (n = 1). There were no significant differences in outcomes between patients for whom induction I MRD data were and were not available (5-year OS, 93.8% ± 4.0% vs 91.0% ± 7.7%, respectively [log-rank P = .449]; 5-year DFS, 90.3% ± 7.4% vs 89.2% ± 8.3%, respectively [log-rank P = .715]).
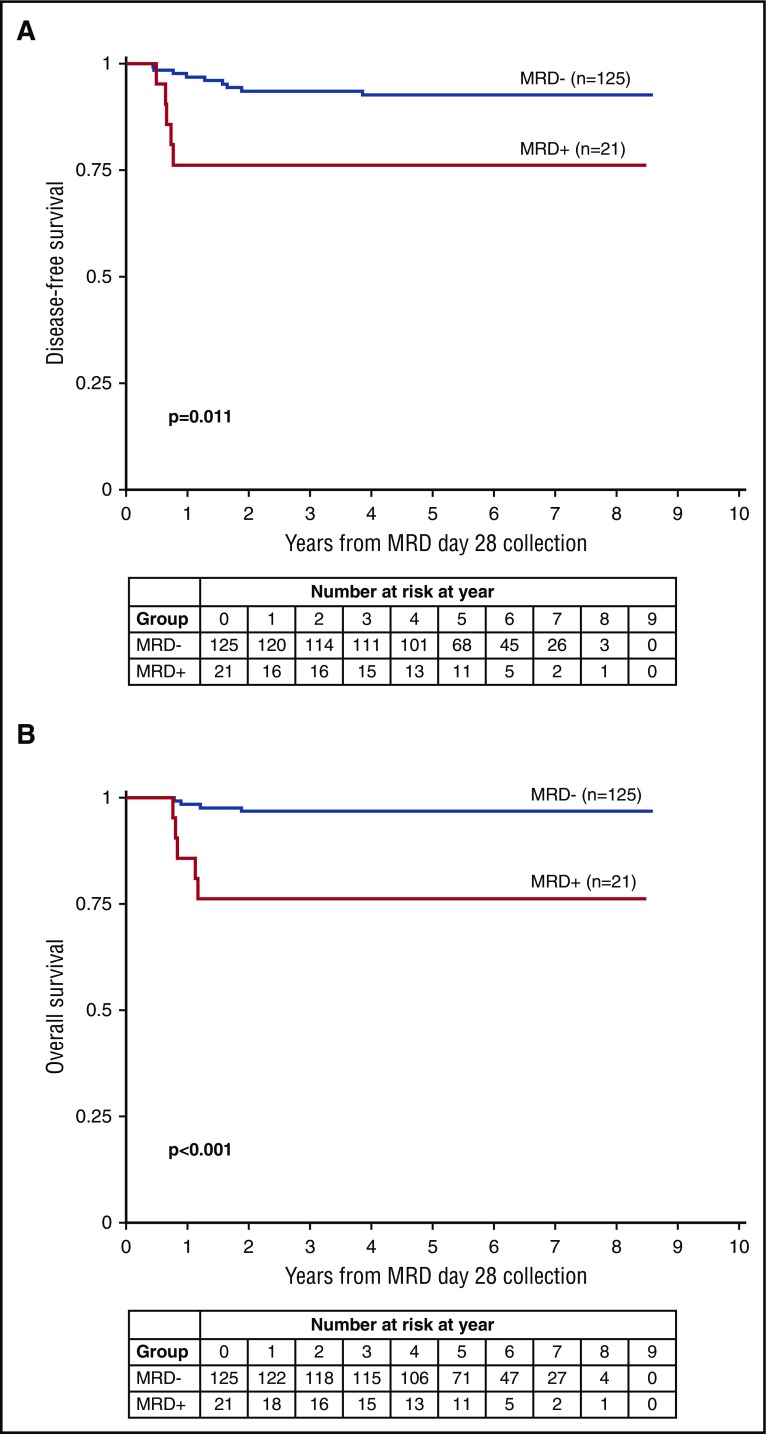
MRD (>0.01%) was detected in 21 patients (14.4%) at day 28 after induction I. These patients had a significantly worse 5-year DFS (76.2%; 95% CI, 51.9%-89.3%) than the 125 patients with no detectable MRD (92.7%; 95% CI, 86.3%-96.1% [log-rank P = .011]). The 5-year OS was also significantly worse for MRD-positive patients than MRD-negative patients (Figure 4; Table 3). Of the 5 patients for whom MRD levels were 0.01% to 0.1%, 2 relapsed, 1 electively withdrew from protocol therapy, and 2 remain in remission.
