Abstract
Neuronal ensembles are coactive groups of neurons that may represent emergent building blocks of neural circuits. They could be formed by Hebbian plasticity, whereby synapses between coactive neurons are strengthened. Here we report that repetitive activation with two-photon optogenetics of neuronal populations in visual cortex of awake mice generates artificially induced ensembles which recur spontaneously after being imprinted and do not disrupt preexistent ones. Moreover, imprinted ensembles can be recalled by single cell stimulation and remain coactive on consecutive days. Our results demonstrate the persistent reconfiguration of cortical circuits by two-photon optogenetics into neuronal ensembles that can perform pattern completion.
Neuronal ensembles are groups of coactive neurons evoked by sensory stimuli (1–3) or motor behaviors (4–6), and may represent emergent building blocks of cortical function (7, 8). In the absence of external inputs, ongoing cortical ensembles resemble sensory evoked ones (9–11), as if the cortex has an imprinted representation of the world, implemented by groups of neurons with strong synaptic connectivity. Ensembles could result from Hebbian plasticity, whereby connectivity between coactive neurons is strengthened due to overlapped activity (12). Indeed, optogenetic studies in which all expressing neurons and their axons are simultaneously photostimulated have demonstrated Hebbian plasticity (13). However, to artificially generate specific neuronal ensembles with single cell resolution has been experimentally difficult.
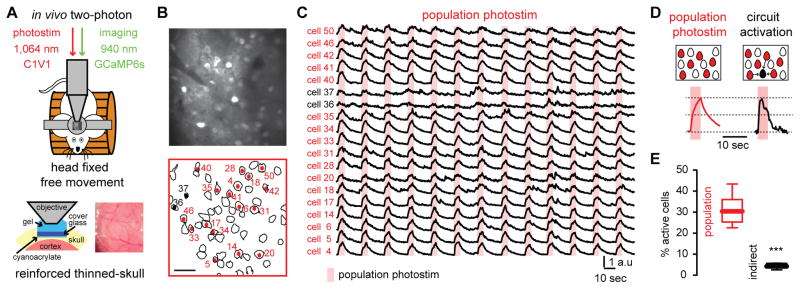
To do so, we used simultaneous two-photon calcium imaging and two-photon photostimulation (14, 15) in primary visual cortex of head-fixed mice running on a treadmill. GCaMP6s signals of layer 2/3 neurons were imaged through a reinforced thinned-skull window, while C1V1 expressing neurons were optogenetically stimulated with a second two-photon laser (16) (Fig. 1, A and B). Two-photon population photostimulation evoked calcium transients reliably in a specific subset of neurons (Fig. 1C). In vivo electrophysiological recordings demonstrated that population photostimulation evoked bursting activity with similar temporal features, independently of the spatial location of the neurons (fig. S1). Neurons responding to direct photostimulation were differentiated from photostimulation light artifacts and from other active neurons (Fig. 1, D and E) by their different temporal responses (fig. S1). This enabled us to distinguish the photostimulated cells from those that became active because of the effect of photostimulation on the circuit.
Fig. 1. Two-photon optogenetic photostimulation reliably activates specific neuronal populations.
(A), Simultaneous two-photon imaging and two-photon optogenetic photostimulation was performed in layer 2/3 over left primary visual cortex (V1) in awake head fixed mice through a reinforced thinned skull window. (B) Automatic contour detection of cortical neurons. Red cells denote neurons that reliably respond to optogenetic population photostimulation. Scale bar 50 μm. (C) Calcium transients of neurons activated by population photostimulation (red) and neurons activated indirectly (black). (D) Calcium transients from directly photostimulated neurons differed from calcium transients evoked indirectly by circuit activation. (E) Indirectly activated neurons represent a small percentage of the population (n = 6 mice; ***P = 0.0006; Mann-Whitney test). Data presented as whisker box plots displaying median and interquartile ranges.
