Abstract
Purpose of review
Clearing of atherogenic lipoprotein particles by the liver requires hepatic LDLR and LRP1. This review highlights recent studies that have expanded out understanding of the molecular regulation and metabolic functions of LDLR and LRP1 in the liver.
Recent findings
Various proteins orchestrate the intracellular trafficking of LDLR and LRP1. After internalization, the receptors are redirected via recycling endosomes to the cell surface. Several new endocytic proteins that facilitate the endosomal trafficking of LDLR and consequently the clearance of circulating LDL cholesterol have recently been reported. Mutations in some of these proteins cause hypercholesterolemia in human. In addition, LRP1 controls cellular cholesterol efflux by modulating the expression of ABCA1, and ABCG1, and hepatic LRP1 protects against diet-induced hepatic insulin resistance and steatosis through the regulation of insulin receptor trafficking.
Summary
LDLR and LRP1 have prominent roles in cellular and organismal cholesterol homeostasis. Their functioning, including their trafficking in the cell, is controlled by numerous proteins. Comprehensive studies into the molecular regulation of LDLR and LRP1 trafficking have advanced our fundamental understanding of cholesterol homeostasis, and these insights may lead to novel therapeutic strategies for atherosclerosis, hyperlipidemia and insulin resistance in the future.
Keywords: Trafficking, CCC complex, DAB2, hypercholesterolemia, glucose metabolism
Introduction
The liver plays a crucial role in cholesterol homeostasis through controlling lipid uptake and synthesis. Clearance of plasma lipids via the liver is mediated by the low-density lipoprotein receptor (LDLR) and LDLR-related protein 1 (LRP1) [1–3]. Both receptors are member of the evolutionarily conserved LDLR family [4]. The core of this family is comprised of seven members (LDLR, LRP1, very-low-density lipoprotein receptor [VLDR], LRP8/Apoer2, LRP4/MEGF7, LRP1B, and LRP2/Megalin). These receptors have one transmembrane domain, a large extracellular domain with one or more ligand binding domains, and a cytoplasmic tail, which contains at least one NPxY motif [2]. Numerous adaptor proteins are binding to this particular motif to mediate endocytosis and signal transduction through the receptors. Hepatic LDLR and LRP1 contribute to the clearance of circulating Apolipoprotein E (ApoE) containing particles, such as the triglyceride carrying chylomicrons and VLDL. Intestinal-derived chylomicrons remnants and hepatic VLDL are removed from the circulation by the liver after extensive peripheral metabolism. Part of VLDL is converted to LDL, a cholesterol rich particle that lacks ApoE but contains one apoB100 molecule. In addition to ApoE, also ApoB-containing particles are taken up by hepatocytes through LDLR and LRP1 (Fig. 1, and Fig. 2A).
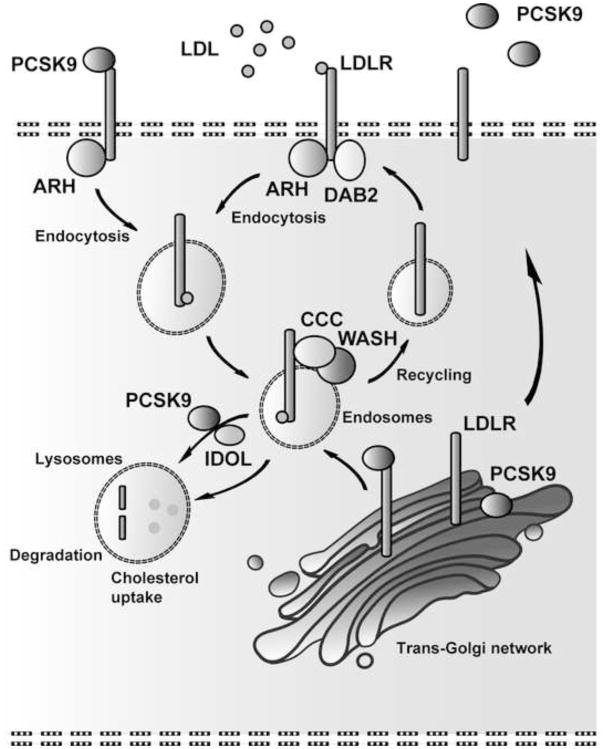
Figure 1. Simplified model of the molecular regulation of cellular LDLR trafficking.
