Abstract
Purpose
To assess changes in retinal nonperfusion (RNP) in patients with retinal vein occlusion (RVO) treated with ranibizumab (RBZ)
Design
Secondary outcome measure in randomized double-masked controlled clinical trial
Subjects
Thirty-nine patients with central RVO (CRVO) and 42 with branch RVO (BRVO)
Methods
Subjects were randomized to 0.5mg or 2.0mg RBZ every month for 6 months and then re-randomized to pro re nata (prn) groups RBZ+scatter photocoagulation (laser) or RBZ alone for an additional 30 months.
Main Outcome Measures
Comparison of percentage of patients with increased or decreased area of RNP in patients with RVO treated with 0.5mg versus 2.0mg RBZ, during monthly injections versus prn RBZ, and in patients treated with prn RBZ versus prn RBZ+laser.
Results
In RVO patients given monthly injections of 0.5mg or 2.0mg RBZ for 6 months there was no significant difference in the percentage who showed reduction or increase in area of RNP. However, regardless of dose, during the 6 month period of monthly injections, a higher percentage of patients showed a reduction in area of RNP and a lower percentage showed an increase in area of RNP compared to subsequent time periods of prn RBZ treatment. After the 6 month period of monthly injections, BRVO, but not CRVO patients randomized to prn RBZ+laser showed significantly less progression of RNP compared to patients treated with prn RBZ.
Conclusions
Regardless of dose of ranibizumab (0.5mg or 2.0mg), monthly injections promote improvement and reduce progression of RNP compared to prn injections. Addition of scatter photocoagulation to prn RBZ may reduce progression of RNP in patients with BRVO, but a statistically significant reduction was not seen in patients with CRVO.
Introduction
Retinal vein occlusion (RVO) is a prevalent retinal vascular disease that is subdivided into central RVO (CRVO), in which there is occlusion of the main outflow vessel of the eye, and branch (BRVO), in which a branch of the central retinal vein is occluded. They differ in the amount of retina affected by the occlusion and on average CRVOs tends to have a worse visual prognosis than BRVOs. There is considerable overlap in molecular pathogenesis, because in both, retina drained by occluded vessels becomes ischemic and produces hypoxia-regulated gene products, including vascular endothelial growth factor (VEGF). A pilot trial indicated that VEGF is a major contributor to macular edema, because suppression of VEGF by intraocular injections of ranibizumab (RBZ) reduced edema and improved visual acuity.1 This was confirmed in large multicenter phase 3 trials.2, 3 Injections of another VEGF antagonist, aflibercept, have shown similar effects.4
Studies with RBZ have uncovered additional deleterious effects of high intraocular levels of VEGF that are reversed by RBZ. Patients with RVO treated with monthly injections of RBZ show more rapid resolution of retinal hemorrhages indicating that VEGF promotes ongoing hemorrhaging that is blocked by RBZ.5, 6 Measurement of the area of retinal nonperfusion (RNP) in the macula by masked grading of fluorescein angiograms (FAs) at an independent reading center demonstrated progression of central RNP in sham-treated patients with RVO that was significantly reduced in patients given monthly injections of RBZ for 6 months.7 Some patients in the RBZ treatment group showed reduction in RNP in the macula over the first 6 months. After 6 months, RBZ injections were given to patients who previously received sham injections and the differences from baseline RNP between the groups was eliminated. This suggests that high levels of VEGF promote closure of retinal vessels and that neutralization of VEGF can prevent additional vessel closure and can even cause recently closed vessels to reopen. This is a revolutionary concept and as is usually the case with new and unexpected findings, it is difficult for many clinicians and researchers to accept. One possible concern is that 30° FAs were used to visualize and grade RNP and therefore only the macula and surrounding area of the retina was assessed. There is no reason to believe that vessels in the posterior retina should differ from those in the peripheral retina in their response to high levels of VEGF, but it would be useful to demonstrate this.
After initiation of the Ranibizumab DosE Comparison (0.5mg and 2.0mg) and the Role of LAser in the ManagemenT of REtinal Vein Occlusion (RELATE) Trial,8 the study protocol was amended to include as a secondary endpoint, the effect of VEGF neutralization on RNP of peripheral as well as central retinal vessels using ultra-wide field FA. The following experimental questions were addressed. (1) During a 6 month period of monthly injections of RBZ, compared to RVO patients treated with 0.5mg RBZ, do a higher percentage of patients treated with 2.0 mg RBZ show a reduction in area of RNP and/or a lower percentage show an increase in area of RNP? (2) During a period when RVO patients are given an injection of RBZ every month regardless of dose, is there a higher percentage with reduction in area of RNP and/or a lower percentage with increase in area of RNP compared to a period when the same patients are given pro re nata (prn) injections? (3) Compared to patients with RVO treated with prn RBZ, do patients treated with prn RBZ+laser show a higher percentage with reduction in the area of RNP and/or a lower percentage with an increase in area of RNP? The results are reported herein.
Methods
The RELATE trial (ClinicalTrials.gov identifier: NCT01003106) was an investigator-initiated double-masked randomized trial sponsored by Genentech, Inc. (South San Francisco, CA) designed to compare the effects of monthly injections of 0.5mg RBZ with monthly injections of 2.0mg RBZ for 6 months in patients with macular edema due to RVO and to also determine if scatter/grid laser photocoagulation (laser) reduces the need for injections and improves long-term outcomes. The study was conducted in accordance with the Declaration of Helsinki, applicable US Food and Drug Administration (FDA) regulations, and the Health Insurance Portability and Accountability Act. The study protocol was approved by the Johns Hopkins University institutional review board before study initiation, and all participating patients provided informed consent. The design and details of the treatment protocol of the RELATE trial have been previously published and are not repeated here.8 After initiation of the trial, ultra-wide field FA using the Optos 200Tx imaging system (Optos PLC, Dunferlmine, Scotland) was provided to evaluate the effect of VEGF suppression on retinal vessel perfusion in the periphery by providing a 200° view of the retina in a single photograph. Some patients had already entered the trial and in those patients seven field images with a 30° fundus camera had been obtained for baseline FAs, but all subsequent FAs in those patients were obtained with an Optos 200Tx imaging system and all patients enrolled after that point had ultra-wide field FA at baseline and months 6, 12, 24, and 36.