Figure 4.
Patient outcome in relation to MRD status at day 28 of Induction I. (A) DFS comparing patients with MRD vs without MRD at day 28 collection. (B) OS comparing patients with MRD vs without MRD at day 28 collection.
Table 3.
Characteristics and outcome for patients with MRD at day 28
| Characteristic | Day 28 MRD−, n = 125 | Day 28 MRD+, n = 21 | P | ||
|---|---|---|---|---|---|
| N | % or 95% CI | N | % or 95% CI | ||
| Sex | |||||
| Male | 51 | 40.8 | 17 | 81.0 | <.001* |
| Female | 74 | 59.2 | 4 | 19.0 | |
| AML/MDS | |||||
| AML | 89 | 71.2 | 18 | 85.7 | .164 |
| MDS | 36 | 28.8 | 3 | 14.3 | |
| History of TMD | |||||
| No | 89 | 71.2 | 15 | 71.4 | .983 |
| Yes | 36 | 28.8 | 6 | 28.6 | |
| Trisomy 8 | |||||
| Absent | 78 | 67.8 | 18 | 94.7 | .016* |
| Present | 37 | 32.2 | 1 | 5.3 | |
| Unknown | 10 | 2 | |||
| Isolated trisomy | |||||
| Absent | 97 | 86.6 | 12 | 63.2 | .050 |
| Present | 15 | 13.4 | 7 | 36.8 | |
| Unknown | 10 | 2 | |||
| Induction I response by BM morphology | |||||
| CR | 110 | 91.7 | 13 | 68.4 | .010* |
| PR | 9 | 7.5 | 3 | 15.8 | .213 |
| RD | 1 | 0.8 | 3 | 15.8 | .008* |
| Unevaluable | 5 | 2 | |||
| Outcome, from day 28 MRD | |||||
| 5-y DFS | 92.7% | 86.3-96.1 | 76.2% | 51.9-89.3 | .011* |
| 5-y OS | 96.8% | 91.6-98.8 | 76.2% | 51.9-89.3 | <.001* |
BM, bone marrow.
Bold P values represent statistical significance <.05.
MRD positivity was significantly higher in male than female patients (P < .001; Table 3) and in those with isolated trisomies (other than trisomy 8) (P = .05; Table 3). There was no significant difference in MRD status based on race/ethnicity, classification of MDS or AML, or history of TMD. In both univariable and multivariable analyses, only MRD on day 28 after induction I was significantly correlated with DFS (Table 4).
Table 4.
Cox analyses of DFS for patients with day 28 MRD data
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P | N | HR | 95% CI | P | |
| MRD day 28 | ||||||||
| MRD− | 125 | 1 | 115 | 1 | ||||
| MRD+ | 21 | 3.78 | 1.27-11.29 | .017* | 19 | 4.14 | 1.15-14.9 | .030* |
| Sex | ||||||||
| Male | 68 | 1 | 61 | 1 | ||||
| Female | 78 | 0.82 | 0.29-2.33 | .703 | 73 | 1.14 | 0.37-3.56 | .824 |
| Trisomy 8 | ||||||||
| Absent | 96 | 1 | 96 | 1 | ||||
| Present | 38 | 0.68 | 0.19-2.44 | .555 | 38 | 1.00 | 0.26-3.92 | .998 |
| Isolated trisomy | ||||||||
| No | 109 | 1 | 109 | 1 | ||||
| Yes | 25 | 1.29 | 0.36-4.364 | .692 | 25 | 0.98 | 0.26-3.63 | .972 |
HR, hazard ratio.