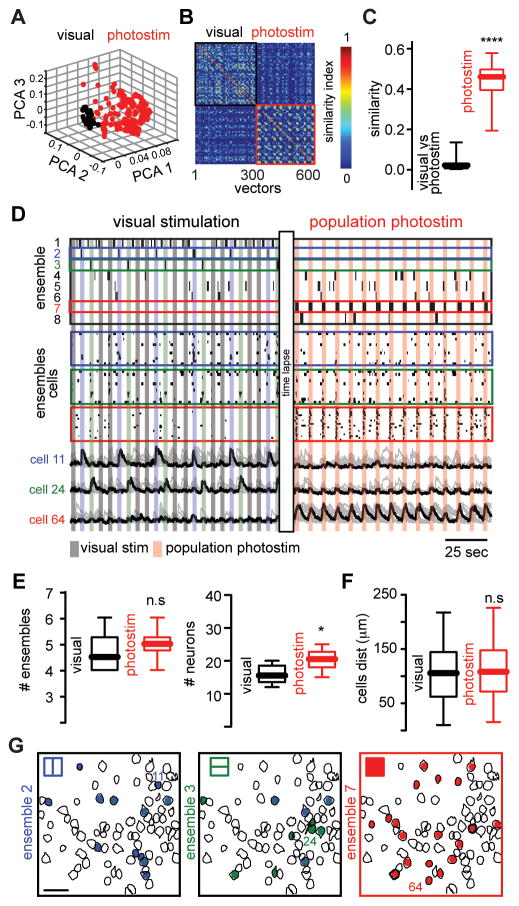
Repeated optogenetic stimulation reliably recruited specific groups of neurons, generating an artificial “photoensemble”, i.e., a group of neurons that were optically stimulated together. To measure ensembles, we analyzed population activity using multidimensional population vectors to quantitatively define clusters of coactive neurons (17–19) and found that photoensembles engaged a different population of neurons than visually evoked ensembles, with only 20.17 ± 9.4 % neurons in common (Fig. 2, A to D. and fig. S2). Although the number of ensembles was similar in both experimental conditions, photoensembles activated more neurons than visually evoked ones (Fig. 2E). Neurons belonging to photoensembles or visual ensembles had a widespread spatial distribution and were spatially intermingled (Fig. 2, F and G). Visual ensembles remained stable after population photostimulation (fig. S3) indicating that repetitive photostimulation did not disrupt preexistent cortical ensembles.
Fig. 2. Population photostimulation generates artificial cortical ensembles.
(A) Principal component analysis (PCA) of population vectors evoked by visual stimuli (black) and optogenetic photostimulation (red). (B) Similarity map representing the angle between population vectors during visual stimuli (black) or population photostimuli (red). (C) Population similarity between visually and photostimulated evoked activity (n = 6 mice; ****P < 0.0001). (D) Time course activation of evoked cortical ensembles (top) aligned with raster plots representing the activity of visually evoked ensembles and photoensemble (middle) and calcium transients (bottom) of the most representative neurons of each ensemble. Colored boxes indicate ensemble label. (E) The total number of ensembles remained stable in both conditions (top; n = 6 mice; n.s P=0.4315). The number of cells defining photoensembles is significantly higher than neurons defining each visually evoked ensemble (bottom, n = 6 mice; *P = 0.0446). (F) Spatial maps of cortical ensembles in both experimental conditions. Scale bar 50 μm. (G) Distance between all neurons belonging to each ensemble (n = 6 mice; n.s. P = 0.3720). Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann-Whitney test.
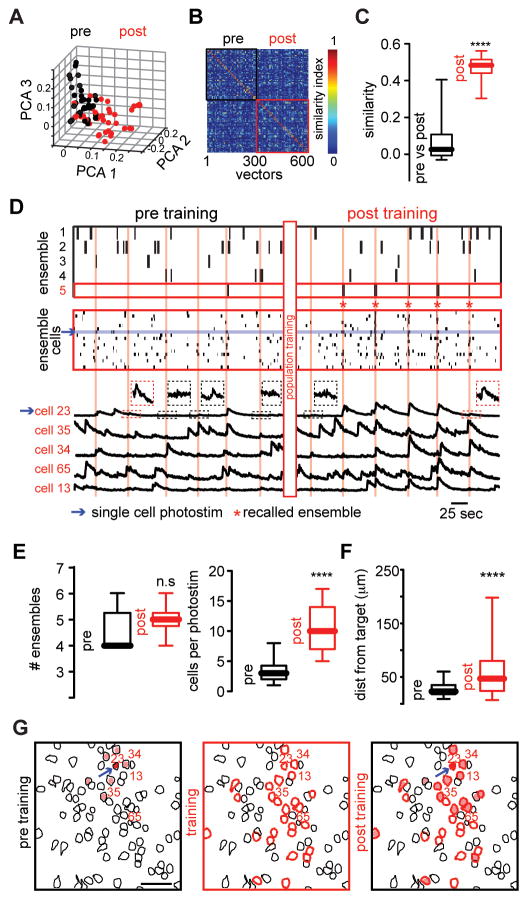
Interestingly, we noted that some photostimulated neurons became active spontaneously (see below). We named these spontaneously active photostimulated neurons “imprinted” ensembles, as if the artificial ensemble had been imprinted into the cortex. Moreover, the activation of a single cell was able to recall imprinted ensembles (Fig. 3A), demonstrating pattern completion. Pattern completion (20) is found in hippocampus (21–23) and is a property of attractor neural networks (22, 24). Single cell activation before population photoactivation did not produce alterations of overall network activity and was unable to consistently recall cortical ensembles (Fig. 3B–D, left; and fig. S4). Nevertheless, after population training, photoactivation of selected members (8 ± 2.5 %) of the imprinted ensemble (Fig. S5) activated a group of cells (Fig. 3, C and D, right). These recalled ensembles, evoked by single cell stimulation, did not disrupt the overall network activity and were interspersed in time with ongoing cortical ensembles (Fig. 3D; top). While the number of ensembles after population training remained stable (Fig. 3E), single cell photostimulation reliably recalled a specific group of neurons that was not coactive before (Fig. 3E; 64.5 ± 12.63 % recalling after population training). The spatial location of neurons in recalled ensembles had a broader distribution than the occasional neurons that were indirectly activated before population training (Fig. 3, F and G). On the other hand, the number of calcium transients during ongoing activity in non-photostimulated neurons remained constant after population training whereas it increased in photostimulated neurons of imprinted ensembles (Fig. 4, A and B), ruling out the possibility that population photostimulation changed the basal level of activity in the whole network. This modification of the functional connectivity between photostimulated neurons also required a minimal number of trials (Fig. 4C) indicating that the observed changes were driven by a change in the circuit triggered by repeated photostimulation of a specific population of neurons.
