Abstract
This network meta-analysis was conducted to assess whether the efficacy of surgery with adjuvant therapies, including radiotherapy (RT+S), chemotherapy (CT+S), and chemoradiotherapy (CRT+S) have better performance in esophageal cancer treatment and management. PubMed and EMBASE were used to search for relevant trials. Both conventional pair-wise and network meta-analyses were carried out. The surface under the cumulative ranking curve (SUCRA) was used to rank interventions based on the efficacy of the treatment method. As for 3-year overall survival (OS), CRT+S showed the highest efficacy (CRT+S vs. surgery: HR=0.81, 95% CrI =0.73-0.90; CRT+S vs. CT+S: HR=0.82, 95% CrI =0.70-0.95; CRT+S vs. RT+S: HR=0.77, 95% CrI =0.62-0.95). For disease-free survival, CRT+S showed efficacy over CT+S ((HR =0.70, 95% CrI =0. 59-0.83). In conclusion, CRT+S showed a better performance for survival outcomes and ranks best among all therapies. The results of our study can provide guidance for medical decisions and treatment options that may help clinical practitioners improve the efficacy of EC treatment.
Keywords: esophageal cancer, surgery, adjuvant therapies, chemotherapy, radiotherapy
INTRODUCTION
Esophageal cancer (EC) is a typical malignant tumor which is often lethal for patients [1]. It is estimated that 16,910 new cases of EC would be diagnosed in 2016 in the USA alone with 15,690 EC deaths [2]. The incidence rate of EC varies from region to region, while some regions including Asia, southern and eastern Africa exhibit a higher rate [3, 4]. Researchers suggested that EC has become one of the most severe malignant tumors in western countries and more than half of new EC cases in the US were diagnosed as adenocarcinoma [5, 6]. Smoking, alcohol consumption, opium abuse and poor dietary habits etc. have been found to be the risk factors of EC [7, 8].
Surgical resection is a common choice for patients with EC [9]. However, patients underwent surgery appeared to have higher mortality rates compared with those who with alternative treatments [10]. The efficacy of surgery are not satisfactory, as studies suggested that these patients had a median survival period of only 18 months [11]. Radiotherapy (RT) is an important option which is commonly used in patients with advanced or metastasized EC [12]. The monotherapy of RT appears to have limited effectiveness and the five-year overall survival rate is approximately 10% [13]. Chemotherapy (CT) is another important therapy for cancers, and researchers have investigated the curative efficacy of CT on EC since 1990s [14]. As suggested by previous studies, combined CT appeared to have more favorable effects compared to single-agent CT [15]. Moreover, chemoradiotherapy (CRT) has been developed as a new approach for metastasis prevention and has recently become a more popular treatment option [16].
A large number of randomized clinical trials (RCTs) have been conducted to evaluate the relative usefulness of the above-mentioned approaches for controlling EC. However, there is substantial variation in the conclusions of these investigations. For example, Ando et al. demonstrated that preoperative CT followed by surgery can improve the survival status significantly compared to postoperative CT [17]. Nevertheless, this conclusion was controversial, and has been challenged by different researchers [18, 19]. Although several pair-wise meta-analyses based on a large number of trials have been carried out to address this inconsistency, the lack of indirect evidence prevented researchers from comparing multiple therapies simultaneously [20–22]. Therefore, we conducted this network meta-analysis to introduce indirect evidence as a potential solution to address the limitations of accurate estimates in EC treatment. In our study, we attempted to determine the relative efficacy of surgical resections and adjuvant therapies. Using a network meta-analysis approach, we compared the efficacy of surgery alone with surgery combined adjuvant therapies RT+S, CT+S and CRT+S.
RESULTS
Characteristics of the studies included in analysis
Characteristics of all involved studies were presented in Table 1, including the original country, sample size, the intervention and control groups, histology and clinical outcomes. A detailed list of included studies, patients, and diagnostic criteria characteristics of each individual study was provided in the analyzed report. All included studies [16, 17, 23–62] were published between 1981 and 2016, and covered a broad geographic area including countries in Asia and Europe, as well as the USA and Australia, and the selection process was presented in Figure 1. The intervention group involved a total of 3,206 patients while the control group contained 3,270 patients.
Table 1. Main characteristics of included studies.
| Study or Subgroup | Country | Histology | Intervention Group | Control Group | Overall Survival | Metastasis/Recurrence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size | Type | Dose (mg/m2) | Size | Type | Follow-up (mo) | HR and 95%Cl | Intervention | Control | |||
| Law et al., 1997 | China | SCC | 74 | CT+S | C:100 d1; F:500 d1-5 | 73 | S | 17 | 0.73 (0.53, 1.00) | 12/29 | 19/50 |