Nonperfusion was evaluated throughout the entire retina including both the posterior and peripheral retina on ultra-wide field FA images. Experienced graders were masked with respect to randomization groups. The ultra-wide field FA images obtained at each time point starting with month 6 were compared to those obtained at the previous time point. While it is clearly advantageous to assess a larger area of the retina, one disadvantage of the ultra-wide angle images is that many of the images have blurring and artifact in the far periphery and the amount of blurring varies from patient to patient; some patients are able to keep their head in the right position and their eyes wide open and others have difficulty doing so resulting in more edge artifact. Thus, the amount of retina that can be assessed varies from patient to patient and measurement of amount of RNP cannot be compared between patients. However, it is feasible to make longitudinal assessments in the same patient and determine if the amount of RNP at one time point is increased, decreased, or the same at the next time point for the same area of retina assessed. It is for this reason that categorical grading was done rather than attempting to provide an absolute amount of RNP in each image because the latter would imply a level of precision that is not possible and would be misleading. In addition, our experimental questions required categorical grading and not absolute measurements. In order to make a change assessment between 2 time points, it is was necessary to have FA images at each time point that were sufficiently clear with only small areas of edge artifact allowing the grading to be made with a high level of confidence. In general, if there was more than a 10% difference in retinal vascular area that could be graded between images, they were judged ungradable. Also, if a region that showed RNP on one image was obscured on the other image due to edge artifact and the region was sufficiently large so that a difference in that region could affect the grading, the images were listed as ungradable.
All statistical tests were performed using IBM SPSS software version 19.0 (IBM Corp., Armonk, NY) and Stata 13.1 (College Station, Texas). For categorical variables, comparison between groups were made using the chi-square test for a large sample size and Fisher’s exact test for a small sample size. Association between variables was sought using the Spearman’s rank correlation coefficient (rho). Independent analysis was run for each variable at month 6, 12, 24 and 36. To compare the change in area of RNP on consecutive FAs, multinomial logistic regression models with robust standard error estimation (Huber/White/sandwich estimator of the variance) were used to account for the correlation among the repeated measures from the same patients.9 For patients who missed one or more of the endpoint visits, ultra-wide field FA images done within 3 months of the endpoint visit were used for grading.
Results
In the RELATE trial, 81 patients with RVO (39 with CRVO and 42 with BRVO) were enrolled at a single center (The Wilmer Eye Institute, Johns Hopkins Hospital) and randomized to receive injections of 0.5mg or 2.0mg of RBZ every month with primary endpoint at 6 months. Seventy-seven patients completed through the primary endpoint and were re-randomized to prn arms, RBZ+laser or RBZ for recurrent macular edema due to RVO. Patient demographics, disposition, and primary outcomes have been previously published.8 After initiation of the trial, an Optos 200Tx imaging system was obtained and the protocol was amended to include an additional group of secondary endpoints related to the effect of VEGF suppression and laser on RNP assessed by ultra-wide field FA.
Patient Disposition with Regard to Change in RNP Outcome
In order for change in RNP to be assessed for a patient during one of the 4 time periods (baseline to month 6, month 6 to month 12, month 12 to month 24, and month 24 to month 36), it was necessary to have a gradable FA at the beginning and end of the time period. The number of BRVO or CRVO patients that fulfilled this criterion for each of the time points was: baseline to month 6, 35 and 34; month 6 to month 12, 34 and 36; month 12 to month 24, 31 and 32, and month 24 to month 36, 32 and 27. It is important to know the status of patients who dropped out or had ungradable FAs for each time period to determine any potential impact they might have had on outcome. Patient disposition relevant to RNP secondary endpoints is included in Table 1 (available at http://aaojournal.org). The table shows all gradings that were done for these patients and also shows all time points for which data were not available because of an ungradable FA or prior exit from the study. Patients with incomplete data were well-balanced among treatment groups and the limited data set from these patients was very similar to the complete data set; therefore, it appears unlikely that patient drop out or inability to obtain a gradable FA at a particular time point for some patients had any effect on outcomes.
Effect of Dose of Ranibizumab on RNP
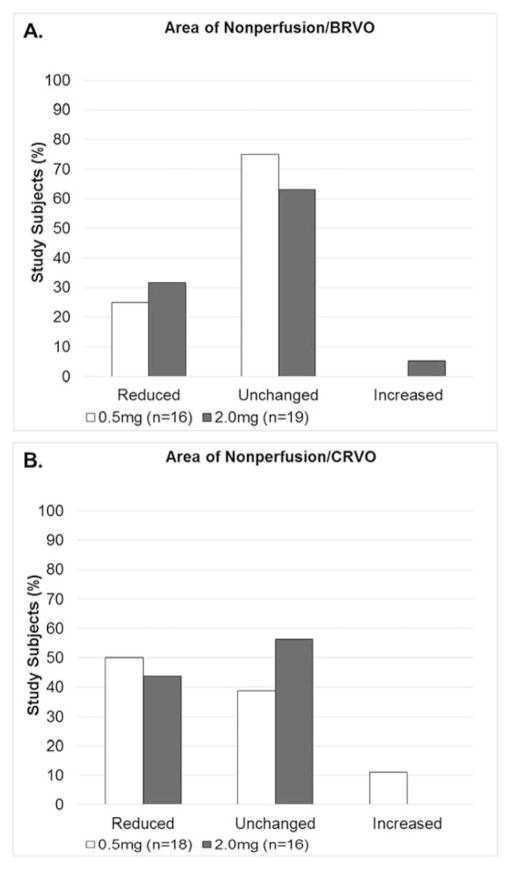
Comparisons between the 2.0mg and 0.5mg RBZ groups at the month 6 primary endpoint in patients with BRVO showed reduction in area of RNP in 31.6% versus 25.0%, no change in 63.2% versus 75.0%, increased RNP in 5.3% versus 0% (Figure 1A). These differences were not statistically significant. In subjects with CRVO, comparisons between the 2.0mg and 0.5mg RBZ groups at the month 6 primary endpoint showed improvement in RNP in 43.8% versus 50.0%, no change in 56.3% versus 38.9%, and increased RNP in 0% versus 11.1% (Figure 1B) and these differences were not statistically significant. These data suggest that in patients with RVO, monthly injections of 2.0mg of RBZ have a similar effect on RNP as monthly injections of 0.5mg of RBZ.
