Abstract
The evolution of Staphylococcus aureus during the modern antibiotic era has been delineated by distinct strain emergence events, many of which include acquisition of antibiotic resistance. The relative high burden of methicillin-resistant S. aureus (MRSA) in healthcare and community settings is a major concern worldwide. Vancomycin, a glycopeptide antibiotic that inhibits cell wall biosynthesis, remains a drug of choice for treatment of severe MRSA infections. S. aureus strains exhibiting increased resistance to vancomycin, known as vancomycin intermediate-resistant S. aureus (VISA) (MIC = 4-8 µg/mL), were discovered in the 1990s. The molecular basis of resistance in VISA is polygenic and involves stepwise mutations in genes encoding molecules predominantly involved in cell envelope biosynthesis. S. aureus isolates with complete resistance to vancomycin (MIC ≥ 16 µg/mL) are termed vancomycin-resistant S. aureus (VRSA)—they were first reported in the U.S. in 2002. Resistance in VRSA is conferred by the vanA gene and operon, which is present on a plasmid. Although treatment of VRSA infections is challenging, the total number of human VRSA infections to date is limited (14 in the U.S.). By comparison, the burden of VISA is relatively high and the molecular mechanisms of resistance are less well-defined. VISA are associated with persistent infections, vancomycin treatment failure, and poor clinical outcomes. Here, we review in brief progress made toward understanding the acquisition of antibiotic resistance in S. aureus, with an emphasis on the molecular mechanisms underlying vancomycin resistance.
Keywords: Staphylococcus aureus, antibiotic resistance, vancomycin, VISA, VRSA
Introduction
Staphylococcus aureus is an important human pathogen and was first recognized as the etiological agent of suppurative abscesses more than 130 years ago [1]. S. aureus infections range from mild skin and soft-tissue infections to life-threatening endocarditis, chronic osteomyelitis, pneumonia, or bacteremia, which are associated with significant morbidity and mortality [2-6]. The advent and use of antibiotics such as penicillin and methicillin in the mid-20th century initially proved effective against S. aureus. However, S. aureus rapidly acquired resistance to these antibiotics and infections with penicillin-resistant S. aureus (PRSA), and in turn methicillin-resistant S. aureus (MRSA), were difficult to treat. Although progress has been made, MRSA remains a significant threat to human health globally. For example, S. aureus isolates represent 29 percent of all reported bacterial isolates in Europe, and an estimated 72,444 cases of invasive MRSA infections occurred in the United States in 2014 [7-9]. Importantly, the glycopeptide antibiotic vancomycin has proven effective in treating severe MRSA infections [10]. However, S. aureus clinical isolates with reduced susceptibility to vancomycin, and less commonly, with complete resistance to vancomycin have emerged within the past 20 years [11-13]. This review highlights features of vancomycin intermediate-resistant S. aureus (VISA, MIC = 4-8 µg/mL) and vancomycin-resistant S. aureus (VRSA, MIC ≥ 16 µg/mL) in the context of epidemiology, mechanisms of resistance, and human infections.
Antimicrobial Resistance
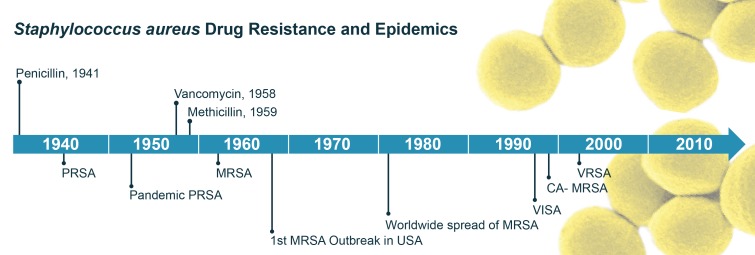
The modern antibiotic era began with the discovery of penicillin by Sir Alexander Fleming. Many infectious diseases became treatable with antibiotics, including those caused by S. aureus [14]. However, the rapid acquisition of antibiotic resistance by S. aureus is a significant problem for treatment of human infections caused by this organism. A timeline illustrating emergence of antibiotic-resistant S. aureus following the introduction of key antibiotics is provided in Figure 1.
Figure 1.
Timeline delineating the advent of antibiotic therapies and subsequent emergence of antibiotic-resistant S. aureus.
Mobile genetic elements (MGEs) play an integral part in the ability of S. aureus to adapt to environmental stresses, which include exposure to antibiotics. MGEs are a primary means by which genetic information is exchanged between bacteria via horizontal gene transfer. S. aureus strains in general contain a relatively large variety of MGEs, including plasmids, transposons, bacteriophages, pathogenicity islands, and staphylococcal cassette chromosomes. Plasmids and staphylococcal cassette chromosomes in particular have played a central role in conferring resistance to β-lactam antibiotics and vancomycin [15-18].
Penicillin & Methicillin Resistance
PRSA was reported in the early 1940s, a few years after the first use of penicillin for treatment of human infections [19,20]. Infections caused by PRSA were widespread among hospitals and in community settings throughout the 1950s and early 1960s [20,21]. A large number of clinical isolates at this time were categorized by phage-type as 80/81 (the pandemic S. aureus phage-type 80/81 strain), which later were predominantly classified as multilocus sequence type (MLST or ST) 30 and clonal complex 30 (CC30) [22,23]. S. aureus resistance to penicillin is primarily conferred by the blaZ gene, which encodes a β-lactamase. β-lactamase inactivates penicillin by hydrolyzing the β-lactam ring of penicillin [24]. Methicillin, a semi-synthetic beta-lactam antibiotic, was introduced in the late 1950s as a therapy for PRSA infections. Despite efficacy of methicillin for treatment of PRSA infections, the first methicillin-resistant S. aureus (MRSA) strains were reported within two years of clinical use [25]. The burden of MRSA worldwide has increased over many decades [26]. Currently, MRSA accounts for a large proportion of hospital-associated S. aureus infections and is associated with significant morbidity and mortality [7,27-29]. The recent emergence of community-associated MRSA (CA-MRSA) further underscored S. aureus as a serious infectious disease threat globally. Notably, CA-MRSA causes infections in otherwise healthy individuals outside of healthcare settings—thus anyone is at risk for infection [30-32]. The mecA gene, which encodes a low-affinity penicillin-binding protein (PBP2a or PBP2’), confers resistance to methicillin. mecA was discovered more than twenty years after the first reported cases of MRSA [25,33]. It is encoded on a mobile genetic element called staphylococcal cassette chromosome (SCC) [34]. Prototype hospital-associated and community-associated MRSA strains harbor distinct SCCmec variants, highlighting the central role that MGEs play in facilitating the evolution of S. aureus antibiotic resistance [33,35]. Inasmuch as there is a high burden of CA-MRSA in the U.S., selected CA-MRSA strains and most notably the epidemic USA300 strain, have moved into the healthcare setting [36-38]. Interestingly, the most prominent healthcare-associated MRSA strains have largely failed to move into the community setting. An explanation for this phenomenon is multifactorial, but involves limited transmission among healthy individuals, fitness burden imparted by the SCCmec element, and/or limited strain virulence capacity [39].