Cholesterol-rich ApoE and ApoB containing lipoprotein particles bind to LDLR and are together with LDLR internalized and accordingly directed to the endosomes. Endocytosis is mediated by ARH in hepatocytes and recent data indicate that DAB2 facilitates LDLR internalization in sinusoid endothelial cells [13**]. At the endosomes, the CCC and WASH complexes recognize LDLR, sort and redirect LDLR back to the cell surface [19**]. Alternatively, LDLR is sent to the lysosomes for proteolysis, which is mediated by PCSK9 and/or IDOL. Esterified cholesterol is hydrolyzed by lysosomal acid lipase in the lysosomes, from where free cholesterol is further distributed to the plasma membrane and the endoplasmic reticulum [55*]. ARH: autosomal recessive hypercholesterolemia; CCC: COMMD1, CCDC22, CCDC93, C16orf62; DAB2: Disabled homolog 2; IDOL: Inducible degrader of the LDLR; PCSK9: Proprotein convertase subtilisin/kexin type-9; WASH: WASHC1, WASHC2A, WASHC3, WASHC4, and WASHC5.
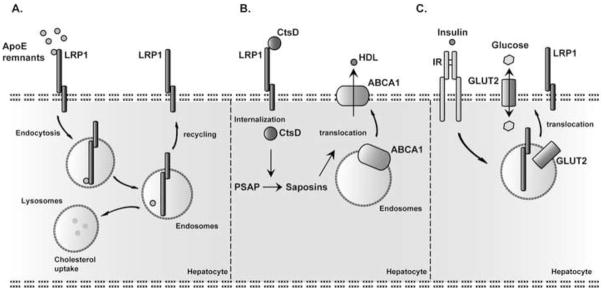
Figure 2. Hepatic LRP1-mediated pathways.
A. Like LDLR (Fig. 1), LRP1 clears cholesterol-rich ApoE containing particles from the circulation. B. LRP1-mediated Cathepsin D (CtsD) uptake is required for processing of Prosaposin (PSAP) into Saposins. Saposins positively controls the translocation of ABCA1 to the transmembrane. ABCA1 is an important hepatic cholesterol efflux transporter, and controls plasma HDL levels. C. Insulin-induced GLUT2 translocation to the plasma membrane depends on LRP1 [52**]. Hepatocyte LRP1 deficiency diminishes surface IR levels and consequently attenuates insulin induced GLUT2 translocation. IR: Insulin receptor.
The role of LDLR in clearing circulating atherogenic lipoprotein particles, such as VLDL and LDL, is well established. A large number of mutations in LDLR have been identified that cause familial hypercholesteromia (FH) [5] and predispose to atherosclerosis and cardiovascular disease. Genetic ablation of the LDL receptor in mice also leads to elevation in plasma LDL, and make these animals susceptible to atherosclerosis upon high cholesterol feeding [6,7]. However, the contribution of hepatic LRP1 in preserving cholesterol homeostasis has been ambiguous. In contrast to LDLR deficient mice, mice lacking LRP1 in the germline are not viable, but die at various stages of post-implantation embryonic development, depending upon strain background [8,9]. An initial study with conditional Lrp1 knockout mice showed that mice deficient for hepatocyte LRP1 are viable but don’t have clear alterations in plasma lipid levels [3]. Interestingly, however, the protein levels of hepatocyte LDLR were markedly increased in liver-specific Lrp1 knockout mice. Hepatic deletion of both Ldlr and Lrp1 resulted in a marked elevation in plasma chylomicron remnants and LDL levels [3]. These data imply that LDLR can partially compensate for the loss of LRP1 in hepatocytes, and demonstrate the importance of both receptors for the clearance of circulating atherogenic lipoprotein particles. Here, we describe recent findings contributing to the understanding of the molecular regulation and function of LDLR and LRP1, with a focus on their function in the liver, and lipid and glucose homeostasis.