| Ancona et al., 2001 | Italy | SCC | 47 | CT+S | C:100 d1; F:500 d1-5 | 47 | S | 24 | 0.84 (0.58, 1.10) | 19/28 | 19/29 |
| Kelsen et al., 2007 | USA | SCC&AC | 216 | CT+S | C:100 d1; F:1000 d1-5 | 227 | S | 56 | 1.07 (0.87, 1.32) | NR | NR |
| Allum et al., 2009 | UK | SCC | 400 | CT+S | C:100 d1; F:1000 d1-5 | 402 | S | 37 | 0.84 (0.72, 0.98) | 68/82 | 60/101 |
| Boonstra et al., 2011 | Netherland | SCC | 85 | CT+S | C:80 d1; Eto:100 d1,2 | 84 | S | 60 | 0.71 (0.51, 0.98) | 14/25 | 15/31 |
| Ando et al., 2012 | Japan | SCC | 164 | CT+S | C:80 d1; F:800 d1-5 | 166 | S | 62 | 0.64 (0.45, 0.91) | NR/51 | NR/41 |
| Maipang et al., 1994 | Thailand | SCC | 24 | CT+S | C:100 d1; Vinblastine:3 d1-4; B:10 d1-5 | 22 | S | 17 | 1.61 (0.79, 3.27) | NR | NR |
| Nygaard et al., 1992* | Norway | SCC | 50 | CT+S | C:20 d1-5; B:10mg, d1-5 | 41 | S | 18 | 1.10 (0.93, 1.30) | NR | NR |
| Nygaard et al., 1992* | Norway | SCC | 47 | CRT+S | C:20 d1-5; B:10mg, d1-5; 35GY | 41 | S | 18 | 0.76 (0.45, 1.28) | NR | NR |
| Nygaard et al., 1992* | Norway | SCC | 48 | RT+S | 35Gy | 41 | S | 18 | 0.80 (0.63, 1.02) | NR | NR |
| Schlag et al., 1992 | German | SCC | 22 | CT+S | C:20 d1-5;F:1000, d1-d5 | 24 | S | 75 | 0.97 (0.60, 1.57) | NR | NR |
| Ychou et al., 2011 | France | AC, GEJ | 113 | CT+S | C:100 d1; F:800, d1-5 | 111 | S | 60 | 0.69 (0.50, 0.95) | 49/63 | 62/71 |
| Pouliquen et al., 1996 | France | SCC | 52 | CT+S | C:100, d1; F:20, d1-5 | 68 | S | NR | 1.03 (0.89, 1.13) | NR | NR |
| Ando et al., 1997 | Japan | SCC | 105 | CT+S | C:70 d1,21; V: 3 d1.21 | 100 | S | 59.2 | 1.08 (0.87, 1.34) | NR/57 | NR/55 |
| Ando et al., 2003 | Japan | SCC | 120 | CT+S | C:80 d1; F:800, d1-5 | 122 | S | NR | 1.20 (0.96, 1.51) | NR/63 | NR/45 |
| Lee et al., 2005 | Korea | SCC | 40 | CT+S | C:60 d1-4; F:1000, d1-3 | 52 | S | 25 | 0.60 (0.47, 0.77) | 18/28 | 9/19 |
| Heroor et al., 2003 | Japan | SCC | 94 | CT+S | C:70 d1; F:700, d1-4, V 3, d1 | 117 | S | 80 | 1.46 (1.21, 1.71) | NR | NR |
| Shiozaki et al., 2004 | Japan | SCC | 98 | CT+S | C:10, d1-5; F250-500, d1-5 | 52 | S | NR | 0.48 (0.35, 0.66) | NR | NR |
| Zhang et al., 2008 | China | SCC&AC | 66 | CT+S | C:25, d1-3; F:375, d1-5; L:135 d1-5 | 160 | S | NR | 1.36 (0.93, 1.98) | NR | NR |
| Walsh et al., 1996 | Ireland | AC | 55 | CRT+S | C:75; F:15mg/kg/d; 45Gy | 55 | S | 10 | 0.53 (0.33, 0.84) | NR | NR |
| Urba et al., 2001 | USA | SCC&AC | 47 | CRT+S | C:20; F:300; 35Gy | 50 | S | 98 | 0.75 (0.46, 1.22) | NR | NR |
| Stahl et al., 2009 | German | SCC | 60 | CRT+S | C:50; Eto:80; 30Gy | 59 | CT++S | 45.6 | 0.67 (0.41, 1.09) | NR/19 | NR/27 |
| Burmeister et al., 2011 | Australia | AC | 39 | CRT+S | C:80; F:1000 /d; 35Gy | 36 | CT#+S | 65 | 0.79 (0.41, 1.54) | NR/18 | NR/21 |
| Tepper et al., 2008 | USA | SCC&AC | 30 | CRT+S | C:100; F:1000; 41.5Gy | 26 | S | 60 | 0.51 (0.38, 0.68) | NR/9 | NR/12 |
| van Hagen et al., 2012 | Netherlands | SCC&AC | 178 | CRT+S | Carboplatin:2mg/ ml/min; P:50 ;41.4Gy | 188 | S | 45.4 | 0.73 (0.54, 1.00) | NR/62 | NR/188 |
| Burmeister et al., 2005 | Australia | SCC&AC | 128 | CRT+S | C:80; F:1800; 35Gy | 128 | S | 65 | 0.89 (0.67, 1.19) | 48/61 | 54/68 |
| Lv et al., 2010** | China | SCC | 80 | CRT+S | P:135 d1,22; C 20 d1-3 and 22-25; 40Gy | 80 | S | 45 | 0.71 (0.60, 0.85) | NR | NR |
| Lv et al., 2010** | China | SCC | 78 | CRT+S | P:135 d1,22; C 20 d1-3 and 22-25; 40Gy | 80 | S | 45 | 0.68 (0.58, 0.82) | NR | NR |
| Apinop et al., 1994 | Thailand | SCC | 35 | CRT+S | C 100 d1,22; F: 1000 d1-4and22-25; 40Gy | 34 | S | NR | 0.80 (0.48, 1.34) | NR | NR |
| Le Prise et al., 1994 | France | SCC | 41 | CRT+S | C 100 d1,22; F: 600 d1-4and22-25; 20Gy | 45 | S | 16 | 0.85 (0.50, 1.46) | 8/17 | 10/17 |
| Walsh et al., 1995 | Ireland | AC | 58 | CRT+S | C:75; F:15mg/kg/d; 45Gy | 55 | S | 10 | 0.58 (0.38, 0.88) | NR | NR |
| Mariette et al., 2014 | France | SCC | 98 | CRT+S | C 75 d1; F:800 on d1-4; 45Gy | 97 | S | 93.6 | 0.99 (0.69, 1.40) | 22/28 | 28/43 |
| Kobayashi et al., 2000 | Japan | NR | 91 | CT+S | F:600 | 80 | S | NR | 1.10 (0.67, 1.81) | NR | NR |
| Launois et al., 1981 | France | SCC | 67 | RT+S | 64-90Gy preop | 57 | S | NR | 1.17 (1.04, 1.32) | NR | NR |
| Gignoux et al., 1987 | Europe | SCC | 115 | RT+S | 33 Gy preop | 114 | S | 43.2 | 1.12 (0.95, 1.32) | NR | NR |
| Arnott et al., 1992 | Scotland | SCC | 90 | RT+S | 20 Gy Preop | 86 | S | NR | 1.02 (0.87, 1.19) | NR | NR |
| Lee et al., 2004 | Korea | SCC | 51 | CRT+S | C:60; F:1000; 45.6Gy | 50 | S | 25 | 0.88 (0.48, 1.62) | 6/19 | 12/18 |
Intervention: NR-not report C - Cisplatin, F-Fluorouracil, Eto-Etoposide, B-Bleomycin, V-Vindesine, P-Paclitaxel; Treatment: CRT-chemoradiotherapy, S-surgery, CT-chemotherapy, RT-radiotherapy; Tumor: SCC-Squamous Cell Carcinoma, AC-Adenocarcinoma, GEJ-Gastroesophageal Junction; CI-Confidence Interval; HR-Hazard Ratio
Note:*: These three studies are from the same paper. **: These two studies are from the same paper. The first one is the preoperative group and the latter one is the postoperative group. +: The dose is F: 2000mg/m2, leucovorin: 500mg/m2, C: 50mg/m2; #: The dose is C:80mg/m2, F:1000mg/m2.
Figure 1. Flow chart.
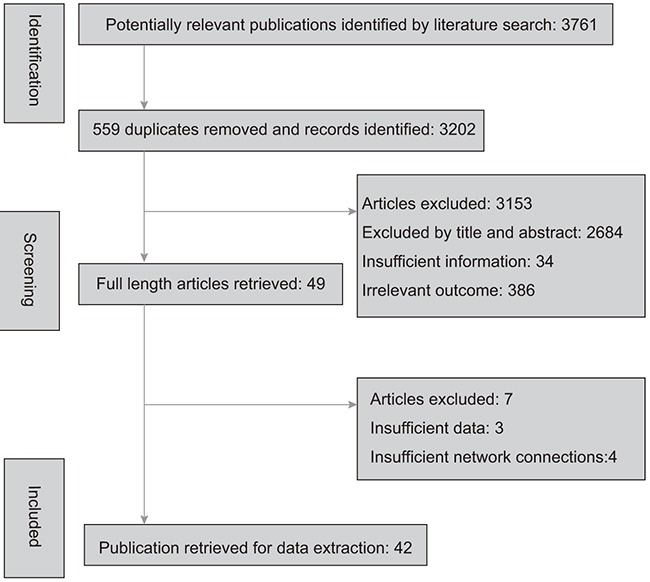
There are 42 studies included at last.
