Abstract
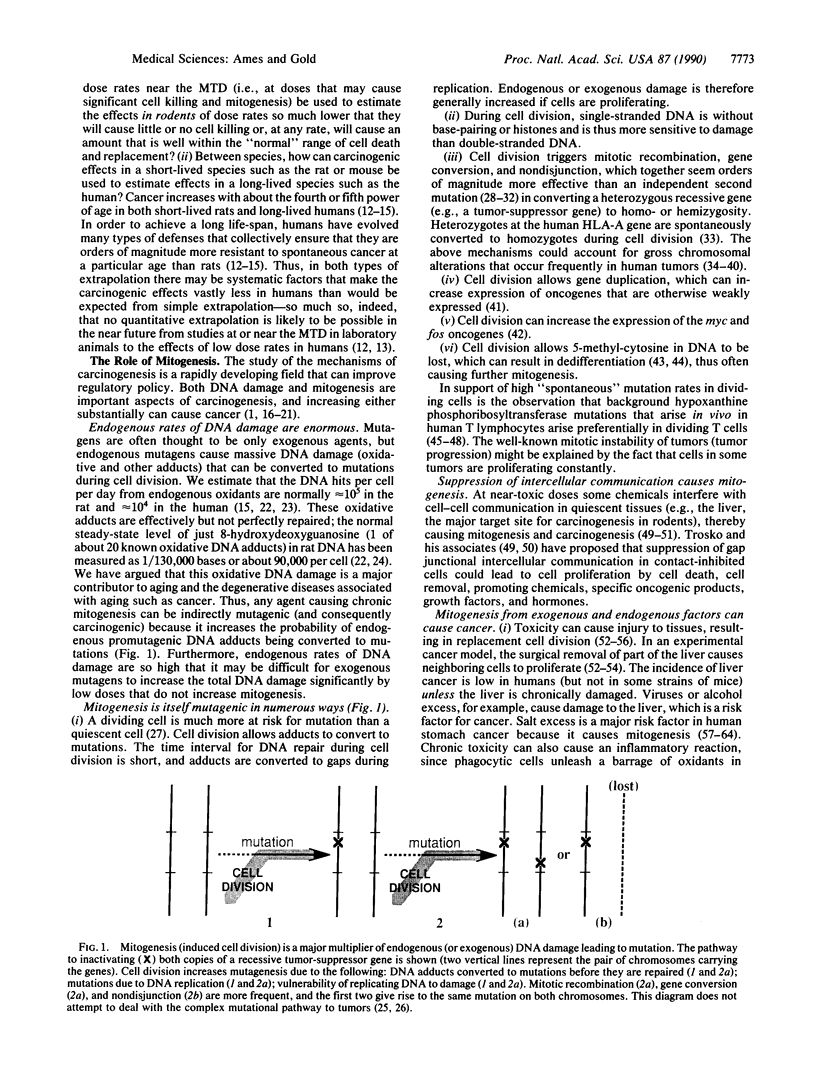
The administration of chemicals at the maximum tolerated dose (MTD) in standard animal cancer tests is postulated to increase cell division (mitogenesis), which in turn increases rates of mutagenesis and thus carcinogenesis. The animal data are consistent with this mechanism, because a high proportion--about half--of all chemicals tested (whether natural or synthetic) are indeed rodent carcinogens. We conclude that at the low doses of most human exposures, where cell killing does not occur, the hazards to humans of rodent carcinogens may be much lower than is commonly assumed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Magaw R., Gold L. S. Ranking possible carcinogenic hazards. Science. 1987 Apr 17;236(4799):271–280. doi: 10.1126/science.3563506. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen. 1989;14 (Suppl 16):66–77. doi: 10.1002/em.2850140614. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Profet M., Gold L. S. Dietary pesticides (99.99% all natural). Proc Natl Acad Sci U S A. 1990 Oct;87(19):7777–7781. doi: 10.1073/pnas.87.19.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res. 1988 Jan;204(1):17–115. doi: 10.1016/0165-1218(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Bernstein L., Gold L. S., Ames B. N., Pike M. C., Hoel D. G. Some tautologous aspects of the comparison of carcinogenic potency in rats and mice. Fundam Appl Toxicol. 1985 Feb;5(1):79–86. doi: 10.1016/0272-0590(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Cai L., Liu S. Z. Induction of cytogenetic adaptive response of somatic and germ cells in vivo and in vitro by low-dose X-irradiation. Int J Radiat Biol. 1990 Jul;58(1):187–194. doi: 10.1080/09553009014551541. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Chan T. M., Chen E., Tatoyan A., Shargill N. S., Pleta M., Hochstein P. Stimulation of tyrosine-specific protein phosphorylation in the rat liver plasma membrane by oxygen radicals. Biochem Biophys Res Commun. 1986 Sep 14;139(2):439–445. doi: 10.1016/s0006-291x(86)80010-9. [DOI] [PubMed] [Google Scholar]
- Charnley G., Tannenbaum S. R. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer Res. 1985 Nov;45(11 Pt 2):5608–5616. [PubMed] [Google Scholar]
- Coggon D., Barker D. J., Cole R. B., Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989 Aug 2;81(15):1178–1182. doi: 10.1093/jnci/81.15.1178. [DOI] [PubMed] [Google Scholar]
- Columbano A., Ledda-Columbano G. M., Ennas M. G., Curto M., Chelo A., Pani P. Cell proliferation and promotion of rat liver carcinogenesis: different effect of hepatic regeneration and mitogen induced hyperplasia on the development of enzyme-altered foci. Carcinogenesis. 1990 May;11(5):771–776. doi: 10.1093/carcin/11.5.771. [DOI] [PubMed] [Google Scholar]
- Coni P., Pichiri-Coni G., Ledda-Columbano G. M., Rao P. M., Rajalakshmi S., Sarma D. S., Columbano A. Liver hyperplasia is not necessarily associated with increased expression of c-fos and c-myc mRNA. Carcinogenesis. 1990 May;11(5):835–839. doi: 10.1093/carcin/11.5.835. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987 Feb;79(2):532–541. doi: 10.1172/JCI112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal F. H., Richardson F. C., Swenberg J. A. Dose response of hepatocyte replication in rats following continuous exposure to diethylnitrosamine. Cancer Res. 1989 Dec 15;49(24 Pt 1):6985–6988. [PubMed] [Google Scholar]
- Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981 Jun;66(6):1191–1308. [PubMed] [Google Scholar]
- Dunsford H. A., Sell S., Chisari F. V. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990 Jun 1;50(11):3400–3407. [PubMed] [Google Scholar]
- Erisman M. D., Scott J. K., Astrin S. M. Evidence that the familial adenomatous polyposis gene is involved in a subset of colon cancers with a complementable defect in c-myc regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4264–4268. doi: 10.1073/pnas.86.11.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrig R. The effect of dose and time on the induction of genetic alterations in Saccharomyces cerevisiae by aminoacridines in the presence and absence of visible light irradiation in comparison with the dose-effect-curves of mutagens with other type of action. Mol Gen Genet. 1976 Mar 22;144(2):131–140. doi: 10.1007/BF02428101. [DOI] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem Pharmacol. 1990 Jun 15;39(12):1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Farber E. Possible etiologic mechanisms in chemical carcinogenesis. Environ Health Perspect. 1987 Nov;75:65–70. [PMC free article] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata C., Sato Y., Hosaka M., Matsushima T., Furukawa F., Takahashi M. NaCl induced ornithine decarboxylase and DNA synthesis in rat stomach mucosa. Biochem Biophys Res Commun. 1984 Jun 29;121(3):1027–1032. doi: 10.1016/0006-291x(84)90780-0. [DOI] [PubMed] [Google Scholar]
- Furihata C., Sudo K., Matsushima T. Calcium chloride inhibits stimulation of replicative DNA synthesis by sodium chloride in the pyloric mucosa of rat stomach. Carcinogenesis. 1989 Nov;10(11):2135–2137. doi: 10.1093/carcin/10.11.2135. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Backman G. M., Hooper N. K., Peto R. Ranking the potential carcinogenic hazards to workers from exposures to chemicals that are tumorigenic in rodents. Environ Health Perspect. 1987 Dec;76:211–219. doi: 10.1289/ehp.8776211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Bernstein L., Magaw R., Slone T. H. Interspecies extrapolation in carcinogenesis: prediction between rats and mice. Environ Health Perspect. 1989 May;81:211–219. doi: 10.1289/ehp.8981211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Sawyer C. B., Magaw R., Backman G. M., de Veciana M., Levinson R., Hooper N. K., Havender W. R., Bernstein L., Peto R. A carcinogenic potency database of the standardized results of animal bioassays. Environ Health Perspect. 1984 Dec;58:9–319. doi: 10.1289/ehp.84589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Backman G. M., Eisenberg S., Da Costa M., Wong M., Manley N. B., Rohrbach L., Ames B. N. Third chronological supplement to the carcinogenic potency database: standardized results of animal bioassays published through December 1986 and by the National Toxicology Program through June 1987. Environ Health Perspect. 1990 Mar;84:215–286. doi: 10.1289/ehp.9084215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Backman G. M., Magaw R., Da Costa M., Lopipero P., Blumenthal M., Ames B. N. Second chronological supplement to the Carcinogenic Potency Database: standardized results of animal bioassays published through December 1984 and by the National Toxicology Program through May 1986. Environ Health Perspect. 