Low-density lipoprotein receptor (LDLR)
In the 1970’s, Goldstein and Brown reported for the first time the existence of a specific receptor for LDL [1]. The expression of LDLR at the cell surface is regulated by cellular membrane cholesterol content. Low intracellular cholesterol concentrations result in increased expression of LDLR, which is mediated by the transcription factor sterol regulatory element binding protein 2 (SREBP2) [10]. At the cell surface, LDL binds through ApoB100 to LDLR. LDL and LDLR are endocytosed together (Fig. 1), which is mediated by several adaptor proteins such as autosomal recessive hypercholesterolemia (ARH) protein, and Disabled homolog 2 (DAB2) protein (reviewed in [11*]) (Fig. 1A). Both adaptor proteins bind to the NPxY motif of LDLR. Mutations in ARH have been identified in patients with FH. Like FH patients carrying mutations in ARH, mice deficient for ARH show elevated LDL plasma levels. DAB2 mutations correlated with hypercholesterolemia have not been reported yet, and DAB2 deficient mice are not viable. In mice, DAB2 is likely important for normal development of the placenta as Dab2 knockout mice with only normal expression of Dab2 in extra-embryonic tissues are born alive [12]. These mice have a slight increase in circulating plasma cholesterol. Since DAB2 is not expressed in hepatocytes, its contribution to LDLR-mediated cholesterol clearance is likely very restricted, but a recent study has demonstrated that in the liver DAB2 is mainly expressed in sinusoid endothelial cells [13**]. To further investigate the contribution of DAB2 in cholesterol homeostasis the authors of this study depleted both Dab2 and Arh in mice. These mice were fed a high sucrose diet to stimulate the production/secretion of VLDL by the liver. Plasma cholesterol levels were determined and compared with high-sucrose diet fed Ldlr−/− and Arh−/− mice. Plasma cholesterol levels in DAB2 deficient mice were slightly increased, and only Dab2-Arh double knockout mice had cholesterol levels comparable to that of Ldlr−/− mice. Furthermore, the degree of HMG-CoA reductase (HMGCR) and cholesterol increase in the liver of Dab2-Arh double knockout mice was comparable to that of Ldlr−/− mice. Based on these findings the authors concluded that ARH is mainly required for the endocytosis of LDLR in hepatocytes, whereas DAB2 facilitates the intracellular trafficking of LDLR in liver sinusoid endothelial cells. Since hepatocyte HMGCR levels were only elevated in Dab2-Arh double knockout mice and in Ldlr−/− mice, but not in Arh−/− mice, could suggest that LDLR expression in sinusoid endothelial cells has a significant role in the liver uptake and sensing of cholesterol to preserve homeostatic cholesterol levels. Alternatively, a small degree of DAB2 expression by hepatocytes, below the level of detection by conventional methods, might be able to partially compensate for the loss of ARH to allow residual uptake of LDL cholesterol sufficient to suppress full-blown upregulation of HMGCR expression.
Internalized LDLR is delivered to the endosomes and sorted either back to the plasma membrane, for reuse, or to the lysosomes where LDLR is degraded [14] (Fig. 1). In an ARH-dependent fashion, proprotein convertase subtilisin/kexin type 9 (PCSK9) directs LDLR via the endocytic pathway to the lysosomes and prevents the recycling of LDLR [15,16*] (Fig. 1). Like PCSK9, ubiquitin ligase inducible degrader of the LDLR (IDOL) stimulates the proteolysis of LDLR in a variety of tissues including the brain (reviewed in [17*]) (Fig. 1). Although the transport of IDOL-mediated LDLR degradation is ARH-independent, it requires the endosomal-sorting complex required for transport machinery (ESCRT) to direct LDLR towards the lysosomes (reviewed in [11*,17*]). Intriguingly, however, for reasons that are not understood, IDOL has no significant effect on the degradation of LDLR in murine livers, but might have a potential role in LDLR proteolysis in human and monkey livers [18*].