Systematic reviews are presented in Supplementary Figure 1, indicating that no obvious publication bias was observed. A Jadad scale table was also generated and is presented in Supplementary Table 1. The width of the lines in Figure 2 represent the number of trials comparing each pair of treatments and the area of circles indicate the cumulative number of patients for each intervention.
Figure 2. Network of randomized controlled trials comparing different treatments of EC.
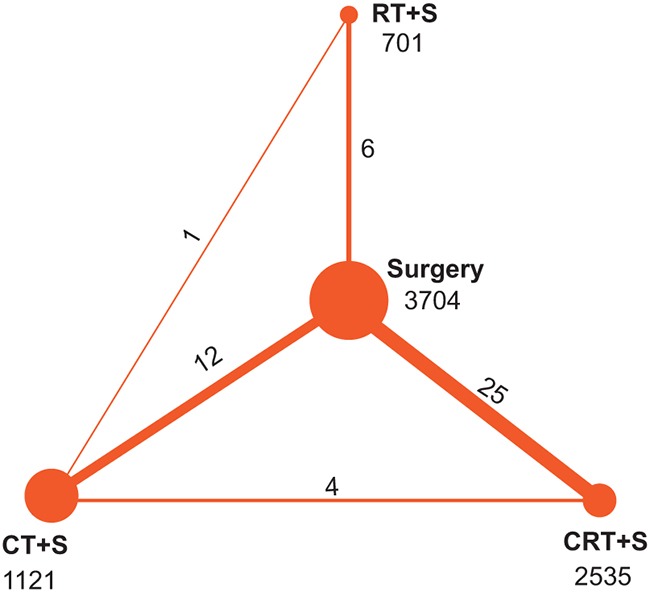
Treatment: CRT+S-chemo-radiotherapy plus surgery, CT+S-chemotherapy plus surgery, RT+S-radiotherapy plus surgery. Numbers above lines represent direct comparisons between two treatments. Numbers above dots represent total size of the treatment.
Pair-wise comparisons
The original data of 3-year OS, 5-year OS, and DFS are shown in Table 2. We conducted pair-wise meta-analysis and calculated the hazard ratio (HR) and 95% confidence interval (CI).
Table 2. Overall survival in 3 years and 5 years and disease-free survival of included studies.
| Study or Subgroup | Intervention | OS (HR and 95% CI) | DFS (HR and 95%CI) | |||
|---|---|---|---|---|---|---|
| Treatment (Size) | 3-year | 5-year | ||||
| Law et al., 1997 | CT+S | /S | (74/73) | - | 0.73 (0.53, 1.00) | - |
| Ancona et al., 2001 | CT+S | /S | (47/47) | - | 0.84 (0.58, 1.10) | - |
| Kelsen et al., 2007 | CT+S | /S | (216/227) | 1.05 (0.88, 1.26) | 1.06 (0.89, 1.25) | - |
| Allum et al., 2009 | CT+S | /S | (400/402) | 0.84 (0.70, 1.01) | 0.84 (0.58, 1.10) | 0.82 (0.71, 0.95) |
| Boonstra et al., 2011 | CT+S | /S | (85/84) | 0.65 (0.53, 0.80) | 0.66 (0.55, 0.80) | 0.72 (0.52, 1.00) |
| Ando et al., 2012 | CT+S | /S | (164/166) | 0.71 (0.53, 0.95) | 0.67 (0.52, 0.87) | 0.73 (0.54, 0.99)*** |
| Maipang et al., 1994 | CT+S | /S | (24/22) | 1.08 (0.79, 1.48) | - | - |
| Nygaard et al., 1992 * | CT+S | /S | (50/41) | 1.10 (0.93, 1.30) | - | - |
| Nygaard et al., 1992 * | CRT+S | /S | (47/41) | 0.8 (0.63, 1.02) | - | - |
| Nygaard et al., 1992 * | RT+S | /S | (48/41) | 0.76 (0.45, 1.28) | - | - |
| Schlag et al., 1992 | CT+S | /S | (22/24) | 0.97 (0.60, 1.57) | 0.97 (0.60, 1.57) | - |
| Ychou et al., 2011 | CT+S | /S | (113/111) | 0.69 (0.50, 0.95) | - | 0.69 (0.50, 0.95) |
| Pouliquen et al., 1996 | CT+S | /S | (52/68) | 0.99 (0.84, 1.17) | 1.00 (0.86, 1.18) | - |
| Ando et al., 1997 | CT+S | /S | (105/100) | 0.87 (0.65, 1.18) | 1.00 (0.86, 1.18) | - |
| Ando et al., 2003 | CT+S | /S | (120/122) | 0.77 (0.56, 1.07) | 0.75 (0.56, 0.99) | 0.73 (0.54, 0.99) |
| Lee et al., 2005 | CT+S | /S | (40/52) | 0.85 (0.66, 1.08) | 0.86 (0.68, 1.09) | 0.68 (0.55, 0.83) |
| Heroor et al., 2003 | CT+S | /S | (94/117) | 1.24 (0.94, 1.65) | 1.31 (1.03, 1.67) | - |
| Shiozaki et al., 2004 | CT+S | /S | (98/52) | - | 0.48 (0.35, 0.66) | - |
| Zhang et al., 2008 | CT+S | /S | (66/160) | 1.36 (0.93, 1.98) | - | 1.80 (1.26, 2.59) |
| Walsh et al., 1996 | CRT+S | /S | (55/55) | - | 0.53 (0.33, 0.84) | - |
| Urba et al., 2001 | CRT+S | /S | (47/50) | 0.75 (0.46, 1.22) | - | - |
| Stahl et al., 2009 | CRT+S | /CT+S | (60/59) | - | 0.67 (0.41, 1.09) | - |
| Burmeister et al., 2011 | CRT+S | /CT+S | (39/36) | 0.59 (0.46, 0.77) | 0.63 (0.49, 0.81) | 0.74 (0.53, 1.02)*** |
| Tepper et al., 2008 | CRT+S | /S | (30/26) | 0.55 (0.40, 0.76) | 0.52 (0.39, 0.70) | 0.35 (0.27, 0.46)*** |
| van Hagen et al., 2012 | CRT+S | /S | (178/188) | - | 0.73 (0.53, 1.00) | - |
| Burmeister et al., 2005 | CRT+S | /S | (128/128) | 0.87 (0.72, 1.05) | 0.88 (0.73, 1.06) | 0.82 (0.71, 0.95)*** |
| Lv et al., 2010 ** | CRT+S | /S | (80/80) | 0.85 (0.61, 1.18) | 0.83 (0.63, 1.08) | 0.66 (0.55, 0.78)*** |
| Lv et al., 2010 ** | CRT+S | /S | (78/80) | 0.73 (0.55, 0.98) | 0.76 (0.60, 0.97) | 0.70 (0.59, 0.83)*** |
| Apinop et al., 1994 | CRT+S | /S | (35/34) | 0.80 (0.48, 1.34) | 0.80 (0.48, 1.34) | - |
| Le Prise et al., 1994 | CRT+S | /S | (41/45) | 0.85 (0.50, 1.46) | 0.85 (0.50, 1.46) | 0.75 (0.64, 0.90) |
| Walsh et al., 1995 | CRT+S | /S | (58/55) | 0.58 (0.38, 0.88) | 0.58 (0.38, 0.88) | - |
| Mariette et al., 2014 | CRT+S | /S | (98/97) | 0.99 (0.69, 1.40) | - | - |
| Kobayashi et al., 2000 | CT+S | /S | (91/80) | 1.10 (0.67, 1.81) | 1.10 (0.67, 1.81) | - |
| Launois et al., 1981 | RT+S | /S | (67/57) | 1.17 (1.04, 1.32) | 1.17 (1.04, 1.32) | - |
| Gignoux et al., 1987 | RT+S | /S | (115/114) | 1.07 (0.89, 1.29) | 1.04 (0.87, 1.24) | - |
| Arnott et al., 1992 | RT+S | /S | (90/86) | - | 1.02 (0.87, 1.19) | - |
| Lee et al., 2004 | CRT+S | /S | (51/50) | 1.19 (0.92, 1.56) | - | 0.98 (0.55, 1.72) |