Vancomycin-resistant S. aureus (VRSA)
Despite being approved for use in humans in 1958, vancomycin became an antibiotic of choice for treatment of MRSA infections in hospital settings in the late 1980s [10,40,41]. Resistance to vancomycin was discovered in enterococci in the 1980s, and this finding elicited significant concern with regard to the future use of vancomycin as an effective treatment for MRSA [42]. Shortly thereafter, S. aureus isolates with reduced susceptibility to teicoplanin—a structural relative of vancomycin—emerged in Europe [43,44]. The first VRSA isolate in the United States was reported in 2002 [45,46]. Since that time, there have been a total of 14 isolates reported in the United States [47].
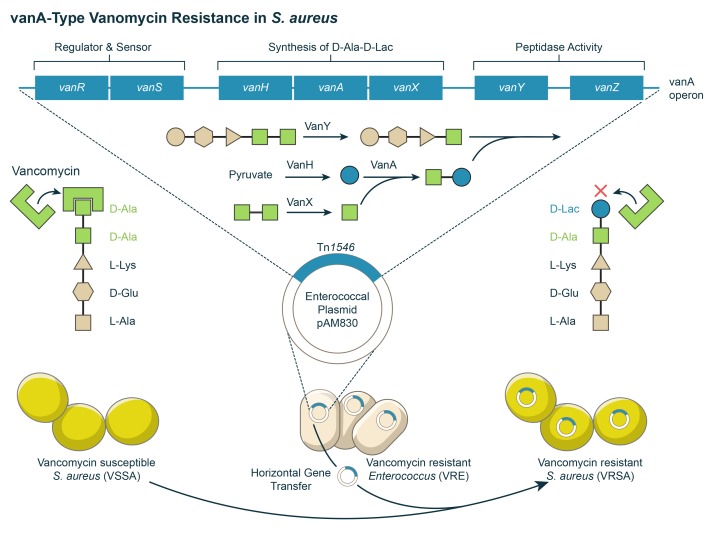
Complete vancomycin resistance in S. aureus (MIC ≥ 16 µg/ml) is conferred by the vanA operon encoded on transposon Tn1546, originally a part of a vancomycin-resistant enterococci (VRE) conjugative plasmid [48]. S. aureus can acquire enterococcal plasmids during discrete conjugation events. Vancomycin resistance in S. aureus is maintained by retaining an original enterococcal plasmid or by a transposition of Tn1546 from the VRE plasmid into a staphylococcal resident plasmid (Figure 2) [49,50]. To better understand the molecular mechanism by which the vanA operon confers resistance, it is necessary to understand primary components of the S. aureus cell wall and the mechanism of action of vancomycin. The S. aureus cell wall lies just beneath the outermost polysaccharide capsule layer. The cell wall is essential for preserving cell integrity as well as facilitating host-pathogen interactions [51]. The principle component of the cell wall is heavily cross-linked peptidoglycan, which is itself made up of glycan chains NAG (N-acetylglucosamine) and NAM (N-acetylmuramic acid) cross-linked to one another by glycine bridges and stem pentapeptides (UDP-MurNAc-L-Ala-D-iso-Gln-L-Lys-D-Ala-D-Ala). As a new cell wall is formed, each precursor component is synthesized in the cytoplasm and transported to the division septum of the growing cell wall for further assembly [52].
Figure 2.
Schematic model illustrating the acquisition and molecular mechanism of vanA-type vancomycin resistance in S. aureus.
In Gram-positive bacteria, vancomycin interferes with late-stage peptidoglycan synthesis by forming non-covalent hydrogen bonds with the penultimate D-Ala-D-Ala residues of newly synthesized UDP-MurNAc-pentapeptides, thereby disrupting downstream peptidoglycan assembly. Ultimately, cell wall synthesis is inhibited and bound vancomycin-pentapeptide complexes accumulate within the cell [53,54]. Two key events are necessary for vanA operon-mediated vancomycin resistance: 1) hydrolysis of dipeptide D-Ala-D-Ala peptidoglycan precursors, which bind vancomycin, and 2) synthesis of D-Ala-D-lactate peptidoglycan precursors, which cannot bind vancomycin [55]. A schematic diagram that depicts acquisition and molecular mechanism of vanA-type vancomycin resistance is provided by Figure 2. The vanA operon is comprised of vanA, vanH, vanX, vanS, vanR, vanY, and vanZ genes. It is controlled via a two-component sensor-regulator system encoded by vanS and vanR that sense vancomycin and activate transcription of the operon respectively [56]. VanA, VanH, and VanX together are essential for the vancomycin resistance phenotype. VanA and VanH are responsible for synthesizing the depsipeptide D-Ala-D-Lac. VanA is a ligase that catalyzes the ester-bond formation of the D-Ala-D-Lac depsipeptide and VanH is a dehydrogenase that forms D-Lac by reducing pyruvate [55]. VanX is a D,D-dipeptidase that hydrolyzes the D-Ala-D-Ala ester bond, ensuring the newly formed D-Ala-D-Lac depsipeptide has little competition to bind the UDP-linked tripeptide peptidoglycan precursor [57]. VanY is a D,D-carboxylpeptidase that performs a similar—but not essential—function by facilitating the cleavage of D-Ala-D-Ala dipeptides already attached to the C-terminal end of stem pentapeptide structures [58]. The role of VanZ is not well understood, but it may confer S. aureus resistance to teicoplanin. Incorporation of altered D-Ala-D-Lac into peptidoglycan yields a cell wall that is no longer susceptible to vancomycin.