A recent study has identified a large number of proteins involved in the removal of plasma cholesterol by coordinating the endosomal trafficking of LDLR [19**]. Here, Bartuzi and colleagues linked the coiled-coil domain-containing protein 22 (CCDC22) to plasma LDL cholesterol clearance. Mutations in CCDC22 cause X-linked intellectual disability (XLID) syndrome [20,21]. XLID syndrome is characterized by developmental defects, which includes intellectual disability, cerebellar malformations, cardiac defects and limb abnormalities, but this study reports for the first time that these patients also suffer from hypercholesterolemia. CCDC22 participates in a multiprotein complex, named the CCC complex [19***,22,23*,24]. CCC complex is compromised of 4 proteins, COMMD1, CCDC22, CCDC93 and C16orf62. COMMD1 physically associates with LDLR, through the binding with the NPxY motif of LDLR [19**]. COMMD1 deficiency dramatically decreases the levels of the CCC complex components CCDC22 and CCDC93 in the liver of dogs and mice [19**]. This compromised integrity of the CCC complex coincides with elevated plasma cholesterol levels in these animals, in particularly LDL cholesterol. Studies with primary hepatocytes and mouse embryonic fibroblast (MEFs) cells lacking COMMD1 revealed that the CCC complex is needed for normal trafficking of LDLR. The amount of LDLR localized to endosomes was increased, whereas its level in the plasma membrane was diminished by the loss of COMMD1. Consequently, mislocalization of LDLR in COMMD1 deficient cells impaired LDL uptake. The CCC complex has previously been linked to copper homeostasis by coordinating the trafficking of copper transporting proteins ATP7A and ATP7B [23*,25–29]. CCC complex is physically associated with Wiskott-Aldrich Syndrome Protein and SCAR Homolog (WASH) complex [23*], a multiprotein complex composed of five proteins, WASHC1, WASHC2A, WASHC3, WASHC4, and WASHC5, also known as WASH1, FAM21, CCDC53, KIAA1033 (SWIP), and KIAA0196 (Strumpellin), respectively [30]. WASH facilitates the endosomal trafficking of an array of transmembrane proteins, including ATP7A [23*,30]. The observation that CCC and WASH are physically connected and are both required for the endosomal trafficking of ATP7A suggests that they likely act together. This notion was further supported by the study of Bartuzi and colleagues, in which they showed that LDLR trafficking and its surface expression in MEFs also rely on the WASH complex. A mutation in WASHC5 has been associated with Ritscher-Schinzel/3C syndrome (RSS), and phenotypically RSS patients resemble XLID patients. This study now shows that both XLID and RSS patients have elevated plasma LDL cholesterol levels, which further implies that the CCC and WASH complexes are both involved in endosomal trafficking of LDLR to direct LDLR back to the cell surface for efficient LDL uptake (Fig. 1).
The importance of the endocytic system for LDL clearance is further supported by other recent studies in which the small GTPase Rab5 was down regulated in adult mouse livers using RNA interference technology [31*,32]. Rab5 has been identified as a crucial hub in a large protein network in endosome biogenesis. Rab5 insufficiency results in a reduced number of early and late endosomes and lysosomes. Interestingly, the total amount of LDLR was increased in Rab5 insufficient livers, but despite these elevated LDLR levels plasma LDL cholesterol levels of these mice were markedly increased. Further analyses revealed that LDL uptake in primary hepatocytes cells with low levels of Rab5 was impaired, supporting the notion that LDLR trafficking highly depends on the endocytic system.
As mentioned previously, both PCSK9 and IDOL-mediated LDLR degradation pathways make use of the endocytic system and it would be therefore informative to assess, whether the CCC and WASH complexes also participate in the sorting of LDLR to lysosomes. Preventing proteolysis of LDLR or increasing endocytosis/recycling of LDLR would be beneficial to increase LDL uptake. For example, high expression of the endosome-associated protein Sortin nexin 17 (SNX17) enhances LDL uptake due to increased endocytosis of LDLR [33]. SNX17 also facilitates the recycling of LRP1 and ApoER2 [34,35], but whether SNX17 acts in conjunction with the CCC and WASH complexes, and whether these two complexes are also required for endosomal sorting of LRP1 or other members of the LDLR family has yet to be determined.