Bold P values represent statistical significance <.05.
The expression of several genes that encode proteins linked to araC and daunorubicin activity was studied to examine potential differences in expression levels by patient characteristics and MRD group. Quantitative reverse transcription polymerase chain reaction analysis of diagnostic blasts found no significant differences in the expression of genes related to araC metabolism/transport-related genes (eg, deoxycytidine kinase, cytidine deaminase, HENT1) between female and male patients overall, or, within the male group, between MRD-negative and MRD-positive patients. Expression of TOP2A, which encodes topoisomerase IIα (a primary daunorubicin target), was significantly lower in MRD-positive patients, which could contribute to relative resistance to daunorubicin (data not shown).
Bone marrow morphologic response
Morphologic bone marrow responses after induction I, as reported by individual institutions, indicated CR in 177 (87.2%), PR in 15 (7.4%), and RD in 10 patients (4.9%). One patient died before evaluation and 1 patient had an unevaluable bone marrow. For 7 patients, bone marrow results for day 28 after induction I were not available, but were available for day 14 of induction I (6 patients with CR and 1 patient with RD) and were included in the induction I response.
Table 3 compares the results of MRD analysis after induction I for the 146 patients with evaluable MRD data and morphologic response after day 28. Of the MRD-negative patients, 10 of 120 (8.3%) with evaluable response were classified as not being in CR by morphology. Of the MRD-positive patients, 13 patients (68.4%) were classified as being in CR.
Morphologic classification and cytogenetics
This trial used institutional reporting to classify cases as either MDS or AML and opened before implementation of the WHO 2008 criteria, which uses the collective ML-DS designation. Of the AML patients, 85 had FAB M7 AMKL, whereas 19 patients had M0-M6 AML. This is in keeping with the prior COG ML-DS trial, A2971, where only 39% of patients had M7 morphology.12 Of the 164 cases for whom adequate diagnostic bone marrow slides were available and retrospectively reviewed centrally by pediatric hematopathologists (K.M., D.H.), 163 met the criteria for ML-DS, whereas 1 patient appeared to be a standard case of AML. According to the classification used before the implementation of the WHO 2008 criteria, 60 would be classified as MDS and 104 as AML based on central review.
Although 5-year EFS was not different between these 2 groups (90.4% ± 6.5% for M7 vs 79.0% ± 18.7% for M0-M6 [log-rank P = .143]), M7 patients had improved 5-year OS of 95.2% ± 4.6% compared with 79.0% ± 18.7% for M0-M6 patients (P = .014; supplemental Figure 2A-B). We also examined cytogenetics among AML patients to determine whether cytogenetic subgroups had adverse outcomes. Compared with all other patients for whom cytogenetic data were available, ML-DS patients with +8, −7, or normal karyotype had no difference in EFS or OS (supplemental Table 3).
In a subset of 46 patients, GATA1 mutations, the signature somatic genetic abnormality in ML-DS, were identified in 41 patients using Sanger sequencing. However, using next-generation sequencing technology as highlighted by the study by Roberts et al,3 a higher number of cases with low blast percentages would be expected to have GATA1 mutations detected. In addition to the WHO 2008 criteria, next-generation sequencing could be used to complement the diagnosis of ML-DS based on the detection of GATA1 mutations.