Abbreviation: OS-Overall survival, DFS-Disease-free survival, HR-Hazard ratio, CI-Confidence interval, CRT-chemoradiotherapy, S-surgery, CT-chemotherapy, RT-radiotherapy
Note: *: These three studies are from the same paper. **: These two studies are from the same paper. The first one is the preoperative group and the latter one is the postoperative group. ***: Progression-Free Survival
As shown in Table 3, the comparison between surgery alone and the combination of CT+S demonstrated that CT+S had better performance compared to surgery alone with respect to 3-year OS (HR = 0.88, 95% CI = 0.82-0.93), 5-year OS (HR = 0.81, 95% CI = 0.76-0.86) and DFS (HR = 0.76, 95% CI = 0.69-0.83). Similarly, surgery tended to present a more promising result when CRT was also involved in the treatment, with an significant promotion of 3-year OS (HR = 0.77, 95% CI = 0.69-0.85), 5-year OS (HR = 0.70, 95% CI = 0.63-0.78) and DFS (HR = 0.59, 95% CI = 0.53-0.65). On the contrary, RT+S presented a poor treatment effect compared to surgery with regards to 3-year OS (HR = 1.05, 95% CI = 0.95-1.15) and 5-year OS (HR = 1.09, 95% CI = 0.99-1.18). These statistics indicated that RT+S was unable to noticeably enhance the prognosis features of surgical treatment of EC. CT+S had a poorer performance rate than CRT+S with respect to the survival rates of 3-year OS (HR = 0.60, 95% CI = 0.44-0.75), 5-year OS (HR = 0.64, 95% CI = 0.49-0.78) and DFS (HR = 0.74, 95% CI = 0.50-0.99).
Table 3. Direct pairwise comparison results of esophageal cancer treatments.
| Comparison | 3-year OS | 5-year OS | DFS | Recurrence | Metastasis |
|---|---|---|---|---|---|
| CT+S vs S | 0.88 (0.82, 0.93) | 0.81 (0.76, 0.86) | 0.76 (0.69,0.83) | 0.85 (0.73, 1.00) | 0.91 (0.72, 1.15) |
| CRT+S vs S | 0.77 (0.69, 0.85) | 0.70 (0.63, 0.78) | 0.59 (0.53, 0.65) | 0.70 (0.45, 1.08) | 0.81 (0.58, 1.12) |
| RT+S vs S | 1.05 (0.95, 1.15) | 1.09 (0.99, 1.18) | - | - | - |
| CRT+S vs CT+S | 0.60 (0.44, 0.75) | 0.64 (0.49, 0.78) | 0.74 (0.50, 0.99) | 0.73 (0.44, 1.23) | - |
Abbreviation: OS-Overall survival, DFS-Disease-free survival.
Note: The data of 3-year OS, 5-year OS and DFS is HR (hazard ratio) and 95% confidence interval. The results of reccurence and matastasis are OR (odds ratio) and 95% confidence interval.
In conclusion, CT+S and CRT+S had better performance than surgery alone with respect to prognostic indicators, including 3-year OS, 5-year OS and DFS, while CRT+S surpassed the efficacy of CT+S. However, insufficient information provided by a pair-wise meta-analysis and indirect evidence could not be created.
Network meta-analysis
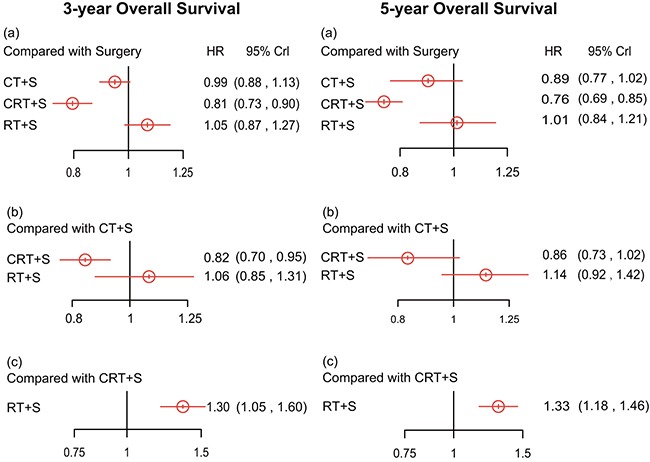
As for 3-year OS shown in Table 4, CRT+S showed the highest efficacy among the four (CRT+S vs. S: HR=0.81, 95% credential interval (CrI) =0.73-0.90; CRT+S vs. CT+S: HR=0.82, 95% CrI =0.70-0.95; CRT+S vs. RT+S: HR=0.77, 95% CrI =0.62-0.95) and CT+S was inferior to RT+S (HR=0.95, 95% CrI =0.76-1.18) while the two comparisons concerning surgery alone showed no significant statistical difference. Compared to the pair-wise meta-analysis, the network meta-analysis provided us with more comprehensive results such as the comparison between CRT+S and RT+S. Overall, CRT+S acted as the most effective intervention in the treatment of EC with respect to 3-year OS.
Table 4. Network meta-analysis results of esophageal cancer treatments.
| (a) 3-year Overall Survival | |||
| S | 0.94 (0.89, 1.00) | 1.08 (0.99, 1.18) | 0.80 (0.74, 0.87) |
| 1.06 (1.00, 1.13) | CT+S | 1.14 (1.02, 1.28) | 0.85 (0.77, 0.94) |
| 0.93 (0.84, 1.02) | 0.87 (0.78, 0.98) | RT+S | 0.74 (0.66, 0.84) |
| 1.25 (1.15, 1.35) | 1.18 (1.07, 1.30) | 1.35 (1.19, 1.52) | CRT+S |
| (b) 5-year Overall Survival | |||
| S | 0.90 (0.85, 0.95) | 1.10 (1.01, 1.19) | 0.75 (0.70, 0.81) |
| 1.11 (1.05, 1.18) | CT+S | 1.22 (1.10, 1.35) | 0.84 (0.77, 0.92) |
| 0.91 (0.84, 0.99) | 0.82 (0.74, 0.91) | RT+S | 0.69 (0.62, 0.77) |
| 1.33 (1.23, 1.43) | 1.19 (1.09, 1.30) | 1.45 (1.30, 1.62) | CRT+S |
| (c) Disease-free Survival | |||
| S | 0.81 (0.74, 0.88) | - | 0.70 (0.65, 0.76) |
| 1.24 (1.13, 1.36) | CT+S | - | 0.87 (0.78, 0.97) |
| - | - | RT+S | - |
| 1.43 (1.32, 1.54) | 1.15 (1.03, 1.29) | - | CRT+S |
| (d) Recurrence | |||
| S | 0.70 (0.29, 1.64) | - | 0.34 (0.11, 0.92) |
| 1.43 (0.61, 3.46) | CT+S | - | 0.49 (0.14, 1.58) |
| - | - | RT+S | - |
| 2.95 (1.08, 8.79) | 2.05 (0.63, 7.19) | - | CRT+S |
| (e) Metastasis | |||
| S | 0.82 (0.55, 1.15) | - | 0.72 (0.44, 1.12) |
| 1.22 (0.87, 1.82) | CT+S | - | 0.89 (0.48, 1.62) |
| - | - | RT+S | - |
| 1.38 (0.89, 2.25) | 1.12 (0.62, 2.08) | - | CRT+S |
Abbreviation: CRT-chemoradiotherapy, S-surgery, CT-chemotherapy, RT-radiotherapy.