Fig. 3. Pattern completion of artificially imprinted ensembles.
(A) PCA projection of population vectors during single cell photostimulation before and after population training. (B) Similarity map of population vectors from ongoing cortical activity. (C) Single cell photostimulation after population training recalled population vectors with high similarity (n = 6 mice; ****P < 0.0001). (D) Time course activation of cortical ensembles (top) aligned with raster plot of all the cells that belong to recalled ensemble (middle) and calcium transients (bottom) of representative neurons from recalled ensemble (red labels) before and after population training. (E) The number of ensembles before and after population training remains stable (top; n = 6 mice; n.s. P = 0.2259). After population training single cell photostimulation consistently recruits a group of neurons significantly larger than control conditions (bottom; n = 6 mice; ****P < 0.0001). (F) Spatial maps of neurons recruited by single cell photostimulation before (left) during (middle) and after population training (right). Arrow indicates stimulated neuron. Scale bar 50 μm. (G) After population training the distance from the target cell and activated neurons is increased (n = 6 mice; **** P < 0.0001). Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann-Whitney test.
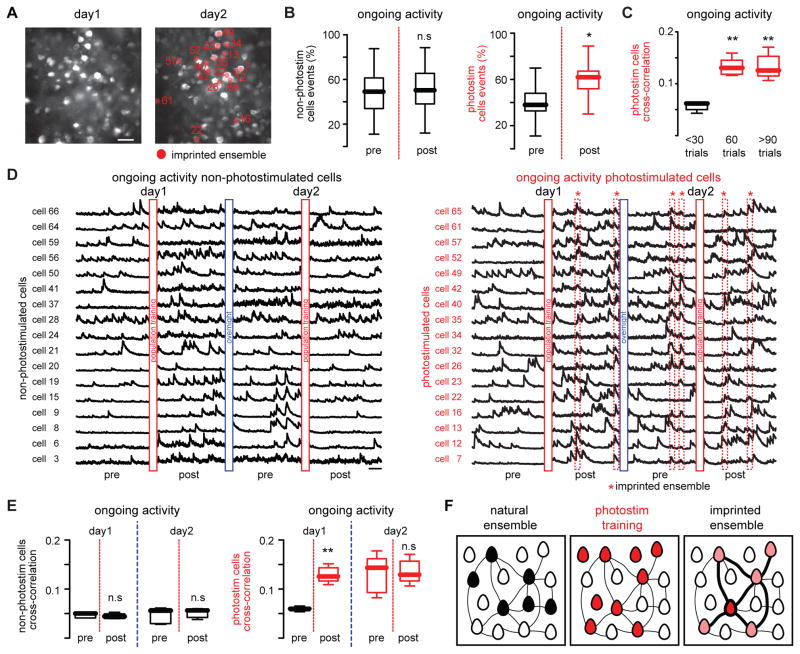
Fig. 4. Imprinted ensembles persist after consecutive days.
(A) Images showing the same optical field at two different days. Scale bar 50 μm. (B) Percentage of events during ongoing activity of non-photostimulated cells remains stable (left; n = 5 mice; n.s P=0.5664; Wilcoxon matched-pairs signed rank test) whereas photostimulated cells increased their activity (right; n = 5 mice; *P = 0.0147; Wilcoxon matched-pairs signed rank test) after population training. Red line denotes population training. (C) The enhancement of cross-correlation between photostimulated cells depends on the number of training trials (n = 5 mice; **P = 0.0092; Kruskal-Wallis test). (D) Calcium transients of non-photostimulated neurons (left) and photostimulated neurons (right) during ongoing cortical activity at two different days before and after population training. Imprinted ensembles recur spontaneously at consecutive days (dotted red boxes). (E) Cross-correlation between non-photostimulated neurons (left; n = 5 mice; day 1: n.s. P = 0.5476; day 2: n.s. P = 0.8413; Mann-Whitney test) and photostimulated neurons (right; n = 5 mice; day 1: **P = 0.079; day 2: n.s. P = 1; Mann-Whitney test) during ongoing activity at consecutive days. (F) Population photostimulation enhances the functional connectivity between responsive neurons. Lines widths represent the strength of the functional connectivity between neurons. Data presented as whisker box plots displaying median and interquartile ranges.