Discussion
Although children with DS and AML have an overall favorable prognosis, determining the appropriate curative chemotherapy dose intensity while minimizing treatment-related toxicity remains a clinical challenge. Given the increased ex vivo sensitivity of ML-DS blasts to both araC and daunorubicin, the AAML0431 trial was designed using the treatment schema of the COG A2971 protocol with the following modifications: (1) administration of HD-araC in induction II rather than the fifth and final systemic intensification cycle; (2) a 25% reduction in the cumulative daunorubicin dose; and (3) a reduction in the number of prophylactic intrathecal treatments from 7 to 2, based on the very low incidence of CNS leukemia in ML-DS.12 The cumulative araC and daunorubicin doses for the first 2 cycles of induction therapy were 24 800 mg/m2 and 80 mg/m2, respectively, compared with 1600 mg/m2 and 160 mg/m2 for the A2971 trial.
The 5-year EFS and OS from study entry for the 204 eligible patients were 91% and 93%, respectively (Figure 1A-B), which represent a significant improvement over rates of 79% and 84%, respectively, for patients in the COG A2971 trial.12 However, there were differences in patient characteristics between AAML0431 and A2971 trials with respect to race/ethnicity, history of TMD, presenting platelet, and peripheral blast count (supplemental Table 4). Although these factors may have contributed to improved patient outcomes in the AAML0431 trial, the modifications made in the trial design likely played a major role in better patient outcomes than those in the A2971 and other legacy COG trials in which ML-DS patients were treated concurrently with the general pediatric AML population (EFS of 80% and 77% in the POG 9421 and Children’s Cancer Group 2891 trials, respectively).8,9
After induction I, the highest percentage of adverse events, including infectious complications, was seen during induction II, in which HD-araC was used. Interestingly, the microbiologically documented sterile-site bacterial infection rate using the identical HD-araC regimen was significantly lower for DS patients (induction II; 45 of 199; 22.6%) than that for non-DS AML patients treated on AAML0531 (intensification III; 299 of 517 patients; 57.8%) (P < .001). Three patients died of infections, of whom 1 patient was off therapy. The treatment-related mortality that occurred during protocol therapy (2 of 204; 1.0%) was comparable to that of A2971 (3 of 126 patients; 2.4%). There were 7 cardiac-related adverse events ≥grade 3, but no deaths occurred. Of the ML-DS patients treated with reduced intensity on the BFM-2004 trial, 3 died of viral infections but there were no deaths from bacterial or fungal infections.27 Our study highlights the need for continued monitoring of ML-DS patients, including the time of chemotherapy cycles after adequate ANC recovery. Moreover, the risk of prolonged myelosuppression raises the question of whether HD-araC is necessary in ML-DS, an issue being addressed in the current COG ML-DS trial (see the following paragraphs).
To identify new and more accurate prognostic factors for children with ML-DS, we assessed the clinical significance of MRD, focusing on analyzing data collected on day 28 of induction I. Of all prognostic factors studied, MRD on day 28 of induction I was the only significant predictor of outcome in both univariable and multivariable analyses. The 5-year DFS for MRD-negative patients was 92.7% vs 76.2% for MRD-positive patients. For 19 of the 21 MRD-positive patients, MRD was assessed after induction IV. Of these 19 patients, 16 were MRD negative and remain alive. The 3 patients who remained MRD positive relapsed, with 1 patient remaining alive after SCT. It can be speculated that the MRD-positive patients benefited from HD-araC treatment in induction II because treatments in inductions III and IV were identical to those in induction I and used a much lower araC dose. Thus, as previously confirmed for cases of non-DS AML, MRD appears to be a new important prognostic for ML-DS that can help in risk assessment and guide the intensity of treatment.
A comparison of marrow morphology and MRD analysis after induction I revealed that 23 of 139 patients (16.5%) classified by morphology would have been reclassified as positive or negative by MRD. MRD therefore appears to be a more highly sensitive and objective indicator of response compared with marrow morphology and should become the standard of care for assessing treatment response for ML-DS. The outcomes for these 23 patients showed a trend toward better DFS stratification by MRD than morphology though the results were not statistically significant, likely due to the small case numbers (supplemental Figure 3).