Note: In the overall survival of 3 years and 5 years and the disease-free survival, the data are presented in HR (hazard ratio) and 95% CrI. In the results of reccurence and matastasis, the data are presented in OR (odds ratio) and 95% CrI.
Likewise, the results from the network meta-analysis with respect to 5-year OS, as displayed in Figure 3, show that all acquired data express a statistical difference. More specifically, CRT+S showed more promising results than all the other three (CRT+S vs. S: HR=0.76, 95% CrI =0.69-0.85; CRT+S vs. CT+S: HR=0.86, 95% CrI =0.73-1.02; CRT+S vs. RT+S: HR=0.75, 95% CrI =0.61-0.93) surgery. The 5-year OS data was almost in accord with 3-year OS, while the comparisons between surgery versus CT+S and surgery versus RT+S showed statistical veracity.
Figure 3. Hazard ratios (95% credential intervals) of overall survival in 3 years and 5 years for network comparison of EC treatments.
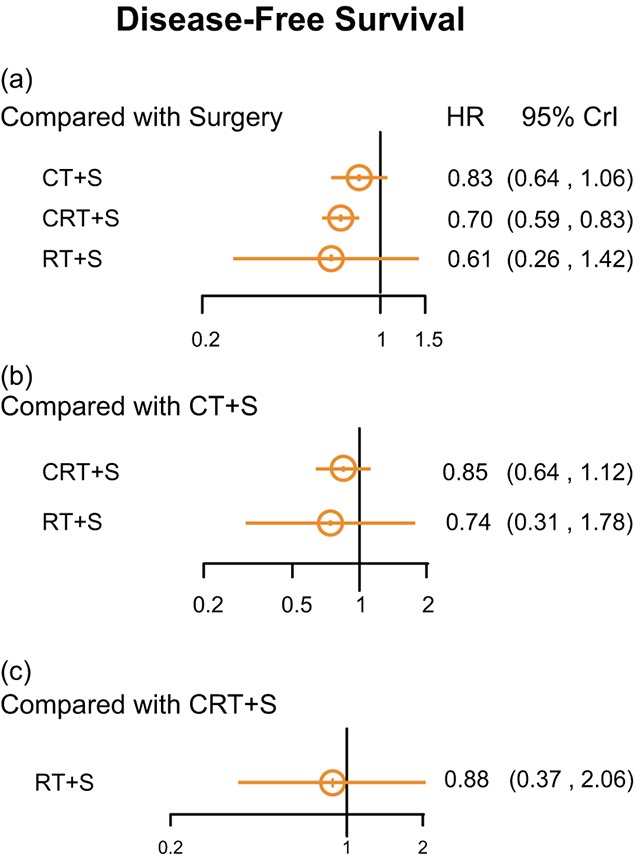
Concerning DFS, CRT+S showed an increased efficacy over surgery (HR =0.70, 95% CrI =0. 595-0.83 Figure 4).
Figure 4. Hazard ratios (95% credential intervals) of disease-free survival for network comparison of EC treatments.
The only confident result concerning recurrence lay in that CRT+S presented a lower rate of recidivism than common surgery (OR=0.33, 95% CrI=0.11-0.92). Meanwhile, CRT+S demonstrated a superior potential than CT+S (OR=0.46, 95% CrI=0.15-1.50). Such a relation was also seen between CT+S and surgery as shown in the node-splitting results in Figure 5. The heat plot showed the robustness of our results Figure 6.
Figure 5. Node Splitting results according to type of treatments for recurrence.
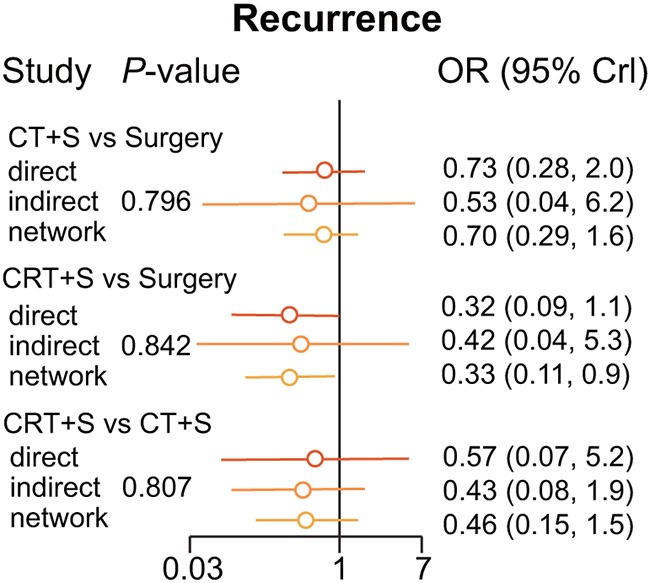
Figure 6. Heat plot for EC treatments.