Figure 1. Change from baseline area of retinal nonperfusion at month 6 for 0.5mg versus 2.0mg ranibizumab in patients with (A) branch retinal vein occlusion and (B) central retinal vein occlusion.
Patients with macular edema due to branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO) were randomized at baseline to receive monthly injections of 0.5mg versus 2.0mg of ranbizumab for 6 months. Month 6 ultra-wide field fluorescein angiograms (FAs) were compared to those at baseline and change in area of retinal nonperfusion (RNP) was graded as reduced, unchanged, or increased for the two dosing groups. There was no significant difference between the percentage of study subjects that showed a change in area of RNP between the 0.5mg and 2.0mg groups for subjects with BRVO or CRVO. In (A) 0% of BRVO patients treated with 0.5mg ranibizumab showed an increase in RNP and hence no white bar in the increased category. In (B) 0% of CRVO patients treated with 2.0mg ranibizumab showed an increase in RNP and hence no black bar in the increased category.
Effect of Injection Frequency on RNP
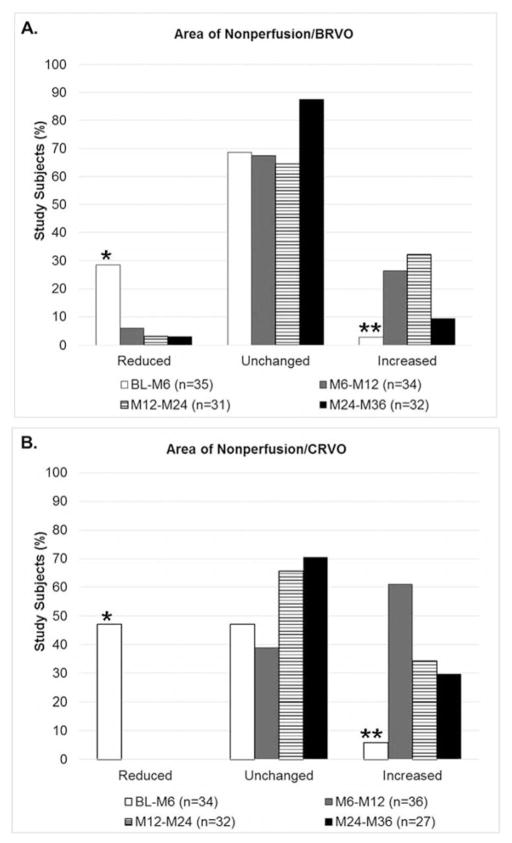
During the first 6 months, there was good compliance with monthly injections; the mean number of injections was 5.8 in patients with BRVO and 5.9 in patients with CRVO. In patients with BRVO, the mean number of injections per month was reduced from 0.97 in the first 6 months to 0.62 between months 6 and 12, 0.57 between months 12 and 24, and 0.36 between months 24 and 36. In patients with CRVO, the mean number of injections per month was reduced from 0.98 in the first 6 months, to 0.58 between months 6 and 12, 0.55 between months 12 and 24, and 0.44 between months 24 and 36. The area of RNP was reduced in 28.6% of patients with BRVO during the first 6 months and a significantly smaller percentage had reduction of RNP at all subsequent time points (Figure 2A, p=0.008 by multinomal logistic regression model with Huber/White/sandwich estimator of variance). The percentage of BRVO patients with an increase in RNP was 2.9% in the first 6 months and a significantly greater percentage had an increase in RNP at all subsequent time points (p=0.009). These differences were even greater in patients with CRVO, in whom 47.1% showed a reduction in area of RNP in the first 6 months compared to 0% in subsequent time periods (Figure 2B, p=0.0001). The percentage of CRVO patients who showed an increase in the area of RNP was 5.9% in the first 6 months, 61.1% between months 6 and 12, 34.4% between months 12 and 24, and 29.6% between months 24 and 36 (p=0.0006). Thus, during a period of monthly RBZ injections, a significantly higher percentage of patients with BRVO or CRVO showed a reduction in the area of RNP and a significantly lower percentage showed an increase in area of RNP than during periods when injections were given less frequently.
Figure 2. Change in area of retinal nonperfusion following monthly injections of ranibizumab between baseline and month 6 versus pro re nata ranibizumab thereafter in subjects with (A) branch retinal vein occlusion (B) central retinal vein occlusion.
Patients with macular edema due to branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO) were treated with monthly injections of ranibizumab between baseline and month 6 (BL-M6) followed by pro re nata (prn) injections of ranibizumab between M6 and month 12 (M6–M12), between M12 and month 24 (M12–M24), and between M24 and month 36 (M24–M36). Change in area of retinal nonperfusion (RNP) was graded as reduced, unchanged or increased between each time point. A greater percentage of BRVO (A) and CRVO (B) patients showed a reduction in area of RNP and a lower percentage showed an increase in area of RNP when receiving monthly injections of ranibizumab (BL-M6) than during subsequent periods when receiving prn ranibizumab. In (B), 0% of patients in the M6–M12, M12–M24, and M24–M36 time points showed reduction in RNP and hence only the white bar (BL-M6) bar appears in the reduced category.
(A) *p=0.008 for difference in percentage of patients with a reduction in area of RNP in BL-M6 period versus each of the other periods; **p=0.009 for difference in percentage of patients with an increase area of RNP in BL-M6 period and each of the other periods using a multinomal logistic regression model with Huber/White/sandwich estimator of variance to account for the correlation among repeated measures from the same patients.
(B) *p<0.0001 for difference in percentage of patients with a reduction in area of RNP in BL-M6 period versus each of the other periods; ** p=0.0006 for difference in percentage of patients with an increase area of RNP in BL-M6 period and each of the other periods using a multinomal logistic regression model with Huber/White/sandwich estimator of variance to account for the correlation among repeated measures from the same patients.