To investigate whether imprinted ensembles were persistently integrated in ongoing cortical activity, we imaged the same area on consecutive days. Indeed, single cell photostimulation was still able to recall previously imprinted ensembles on consecutive days (fig. S6). The analysis of ongoing activity from non-photostimulated (Fig. 4D, left) and photostimulated neurons showed that imprinted ensembles recurred spontaneously even on consecutive days (Fig. 4D, right). While cross-correlations between non-responsive neurons were not altered (Fig. 4E, left), they were enhanced between photostimulated neurons and remained stable the next day even after further photostimulation (Fig. 4E, right). Thus, optogenetic activation of identified neurons enhanced their functional connections for at least one day (Fig. 4F).
Recalled ensembles shared similar characteristics to ongoing ones, such as number of neurons and spatial distribution (fig. S7), but the mean distance between active neurons was smaller (fig. S7D), demonstrating that the effect of the photostimulation is local. Recalled ensembles were also composed of combinations of neurons which may or may not belong to ongoing ensembles (fig. S7, D and E), demonstrating that recalled ensembles are indeed novel and not just dormant preexisting ensembles. However, given that cortical connections are not in a tabula rasa state, we expect that imprinted ensembles may recruit segments of physiologically relevant circuit motifs.
Previously, electrical or optogenetic stimulation (25) showed that co-activation of neuronal groups can produce physiologically relevant behaviors (13,26). Here, we show the possibility to train individual neurons to build artificial neuronal ensembles (13), which then become spontaneously active (Fig. 4D, right). Our results can explain the similarity between visually evoked and spontaneous ensembles (9) and the finding that neurons responding to similar visual stimuli have a higher interconnectivity (27). In both cases, recurrent co-activation of a neuronal group would enhance their functional connectivity, imprinting ensembles into the circuit.
More than sixty years ago, Hebb proposed that repeated co-activation of a group of neurons might create a memory trace through enhancement of synaptic connections (12). Because of technical limitations, this hypothesis has been difficult to test in awake animals with single cell resolution. Our work, combining novel imaging and photostimulation techniques (14, 15) and analytical tools (19), can be interpreted as a confirmation of the Hebbian postulate and as a demonstration that cortical microcircuits can perform pattern completion.
Supplementary Material
Fig. S1. Two-photon population photostimulation of cortical neurons in awake mice.
(A) Temporal profile of evoked responses. Light artifacts are different from calcium transients induced by neuron activity (left). Principal component analysis (PCA) of evoked responses taking each neuron as the independent variable. Note that photostimulation responses can be separated from indirect responses and light artifacts (right). (B) Percentage of responses per photostimulated neuron as a function of photostimulation laser power. Red box represents the parameters used for population training. (C) Population photostimuli protocol evoked by raster scanning the whole field of view with the 1064 nm laser. Each pixel was photostimulated at ~4Hz for 4–6 seconds with intervals of 5–10 seconds without photostimulation (60–90 trials). δ represents dwell time (2 μsec/pixel). (D) Population photostimulation protocol induced bursting activity with similar latencies independently of the spatial location of cells. Schematic representation of the same neuron recorded at different locations in the recorded field. Extracellular action currents evoked by photostimulation demonstrate that the latency firing is not significantly different in the same neuron at different locations. Note that the characteristic calcium transients of photoactivated neurons can be explained by the higher firing frequency during the first half of the stimuli that will be reflected as a steeper slope of calcium signals and higher values of the first time derivative compared with the second half of the responses. Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann-Whitney test.
Fig. S2. Identification of neuronal ensembles using population vectors.
(A) Binary arrays representing the first time derivative from calcium signals. (B) Raster plot representing overall network activity from active neurons during visual stimulation with drifting-gratings. (C) Schematic representation of vectorization procedure. Binary vectors represent active cells (ones) and inactive cells (zeros) at different time. (D) Term frequency inverse document frequency normalization (tf-idf). Note that neurons with high activity rates (red) are discarded by this normalization. (E) Similarity map representing the angle between all possible population vector pairs (top). Factorization of similarity maps (bottom) using hard thresholding of the most significant values obtained with singular value decomposition (SVD). (F) Normalized magnitude of each factor taken from SVD. The cutoff value signals the number of neuronal ensembles that repeat above chance levels. (G) Factors representing neuronal ensembles sorted in time. First and fifth factors represent vertical and horizontal drifting-gratings respectively. (H) The most representative neurons from each ensemble defined as the highest ranked values from all the population vectors that belong to a given ensemble (colored rectangles). (I) Time course activation of cortical ensembles obtained from SVD factorization. Colors indicate the time when a given orientation was presented to the animal. (J) Zero lag cross-correlation of binary arrays taken from neurons belonging to neuronal ensembles representing drifting-gratings.