LDLR-related protein 1 (LRP1)
LRP1 is the only other core member of the LDL receptor gene family that is expressed at functionally significant levels in hepatocytes and thus can mediate the bulk transport of ApoE-containing lipoproteins that have entered the Space of Disse, following extensive metabolism by lipolysis or lipid transfer in the periphery and in the circulation (Fig. 2A). Early genetic insights, gathered from the work by Kita, Brown and Goldstein (1982) [36] implied the existence of a hepatic ApoE-binding chylomicron remnant receptor distinct from the LDL receptor. The discovery of LRP1, its structural similarity with the LDL receptor, its expression on the hepatocyte cell surface [2], and its ability to bind ApoE [37,38] and deliver internalized remnant lipoproteins to the lysosome [38] were highly suggestive that LRP1 was the elusive chylomicron remnant receptor. However, the apparent absence of human mutations in LRP1 causing remnant clearance defects and early embryonic lethality in whole animal mouse knockout models [8,9,39] prevented conclusive genetic proof of this hypothesis. The introduction of tissue-specific gene disruption technologies in mice ultimately showed that LDLR and LRP1 jointly mediate the hepatocellular uptake of chylomicron remnants [3,40].
In these initial studies of the hepatic LRP1 function in lipid metabolism no major effect of an isolated, hepatic-only LRP1 deficiency on plasma lipid levels were reported [3]. This is due to a large extent to an approximately 2-fold increase in the protein expression of hepatic LDLR in the absence of LRP1. However, in another study Basford and colleagues noted reduced levels of plasma HDL in liver-specific LRP1 deficiency consistent with a diminished expression of the ATP-binding cassette, subfamily A member 1 (ABCA1) at the liver cell surface [41*]. A similar, but much more pronounced reduction of ABCA1 had previously been reported in LRP1-deficient vascular smooth muscle cells [42]. ABCA1 acts as an important hepatic cholesterol efflux transporter, and its activity determines plasma HDL levels. Congenital deficiency of ABCA1 causes Tangier Disease (OMIM#205400), a rare genetic disorder characterized by a severe reduction in circulating HDL cholesterol. Translocation of ABCA1 to the plasma membrane has been reported to be dependent on the precursor of the glycosphingholipid-hydrolyzing saposins, Prosaposin (PSAP), which is itself a ligand of LRP1 [43]. Furthermore, Cathepsin D (CtsD) mediates the processing of PSAP. Since LRP1 can also mediate the internalization of CtsD, a possible mechanism by which LRP1 deficiency might adversely affect the translocation of ABCA1 to the plasma membrane is due to impaired PSAP trafficking and CtsD-mediated PSAP activation (Fig. 2B). Moreover, GWAS found an association between the LRP1 locus and plasma HDL levels [44]. LRP1 might therefore have an additional role in lipid metabolism, independent of its role as a chylomicron clearance receptor, and thus could contribute to cardiovascular events in humans, similar to what has previously been reported for SR-B1 [45**].
In smooth muscle cells (SMCs) LRP1 deficiency results in reduced LXR-mediated ABCA1 expression that coincides with lipid accumulation [42,46]. Recently, El Asmar and colleagues further investigated the molecular mechanism by which LRP1 controls ABCA1 expression [47**]. In this study, the second NPXY motif within the cytoplasmic tail of LRP1 was found to be important for LRP1-mediated expression of ABCA1. This motif is important for the binding of Erk2 and subsequently for the phosphorylation of Erk1/2 during low intracellular cholesterol conditions. ERK activation leads to phosphorylation and activation of cPLA2 [46]. Activation of cPLA2 results in increased production of arachidonic acid, which inhibits LXR [46,48] and consequently diminishes ABCA1 expression. Furthermore, the study also reported that activation of the Wnt5a signaling pathway requires LRP1 and that LRP1 deficiency impairs TGFβ-mediated Wnt5a expression, which in turn mediates cholesterol export through controlling the expression of the cholesterol efflux transporter ABCG1, and by blocking SREBP-2 and cholesterol biosynthesis. Taken together, these data may explain the massive cholesterol accumulation observed in the vascular wall of mice lacking LRP1 in SMCs [42,46]. These intriguing LRP1-dependent mechanisms were observed in either SMCs or in MEFs treated with an adipogenic cocktail, but whether these signaling pathways also occur in other cell types, such as hepatocytes, has yet to be determined.