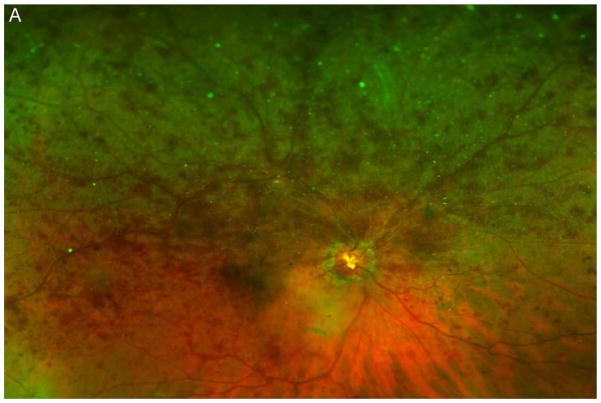
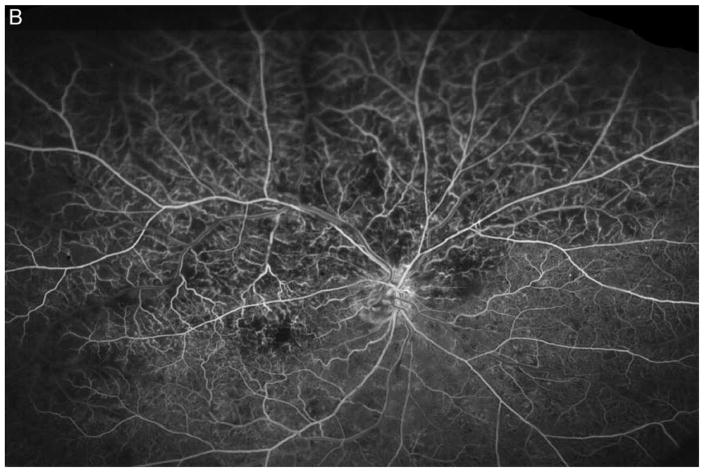
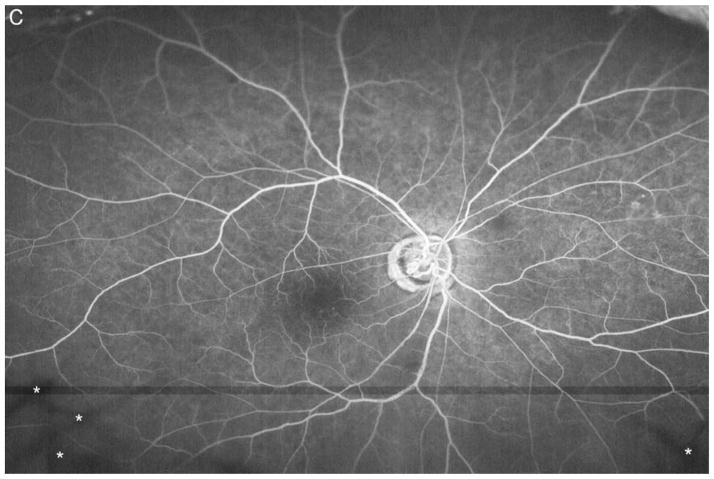
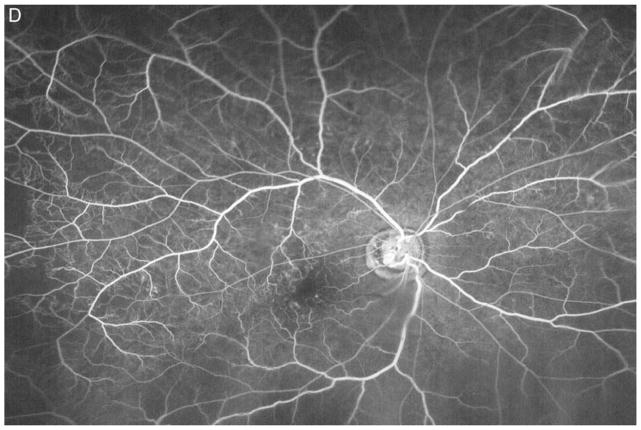
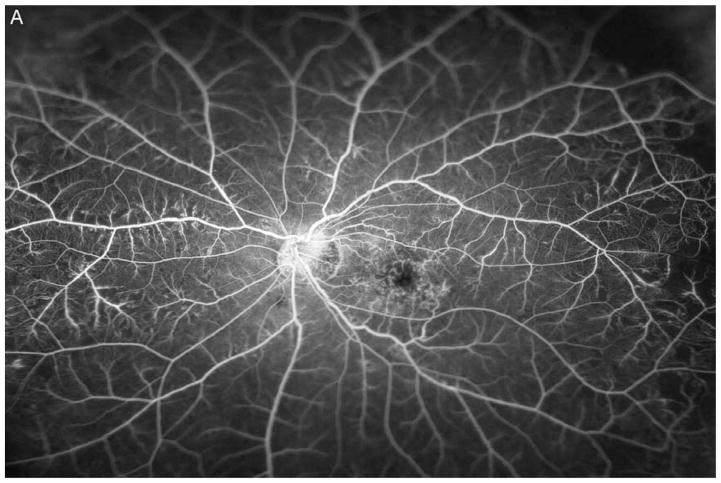
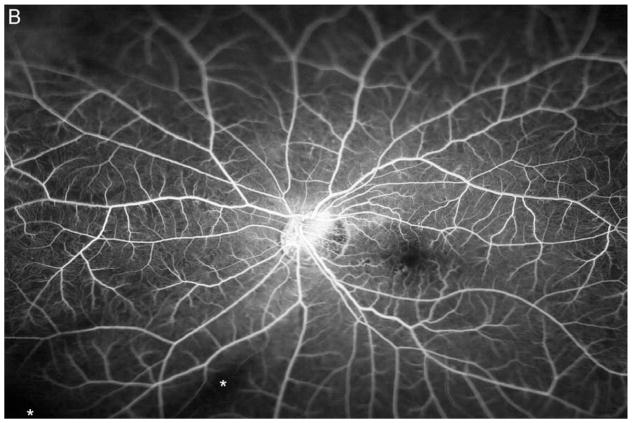
Figure 3A shows a baseline fundus photograph from a 71 year old male with a superior BRVO of 1 month duration who had not received any anti-VEGF injections prior to enrollment. There was fairly mild intraretinal hemorrhage. An ultra-wide angle FA shows many areas of hypofluorescence, some that correspond to hemorrhages and represent blocked fluorescence, but most that do not correspond to hemorrhages and represent RNP (Figure 3B). A repeat FA after 6 injections of RBZ a month apart shows a marked decrease in the area of RNP (Figure 3C). Six months after switching to prn RBZ, there were recurrent areas of RNP, but overall perfusion was still better than baseline (Figure 3D). A 61 year old male was enrolled 5 months after onset of a CRVO in the left eye for which he had received 2 anti-VEGF injections prior to enrollment. Ultra-wide angle FA shows large patches of RNP in the temporal periphery and small patches in the midperiphery nasally (Figure 4A). After 6 injections of 0.5mg RBZ a month apart, many of the areas of RNP in the temporal periphery were smaller, but there were still small patches nasally (Figure 4B). At month 12, after 6 months of prn RBZ, there was an increase in RNP temporally and nasally (Figure 4C).