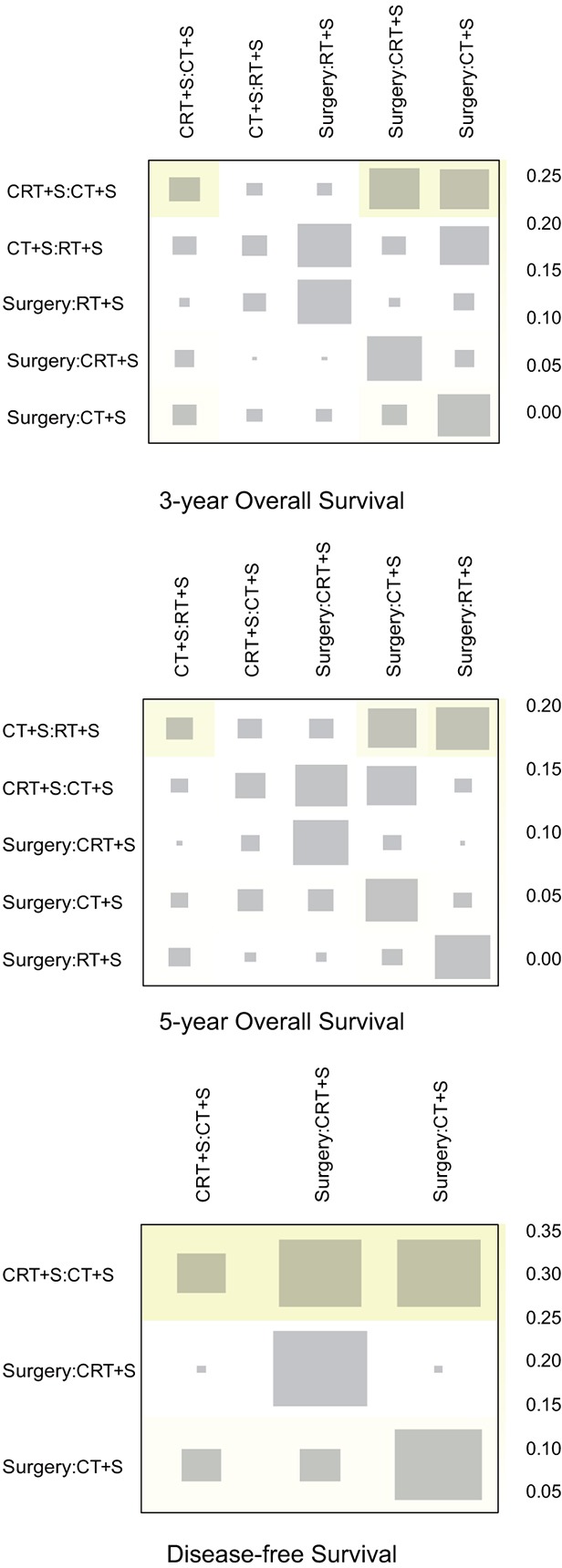
The area of the gray squares displays the contribution of the direct estimate in design d (shown in the column) to the network estimate in design d (shown in the row). The colors are associated with the change in inconsistency between direct and indirect evidence (shown in the row) after detaching the effect (shown in the column). Blue colors indicate an increase and warm colors indicate a decrease (the stronger the intensity of the color, the stronger the change).
Finally, Supplementary Table 2 provided the SUCRA values for each strategy and its clinical outcomes. In general, CRT+S ranked best among the tested therapies (surface under the cumulative ranking curve (SUCRA): 3-year OS=0.99; 5-year OS=0.99; DFS=0.76; recurrence=0.93; metastasis=0.80) while surgery alone proved to be the least efficacious treatment concerning recurrence rates (SUCRA=0.11) and metastasis (SUCRA=0.09).
DISCUSSION
Currently, surgical resection is the preferable treatment for patients without distant metastases (cT1-3 N0-1 M0) [63]. However, the prognoses of patients treated with surgery alone remained poor and could be improved using s adjuvant therapies [21, 63, 64]. Concurrent CRT was found to improve overall survival significantly as well as reduce persistence and recurrence or patients with resectable esophago-gastric adenocarcinoma [64, 65]. Based on previous studies, the advantage of CRT in the treatment of EC has been widely verified, although few studies have been conducted to detect the similarities and differences between other adjuvant therapies and CRT. And according to the current network meta-analysis Pasquali et al. [66], CRT+S was the best option, which is consistent to former studies and our results. However, there were some limitations in this study, firstly, no indirect comparison results were provided; besides, the prognosis outcomes were not taken follow-up periods into consideration; additionally, some studies contained duplicate trials such as Natsugoe et al. [60] and Tachibana et al. [67].
In our analysis, OS was considered as the primary endpoint. The results showed that CRT+S contributed to the better long-term survival compared with surgery, CT+S and RT+S while RT+S seemed to have no positive effect on survival time, and even resulted in a worsened prognosis. Treatment of CT+S was comparatively superior to surgery and RT+S but inferior to CRT+S with regards to 3-year OS and 5-year OS. The only difference between 3-year OS and 5-year OS was the statistical significance observed between CT+S and RT+S compared to surgery in 5-year OS. The harmful effects of RT+S might be explained by its toxicity to patients. The exposure to RT might lead to acute toxicity which overwhelmed the treatment effects and made the prognosis worse for EC patients [68]. Thus, when it comes to the treatment options, RT alone without any other adjuvant therapies may be considered mainly a palliative tool rather than a curative option for EC patients [69].
DFS was another important endpoint in clinical trials. We detected that CT+S and CRT+S were both beneficial to DFS compared with S, but treatment with CRT+S was better in promoting DFS. Besides survival rate, adverse effects included recurrence and metastasis also compared comprehensively. The results indicated that compared with surgery, CT+S and CRT+S were apt to lower the recurrence and metastasis rates. Furthermore, CRT+S performed better in the prevention of adverse effects ranking first of the treatments analyzed, while CT+S ranked second and surgery third. The results mentioned above are consistent with previous studies [64, 70], which have indicated that CRT+S provides local control of tumors and prevents metastasis. These could be considered reasons for the increased survival rate of patients who have undergone CRT an additional adjuvant treatment.
The results suggest that CRT+S should be the first option taken into consideration, while RT+S should be an option reserved for those who are medically fit for an aggressive modality. Inversely, for patients who are not fit to be exposed to such an aggressive modality, RT may a good choice as a palliative tool. If patients are in the early stages of cancer, therapies with less serious adverse effects are a more pragmatic choice in treatment aimed the eradication of the disease. CRT+S should still be considered a preferred choice in these regards.
However, our study still has several limitations that might affect the interpretation of the results. First of all, the trials on RT are quite rare in our study despite the fact that the study involves a large number of RCTs. And squamous cell cancer is known to be completely different in terms of risk factors, disease biology, tumour location, surgical management from adenocarcinoma of the oesophagus, however we lumped together to provided sufficient data. Additionally, the study includes papers published from 1981 to 2016, spanning 35 years. The time span is long enough that errors are inevitable in our study, considering the development of medical technology. Besides, baselines such as patient clinical stage were not taken into consideration, which may influence the final conclusion. Finally, our study does not take into account the type and dose of chemotherapeutic medications. However, different types and doses of chemotherapeutic drugs do have different effects on patients suffering EC that may result in errors in our analysis.
In summary, we assessed the efficacy and adverse effects of surgery with different adjuvant therapies for EC and drew the conclusion that CTR+S is the most effective option. Patients treated with CRT+S have the best prognosis including long-term survival and low risk of recurrence compared to the other treatments studied here. Furthermore, CT+S is able to reduce adverse effects with an efficacy rate second only to CTR+S. The results of our study may act as guidelines for medical decision and treatment options in the future.
MATERIALS AND METHODS
Search strategy
Databases were systematically searched for relevant literature, including PubMed and EMBASE. Key words were used as follows: “esophageal neoplasms”, “surgery”, “chemotherapy”, “radiotherapy”, “chemoradiotherapy”, and “randomized clinical trials”. The results included 3,761 records, 559 were identified as duplicates and hence removed after assessment. 3,153 studies were excluded after identified as irrelevant based on titles and abstracts. Among the 49 studies remaining, full-text articles were reviewed and included if they met the inclusion criteria listed below. This process resulted in 42 studies available and qualified for analysis in this research. They are presented in Table 1.
Inclusion criteria and data extraction
Articles were included if they: (1) were RCTs with a total of more than 30 samples, had follow-up rates above 90% and follow-up periods of not less than 3 years; (2) contained sufficient information about histology and interventions; (3) provided data on disease-free survival (DFS), 3-year overall survival (OS) and 5-year OS; (4) had at least one pair-wise comparison among surgery alone, or surgery combined with RT, CT and CRT.