Fig. S3. Visually evoked ensembles remain stable after population photostimulation.
(A) PCA analysis of population vectors during visual stimuli before (black) and after (blue) population photostimuli. (B) Similarity map of all significant frames before and after population training. Red lines denote the separation between visual stimuli. Time between visually evoked activity recordings was ~50 min. (C) The population similarity before and after photostimulation remained stable (n = 6 mice; P = 0.8438; Wilconxon matched-pairs signed rank test). (D) Time course activation of visually evoked cortical ensembles (top) aligned with calcium transients (bottom) of representative cells belonging to cortical ensembles evoked by visual stimuli before and after population training. Photostimulation period lasted ~40 min. Note that the same visually evoked ensembles appeared before and after photostimulation demonstrating robust cortical microcircuits for visually evoked responses. Note that exactly the same ensembles appeared before and after photostimulation. (E) The total number of ensembles remained stable before and after population photostimulation. (F) Spatial maps of cortical ensembles evoked by visual stimuli. Scale bar 50 μm. (G) The number of cells defining visually evoked ensembles remained stable (n = 6 mice; n.s P = 0.3289; Mann Whithney test; left). (H) The percentage of coactive cells remained stable (n = 6 mice; n.s P = 0.7179; Mann Whitney test; right). Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S4. Single cell photostimulation of cortical neurons in vivo.
(A) Single cell photostimulation protocol consist on 20 spirals delivered at ~20Hz each spiral follow a trajectory from the center of the neuron to the boundaries (top). Normalized calcium transients evoked by single cell photostimulation at different times demonstrating that single cell photostimulation reliably activates the same neuron several times. (B) Single cell photostimuli evoked calcium transients as a function of laser power. Red box represents the parameters used for the present experiments. (C) Normalized amplitude of calcium transients to the maximum response evoked by visual stimuli. Single cell photostimulation evoke calcium transients with similar amplitudes to population photostimulation and visually evoked responses. (D) Normalized responses of all the cells during single cell photostimulation versus their distance to target cells. Distance binned 10μm. (E) Single cell photostimulation responses evoked in targeted neurons (left) and non-targeted neurons (right) in randomly selected neurons from the optical field (left). (F) Spatial maps of different neurons individually photostimulated showing that before population training single cell photostimulation is spatially restricted and was not able to reliably recall preexistent cortical ensembles. Scale bar 50 μm. Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S5. Partial recalling evoked by single cell targeting of different neurons from an imprinted ensemble.
(A) Single cell photostimulation of different neurons belonging to the imprinted ensemble. Red lines indicate photostimulation epochs. Each box shows five stimulation trials of different single cells highlighted in blue. Ensemble recalling can be evoked by single cell photostimulation of a given neuron whereas the photostimulation of different neurons evoked partial recalling or non-recalling. (B) Spatial maps of neurons recalled by single cell photostimulation. Scale bar 50 μm. (C) Total number of activated cells per photostimulus. In each trial the number of partially recalled cells and recalled cells are significantly different from non-recalled trials. (n = 5 mice; ****P < 0.0001; Kruskal-Wallis test). (D) The percentage of recalled neurons by single cell photostimulation for all trials is significantly different for non-recalled and partially recalled ensembles (n = 5 mice; ***P = 0.0003; Mann Whithney test). (E) The distance from targeted neurons is not significantly different in non-recalling, partially recalled and recalled cells indicating that non-recalled and partially recalled conditions recruit a specific subpopulation of the recalled ensemble (n = 5 mice; n.s. P = 0.3635; Kruskal-Wallis test). Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S6. Recalling of imprinted ensembles by single cell photostimulation at consecutive days.
(A) Temporal course of cortical ensembles (top) and raster plot of neurons belonging to imprinted ensemble (bottom) demonstrate that single cell photostimulation is able to recall a given ensemble at consecutive days. Vertical red bars indicated recalled neurons by single cell photostimulation. (B) The total number of ensembles remains constant at consecutive days indicating that ongoing cortical ensembles and imprinted ensemble are stable (n = 5 mice; n.s. P = 0.7180). (C) Spatial maps of recalled ensemble at consecutive days showing that recalled neurons have the same spatial characteristics across days. Scale bar 50 μm. (D) The number of recalled cells per photostimuli remains stable at consecutive days (n = 5 mice; n.s. P = 0.6620). (E) The distance between recalled neurons and the target cell remains stable at consecutive days (n = 5 mice; n.s. P = 0.7776). Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann Whithney test.
Fig. S7. Comparison between recalled and ongoing cortical ensembles.