Figure 3. Reduction in area of nonperfusion during monthly ranibizumab injections and partial recurrence of nonperfusion during pro re nata injections in a patient with branch retinal vein occlusion.
(A) Baseline fundus photograph in a patient with a superior branch retinal vein occlusion shows mild intraretinal hemorrhages. (B) Image from early recirculation phase of baseline ultra-wide field fluorescein angiogram (FA) shows many areas of hypofluorescence, some that corresponding to hemorrhages (blocked fluorescence), but most not corresponding to hemorrhages indicating areas of nonperfusion. (C) Image from early recirculation phase of FA at month 6 after monthly injections for 6 months shows a marked decrease in the area of retinal nonperfusion. (D) Image from early recirculation phase of FA at month 12, six months after switching to pro re nata ranibizumab shows some recurrent areas of nonperfusion, but perfusion is better than at baseline. (*) Asterisks indicate artifacts secondary to eye lashes/eyelids.
Figure 4. Reduction in area of nonperfusion during monthly ranibizumab injections and partial recurrence of nonperfusion during pro re nata injections in a patient with central retinal vein occlusion.
(A) Image from early recirculation phase of an ultra-wide field fluorescein angiogram (FA) in patient with central retinal vein occlusion shows large patches of RNP in the temporal periphery and small patches in the mid-periphery nasally. (B) Image from early recirculation phase of FA at month 6 after monthly injections of 0.5mg RBZ shows improvement in RNP with many of the areas of RNP in the temporal periphery smaller than baseline. However, there are still small patches of RNP nasally. (C) Image from early recirculation phase of month 12 FA after 6 months of prn RBZ injections, shows worsening of RNP temporally and nasally *artifacts secondary to eye lashes/eyelids.
Effect of Scatter Photocoagulation on RNP
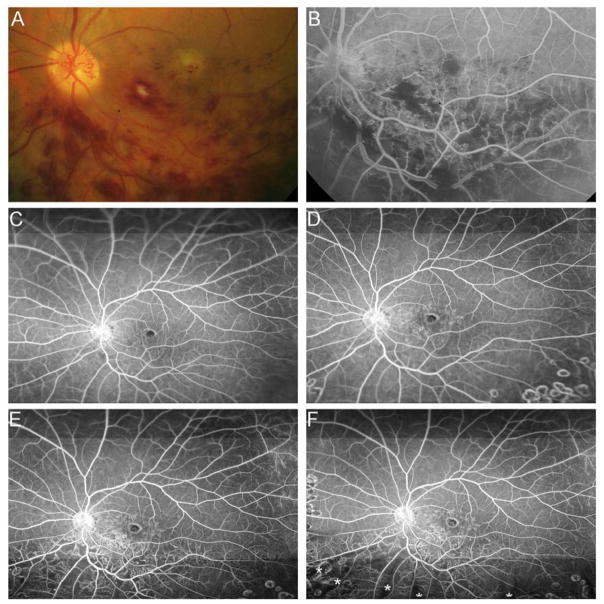
After month 6, patients were re-randomized to prn RBZ or prn RBZ+laser. In patients with BRVO, the change in RNP between 6 and 12 months was similar in the prn RBZ and the prn RBZ+laser groups (Figure 5A); however, between 12 and 24 months and between 24 and 36 months there was a greater percentage of patients in the prn RBZ group that showed an increase in area of RNP and by chi square analysis, there were significant differences in distribution among the groups (Figure 5B and C, p=0.003 and p=0.04). In patients with CRVO, the change in RNP was similar in the prn RBZ and the prn RBZ+laser groups in all three time periods with no significant differences in distribution among the groups (Figure 6). Thus, while the addition of laser to prn RBZ did not result in an identifiable change in progression of RNP in patients with CRVO, there was less progression of RNP in patients with BRVO who had laser+RBZ starting 6 months after randomization. However, the ameliorative effect of laser on progression of RNP in patients with BRVO, was not universal because there was one clear exception. A 65 year old male had an inferior BRVO for 2 months prior to enrollment and had received no anti-VEGF injections. This patient was enrolled prior to the change in protocol allowing use of ultra-wide angle FA and at baseline a 30° fundus photograph show some hemorrhages in the inferior macula (Figure 7A). A 30° FA shows blocked fluorescence corresponding to hemorrhages and areas of RNP inferior to the optic nerve (Figure 7B). After 6 injections of 2.0 mg RBZ, an ultra-wide angle FA shows reduced RNP below the optic nerve and arcade vessel (Figure 7C). At month 12, after 6 months of prn RBZ+laser, there was no change in RNP (Figure 7D), however at month 24, there was a marked increase in RNP inferior and superior to the inferotemporal arcade vessel (Figure 7E). At month 36, there was persistent RNP that was unchanged (Figure 7F).
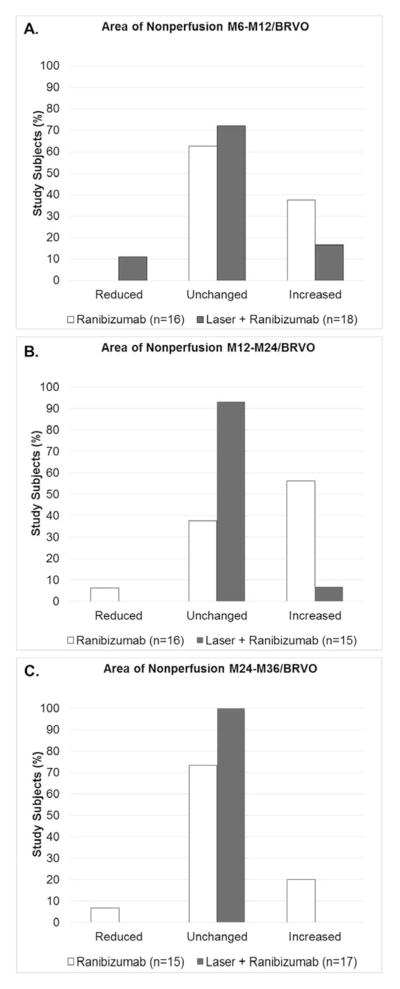
Figure 5. Change in area of retinal nonperfusion in patients with branch retinal vein occlusion in 3 sequential time periods after randomization to pro re nata ranibizumab or ranibizumab+laser.