The 5-year OS (34.3%) for the 17 patients with refractory/relapse leukemia or SMN, was similar to prior studies.17,18 Of 6 patients who received SCT, 4 are alive and 2 died. Thus, new therapies such as histone deacetylase inhibitors and Wee1 inhibitors need to be developed for patients with refractory or relapsed ML-DS.28 In 2 patients, ALL developed potentially due to the increased incidence of ALL in children with DS and not as a “classic” treatment-induced SMN.
In summary, the earlier use of HD-araC and reduction in daunorubicin dose in AAML0431 was associated with better patient outcomes compared with those seen in past COG trials. MRD was identified as a new prognostic factor for the ML-DS population which previously has been identified for non-DS AML patients. By identifying the appropriate patient population (eg, MRD negative after induction I), a reduction in araC dose intensity (eg, elimination of HD-araC) presents a logical approach to reduce potential toxicity, particularly infectious complications, in ML-DS patients. This concept is being tested prospectively for the current COG ML-DS AAML1531 trial.
Acknowledgments
The authors thank Anders Kolb, Holly Pitman, and Vani Shanker for their constructive review of the manuscript.
This work was supported by the National Cancer Institute of the National Institutes of Health National Clinical Trials Network (NCTN) Operations Center grant U10CA180886, NCTN Statistics & Data Center U10CA180899, Chair’s grant U10CA098543, Statistics and Data Center grant U10CA098413. The correlative biology studies were supported by grant R01 CA120772.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.W.T., J.N.B., J.K.H., A.D.S., N.J.L., P.M., T.A.A., and A.S.G. were involved in the design and planning of the trial; J.W.T., J.N.B., J.K.H., A.D.S., N.J.L., P.M., R.B.G., T.A.A., D.C., and A.S.G. were involved in the manuscript writing; S.R. and B.H. performed the cytogenetic analysis; Y.G. performed and analyzed the gene expression studies; K.M. and D.H. performed the hematopathology review; R.B.G., Y.-C.W., and T.A.A. oversaw the data collection and statistical analysis; and D.C. and E.C.-S. performed and analyzed the MRD data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey W. Taub, Division of Hematology/Oncology, Children's Hospital of Michigan, 3901 Beaubien Blvd, Detroit, MI 48201; e-mail: jtaub@med.wayne.edu.
References
- 1.Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn. 2009;11(5):371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipursky A, Thorner P, De Harven E, Christensen H, Doyle J. Myelodysplasia and acute megakaryoblastic leukemia in Down’s syndrome. Leuk Res. 1994;18(3):163-171. [DOI] [PubMed] [Google Scholar]
- 3.Roberts I, Alford K, Hall G, et al. ; Oxford-Imperial Down Syndrome Cohort Study Group. GATA1-mutant clones are frequent and often unsuspected in babies with Down syndrome: identification of a population at risk of leukemia. Blood. 2013;122(24):3908-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechsler J, Greene M, McDevitt MA, et al. . Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32(1):148-152. [DOI] [PubMed] [Google Scholar]
- 5.Rainis L, Bercovich D, Strehl S, et al. . Mutations in exon 2 of GATA1 are early events in megakaryocytic malignancies associated with trisomy 21. Blood. 2003;102(3):981-986. [DOI] [PubMed] [Google Scholar]
- 6.Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003;101(11):4301-4304. [DOI] [PubMed] [Google Scholar]
- 7.Ravindranath Y, Abella E, Krischer JP, et al. . Acute myeloid leukemia (AML) in Down’s syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80(9):2210-2214. [PubMed] [Google Scholar]
- 8.Gamis AS, Woods WG, Alonzo TA, et al. ; Children’s Cancer Group Study 2891. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891. J Clin Oncol. 2003;21(18):3415-3422. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien MM, Taub JW, Chang MN, et al. ; Children’s Oncology Group Study POG 9421. Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children’s Oncology Group Study POG 9421. J Clin Oncol. 2008;26(3):414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005;19(8):1355-1360. [DOI] [PubMed] [Google Scholar]