Intermediate Resistance to Vancomycin
In 1997, a S. aureus clinical isolate from a patient in Japan was found to have reduced susceptibility to vancomycin [59]. This was the first reported VISA isolate. However, retrospective studies suggest that reduced S. aureus susceptibility to vancomycin dates back at least to 1987 in the United States [60]. VISA is typically associated with hospitalization, persistent infection, prolonged vancomycin treatment and/or treatment failure. The VISA phenotype is frequently preceded by an intermediate phenotype known in the clinical laboratory as heterogenous VISA (hVISA) [61-63]. An hVISA phenotype refers to a mixed cell population—derived originally from a single colony of S. aureus—in which the majority of cells have little or no resistance to vancomycin (MIC ≤ 2 µg/ml) and a sub-population of cells is resistant to the antibiotic at the level of VISA (MIC ≥ 4 µg/ml) [64]. The molecular mechanisms that underlie development of hVISA are incompletely defined, although progress has been made. For example, Roch and colleagues demonstrated that an hVISA phenotype can be triggered by exposure of S. aureus to non-glycopeptide antibiotics such as β-lactams [65]. Consistent with these findings, Haaber et al. reported recently that exposure of strain USA300 to colistin caused enhanced resistance to vancomycin, a phenomenon that is regulated at the level of gene expression and thus reversible [66]. Collectively, these studies provide support to the notion that development of hVISA is an epigenetic process rather than one based on gene mutation [65,66]. A current accepted hypothesis is that VISA, which has homogeneous resistance to vancomycin, develops from hVISA in individuals treated with glycopeptide antibiotics over extended time periods [64,65].
Fundamental characteristics of the VISA phenotype include increased cell wall thickness, caused by differentially regulated cell wall biosynthesis and stimulatory pathways [67-70], reduced cross-linking of peptidoglycan, decreased autolytic activity of the enzymes responsible to cell-wall turnover [61,71-76], altered surface protein profile, dysfunction of the agr system and changes to growth characteristics [73,77-81]. Multiple approaches have been used to investigate the molecular genetic basis of the VISA phenotype. Such studies have employed transcriptome, proteome, and comparative genomics profile analyses to compare VISA and vancomycin susceptible S. aureus (VSSA) isolates, including comparisons between closely related VSSA and VISA, sequentially isolated strains from patients undergoing vancomycin therapy, and analysis of laboratory-derived VISA strains [62,67-79,75,80]. These studies identified several genes and/or mutations in genes that contribute to the vancomycin intermediate phenotype (Table 1). VSSA strains most likely develop vancomycin intermediate-resistance in a step-wise manner, acquiring mutations that each play a role in reducing susceptibility to vancomycin (reviewed in [64]). As proof of this concept, Katayama et al. generated a laboratory-derived VISA strain by introducing sequential mutations in six different genes in the VSSA strain, N315 [82].
Table 1. Genes associated with the VISA phenotype.
| Phenotype | Associated Genes | Role in VISA Phenotype | Reference |
| Cell Wall Thickening and Reduced Autolytic Activity | |||
| graSR | Up-regulates vraSR, dlt operon, capsule operon, mprF/fmtC, mgrA and rot. Down-regulates walKR and agr. Associated with nucleotide metabolism. | [83,87,88] | |
| walkKR (yycFG) | Limited walKR activity lowers rates of autolysis and increases cell wall thickness. | [84,124,126] | |
| yycH | Lowered expression of genes associated with autolysis. | [62] | |
| pbp4 | Reduced rates of peptidoglycan cross-linking and transpeptidation. | [137] | |
| sarA | Reduced production of autolysins responsible for cell wall recycling. | [138] | |
| mgrA | Reduced production of autolysins responsible for cell wall recycling. | [138] | |
| clpP | Cell wall thickening, slow growth, and reduced autolysis. | [85] | |
| stp1 | Cell wall thickening, slow growth, and reduced autolysis. | [139] | |
| Up-regulated Cell Wall Stimulon | |||
| vraSR | Up-regulation of VraSR, reduced susceptibility to vancomycin. | [88,95] | |
| vraFG | Associated with reduced susceptibility to vancomycin. | [83] | |
| mprF/fmtC | Increased net negative charge of cell wall and reduced peptidoglycan cross-linking. | [89,91] | |
| spoVG | Increased capsule production. | [140] | |
| capA-capP | Increased capsule production. | [88,95] | |
| isdE | Associated with reduced susceptibility to vancomycin. | [62] | |
| prsA | Associated with reduced susceptibility to vancomycin. | [62] | |
| Down-regulated Global Regulators | |||
| agr | Attenuation of virulence and reduced susceptibility to vancomycin. | [62,81] | |
| rot | Attenuation of virulence and reduced susceptibility to vancomycin. | [87] | |
| rpoB | Associated with reduced susceptibility to vancomycin. | [85,86] | |
| rsbU | Associated with reduced susceptibility to vancomycin. | [64,140] | |
| yjbH | Associated with reduced susceptibility to vancomycin. | [139] | |
| yvqF | Up-regulation of vraSR, reduced susceptibility to vancomycin. | [92] | |
| Decreased Production of Virulence Factors | |||
| spa | Decreased production of Spa, observed alterations in opsonization and phagocytosis. | [88,95] | |
| sbi | Decreased transcription of sbi and altered IgM binding. | [88,95] | |
| Unknown Function | |||
| trfA/trfB | Associated with reduced susceptibility to vancomycin. | [141] |
Although our understanding of the molecular basis of the VISA phenotype is incomplete, several genes / mutations are known to contribute to the development of VISA. Of particular significance are mutations within genes encoding two-component regulatory systems, such as graRS and walKR, which have been linked to glycopeptide resistance [83,84]. A gene encoding the DNA-dependent RNA polymerase β–subunit (rpoB) is also commonly associated with increased resistance to vancomycin, prolonged propagation time, and increased cell wall thickness [62,85,86]. GraRS differentially regulates transcription of cell wall biosynthesis genes and has been associated with a broad array of genes and regulators that play a role in the intermediate resistance phenotype [87]. Specifically, GraRS up-regulates genes in the capsule biosynthesis operon, leading to increased capsule production [87]. Two separate studies found that point mutations within graRS reduced susceptibility to vancomycin [83,88]. Additionally, GraRS up-regulates the dlt operon and the mprF/fmtC genes, which are linked to teichoic acid alanylation and alteration of cell wall charge [89-91]. Moreover, graRS mutations are linked to modified expression of global regulators, rot (repressor of toxins) and agr (accessory gene regulator) [81,87]. Altered expression of global gene regulators has a tremendous downstream effect, and thus could play a role in a VISA phenotype. VISA isolates have been shown to have non-silent mutations in vraSR. Such mutations could lead to downstream up-regulation of