At month 6, patients with branch retinal vein occlusion (BRVO) were re-randomized to pro re nata (prn) ranibizumab or prn ranibizumab+laser. Ultra-wide field fluorescein angiograms (FA) were used to grade the change in area of retinal nonperfusion as reduced, unchanged, or increased between M6 and month 12 (M6–M12, A), between M12 and month 24 (M12–M24, B), and between M24 and month 36 (M24–M36, C). The distribution among the 3 grades (reduced, unchanged, or increased) was compared by Fisher’s exact test and there was no significant difference in ranibizumab versus laser+ranibizumab groups in the M6–M12 period (A), but significant differences were present in the M12–M24 period (B, p=0.003) and the M24–M36 period (C, p=0.04).
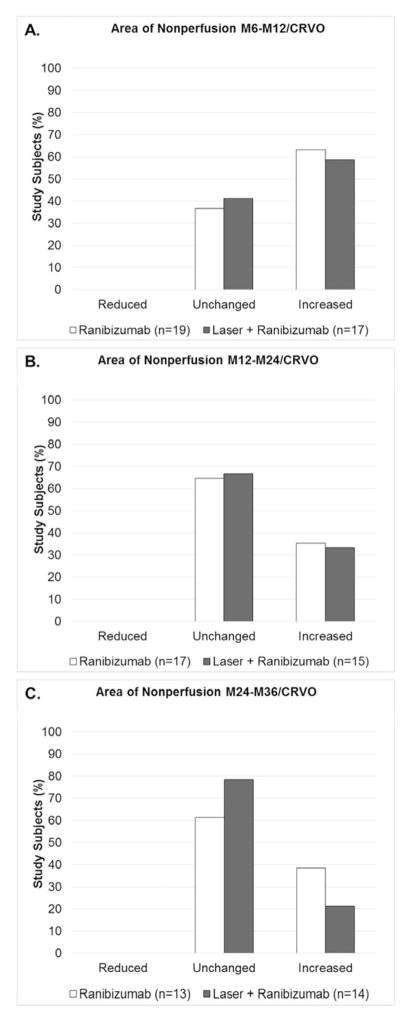
Figure 6. Change in area of retinal nonperfusion in patients with central retinal vein occlusion in 3 sequential time periods after randomization to pro re nata ranibizumab or ranibizumab+laser.
At month 6, patients with central retinal vein occlusion (CRVO) were re-randomized to pro re nata (prn) ranibizumab or prn ranibizumab+laser. Ultra-wide field fluorescein angiograms (FA) were used to grade the change in area of retinal nonperfusion as reduced, unchanged, or increased between M6 and month 12 (M6–M12, A), between M12 and month 24 (M12–M24, B), and between M24 and month 36 (M24–M36, C). The distribution among the 3 grades (reduced, unchanged, or increased) was compared by Fisher’s exact test and there was no significant difference in ranibizumab versus laser+ranibizumab groups in any of the 3 time periods.
Figure 7. Reperfusion of nonperfused retina in a patient with branch retinal vein occlusion during monthly injections of ranbizimab and failure to prevent recurrent nonperfusion by scatter photocoagulation and pro re nata ranibizumab.
(A) Baseline 30° fundus photograph of patient with inferior branch retinal vein occlusion (BRVO) showing some hemorrhages in the inferior macula. (B) Early recirculation phase of a 30° fluorescein angiogram shows blocked fluorescence corresponding to hemorrhages and areas of retinal nonperfusion (RNP) inferior to the optic nerve. (C) Early recirculation phase of an ultra-wide field (FA) at month 6 after monthly injections of 2.0mg ranibizumab for 6 months shows reperfusion of some previously nonperfused areas. (D) Early recirculation phase of FA at month 12, after 6 months of prn ranibizumab+laser, show no change in RNP (E). Month 24 FA shows a marked increase in RNP inferior and superior to the inferotemporal arcade vessel (F). Month 36 FA shows persistent RNP that was unchanged from the previous visit. (*) Asterisks indicate artifacts secondary to eye lashes/eyelids.
Correlation of Central and Peripheral RNP
Previous studies measured the effect of treatments on RNP in the ETDRS center, inner, and outer subfields and assumed that central RNP was representative of RNP in the periphery and hence throughout the entire retina. We compared grading of RNP centrally with that in the periphery. There was a statistically significant correlation between central and peripheral RNP grade at each time point in BRVO (Table 2) and CRVO (Table 3) patients.
Table 2.
Comparison of Central and Peripheral Retinal Nonperfusion in Patients with Branch Retinal Vein Occlusion
| Retinal Nonperfusion at Baseline | ||||
|---|---|---|---|---|
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 6(22.2) | 3(7.9) | 0.55 | 0.003 |
| Minimal | 0(0.0) | 1(2.6) | ||
| Mild | 12(44.4) | 16(42.1) | ||
| Moderate | 5(18.5) | 6(15.8) | ||
| Severe | 4(14.8) | 12(31.6) | ||
| Retinal Nonperfusion at Month 6 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 6(18.8) | 4(11.1) | 0.73 | <0.001 |
| Minimal | 2(6.3) | 4(11.1) | ||
| Mild | 12(37.5) | 13(36.1) | ||
| Moderate | 10(31.3) | 5(13.9) | ||
| Severe | 2(6.3) | 10(27.9) | ||
| Retinal Nonperfusion at Month 12 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 6(18.2) | 6(17.1) | 0.44 | 0.01 |
| Minimal | 3(9.1) | 6(17.1) | ||
| Mild | 14(42.4) | 9(25.7) | ||
| Moderate | 9(27.3) | 6(17.1) | ||
| Severe | 1(3.0) | 8(22.9) | ||
| Retinal Nonperfusion at Month 24 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 6(19.4) | 4(12.9) | 0.35 | 0.05 |
| Minimal | 11(35.5) | 4(12.9) | ||
| Mild | 4(12.9) | 9(29.0) | ||
| Moderate | 8(25.8) | 7(22.6) | ||
| Severe | 2(6.5) | 7(22.6) | ||
| Retinal Nonperfusion at Month 36 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 6(19.4) | 5(15.6) | 0.68 | <0.001 |
| Minimal | 4(12.9) | 4(12.5) | ||
| Mild | 12(38.7) | 10(31.3) | ||
| Moderate | 7(22.6) | 5(15.6) | ||
| Severe | 2(6.5) | 8(25.0) | ||
Table 3.