(A) Temporal course of cortical ensembles (top) and neurons belonging to each ensemble (bottom) before and after population training. Red box highlight the recalled ensemble. (B) The total number of neurons (top; n = 6 mice; n.s. P = 0.2080) and the percentage of coactive cells between ongoing ensembles and recalled ensembles are similar (bottom; n = 6 mice; n.s. P = 0.2463) demonstrating that ongoing and recalled ensembles have similar characteristics. (C) Spatial maps of ongoing ensembles and recalled ensemble. Scale bar 50 μm. (D) The distance between neurons from recalled ensembles and ongoing ensembles is significantly different demonstrating that ensemble imprinting is spatially restricted (n = 6 mice; ****P < 0.0001). (E) The recalled ensemble is composed by a combination of different neuronal subsets taken from ongoing ensembles and also by neurons that just belong to the recalled ensemble itself (top; n = 6 mice; **P = 0.0079). (F) Cell identity map of neurons belonging to ongoing ensembles and recalled ensemble. Red box highlight recalled ensemble. Note that some neurons from the recalled ensemble can be part of multiple ongoing ensembles but some other neurons just belong to the recalled ensemble. Numbers in the abscissa represent neurons belonging to recalled ensemble. Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann Whithney test.
Acknowledgments
Laboratory members for help and virus injections, A. Fairhall for comments and the Stanford Neuroscience Gene Vector and Virus Core for AAVdj virus. Supported by the National Eye Institute (DP1EY024503), National Institute of Mental Health (R01MH101218) and the Defense Advanced Research Projects Agency (SIMPLEX N66001-15-C-4032). Y.B. holds a fellowship from Uehara Memorial Foundation. This material is based upon work supported by, or in part by, the U. S. Army Research Laboratory and the U. S. Army Research Office (Contract W911NF-12-1-0594, MURI). The authors declare no competing financial interests. Conceptualization, L.C.-R. and R.Y.; Methodology, L.C.-R., D.S.P., W.Y. and R.Y.; Investigation, L.C.-R., W.Y., and Y.B.; Writing – Original Draft, L.C.-R.; Writing – Review Editing, L.C.-R. and R.Y.; Resources, D. S. P., W. Y. and L.C.-R.; Funding Acquisition, R.Y. All the data are archived in the NeuroTechnology Center, Sherman Fairchild Imaging Floor, NWC Building. Columbia University in the City of New York.
Footnotes
References and Notes
- 1.Kampa BM, Roth MM, Gobel W, Helmchen F. Representation of visual scenes by local neuronal populations in layer 2/3 of mouse visual cortex. Frontiers in neural circuits. 2011;5:18. doi: 10.3389/fncir.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 3.Sawinski J, et al. Visually evoked activity in cortical cells imaged in freely moving animals. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchland MM, et al. Neural population dynamics during reaching. Nature. 2012;487:51–56. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 6.Cao VY, et al. Motor Learning Consolidates Arc-Expressing Neuronal Ensembles in Secondary Motor Cortex. Neuron. 2015;86:1385–1392. doi: 10.1016/j.neuron.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 8.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 9.Miller JE, Ayzenshtat I, Carrillo-Reid L, Yuste R. Visual stimuli recruit intrinsically generated cortical ensembles. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4053–4061. doi: 10.1073/pnas.1406077111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- 11.Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62:413–425. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebb DO. A Wiley book in clinical psychology. Wiley; New York: 1949. The organization of behavior; a neuropsychological theory; p. xix.p. 335. [Google Scholar]
- 13.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer AM, Russell LE, Dalgleish HW, Hausser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nature methods. 2015;12:140–146. doi: 10.1038/nmeth.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rickgauer JP, Deisseroth K, Tank DW. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nature neuroscience. 2014;17:1816–1824. doi: 10.1038/nn.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drew PJ, et al. Chronic optical access through a polished and reinforced thinned skull. Nature methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nature neuroscience. 2005;8:1568–1576. doi: 10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- 18.Carrillo-Reid L, et al. Encoding network states by striatal cell assemblies. Journal of neurophysiology. 2008;99:1435–1450. doi: 10.1152/jn.01131.2007. [DOI] [PubMed] [Google Scholar]
- 19.Carrillo-Reid L, Miller JE, Hamm JP, Jackson J, Yuste R. Endogenous Sequential Cortical Activity Evoked by Visual Stimuli. J Neurosci. 2015;35:8813–8828. doi: 10.1523/JNEUROSCI.5214-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 21.O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 22.Rolls ET, Treves A. Neural networks in the brain involved in memory and recall. Prog Brain Res. 1994;102:335–341. doi: 10.1016/S0079-6123(08)60550-6. [DOI] [PubMed] [Google Scholar]
- 23.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko H, et al. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- 29.Packer AM, et al. Two-photon optogenetics of dendritic spines and neural circuits. Nature methods. 2012;9:1202–1205. doi: 10.1038/nmeth.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cossart R, Aronov D, Yuste R. Attractor dynamics of network UP states in neocortex. Nature. 2003;423:283–289. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- 32.Shmiel T, et al. Temporally precise cortical firing patterns are associated with distinct action segments. Journal of neurophysiology. 2006;96:2645–2652. doi: 10.1152/jn.00798.2005. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki T, Matsuki N, Ikegaya Y. Metastability of active CA3 networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:517–528. doi: 10.1523/JNEUROSCI.4514-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber S, Fellous JM, Whitmer D, Tiesinga P, Sejnowski TJ. A new correlation-based measure of spike timing reliability. Neurocomputing. 2003;52–4:925–931. doi: 10.1016/S0925-2312(02)00838-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbarine E, DePasquale J, Gadia V, Polikar R, Rosen G. Information-theoretic approaches to SVM feature selection for metagenome read classification. Comput Biol Chem. 2011;35:199–209. doi: 10.1016/j.compbiolchem.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Islamaj Dogan R, Lu Z. Click-words: learning to predict document keywords from a user perspective. Bioinformatics. 2010;26:2767–2775. doi: 10.1093/bioinformatics/btq459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z, Kim W, Wilbur WJ. Evaluating relevance ranking strategies for MEDLINE retrieval. J Am Med Inform Assoc. 2009;16:32–36. doi: 10.1197/jamia.M2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan M, Tan CL, Su J, Lu Y. Supervised and traditional term weighting methods for automatic text categorization. IEEE Trans Pattern Anal Mach Intell. 2009;31:721–735. doi: 10.1109/TPAMI.2008.110. [DOI] [PubMed] [Google Scholar]
- 40.Margrie TW, et al. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron. 2003;39:911–918. doi: 10.1016/j.neuron.2003.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Two-photon population photostimulation of cortical neurons in awake mice.