However, another signaling mechanism that is also regulated by LRP1 and that is central to the functioning of the liver and the regulation of hepatic glucose and fatty acid metabolism involves the insulin receptor. A functional interaction between LRP1 and insulin signaling was first noted over 20 year ago, when Descamps and colleagues reported on the rapid translocation of LRP1 from intracellular compartments to the cell surface in response to insulin stimulation of rat epididymal adipocytes [49]. Similar observations were later made in hepatocytes [50]. Conversely, LRP1 also regulates the surface expression of the insulin receptor in neurons [51] and in the liver [52**]. In LRP1-deficient livers, reduced surface expression of the insulin receptor, and subsequently diminished expression of the glucose transporter GLUT2 (Fig. 2C), creates a latent state of insulin resistance that is unmasked by high fat diet feeding, leading to a full-blown metabolic syndrome with hepatic steatosis, reduced VLDL secretion, hepatic insulin resistance, impaired glucose tolerance, hyperglycemia, hyperinsulinemia and increased gluconeogenesis [52**]. A moderate reduction in HDL cholesterol levels was also seen, consistent with the observations by Basford and colleagues [41]. Taken together, these studies indicate that LRP1 and insulin receptor mutually regulate their intracellular trafficking and surface expression (Fig. 2C).
Conclusions
Although the genetic evidence for a role of LRP1 in lipid metabolism and cardiovascular risk in humans is less clear, likely because of its pleiotropic function, the contribution of LDLR has been well established. By contrast, a clear genetic basis for the importance of LRP1 in the formation of abdominal aneurysms has emerged [42, 53**]. In recent years novel genes and proteins controlling LDLR function has been identified, resulting in the development of new therapies, such as PCSK9 inhibitors, to lower plasma cholesterol levels. Recent studies identified several novel proteins that are involved in the endosomal trafficking of LDLR. Moreover, from genetic studies in mice a picture emerges that brings LDLR, LRP1, ABC transporters and the insulin receptor under a common umbrella where cellular glucose and lipid homeostasis are integrated to regulate cellular and systemic energy metabolism. Taking further into account that altering the levels of proteins associated with the endocytic machinery, either by overexpressing [33] or by using pharmacological compounds [54], can improve endosomal sorting point towards novel therapeutic opportunities to treat cardiovascular diseases, diabetes and metabolic syndrome.
Key points.
LDLR endocytosis requires DAB2 in liver sinusoid endothelial cells.
Large group of proteins identified facilitating the endosomal trafficking of LDLR.
LRP1 mediates cholesterol efflux through ABCA1 and ABCG1
Hepatocyte LRP1 deficiency increases the susceptibility to diet-induced insulin resistance and steatosis in mice
Acknowledgments
Financial support and sponsorship
BS and MW are supported by TransCard FP7-603091-2. JH is supported by grants from the NIH, the Brightfocus Foundation and the Consortium for Frontotemporal Dementia Research.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- 2.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors–an evolutionarily ancient multifunctional receptor family. Biological Chemistry. 2010;391:1341. doi: 10.1515/BC.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usifo E, Leigh SEA, Whittall RA, Lench N, Taylor A, Yeats C, Orengo CA, Martin ACR, Celli J, Humphries SE. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Annals of Human Genetics. 2012;76:387–401. doi: 10.1111/j.1469-1809.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 9.Herz J, Clouthier DE, Hammer RE. Correction: LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1993;73:428. doi: 10.1016/0092-8674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein Sensors for Membrane Sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 11*.Wijers M, Kuivenhoven JA, van de Sluis B. The life cycle of the low-density lipoprotein receptor. Current Opinion in Lipidology. 2015;26:82–87. doi: 10.1097/MOL.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 12.Tao W, Moore R, Smith ER, Xu X-X. Endocytosis and Physiology: Insights from Disabled-2 Deficient Mice. Front Cell Dev Biol. 2016;4:1311. doi: 10.3389/fcell.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Tao W, Moore R, Meng Y, Smith ER, Xu X-X. Endocytic adaptors Arh and Dab2 control homeostasis of circulatory cholesterol. The Journal of Lipid Research. 2016 doi: 10.1194/jlr.M063065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MS, Herz J, Goldstein JL. LDL-receptor structure. Calcium cages, acid baths and recycling receptors. Nature. 1997;388:629–630. doi: 10.1038/41672. [DOI] [PubMed] [Google Scholar]