Comparison of Central and Peripheral Retinal Nonperfusion in Patients with Central Retinal Vein Occlusion
| Retinal Nonperfusion at Baseline | ||||
|---|---|---|---|---|
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 1(2.8) | 1(2.8) | 0.51 | 0.001 |
| Minimal | 2(5.6) | 3(8.3) | ||
| Mild | 25(69.4) | 16(44.4) | ||
| Moderate | 3(8.3) | 9(25.0) | ||
| Severe | 2(5.6) | 7(19.4) | ||
| Retinal Nonperfusion at Month 6 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 1(2.7) | 0(0.0) | 0.54 | 0.001 |
| Minimal | 2(5.4) | 3(8.1) | ||
| Mild | 24(64.9) | 17(45.9) | ||
| Moderate | 7(18.9) | 9(24.3) | ||
| Severe | 2(5.4) | 8(21.6) | ||
| Retinal Nonperfusion at Month 12 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 1(2.8) | 0(0.0) | 0.48 | 0.003 |
| Minimal | 3(8.3) | 4(11.1) | ||
| Mild | 23(63.9) | 15(41.7) | ||
| Moderate | 6(16.7) | 8(22.2) | ||
| Severe | 3(8.3) | 9(25.0) | ||
| Retinal Nonperfusion at Month 24 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 1(3.3) | 0(0.0) | 0.53 | 0.003 |
| Minimal | 2(6.7) | 3(10.0) | ||
| Mild | 20(66.7) | 14(46.7) | ||
| Moderate | 4(13.3) | 5(16.7) | ||
| Severe | 3(10.0) | 8(26.7) | ||
| Retinal Nonperfusion at Month 36 | ||||
| Central Nonperfusion, n(%) | Peripheral Nonperfusion, n(%) | Spearman’s Correlation Coefficient (rho) | p-value | |
| None | 0(0.0) | 0(0.0) | 0.74 | <0.001 |
| Minimal | 0(0.0) | 0(0.0) | ||
| Mild | 16(66.7) | 13(52.0) | ||
| Moderate | 5(20.8) | 4(16.0) | ||
| Severe | 3(12.5) | 8(32.0) | ||
Discussion
Interventional trials for macular edema in patients with RVO have provided important information in addition to the effect of treatments on macular edema. In the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) trials, measurement of RNP in the center, inner, and outer subfields by masked graders at a reading center showed that a high percentage of control, untreated patients with CRVO or control BRVO patients treated with grid laser experienced large increases in the area of RNP in the central retina over time.9, 10 This documented what had been observed in clinical practice and was generally referred to as “conversion from nonischemic to ischemic RVO”. The cause of this increase in RNP was unknown, but it was generally attributed to worsening of the vascular occlusion. In the CRUISE and BRAVO trials, progression of RNP was seen in the sham groups between baseline and month 6 but was significantly less in the RBZ groups.7 In fact, many RBZ-treated patients showed a reduction in RNP. Thus, high levels of VEGF cause an increase in RNP in patients with RVO whereas suppression of VEGF can reduce progression or reduce the amount of RNP.
In this study, we have used ultra-wide angle FA to address additional questions. We found that during a 6 month period of monthly injections of RBZ, there was no significant difference in the percentage of patients treated with 2.0 mg RBZ who showed a reduction or an increase in area of RNP compared with those treated with 0.5 mg RBZ. As previously published, there was also no significant difference in improvement in BCVA between baseline and 6 months in CRVO or BRVO patients treated with 2.0 mg vs 0.5 mg RBZ, but in patients with CRVO, those treated with 2.0 mg of RBZ had a significantly greater reduction in CST than those treated with 0.5 mg.8 This suggests that in patients with RVO monthly injections of 0.5 mg of RBZ appear to provide sufficient neutralization of VEGF to slow progression of RNP and maximize BCVA, but in some patients with CRVO there is not sufficient neutralization of VEGF to cause maximal resolution of edema.
In contrast to dose of RBZ, frequency of RBZ injection had a major impact on RNP in patients with RVO. At enrollment in the study, the mean duration from onset of BRVO was 13.7 ± 3.0 months with a range of 0 to 102 months, and the mean duration from onset of CRVO was 15.3 ± 2.2 months with a range of 0 to 62 months. During the time prior to enrollment, patients received prn anti-VEGF injections. For six months following enrollment, patients received an injection of 0.5mg or 2mg of ranibizumab every month and then resumed prn treatment. At the end of the first 6 months nearly 30% of patients with BRVO and 50% of patients with CRVO showed a decrease in RNP and approximately 5–7% showed an increase. At the end of the subsequent 6 months of prn treatment, 5% of BRVO patients and 0% of CRVO patients showed improvement in RNP, while 25% of BRVO and 60% of CRVO patients showed an increase in RNP. Thus, when patients went from prn treatment for more than 6 months to every month treatment for 6 months, there was a substantial percentage of patients who showed improvement in RNP and then when they resumed prn treatment over the next 6 months, almost the same percentage of patients showed an increase in RNP. These data suggest that going from a period of intermittent suppression of VEGF to a period of constant suppression of VEGF contributed to the reduction in RNP, and then resumption of intermittent suppression of VEGF contributed to the reversal. We cannot rule out that other factors may have also influenced the results, but time from onset does not explain the results because the patients had on average a period of 12 months of prn treatment prior to enrollment and after 6 ranibizumab injections a month apart, RNP was reduced, while after the next 6 months of prn injections, RNP was increased. During two subsequent 12 month periods there was very little change in the percentages, indicating that RNP did not continue to worsen when the only change was duration of disease with no change in the anti-VEGF treatment regimen. This confirms a previous study that showed that continuous suppression of VEGF was required to prevent an increase in RNP.11
We found that after the first 6 month period of once a month injections, comparison of patients treated with prn RBZ+laser versus those treated with prn RBZ showed that the addition of laser reduced the percentage of patients with progression of RNP in patients with BRVO, but not those with CRVO. The reduction in progression of RNP in patients with BRVO was not seen in the first 6 months after the second randomization, but was seen in the subsequent 2 years. In order to receive laser, patients had to have recurrent edema on 2 consecutive visits, and the earliest a second laser could be given was 3 months after the first treatment. Thus, laser treatment was generally not completed until more than 6 months after the second randomization (month 12), which could explain the discordance between months 6–12 versus months 12–24 and months 24–36. However, the lack of an effect of laser on progression of RNP in patients with CRVO suggests the possibility that despite statistical significance, the effect of laser on progression of RNP in patients with BRVO is due to chance. However, it is also possible that there is a real difference between BRVO and CRVO with regard to the effect of laser on progression of RNP that is due to the larger area of retina affected by CRVO versus BRVO. Scatter photocoagulation leaves about half of the peripheral retina and most of the central retina untreated, and much of the peripheral retina between burns and all of the untreated posterior retina may remain hypoxic and a source of increased VEGF production. Since the area of untreated retina both posteriorly and in the periphery is greater after laser in patients with CRVO versus those with BRVO, this could explain the lack of effect of laser on progression of RNP in patients with CRVO. Interestingly, grid laser therapy provides benefit in patients with BRVO, but not those with CRVO.12, 13