(A) Temporal profile of evoked responses. Light artifacts are different from calcium transients induced by neuron activity (left). Principal component analysis (PCA) of evoked responses taking each neuron as the independent variable. Note that photostimulation responses can be separated from indirect responses and light artifacts (right). (B) Percentage of responses per photostimulated neuron as a function of photostimulation laser power. Red box represents the parameters used for population training. (C) Population photostimuli protocol evoked by raster scanning the whole field of view with the 1064 nm laser. Each pixel was photostimulated at ~4Hz for 4–6 seconds with intervals of 5–10 seconds without photostimulation (60–90 trials). δ represents dwell time (2 μsec/pixel). (D) Population photostimulation protocol induced bursting activity with similar latencies independently of the spatial location of cells. Schematic representation of the same neuron recorded at different locations in the recorded field. Extracellular action currents evoked by photostimulation demonstrate that the latency firing is not significantly different in the same neuron at different locations. Note that the characteristic calcium transients of photoactivated neurons can be explained by the higher firing frequency during the first half of the stimuli that will be reflected as a steeper slope of calcium signals and higher values of the first time derivative compared with the second half of the responses. Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann-Whitney test.
Fig. S2. Identification of neuronal ensembles using population vectors.
(A) Binary arrays representing the first time derivative from calcium signals. (B) Raster plot representing overall network activity from active neurons during visual stimulation with drifting-gratings. (C) Schematic representation of vectorization procedure. Binary vectors represent active cells (ones) and inactive cells (zeros) at different time. (D) Term frequency inverse document frequency normalization (tf-idf). Note that neurons with high activity rates (red) are discarded by this normalization. (E) Similarity map representing the angle between all possible population vector pairs (top). Factorization of similarity maps (bottom) using hard thresholding of the most significant values obtained with singular value decomposition (SVD). (F) Normalized magnitude of each factor taken from SVD. The cutoff value signals the number of neuronal ensembles that repeat above chance levels. (G) Factors representing neuronal ensembles sorted in time. First and fifth factors represent vertical and horizontal drifting-gratings respectively. (H) The most representative neurons from each ensemble defined as the highest ranked values from all the population vectors that belong to a given ensemble (colored rectangles). (I) Time course activation of cortical ensembles obtained from SVD factorization. Colors indicate the time when a given orientation was presented to the animal. (J) Zero lag cross-correlation of binary arrays taken from neurons belonging to neuronal ensembles representing drifting-gratings.
Fig. S3. Visually evoked ensembles remain stable after population photostimulation.
(A) PCA analysis of population vectors during visual stimuli before (black) and after (blue) population photostimuli. (B) Similarity map of all significant frames before and after population training. Red lines denote the separation between visual stimuli. Time between visually evoked activity recordings was ~50 min. (C) The population similarity before and after photostimulation remained stable (n = 6 mice; P = 0.8438; Wilconxon matched-pairs signed rank test). (D) Time course activation of visually evoked cortical ensembles (top) aligned with calcium transients (bottom) of representative cells belonging to cortical ensembles evoked by visual stimuli before and after population training. Photostimulation period lasted ~40 min. Note that the same visually evoked ensembles appeared before and after photostimulation demonstrating robust cortical microcircuits for visually evoked responses. Note that exactly the same ensembles appeared before and after photostimulation. (E) The total number of ensembles remained stable before and after population photostimulation. (F) Spatial maps of cortical ensembles evoked by visual stimuli. Scale bar 50 μm. (G) The number of cells defining visually evoked ensembles remained stable (n = 6 mice; n.s P = 0.3289; Mann Whithney test; left). (H) The percentage of coactive cells remained stable (n = 6 mice; n.s P = 0.7179; Mann Whitney test; right). Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S4. Single cell photostimulation of cortical neurons in vivo.