- 11.Zeller B, Gustafsson G, Forestier E, et al. ; Nordic Society of Paediatric Haematology and Oncology (NOPHO). Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol. 2005;128(6):797-804. [DOI] [PubMed] [Google Scholar]
- 12.Sorrell AD, Alonzo TA, Hilden JM, et al. . Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children’s Oncology Group trial A2971: a report from the Children’s Oncology Group. Cancer. 2012;118(19):4806-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao A, Hills RK, Stiller C, et al. . Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol. 2006;132(5):576-583. [DOI] [PubMed] [Google Scholar]
- 14.Lange BJ, Kobrinsky N, Barnard DR, et al. . Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children’s Cancer Group Studies 2861 and 2891. Blood. 1998;91(2):608-615. [PubMed] [Google Scholar]
- 15.Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18(1):72-77. [DOI] [PubMed] [Google Scholar]
- 16.Hitzler JK, He W, Doyle J, et al. ; CIBMTR Pediatric Cancer Working Committee. Outcome of transplantation for acute myelogenous leukemia in children with Down syndrome. Biol Blood Marrow Transplant. 2013;19(6):893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loew TM, Gamis A, Smith FO, et al. . Down syndrome patients with relapsed acute myelogenous leukemia [abstract]. Blood. 2004;104(11). Abstract 4526. [Google Scholar]
- 18.Taga T, Saito AM, Kudo K, et al. . Clinical characteristics and outcome of refractory/relapsed myeloid leukemia in children with Down syndrome. Blood. 2012;120(9):1810-1815. [DOI] [PubMed] [Google Scholar]
- 19.Taub JW, Matherly LH, Stout ML, Buck SA, Gurney JG, Ravindranath Y. Enhanced metabolism of 1-beta-D-arabinofuranosylcytosine in Down syndrome cells: a contributing factor to the superior event free survival of Down syndrome children with acute myeloid leukemia. Blood. 1996;87(8):3395-3403. [PubMed] [Google Scholar]
- 20.Taub JW, Huang X, Matherly LH, et al. . Expression of chromosome 21-localized genes in acute myeloid leukemia: differences between Down syndrome and non-Down syndrome blast cells and relationship to in vitro sensitivity to cytosine arabinoside and daunorubicin. Blood. 1999;94(4):1393-1400. [PubMed] [Google Scholar]
- 21.Ge Y, Dombkowski AA, LaFiura KM, et al. . Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006;107(4):1570-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Stout ML, Tatman DA, et al. . GATA1, cytidine deaminase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst. 2005;97(3):226-231. [DOI] [PubMed] [Google Scholar]
- 23.Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer. 2005;44(1):33-39. [DOI] [PubMed] [Google Scholar]
- 24.Kalabus JL, Sanborn CC, Jamil RG, Cheng Q, Blanco JG. Expression of the anthracycline-metabolizing enzyme carbonyl reductase 1 in hearts from donors with Down syndrome. Drug Metab Dispos. 2010;38(12):2096-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubnitz JE, Inaba H, Dahl G, et al. . Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Velden VH, van der Sluijs-Geling A, Gibson BE, et al. . Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24(9):1599-1606. [DOI] [PubMed] [Google Scholar]
- 27.Hassler A, Bochennek K, Gilfert J, et al. . Infectious complications in children with acute myeloid leukemia and Down syndrome: analysis of the prospective multicenter trial AML-BFM 2004. Pediatr Blood Cancer. 2016;63(6):1070-1074. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell JT, Edwards H, Buck SA, Ge Y, Taub JW. Targeting the wee1 kinase for treatment of pediatric Down syndrome acute myeloid leukemia. Pediatr Blood Cancer. 2014;61(10):1767-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]