over 40 cell wall synthesis genes, including genes required for producing cell wall derivatives such as D-Ala-D-Ala [92-95]. WalKR is another two-component gene regulatory system associated with the VISA phenotype. Down-regulation of the walKR operon by acquired mutations or insertion of IS256 leads to increased capsule synthesis, cell wall thickness increases, and reduced autolysis [62,84,95,96]. Additionally, VISA strains have altered acetate catabolism compared to VSSA strains, perhaps altering growth characteristics, cell death kinetics, tolerance to antibiotics, and increases in synthesis of polysaccharide intercellular adhesion [97]. The aforementioned genetic modifications that yield a vancomycin intermediate phenotype can vary significantly between isolates, and the predominance of certain mutations is often associated with specific S. aureus lineages. Nonetheless, Vidaillac et al. demonstrated that parental genetic background does not necessarily define the composition of mutations that lead to a VISA phenotype and parallel isolates derived from the same parental isolate can aquire different mutations under various environmental pressure [98]. Interestingly, Berscheid and colleagues demonstrated that sequential mutation of a VSSA strain in vitro can yield an isolate that exhibits a vancomycin MIC of ≥ 32 µg/ml, which exceeds the breakpoint for VRSA [99]. This mechanism is in contrast to vanA-type vancomycin resistance, whereby vancomycin is unable to bind to the modified D-Ala-D-Lac peptides. The hVISA/VISA isolates have a thicker cell wall, reduced peptidoglycan cross-linking, and excess free D-Ala-D-Ala residues that serve as a decoy target for vancomycin within the cell wall. In addition, D-Ala-D-Ala-bound vancomycin accumulates at the cell wall and thereby obstructs further vancomycin diffusion [73,100,101]. However, a number of studies have indicated that VISA strains with higher levels of vancomycin resistance are less stable—impaired growth and significant fitness cost associated with the mutations that enable a VISA phenotype—and often revert to lower levels of resistance associated with hVISA or to full vancomycin-susceptibility [73,102,103].
Clinical Implications of Intermediate Resistance to Vancomycin
The increasing prevalence of hVISA/VISA poses a significant threat, as these organisms often cause infections for which vancomycin treatment fails [104-106]. There are many factors that contribute to the challenges associated with assessing the clinical impact of VISA and hVISA. One important factor is the lack of prospective comparative studies that definitively relate low-level vancomycin resistance in S. aureus to vancomycin treatment failure and poor clinical outcomes. This issue is compounded by the use of multiple testing methods (e.g., eStrip, broth microdilution, etc.). Prior to 2006, Clinical and Laboratory Standards Institute (CLSI) guidelines for isolate classification based upon glycopeptide susceptibility MIC in broth microdilution was 8-16 µg/ml for VISA and ≤ 4 µg/ml for hVISA, whereas after 2006 the CLSI updated the classification to 4-8 µg/ml for VISA and ≤ 2 µg/ml for hVISA [107,108]. These changes have ameliorated some of the previous difficulties; however, hVISA strains remain a concern. These strains are remarkably difficult to detect using international susceptibility testing breakpoints [63,109], and if they are detected there is a lack of optimized treatment options. Clinical studies generally agree that for infections with VISA having an MIC greater than 8 µg/ml, treatment with glycopeptide antibiotics is not optimal [110,111]. In addition, surgical intervention can be considered for treatment of hVISA infections related to deep abscesses, osteomyelitis, and endocarditis for which there are high numbers of bacteria [112,113]. Interestingly, reduced susceptibility to glycopeptide antibiotics, including vancomycin, has been associated with increased susceptibility to beta-lactams [114,115]. Studies of this phenomenon, termed the “see-saw effect,” have produced conflicting clinical reports [116-118] and more work is needed in this area. The treatment guidelines of the Infectious Diseases Society of America (IDSA) stipulate that an alternative to vancomycin should be utilized for the management of persistent MRSA bacteremia and vancomycin treatment failures with an observed reduction in vancomycin susceptibility (MIC > 2 µg/ml). Viable alternatives to vancomycin include a combination of high-dose daptomycin with another antibiotic such as gentamicin, rifampin, linezolid, trimethoprim-sulfamethoxazole (TMP-SMX), or a β-lactam. Similarly, if reduced susceptibility to daptomycin is observed alongside reduced vancomycin susceptibility, then a combination or single use of the following is recommended; quinupristin-dalfopristin, TMP-SMX, linezolid, or telavancin [119].
Conclusions and Outlook
S. aureus is notorious for its ability to acquire and/or develop resistance to antibiotics. This attribute, coupled with the high burden of S. aureus infections is a problem for treatment. Inasmuch as vancomycin is important for treatment of severe MRSA infections, the ability of S. aureus to become completely resistant to vancomycin is disconcerting. Fortunately, strains that have complete resistance to vancomycin (VRSA) are rare, despite wide use of vancomycin for treatment of severe MRSA infections. The paucity of VRSA may be attributed to a fitness cost associated with acquisition of vanA-mediated vancomycin resistance and the infrequency of horizontal gene transfer from enterococci, robust S. aureus restriction modification systems that prevent foreign DNA uptake, and strain-lineage specificity that enable certain strains of S. aureus to more readily take up enterococcal plasmids [49,120]. Also, it is not known why the majority of VRSA strains isolated in the U.S. have similar genetic background—i.e., categorized as belonging to clonal complex (CC) 5 by multilocus sequence typing and USA100 by pulsed field gel electrophoresis [121-123]. Association of VRSA with one S. aureus genetic background might be explained at least in part by the high prevalence CC5 in healthcare settings. The prevalence of hVISA/VISA is much greater than that of VRSA, but the propensity for spread of these strains appears limited at present [105,115,123]. The failure of these strains to spread is perhaps linked to the transient nature of the hVISA phenotype, as the organism can revert rapidly to VSSA in the absence of selective pressure imparted by glycopeptide antibiotics. hVISA/VISA strains are largely hospital-adapted MRSA strains that belong to CC5 and CC8 [124]. These findings are consistent with the notion that selective pressures driving the step-wise evolution of hVISA/ VISA strains are greater in the hospital setting than in the community. Unlike VRSA, hVISA/VISA has been associated with many S. aureus genetic backgrounds, including CC5, CC8, CC30, and CC45 [125,126]. From a practical standpoint, and considering these characteristics collectively, it can be argued that VISA is a much greater problem for the clinic than is VRSA.