The data in Table 1 was extracted from the eligible studies, including the country in which the study was performed, sample size of the intervention and control groups, as well as histology and clinical outcomes. After two investigators reviewed the manuscripts of all the studies independently, the data were extracted into a database. A joint review of the manuscript was performed to solve disagreements until a consensus was reached.
Statistical analysis
Initially a conventional pair-wise meta-analysis was carried out directly. For each study, HR and then merged the data obtained to discern the overall impact level. The impact level was considered significant if the corresponding 95% CI exceeded 1.
We also performed a network meta-analysis for each endpoint within a Bayesian framework using R 3.2.3 software. The treatment effects were compared through direct and indirect evidence by using HRs or ORs with 95% CrI. Moreover, clinical outcomes such as 3-year OS and 5-year OS were evaluated to estimate the efficacy of the respective treatment. The SUCRA was then used to create a ranking scale of the treatment interventions. For each outcome, the efficacy of a certain intervention was more desirable if a larger SUCRA value was obtained.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Abbreviations
- EC
esophageal cancer
- RT
radiotherapy
- CT
chemotherapy
- CRT
chemoradiotherapy
- SUCRA
surface under the cumulative ranking curve
- OS
overall survival
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5:517–526. doi: 10.1038/ncpgasthep1223. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Muir CS, Cancer Incidence in Five Continents Comparability and quality of data. IARC Sci Publ. 1992:45–173. [PubMed] [Google Scholar]
- 4.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Nasrollahzadeh D, Kamangar F, Aghcheli K, Sotoudeh M, Islami F, Abnet CC, Shakeri R, Pourshams A, Marjani HA, Nouraie M, Khatibian M, Semnani S, Ye W, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98:1857–1863. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, Abedi-Ardekani B, Merat S, Vahedi H, Semnani S, Abnet CC, Brennan P, Moller H, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo N, Ohira M, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Shibutani M, Yamazoe S, Kimura K, Nagahara H, Amano R, Ohtani H, et al. The impact of combined thoracoscopic and laparoscopic surgery on pulmonary complications after radical esophagectomy in patients with resectable esophageal cancer. Anticancer Res. 2014;34:2399–2404. [PubMed] [Google Scholar]
- 10.Morita M, Nakanoko T, Fujinaka Y, Kubo N, Yamashita N, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Shirabe K, Maehara Y. In-hospital mortality after a surgical resection for esophageal cancer: analyses of the associated factors and historical changes. Annals of surgical oncology. 2011;18:1757–1765. doi: 10.1245/s10434-010-1502-5. [DOI] [PubMed] [Google Scholar]
- 11.Khushalani N. Cancer of the esophagus and stomach; Mayo Clin Proc; 2008; pp. 712–722. [PubMed] [Google Scholar]
- 12.Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapy. PLoS One. 2014;9:e97225. doi: 10.1371/journal.pone.0097225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan YJ, Song X, Li JL, Li XM, Liu B, Wang R, Fan ZM, Wang LD. Esophageal and gastric cardia cancers on 4238 Chinese patients residing in municipal and rural regions: a histopathological comparison during 24-year period. World journal of surgery. 2008;32:1980–1988. doi: 10.1007/s00268-008-9674-x. [DOI] [PubMed] [Google Scholar]
- 14.Pouliquen X, Levard H, Hay JM, McGee K, Fingerhut A, Langlois-Zantin O. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French Associations for Surgical Research. Annals of surgery. 1996;223:127–133. doi: 10.1097/00000658-199602000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. Journal of clinical oncology. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 16.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 17.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Annals of surgical oncology. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Swisher SG. Preoperative chemotherapy for localized squamous cell carcinoma of the esophagus? We should go back to the drawing board! Annals of surgical oncology. 2012;19:3–4. doi: 10.1245/s10434-011-2101-9. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa Y, Ando N, Nakamura K, Shibata T, Fukuda H. The role of adjuvant chemotherapy for localized squamous cell esophageal cancer: current Japanese standard and the unending role of the drawing board. Annals of surgical oncology. 2012;19:1425–1427. doi: 10.1245/s10434-012-2250-5. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Li Y, Liu X, Sun H, Wang Z, Zhang R. Reevaluation of neoadjuvant chemotherapy for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials over the past 20 years. Medicine. 2015;94:e1102. doi: 10.1097/MD.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu T, Bu ZD, Li ZY, Zhang LH, Wu XJ, Wu AW, Shan F, Ji X, Dong QS, Ji JF. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC cancer. 2015;15:322. doi: 10.1186/s12885-015-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu XH, Peng XH, Yu P, Xu XY, Cai EH, Guo P, Li K. Neoadjuvant chemotherapy for resectable esophageal carcinoma: a meta-analysis of randomized clinical trials. Asian Pacific journal of cancer prevention. 2012;13:103–110. doi: 10.7314/apjcp.2012.13.1.103. [DOI] [PubMed] [Google Scholar]
- 23.Launois B, Dalarue D, Campion JP. Preoperative radiotherapy for carcinoma of the esophagus. Surgery Gynecology and Obstetrics. 1981;153:690–692. [PubMed] [Google Scholar]
- 24.Gignoux M, Roussel A, Paillot B, Gillet M, Schlag P, Favre JP, Dalesio O, Buyse M, Duez N. The value of preoperative radiotherapy in esophageal cancer: Results of a study of the E.O.R.T.C. World Journal of Surgery. 1987;11:426–432. doi: 10.1007/BF01655805. [DOI] [PubMed] [Google Scholar]
- 25.Arnott SJ, Duncan W, Kerr GR, Walbaum PR, Cameron E, Jack WJL, Mackillop WJ. Low dose preoperative radiotherapy for carcinoma of the oesophagus: Results of a randomized clinical trial. Radiotherapy and Oncology. 1992;24:108–113. doi: 10.1016/0167-8140(92)90287-5. [DOI] [PubMed] [Google Scholar]
- 26.Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, Mantyla M, Modig H, Munck-Wikland E, Rosengren B, Tausjø J, Elgen K. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World journal of surgery. 1992;16:1104–1109. doi: 10.1007/BF02067069. discussion 1110. [DOI] [PubMed] [Google Scholar]
- 27.Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Archives of surgery (Chicago, Ill 1960) 1992;127:1446–1450. doi: 10.1001/archsurg.1992.01420120080015. [DOI] [PubMed] [Google Scholar]
- 28.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepato-gastroenterology. 1994;41:391–393. [PubMed] [Google Scholar]
- 29.Le Prise E, Etienne PL, Meunier B, Maddern G, Ben Hassel M, Gedouin D, Boutin D, Campion JP, Launois B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. doi: 10.1002/1097-0142(19940401)73:7<1779::aid-cncr2820730702>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Maipang T, Vasinanukorn P, Petpichetchian C, Chamroonkul S, Geater A, Chansawwaang S, Kuapanich R, Panjapiyakul C, Watanaarepornchai S, Punperk S. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. Journal of surgical oncology. 1994;56:191–197. doi: 10.1002/jso.2930560314. [DOI] [PubMed] [Google Scholar]
- 31.Walsh T. Predicting, defining and improving outcomes for oesophageal carcinoma. Dublin: Trinity College, University of Dublin; 1995. The role of multimodality therapy in improving survival: a prospective randomised trial; pp. 124–150. [MD thesis] [Google Scholar]
- 32.Pouliquen X, Levard H, Hay JM, McGee K, Fingerhut A, Langlois-Zantin O. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French Associations for Surgical Research. Annals of surgery. 1996;223:127. doi: 10.1097/00000658-199602000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. New England Journal of Medicine. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 34.Ando N, Iizuka T, Kakegawa T, Isono K, Watanabe H, Ide H, Tanaka O, Shinoda M, Takiyama W, Arimori M. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. The Journal of thoracic and cardiovascular surgery. 1997;114:205–209. doi: 10.1016/S0022-5223(97)70146-6. [DOI] [PubMed] [Google Scholar]