Figure 7 illustrates a patient with BRVO who had progression of RNP despite laser and may be the exception that supports the rule. In this patient, the investigator left a strip of retina between the posterior extent of the laser and the arcade vessels whereas in other patients, laser treatment extended to the arcade vessel. Progression of RNP occurred in that region of untreated retina anterior to the arcade vessel suggesting that retina was probably hypoxic and may have been a source of residual VEGF that continued to drive progression of RNP. Even with laser extending to superior and inferior arcade vessels there might be sufficient hypoxic posterior retina in some patients with CRVO to drive progression of RNP. This may also be the reason why laser did not improve visual outcomes or reduce treatment burden.8
This study did not address the effects of RNP in patients with RVO, but a previous study has shown that patients may lose vision when there is progression of RNP to involve perifoveal capillaries on all sides of the fovea.11 When the area of RNP is greater than 10 disc areas in the center, inner, and outer subfields, the risks of neovascular complications and a poor outcome are increased.14 Since patients with large areas of RNP are prone to neovascular glaucoma, a complication of retinal ischemia, RNP is felt to be a marker for retinal ischemia, but there may be some instances in which nonperfused retina contains only dead cells that are unable to produced VEGF. Thus, it appears likely that progression of RNP is undesirable and should be avoided if possible, but it is uncertain if it is a threat to vision in all patients.
The demonstration that high levels of VEGF are the cause of progressive RNP in RVO and that continuous suppression of VEGF can result in reperfusion of some recently closed vessels is a revolutionary concept and as is usually the case with new, unexpected findings, there has been a substantial amount of healthy skepticism. The original finding was based upon measurements done in the central retina, a relatively small sample of the entire retina. In the current study, ultra-wide angle FA demonstrates that there is significant correlation between RNP gradings in the central and peripheral retina. This suggests that measurements of RNP in the central retina are representative of changes throughout the entire retina. From a practical standpoint, it is unfortunate that monthly injections of anti-VEGF agents are needed to prevent worsening of RNP, because frequent visits and injections are a substantial burden on patients and their families. Sustained delivery of anti-VEGF agents may be the best approach to achieve this objective and minimize disease progression in patients with RVO.
Supplementary Material
Acknowledgments
This study was funded by Genentech, South San Francisco, CA.
The authors thank Jiangxia Wang, MS, MA and the Wilmer Biostatistics Core for statistical consultation and the support of the Wilmer Biostatistics Core Grant EY01765.
Footnotes
Potential Conflicts of Interest are listed after the references
An ultra-wide angle imaging system was provided for unrestricted institutional research by Optos, Dunfermline, Scotland.
This article contains online-only material (Table 1) available at http://aaojournal.org.
Financial Disclosure(s):
The author(s) have made the following disclosure(s):
P.A.C.: Financial support - Genentech, Inc. (South San Francisco, CA); Regeneron (Tarrytown, NY); Aerpio (Cincinnati, OH); Akebia (Cincinatti, OH), Roche (Basel, Switzerland); Abbvie (North Chicago, IL); Allergan (Irvine, CA); Clearside Biomedical, Inc. (Alpharetta, GA); GlaxoSmithKline (Mid-dlesex, UK); Genzyme (Cambridge, MA); Oxford BioMedica (Oxford, UK); Santen Pharmaceutical Co, Ltd. (Emeryville, CA); AsclepiX (Baltimore, MD); Eleven (Cambridge, MA); Applied Genetic Technologies Corporation (Gainesville, FL); Alimera (Atlanta, GA); Allegro (San Diego, CA); Novaritis (Basel, Switzerland); Kala (Cambridge, MA); Graybug (Baltimore, MD); RXi (Marlborough, MA).
A.W.S.: Financial support – Genenech, Inc. (South San Francisco, CA)
C.J.B.: Financial support - Genentech, Inc. (South San Francisco, CA)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther. 2008;16(4):791–9. doi: 10.1038/mt.2008.10. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology. 2010;117(6):1102–12. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology. 2010;117(6):1124–33. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121(7):1414–20. doi: 10.1016/j.ophtha.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594–602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041–9. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Bhistikul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795–802. doi: 10.1016/j.ophtha.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Hafiz G, Mir TA, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015;122(7):1426–37. doi: 10.1016/j.ophtha.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch vein occlusion. The standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–28. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion. The standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101–14. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sophie R, Hafiz G, Scott A, et al. Long term outcomes in ranibizumab-treated patients with retinal vein occlusion; the role of progression of retinal nonperfusion. Am J Ophthalmol. 2013;156(4):693–705. doi: 10.1016/j.ajo.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Branch Vein Occlusion Study. Argon laser photocoagulation for macular edema in branch vein occlusion. Amer J Ophthalmol. 1984;98(3):271–82. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 13.The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The central vein occlusion study group M report. Ophthalmology. 1995;102(10):1425–33. doi: 10.1016/s0161-6420(95)30849-4. [DOI] [PubMed] [Google Scholar]
- 14.The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115(4):486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.