S. aureus with reduced susceptibility to vancomycin is not restricted to humans. Recently, VRSA and/or VISA have been isolated from pigs, goats, and cattle [127-129]. Although resistance in the livestock-associated VRSA isolates reported by Bhattacharyya et al. was not conferred by presence of the vanA gene and operon, the MIC for vancomycin was ≥ 16 µg/ml for two of the isolates [127]. Inasmuch as vancomycin is not used in livestock in that region of the world, Bhattacharyya et al. suggested that isolates either originated from humans or resistance developed as a result of the continuous exposure of animals to other antibiotics [127]. Consistent with the later hypothesis, these authors reported that the livestock VRSA and VISA isolates were resistant to multiple antibiotics, including β-lactams. The recovery of vancomycin non-susceptible isolates from livestock highlights a potential issue with antibiotic use in livestock and use of antibiotics as a feed supplement [127-129]. Whether an increased burden of such resistance in livestock translates directly to a problem for treatment of human infections remains incompletely determined.
More work is needed to advance identification of hVISA and VISA isolates, which in turn can be used to better assess prevalence of vancomycin intermediate resistance, as well as to facilitate development of optimal treatments. Next-generation sequencing technologies and comparative genomics are tools that can be deployed routinely to aid clinicians in identifying VISA as a cause of infection [130-133]. There a number of hurdles that must be overcome before these approaches can be used for diagnostics on a routine basis, including cost, time, automation, bioinformatics analyses, and standardization (quality control) [130,134]. On a positive note, these tools are available and in use for such purposes at select institutions [135,136].
Acknowledgments
The authors are supported by the Intramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Ryan Kissinger (NIAID) for assistance with graphic illustration.
Glossary
- MRSA
methicillin-resistant S. aureus
- VISA
vancomycin intermediate-resistant S. aureus
- VRSA
vancomycin-resistant S. aureus
- PRSA
penicillin-resistant S. aureus
- MGEs
mobile genetic elements
- MLST or ST
multilocus sequence type
- CC30
clonal complex 30
- CA-MRSA
community-associated MRSA
- SCC
staphylococcal cassette chromosome
Author Contributions
Will A. McGuinness, Natalia Malachowa, and Frank R. DeLeo designed and wrote the manuscript.
References
- Classics in infectious diseases: “on abscesses”: Alexander Ogston (1844-1929). Rev Infect Dis. 1984;6(1):122–128. [PubMed] [Google Scholar]
- Roberts S, Chambers S. Diagnosis and management of Staphylococcus aureus infections of the skin and soft tissue. Intern Med J. 2005;35(s2):S97–S105. doi: 10.1111/j.1444-0903.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Mitchell DH, Howden BP. Diagnosis and management of Staphylococcus aureus bacteraemia. Intern Med J. 2005;35:S17–S24. doi: 10.1111/j.1444-0903.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Corey GR, Hoen B. et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. Staphylococcus aureus infective endocarditis: diagnosis and management guidelines. Intern Med J. 2005;35(s2):S25–S44. doi: 10.1111/j.1444-0903.2005.00978.x. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus Infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Dantes R, Mu Y, Belflower R. et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kraker MEA, Jarlier V, Monen JCM. et al. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2013;19(9):860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Methicillin- Resistant Staphylococcus aureus, 2014. [cited 2017 Apr 21]. Available from: http://www.cdc.gov/abcs/reports-findings/survreports/mrsa14.html .
- Sorrell TC, Packham DR, Shanker S. et al. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann Intern Medd. 1982;97(3):344–350. doi: 10.7326/0003-4819-97-3-344. [DOI] [PubMed] [Google Scholar]
- Hidayat LK, Hsu DI, Quist R. et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Aritaka N, Hanaki H. et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350(9092):1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- Howe RA, Bowker KE, Walsh TR. et al. Vancomycin-resistant Staphylococcus aureus. Lancet. 1998;351(9102):602. doi: 10.1016/S0140-6736(05)78597-4. [DOI] [PubMed] [Google Scholar]
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Fouts DE, Archer GL. et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187(7):2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden MT, Feil EJ, Lindsay JA. et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U. S. A. 2004;101(26):9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JA. Genomic variation and evolution of Staphylococcus aureus. Intern J Med Microbiol. 2010;300(2):98–103. doi: 10.1016/j.ijmm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Musser JM, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30(8):2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plough HH. Penicillin resistance of Staphylococcus aureus and its clinical implications. Am J Clin Pathol. 1945;15(10):446–451. doi: 10.1093/ajcp/15.10.446. [DOI] [PubMed] [Google Scholar]
- Rammelkamp CH, Maxon T. Resistance of Staphylococcus aureus to the action of penicillin. Exp Biol Med. 1942;51(3):386–369. [Google Scholar]
- Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;252(6530):641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- Roundtree PM, Freeman B. Infections caused by a particular phage type of Staphylococcus aureus. Med J Aust. 1955;2(5):157–161. [PubMed] [Google Scholar]
- Robinson DA, Kearns AM, Holmes A. et al. Re-emergence of early pandemic Staphylococcus aureus as a community-acquired meticillin-resistant clone. Lancet. 2005;365(9466):1256–1258. doi: 10.1016/S0140-6736(05)74814-5. [DOI] [PubMed] [Google Scholar]
- Olsen JE, Christensen H, Aarestrup FM. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J Antimicrob Chemother. 2006;57(3):450–460. doi: 10.1093/jac/dki492. [DOI] [PubMed] [Google Scholar]
- Jevons MP. “Celbenin” - resistant Staphylococci. BMJ. 1961;1(5219):124–125. [Google Scholar]
- Ayliffe G. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24(Supplement 1):S74–S79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- Jarvis WR, Schlosser J, Chinn RY. et al. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2006. Am J Infect Control. 2007;35(10):631–637. doi: 10.1016/j.ajic.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Klein E, Smith DL. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis. 2007;13(12):1840. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Tenover FC. et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42(3):389–391. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;9(629):641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Four pediatric deaths from communityacquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep. 1999;48(32):707–710. [PubMed] [Google Scholar]