- 15.Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and inlivers of parabiotic mice. J Clin Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Lagace TA. PCSK9 and LDLR degradation. Current Opinion in Lipidology. 2014;25:387–393. doi: 10.1097/MOL.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Sorrentino V, Zelcer N. Post-transcriptional regulation of lipoprotein receptors by the E3-ubiquitin ligase inducible degrader of the low-density lipoprotein receptor. Current Opinion in Lipidology. 2012;23:213–219. doi: 10.1097/MOL.0b013e3283532947. [DOI] [PubMed] [Google Scholar]
- 18*.Hong C, Marshall SM, McDaniel AL, Graham M, Layne JD, Cai L, Scotti E, Boyadjian R, Kim J, Chamberlain BT, et al. The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice. Cell Metabolism. 2014;20:910–918. doi: 10.1016/j.cmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Bartuzi P, Billadeau DD, Favier R, Rong S, Dekker D, Fedoseienko A, Fieten H, Wijers M, Levels JH, Huijkman N, et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat Comms. 2016;7:10961. doi: 10.1038/ncomms10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voineagu I, Huang L, Winden K, Lazaro M, Haan E, Nelson J, McGaughran J, Nguyen LS, Friend K, Hackett A, et al. CCDC22: a novel candidate gene for syndromic X-linked intellectual disability. Mol Psychiatry. 2011;17:4–7. doi: 10.1038/mp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolanczyk M, Krawitz P, Hecht J, Hupalowska A, Miaczynska M, Marschner K, Schlack C, Emmerich D, Kobus K, Kornak U, et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher-Schinzel/3C syndrome. Eur J Hum Genet. 2014;23:633–638. doi: 10.1038/ejhg.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Starokadomskyy P, Gluck N, Li H, Chen B, Wallis M, Maine GN, Mao X, Zaidi IW, Hein MY, McDonald FJ, et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-κB signaling. J Clin Invest. 2013;123:2244–2256. doi: 10.1172/JCI66466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Phillips-Krawczak CA, Singla A, Starokadomskyy P, Deng Z, Osborne DG, Li H, Dick CJ, Gomez TS, Koenecke M, Zhang J-S, et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Molecular Biology of the Cell. 2015;26:91–103. doi: 10.1091/mbc.E14-06-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Koo Y, Mao X, Sifuentes-Dominguez L, Morris LL, Jia D, Miyata N, Faulkner RA, van Deursen JM, Vooijs M, et al. Endosomal sorting of Notch receptors through COMMD9-dependent pathways modulates Notch signaling. The Journal of Cell Biology. 2015;211:605–617. doi: 10.1083/jcb.201505108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Human Molecular Genetics. 2002;11:165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 26.de Bie P, van de Sluis B, Burstein E, van de Berghe PVE, Muller P, Berger R, Gitlin JD, Wijmenga C, Klomp LWJ. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology. 2007;133:1316–1326. doi: 10.1053/j.gastro.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonk WIM, de Bie P, Wichers CGK, van den Berghe PVE, van der Plaats R, Berger R, Wijmenga C, Klomp LWJ, van de Sluis B. The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell Mol Life Sci. 2011;69:149–163. doi: 10.1007/s00018-011-0743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonk WIM, Bartuzi P, de Bie P, Kloosterhuis N, Wichers CGK, Berger R, Haywood S, Klomp LWJ, Wijmenga C, van de Sluis B. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS ONE. 2011;6:e29183. doi: 10.1371/journal.pone.0029183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD. The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. Journal of Biological Chemistry. 2003;278:41593–41596. doi: 10.1074/jbc.C300391200. [DOI] [PubMed] [Google Scholar]