(A) Single cell photostimulation protocol consist on 20 spirals delivered at ~20Hz each spiral follow a trajectory from the center of the neuron to the boundaries (top). Normalized calcium transients evoked by single cell photostimulation at different times demonstrating that single cell photostimulation reliably activates the same neuron several times. (B) Single cell photostimuli evoked calcium transients as a function of laser power. Red box represents the parameters used for the present experiments. (C) Normalized amplitude of calcium transients to the maximum response evoked by visual stimuli. Single cell photostimulation evoke calcium transients with similar amplitudes to population photostimulation and visually evoked responses. (D) Normalized responses of all the cells during single cell photostimulation versus their distance to target cells. Distance binned 10μm. (E) Single cell photostimulation responses evoked in targeted neurons (left) and non-targeted neurons (right) in randomly selected neurons from the optical field (left). (F) Spatial maps of different neurons individually photostimulated showing that before population training single cell photostimulation is spatially restricted and was not able to reliably recall preexistent cortical ensembles. Scale bar 50 μm. Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S5. Partial recalling evoked by single cell targeting of different neurons from an imprinted ensemble.
(A) Single cell photostimulation of different neurons belonging to the imprinted ensemble. Red lines indicate photostimulation epochs. Each box shows five stimulation trials of different single cells highlighted in blue. Ensemble recalling can be evoked by single cell photostimulation of a given neuron whereas the photostimulation of different neurons evoked partial recalling or non-recalling. (B) Spatial maps of neurons recalled by single cell photostimulation. Scale bar 50 μm. (C) Total number of activated cells per photostimulus. In each trial the number of partially recalled cells and recalled cells are significantly different from non-recalled trials. (n = 5 mice; ****P < 0.0001; Kruskal-Wallis test). (D) The percentage of recalled neurons by single cell photostimulation for all trials is significantly different for non-recalled and partially recalled ensembles (n = 5 mice; ***P = 0.0003; Mann Whithney test). (E) The distance from targeted neurons is not significantly different in non-recalling, partially recalled and recalled cells indicating that non-recalled and partially recalled conditions recruit a specific subpopulation of the recalled ensemble (n = 5 mice; n.s. P = 0.3635; Kruskal-Wallis test). Data presented as whisker box plots displaying median and interquartile ranges.
Fig. S6. Recalling of imprinted ensembles by single cell photostimulation at consecutive days.
(A) Temporal course of cortical ensembles (top) and raster plot of neurons belonging to imprinted ensemble (bottom) demonstrate that single cell photostimulation is able to recall a given ensemble at consecutive days. Vertical red bars indicated recalled neurons by single cell photostimulation. (B) The total number of ensembles remains constant at consecutive days indicating that ongoing cortical ensembles and imprinted ensemble are stable (n = 5 mice; n.s. P = 0.7180). (C) Spatial maps of recalled ensemble at consecutive days showing that recalled neurons have the same spatial characteristics across days. Scale bar 50 μm. (D) The number of recalled cells per photostimuli remains stable at consecutive days (n = 5 mice; n.s. P = 0.6620). (E) The distance between recalled neurons and the target cell remains stable at consecutive days (n = 5 mice; n.s. P = 0.7776). Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann Whithney test.
Fig. S7. Comparison between recalled and ongoing cortical ensembles.
(A) Temporal course of cortical ensembles (top) and neurons belonging to each ensemble (bottom) before and after population training. Red box highlight the recalled ensemble. (B) The total number of neurons (top; n = 6 mice; n.s. P = 0.2080) and the percentage of coactive cells between ongoing ensembles and recalled ensembles are similar (bottom; n = 6 mice; n.s. P = 0.2463) demonstrating that ongoing and recalled ensembles have similar characteristics. (C) Spatial maps of ongoing ensembles and recalled ensemble. Scale bar 50 μm. (D) The distance between neurons from recalled ensembles and ongoing ensembles is significantly different demonstrating that ensemble imprinting is spatially restricted (n = 6 mice; ****P < 0.0001). (E) The recalled ensemble is composed by a combination of different neuronal subsets taken from ongoing ensembles and also by neurons that just belong to the recalled ensemble itself (top; n = 6 mice; **P = 0.0079). (F) Cell identity map of neurons belonging to ongoing ensembles and recalled ensemble. Red box highlight recalled ensemble. Note that some neurons from the recalled ensemble can be part of multiple ongoing ensembles but some other neurons just belong to the recalled ensemble. Numbers in the abscissa represent neurons belonging to recalled ensemble. Data presented as whisker box plots displaying median and interquartile ranges analyzed using Mann Whithney test.