- Herold BC, Immergluck LC, Maranan MC. et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279(8):593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44(6):1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K, Nonoguchi R, Matsuhashi M. et al. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8(6):747–763. doi: 10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Jenkins TC, McCollister BD, Sharma R. et al. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect Control Hosp Epidemiol. 2009;30(3):233–241. doi: 10.1086/595963. [DOI] [PubMed] [Google Scholar]
- Seybold U, Halvosa JS, White N. et al. Emergence of and risk factors for methicillin-resistant Staphylococcus aureus of community origin in intensive care nurseries. Pediatrics. 2008;122(5):1039–1046. doi: 10.1542/peds.2007-3161. [DOI] [PubMed] [Google Scholar]
- Liu C, Graber CJ, Karr M. et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46(11):1637–1646. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- Rudkin JK, Edwards AM, Bowden MG. et al. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J Infect Dis. 2012;205(5):798–806. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata EM, Webb GF, Horn MA. et al. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin Infect Dis. 2009;48(3):274–284. doi: 10.1086/595844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(Supplement 1):S5–S12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342(10):710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- Kaatz GW, Seo SM, Dorman NJ. et al. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis. 1990;162(1):103–108. doi: 10.1093/infdis/162.1.103. [DOI] [PubMed] [Google Scholar]
- Manquat G, Croize J, Stahl J. et al. Failure of teicolpanin treatment associated with an increase in MIC during therapy of Staphylococus aureus septicaemia. J Antimicrob Chemother. 1992;29(6):731–732. doi: 10.1093/jac/29.6.731. [DOI] [PubMed] [Google Scholar]
- Chang S, Sievert DM, Hageman JC. et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;248(14):1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Staphylococcus aureus resistant to vancomycin--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(26):565. [PubMed] [Google Scholar]
- Walters MS, Eggers P, Albrecht V. et al. Vancomycin-resistant Staphylococcus aureus-Delaware, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(37):1056. doi: 10.15585/mmwr.mm6437a6. [DOI] [PubMed] [Google Scholar]
- Arthur M, Molinas C, Depardieu F. et al. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993;175(1):117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrobial Agents Chemother. 2009;53(11):4580–4587. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Murray PR, Huskins WC. et al. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrobial Agents Chemother. 2010;54(10):4314–4320. doi: 10.1128/AAC.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev BA, Toukach FV, Holst O. et al. Tertiary structure of Staphylococcus aureus cell wall murein. J Bacteriol. 2004;186(21):7141–7148. doi: 10.1128/JB.186.21.7141-7148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Gram-positive pathogens. Second Edition. American Society of Microbiology; 2006. The staphylococcal cell wall. pp. 443–455. [Google Scholar]
- Barna JC, Williams DH. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38(1):339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Reynolds PE. Studies on the mode of action of vancomycin. Biochim Biophys Acta. 1961;52(2):403–405. doi: 10.1016/0006-3002(61)90698-9. [DOI] [PubMed] [Google Scholar]
- Bugg TD, Wright GD, Dutka-Malen S. et al. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Hutchings MI, Buttner MJ. et al. Biotechnology and Biological Sciences Research Council, UK. Vancomycin resistance VanS/VanR two-component systems. Adv Exp Med Biol. 2008;631:200–213. doi: 10.1007/978-0-387-78885-2_14. [DOI] [PubMed] [Google Scholar]
- Reynolds PE, Depardieu F, Dutka‐Malen S. et al. Glycopeptide resistance mediated by enterococcal transposon Tn 1546 requires production of VanX for hydrolysis of D‐alanyl‐D‐alanine. Mol Microbiol. 1994;13(6):1065–1070. doi: 10.1111/j.1365-2958.1994.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Gutmann L, Billot-Klein D, Al-Obeid S. et al. Inducible carboxypeptidase activity in vancomycin-resistant enterococci. Antimicrobial Agents Chemother. 1992;36(1):77–80. doi: 10.1128/aac.36.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Hanaki H, Ino T. et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40(1):135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- Jackson MA, Hicks RA. Vancomycin failure in staphylococcal endocarditis. Pediatr Infect Dis J. 1987;6(8):750–751. doi: 10.1097/00006454-198708000-00011. [DOI] [PubMed] [Google Scholar]
- Howden BP, Johnson PD, Ward PB. et al. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50(9):3039–3047. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi MM, Wu SW, Zhou Y. et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U. S. A. 2007;104(22):9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzki K, Roberts RB, Haber SW. et al. The Development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340(7):517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- Howden BP, Davies JK, Johnson PDR. et al. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23(1):99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch M, Clair P, Renzoni A. et al. Exposure of Staphylococcus aureus to subinhibitory concentrations of β-Lactam antibiotics induces heterogeneous vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58(9):5306–5314. doi: 10.1128/AAC.02574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaber J, Friberg C, McCreary M. et al. Reversible antibiotic tolerance induced in Staphylococcus aureus by concurrent drug exposure. mBio. 2015;6(1):e02268. doi: 10.1128/mBio.02268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle-Vavra S, Carey RB, Daum RS. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J Antimicrob Chemother. 2001;48(5):617–625. doi: 10.1093/jac/48.5.617. [DOI] [PubMed] [Google Scholar]
- Daum RS, Gupta S, Sabbagh R. et al. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis. 1992;166(5):1066–1072. doi: 10.1093/infdis/166.5.1066. [DOI] [PubMed] [Google Scholar]
- Hanaki H, Kuwahara-Arai K, Boyle-Vavra S. et al. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42(2):199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- Moreira B, Boyle-Vavra S, Daum RS. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41(8):1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle-Vavra S, Challapalli M, Daum RS. Resistance to autolysis in vancomycin-selected Staphylococcus aureus isolates precedes vancomycin-intermediate resistance. Antimicrob Agents Chemother. 2003;47(6):2036–2039. doi: 10.1128/AAC.47.6.2036-2039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle-Vavra S, Labischinski H, Ebert CC. et al. A spectrum of changes occurs in peptidoglycan composition of glycopeptide-intermediate clinical Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2001;45(1):280–287. doi: 10.1128/AAC.45.1.280-287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Ma X, Sato K. et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol. 2003;41(1):5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni A, Barras C, François P. et al. Transcriptomic and functional analysis of an autolysis-deficient, teicoplanin-resistant derivative of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(9):3048–3061. doi: 10.1128/AAC.00113-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherl A, François P, Charbonnier Y. et al. Exploring glycopeptide-resistance in Staphylococcus aureus: a combined proteomics and transcriptomics approach for the identification of resistance-related markers. BMC Genomics. 2006;7(1):296. doi: 10.1186/1471-2164-7-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P, Francois P, Berger-Bächi B. et al. In vivo emergence of subpopulations expressing teicoplanin or vancomycin resistance phenotypes in a glycopeptide-susceptible, methicillin-resistant strain of Staphylococcus aureus. J Antimicrob Chemother. 2001;47(2):163–170. doi: 10.1093/jac/47.2.163. [DOI] [PubMed] [Google Scholar]