- 35.Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. The Journal of thoracic and cardiovascular surgery. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi T, Kimura T. [Long-term outcome of preoperative chemotherapy with 5′-deoxy-5-fluorouridine (5′-DFUR) for gastric cancer] [Article in Japanese] Gan to kagaku ryoho Cancer & chemotherapy. 2000;27:1521–1526. [PubMed] [Google Scholar]
- 37.Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, Zaninotto G, Bonavina L, Peracchia A. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 38.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. Journal of Clinical Oncology. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 39.Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. Journal of clinical oncology. 2003;21:4592–4596. doi: 10.1200/JCO.2003.12.095. [DOI] [PubMed] [Google Scholar]
- 40.Heroor A, Fujita H, Sueyoshi S, Tanaka T, Toh U, Mine T, Sasahara H, Sudo T, Matono S, Yamana H. Adjuvant Chemotherapy after Radical Resection of Squamous Cell Carcinoma in the Thoracic Esophagus: Who Benefits? Digestive surgery. 2003;20:236–237. doi: 10.1159/000070390. [DOI] [PubMed] [Google Scholar]
- 41.Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, Song HY, Cho KJ, Kim WK, Lee JS, Kim SH, Min YI. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Annals of oncology. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 42.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J, North J, Walpole ET, Denham JW, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncology. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Lee KE, Im YH, Won KK, Park K, Kim K, Young MS. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Annals of Thoracic Surgery. 2005;80:1170–1175. doi: 10.1016/j.athoracsur.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 44.Shiozaki A, Yamagishi H, Itoi H, Fujiwara H, Kikuchi S, Okamoto K, Ichikawa D, Fuji N, Ochiai T, Sonoyama T. Long-term administration of low-dose cisplatin plus 5-fluorouracil prolongs the postoperative survival of patients with esophageal cancer. Oncology Reports. 2005;13:667–672. [PubMed] [Google Scholar]
- 45.Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, Ajani JA, Kocha W, Minsky BD, Roth JA. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. Journal of Clinical Oncology. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 46.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. Journal of Clinical Oncology. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Zhang YW, Chen ZW, Zhou XY, Lu S, Luo QQ, Hu H, Miao LS, Ma LF, Xiang JQ. Adjuvant chemotherapy of cisplatin, 5-fluorouracil and leucovorin for complete resectable esophageal cancer: a case-matched cohort study in east China. Diseases of the Esophagus. 2008;21:207–213. doi: 10.1111/j.1442-2050.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 48.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. Journal of clinical oncology. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 49.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. Journal of Clinical Oncology. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 50.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World journal of gastroenterology. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, Siersema PD, Dinjens WN, van Lanschot JJ, Tilanus HW, van der Gaast A. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, Barbour AP, Gotley DC, Smithers BM. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. European journal of cancer (Oxford, England : 1990) 2011(47):354–360. doi: 10.1016/j.ejca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Geneve J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Journal of clinical oncology. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 54.Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, Bosset JF, Mabrut JY, Triboulet JP, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. Journal of clinical oncology. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 55.A comparison of chemotherapy and radiotherapy as adjuvant treatment to surgery for esophageal carcinoma. Japanese Esophageal Oncology Group. Chest. 1993;104:203–207. doi: 10.1378/chest.104.1.203. [DOI] [PubMed] [Google Scholar]
- 56.Zieren HU, Muller JM, Jacobi CA, Pichlmaier H, Muller RP, Staar S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World journal of surgery. 1995;19:444–449. doi: 10.1007/BF00299187. [DOI] [PubMed] [Google Scholar]
- 57.Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M, Sahmoud T. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. The New England journal of medicine. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 58.Baba M, Natsugoe S, Shimada M, Nakano S, Kusano C, Fukumoto T, Aikou T, Akazawa K. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Diseases of the esophagus. 2000;13:136–141. doi: 10.1046/j.1442-2050.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 59.Xiao ZF, Yang ZY, Liang J, Miao YJ, Wang M, Yin WB, Gu XZ, Zhang DC, Zhang RG, Wang LJ. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. The Annals of thoracic surgery. 2003;75:331–336. doi: 10.1016/s0003-4975(02)04401-6. [DOI] [PubMed] [Google Scholar]
- 60.Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T, Aikou T. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Diseases of the esophagus. 2006;19:468–472. doi: 10.1111/j.1442-2050.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, van Laarhoven HW, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. The Lancet Oncology. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 62.Klevebro F, von Döbeln GA, Wang N, Johnsen G, Jacobsen AB, Friesland S, Hatlevoll I, Glenjen NI, Lind P, Tsai JA, Lundell L, Nilsson M. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Annals of Oncology. 2016;27:660–667. doi: 10.1093/annonc/mdw010. [DOI] [PubMed] [Google Scholar]
- 63.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World journal of gastroenterology. 2009;15:4962–4968. doi: 10.3748/wjg.15.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu LL, Yuan L, Wang H, Ye L, Yao GY, Liu C, Sun NN, Li XJ, Zhai SC, Niu LJ, Zhang JB, Ji HL, Li XM. A Meta-Analysis of Concurrent Chemoradiotherapy for Advanced Esophageal Cancer. PLoS One. 2015;10:e0128616. doi: 10.1371/journal.pone.0128616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Jensen K. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. The Cochrane database of systematic reviews. 2013;5:Cd008107. doi: 10.1002/14651858.CD008107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, Geh JI, Griffiths EA. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Annals of surgery. 2016 doi: 10.1097/SLA.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 67.Tachibana M, Yoshimura H, Kinugasa S, Shibakita M, Dhar DK, Ueda S, Fujii T, Nagasue N. Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. European journal of surgical oncology. 2003;29:580–587. doi: 10.1016/s0748-7983(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 68.Oh D, Noh JM, Nam H, Lee H, Kim TG, Ahn YC. High-dose radiation therapy alone by moderate hypofractionation for patients with thoracic esophageal squamous cell carcinoma. Medicine. 2016;95:e4591. doi: 10.1097/MD.0000000000004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamson D, Blazeby J, Nelson A, Hurt C, Nixon L, Fitzgibbon J, Crosby T, Staffurth J, Evans M, Kelly NH, Cohen D, Griffiths G, Byrne A. Palliative radiotherapy in addition to self-expanding metal stent for improving dysphagia and survival in advanced oesophageal cancer (ROCS: Radiotherapy after Oesophageal Cancer Stenting): study protocol for a randomized controlled trial. Trials. 2014;15:402. doi: 10.1186/1745-6215-15-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J, Wang C, Xiang M, Liu F, Liu Y, Zhao K. Meta-analysis of postoperative efficacy in patients receiving chemoradiotherapy followed by surgery for resectable esophageal carcinoma. Diagnostic pathology. 2014;9:151. doi: 10.1186/1746-1596-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