- 30.Seaman MNJ, Gautreau A, Billadeau DD. Retromer-mediated endosomal protein sorting: all WASHed up! Trends in Cell Biology. 2013;23:522–528. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 32.Zeigerer A, Bogorad RL, Sharma K, Gilleron J, Seifert S, Sales S, Berndt N, Bulik S, Marsico G, D’Souza RCJ, et al. Regulation of Liver Metabolism by the Endosomal GTPase Rab5. Cell Reports. 2015;11:884–892. doi: 10.1016/j.celrep.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Stockinger W. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21:4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotelo P, Farfán P, Benitez ML, Bu G, Marzolo M-P. Sorting Nexin 17 Regulates ApoER2 Recycling and Reelin Signaling. PLoS ONE. 2014;9:e93672. doi: 10.1371/journal.pone.0093672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005;24:2851–2861. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, Havel RJ. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proceedings of the National Academy of Sciences. 1982;79:3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 38.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proceedings of the National Academy of Sciences. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakajima C, Haffner P, Goerke SM, Zurhove K, Adelmann G, Frotscher M, Herz J, Bock HH, May P. The lipoprotein receptor LRP1 modulates sphingosine-1-phosphate signaling and is essential for vascular development. Development. 2014;141:4513–4525. doi: 10.1242/dev.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nature Biotechnology. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- 41.Basford JE, Wancata L, Hofmann SM, Silva RAGD, Davidson WS, Howles PN, Hui DY. Hepatic Deficiency of Low Density Lipoprotein Receptor-related Protein-1 Reduces High Density Lipoprotein Secretion and Plasma Levels in Mice. Journal of Biological Chemistry. 2011;286:13079–13087. doi: 10.1074/jbc.M111.229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boucher P. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 43.Hiesberger T. Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP) EMBO J. 1998;17:4617–4625. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Choi HY, Li W-P, Xu F, Herz J. LRP1 Controls cPLA2 Phosphorylation, ABCA1 Expression and Cellular Cholesterol Export. PLoS ONE. 2009;4:e6853. doi: 10.1371/journal.pone.0006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asmar El Z, Terrand J, Jenty M, Host L, Mlih M, Zerr A, Justiniano H, Matz RL, Boudier C, Scholler E, et al. Convergent Signaling Pathways Controlled by LRP1 (Receptor-related Protein 1) Cytoplasmic and Extracellular Domains Limit Cellular Cholesterol Accumulation. Journal of Biological Chemistry. 2016;291:5116–5127. doi: 10.1074/jbc.M116.714485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proceedings of the National Academy of Sciences. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Descamps O, Bilheimer D, Herz J. Insulin stimulates receptor-mediated uptake of apoE-enriched lipoproteins and activated alpha 2-macroglobulin in adipocytes. Journal of Biological Chemistry. 1993;268:974–981. [PubMed] [Google Scholar]
- 50.Laatsch A, Merkel M, Talmud PJ, Grewal T, Beisiegel U, Heeren J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2009;204:105–111. doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 51.Liu CC, Hu J, Tsai CW, Yue M, Melrose HL, Kanekiyo T, Bu G. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. Journal of Neuroscience. 2015;35:5851–5859. doi: 10.1523/JNEUROSCI.5180-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Ding Y, Xian X, Holland WL, Tsai S, Herz J. Low-Density Lipoprotein Receptor- Related Protein-1 Protects Against Hepatic Insulin Resistance and Hepatic Steatosis. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Guo D-C, Grove ML, Prakash SK, Eriksson P, Hostetler EM, LeMaire SA, Body SC, Shalhub S, Estrera AL, Safi HJ, et al. Genetic Variants in LRP1 and ULK4 Are Associated with Acute Aortic Dissections. The American Journal of Human Genetics. 2016;99:762–769. doi: 10.1016/j.ajhg.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA. Pharmacological chaperones stabilize retromer to limit APP processing. Nature chemical biology. 2014;10:443–449. doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfisterer SG, Peränen J, Ikonen E. LDL–cholesterol transport to the endoplasmic reticulum. Current Opinion in Lipidology. 2016;27:282–287. doi: 10.1097/MOL.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]