- Koehl JL, Muthaiyan A, Jayaswal RK. et al. Cell wall composition and decreased autolytic activity and lysostaphin susceptibility of glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48(10):3749–3757. doi: 10.1128/AAC.48.10.3749-3757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum N, Karauzum H, Getzmann R. et al. In vivo survival of teicoplanin-resistant Staphylococcus aureus and fitness cost of teicoplanin resistance. Antimicrob Agents Chemother. 2006;50(7):2352–2360. doi: 10.1128/AAC.00073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthaiyan A, Jayaswal RK, Wilkinson BJ. Intact mutS in laboratory-derived and clinical glycopeptide-intermediate Staphylococcus aureus strains. Antimicrob Agents Chemother. 2004;48(2):623–625. doi: 10.1128/AAC.48.2.623-625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeltz RF, Singh VK, Schmidt JL. et al. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob Agents Chemother. 2000;44(2):294–303. doi: 10.1128/aac.44.2.294-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoulas G, Eliopoulos GM, Moellering RC. et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46(5):1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Sekine M, Hishinuma T. et al. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrobial Agents Chemother. 2016;60(6):3730–3742. doi: 10.1128/AAC.00420-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl M, Herbert S, Götz F. et al. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(8):2679–2689. doi: 10.1128/AAC.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy CRE, Tsuji B, Gao W. et al. Decreased vancomycin susceptibility in Staphylococcus aureus caused by IS256 tempering of walKR expression. Antimicrob Agents Chemother. 2013;57(7):3240–3249. doi: 10.1128/AAC.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Isii T, Fukuda M. et al. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54(12):5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Hishinuma T, Katayama Y. et al. Mutation of RNA polymerase beta subunit (rpoB) promotes hVISA to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother. 2011;55(9):4188–4195. doi: 10.1128/AAC.00398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert S, Bera A, Nerz C. et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 2007;3(7):e102. doi: 10.1371/journal.ppat.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden BP, Smith DJ, Mansell A. et al. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol. 2008;8(1):1. doi: 10.1186/1471-2180-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Komatsuzawa H, Fujiwara T. et al. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48(12):4800–4807. doi: 10.1128/AAC.48.12.4800-4807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, Otto M, Jack RW. et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- Ruzin A, Severin A, Moghazeh SL. et al. Inactivation of mprF affects vancomycin susceptibility in Staphylococcus aureus. Biochim Biophys Acta. 2003;1621(2):117–121. doi: 10.1016/s0304-4165(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Gardete S, Wu S, Gill S. et al. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(10):3424–3434. doi: 10.1128/AAC.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden BP, Stinear TP, Allen DL. et al. Genomic analysis reveals a point mutation in the two-component sensor gene graS that leads to intermediate vancomycin resistance in clinical Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(10):3755–3762. doi: 10.1128/AAC.01613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Kuroda H, Oshima T. et al. Two‐component system VraSR positively modulates the regulation of cell‐wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49(3):807–821. doi: 10.1046/j.1365-2958.2003.03599.x. [DOI] [PubMed] [Google Scholar]
- McAleese F, Wu SW, Sieradzki K. et al. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J Bacteriol. 2006;188(3):1120–1133. doi: 10.1128/JB.188.3.1120-1133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utaida S, Dunman P, Macapagal D. et al. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology. 2003;149(10):2719–2732. doi: 10.1099/mic.0.26426-0. [DOI] [PubMed] [Google Scholar]
- Nelson JL, Rice KC, Slater SR. et al. Vancomycin-intermediate Staphylococcus aureus strains have impaired acetate catabolism: implications for polysaccharide intercellular adhesin synthesis and autolysis. Antimicrob Agents Chemother. 2007;51(2):616–622. doi: 10.1128/AAC.01057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaillac C, Gardete S, Tewhey R. et al. Alternative mutational pathways to intermediate resistance to vancomycin in methicillin-resistant Staphylococcus aureus. J Infect Dis. 2013;208(1):67–74. doi: 10.1093/infdis/jit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berscheid A, François P, Strittmatter A. et al. Generation of a vancomycin-intermediate Staphylococcus aureus (VISA) strain by two amino acid exchanges in VraS. J Antimicrob Chemother. 2014;69(12):3190–3198. doi: 10.1093/jac/dku297. [DOI] [PubMed] [Google Scholar]
- Cui L, Iwamoto A, Lian J-Q. et al. Novel mechanism of antibiotic resistance originating in vancomycin-Intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(2):428–438. doi: 10.1128/AAC.50.2.428-438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PM, Filipe SR, Tomasz A. et al. Fluorescence ratio imaging microscopy shows decreased access of vancomycin to cell wall synthetic sites in vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3627–3633. doi: 10.1128/AAC.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardete S, Kim C, Hartmann BM. et al. Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLOS Pathogens. 2012;8(2):e1002505. doi: 10.1371/journal.ppat.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle-Vavra S, Berke SK, Lee JC. et al. Reversion of the glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother. 2000;44(2):272–277. doi: 10.1128/aac.44.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys KC, Lagnf AM, Hallesy JA. et al. Pneumonia caused by methicillin-resistant Staphylococcus aureus: does vancomycin heteroresistance matter? Antimicrob Agents Chemother. 2016;60(3):1708–1716. doi: 10.1128/AAC.02388-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin SK, Hageman J, McDougal LK. et al. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis. 2003;36(4):429–439. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sun X, Chang W. et al. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS ONE. 2015;10(8):e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. Ninth edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44(9):1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- Van Hal S, Lodise T, Paterson D. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54(6):755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- Horne K, Howden BP, Grabsch EA. et al. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob Agents Chemother. 2009;53(8):3447–3452. doi: 10.1128/AAC.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise PA, North D, Steenbergen JN. et al. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis. 2009;9(10):617–624. doi: 10.1016/S1473-3099(09)70200-2. [DOI] [PubMed] [Google Scholar]
- Charles PG, Ward PB, Johnson PD. et al. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38(3):448–451. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- Howden BP, Ward PB, Charles PG. et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38(4):521–528. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60(4):788–794. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- Howe RA, Monk A, Wootton M. et al. Vancomycin susceptibility within methicillin-resistant Staphylococcus aureus lineages. Emerg Infect Dis. 2004;10(5):855–857. doi: 10.3201/eid1005.030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwine JK, Werth BJ, Sakoulas G. et al. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: Taking advantage of the back door left open? Drug Resistance Updat. 2013;16(3-5):73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179(8):2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber KE, Ireland CE, Bukavyn N. et al. Observation of “Seesaw Effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther. 2014;3(1):35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Bayer A, Cosgrove SE. et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- Foucault M-L, Courvalin P, Grillot-Courvalin C. Fitness cost of VanA-Type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(6):2354–2359. doi: 10.1128/AAC.01702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbago BM, Kallen AJ, Zhu W. et al. Report of the 13th vancomycin-resistant Staphylococcus aureus isolate from the United States. J Clin Microbiol. 2014;52(3):998–1002. doi: 10.1128/JCM.02187-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Diaz L, Wollam A. et al. Transferable vancomycin resistance in a community-associated MRSA lineage. N Engl J Med. 2014;370(16):1524–1531. doi: 10.1056/NEJMoa1303359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos VN, Desjardins CA, Griggs A. et al. Comparative genomics of vancomycin-resistant Staphylococcus aureus strains and their positions within the clade most commonly associated with methicillin-resistant S. aureus hospital-acquired infection in the United States. mBio. 2012;3(3) doi: 10.1128/mBio.00112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer C, Lin Y, Kornblum J. et al. Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56(11):5845–5851. doi: 10.1128/AAC.01139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JI, Kim JW, Kang GS. et al. Prevalence of amino acid changes in the yvqF, vraSR, graSR, and tcaRAB genes from vancomycin intermediate resistant Staphylococcus aureus. J Microbiol. 2013;51(2):160–165. doi: 10.1007/s12275-013-3088-7. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Cui L, Kim J. et al. comprehensive identification of mutations responsible for heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA)-to-VISA conversion in laboratory-generated VISA strains derived from hVISA clinical strain Mu3. Antimicrob Agents Chemother. 2013;57(12):5843–5853. doi: 10.1128/AAC.00425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya D, Banerjee J, Bandyopadhyay S. et al. First report on vancomycin-resistant Staphylococcus aureus in bovine and caprine milk. Microb Drug Resist. 2016;22(8):675–681. doi: 10.1089/mdr.2015.0330. [DOI] [PubMed] [Google Scholar]
- Kwok GML, O'Donoghue MM, Doddangoudar VC. et al. Reduced vancomycin susceptibility in porcine ST9 MRSA isolates. Front Microbiol. 2013;4:316. doi: 10.3389/fmicb.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno LZ, Dutra MC, Moreno M. et al. Vancomycin-intermediate livestock-associated methicillin-resistant Staphylococcus aureus ST398/t9538 from swine in Brazil. Mem Inst Oswaldo Cruz. 2016;111(10):659–661. doi: 10.1590/0074-02760160276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JC, McCallum N, Sintchenko V. et al. Whole genome sequencing in clinical and public health microbiology. Pathology. 2015;47(3):199–210. doi: 10.1097/PAT.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargis AS, Kalman L, Lubin IM. Assuring the quality of next-generation sequencing in clinical microbiology and public health laboratories. J Clin Microbiol. 2016;54(12):2857–2865. doi: 10.1128/JCM.00949-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasman H, Saputra D, Sicheritz-Ponten T. et al. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J Clin Microbiol. 2014;52(1):139–146. doi: 10.1128/JCM.02452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JZM, Pallen MJ, Oppenheim B. et al. Genome sequencing in clinical microbiology. Nat Biotech. 2012;30(11):1068–1071. doi: 10.1038/nbt.2410. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Suarez CJ, Banaei N. et al. Next-generation sequencing for infectious disease diagnosis and management: a report of the association for molecular pathology. J Mol Diagnost. 2015;17(6):623–634. doi: 10.1016/j.jmoldx.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Köser CU, Holden MTG, Ellington MJ. et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366(24):2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C-M, Lin F-M, Chang T-H. et al. Clinical detection of human probiotics and human pathogenic bacteria by using a novel high-throughput platform based on next generation sequencing. J Clin Bioinforma. 2014;4(1):1. doi: 10.1186/2043-9113-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan J, Archer GL, Pucci MJ. et al. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45(11):3070–3075. doi: 10.1128/AAC.45.11.3070-3075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotonda MP, Xiong YQ, Memmi G. et al. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis. 2009;199(2):209–218. doi: 10.1086/595740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni A, Andrey DO, Jousselin A. et al. Whole genome sequencing and complete genetic analysis reveals novel pathways to glycopeptide resistance in Staphylococcus aureus. PLoS ONE. 2011;6(6):e21577. doi: 10.1371/journal.pone.0021577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulthess B, Meier S, Homerova D. et al. Functional characterization of the σB-dependent yabJ-spoVG operon in Staphylococcus aureus: role in methicillin and glycopeptide resistance. Antimicrob Agents Chemother. 2009;53(5):1832–1839. doi: 10.1128/AAC.01255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni A, Kelley WL, Barras C. et al. Identification by genomic and genetic analysis of two new genes playing a key role in intermediate glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(3):903–911. doi: 10.1128/AAC.01287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]