1987 Oct;74:237–329. doi: 10.1289/ehp.8774237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Bernstein L. Summary of carcinogenic potency and positivity for 492 rodent carcinogens in the carcinogenic potency database. Environ Health Perspect. 1989 Feb;79:259–272. doi: 10.1289/ehp.8979259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., de Veciana M., Backman G. M., Magaw R., Lopipero P., Smith M., Blumenthal M., Levinson R., Bernstein L., Ames B. N. Chronological supplement to the Carcinogenic Potency Database: standardized results of animal bioassays published through December 1982. Environ Health Perspect. 1986 Aug;67:161–200. doi: 10.1289/ehp.8667161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J., Nakamura Y., German J. Molecular evidence that homologous recombination occurs in proliferating human somatic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4315–4319. doi: 10.1073/pnas.87.11.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Genetics of cancer predisposition. Cancer Res. 1987 Nov 1;47(21):5518–5527. [PubMed] [Google Scholar]
- Haseman J. K. Issues in carcinogenicity testing: dose selection. Fundam Appl Toxicol. 1985 Feb;5(1):66–78. doi: 10.1016/0272-0590(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R., Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988 Jan 15;48(2):246–253. [PubMed] [Google Scholar]
- Hoel D. G., Haseman J. K., Hogan M. D., Huff J., McConnell E. E. The impact of toxicity on carcinogenicity studies: implications for risk assessment. Carcinogenesis. 1988 Nov;9(11):2045–2052. doi: 10.1093/carcin/9.11.2045. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. D. A unifying concept for carcinogenic risk assessments: comparison with radiation-induced leukemia in mice and men. Health Phys. 1984 Oct;47(4):533–558. doi: 10.1097/00004032-198410000-00002. [DOI] [PubMed] [Google Scholar]
- Joossens J. V., Geboers J. Nutrition and gastric cancer. Nutr Cancer. 1981;2(4):250–261. doi: 10.1080/01635588109513691. [DOI] [PubMed] [Google Scholar]
- Karube T., Katayama H., Takemoto K., Watanabe S. Induction of squamous metaplasia, dysplasia and carcinoma in situ of the mouse tracheal mucosa by inhalation of sodium chloride mist following subcutaneous injection of 4-nitroquinoline 1-oxide. Jpn J Cancer Res. 1989 Aug;80(8):698–701. doi: 10.1111/j.1349-7006.1989.tb01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociba R. J. Issues of biochemical applications to risk assessment: how should the MTD be selected for chronic bioassays? Environ Health Perspect. 1987 Dec;76:169–174. doi: 10.1289/ehp.8776169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Inflammation, oxidative DNA damage, and carcinogenesis. Environ Health Perspect. 1987 Dec;76:19–27. doi: 10.1289/ehp.877619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988 Jan 15;48(2):235–245. [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Little J. B., Kennedy A. R., McGandy R. B. Effect of dose rate on the induction of experimental lung cancer in hamsters by alpha radiation. Radiat Res. 1985 Aug;103(2):293–299. [PubMed] [Google Scholar]
- Lu J. B., Qin Y. M. Correlation between high salt intake and mortality rates for oesophageal and gastric cancers in Henan Province, China. Int J Epidemiol. 1987 Jun;16(2):171–176. doi: 10.1093/ije/16.2.171. [DOI] [PubMed] [Google Scholar]
- Lu L. J., Liehr J. G., Sirbasku D. A., Randerath E., Randerath K. Hypomethylation of DNA in estrogen-induced and -dependent hamster kidney tumors. Carcinogenesis. 1988 Jun;9(6):925–929. doi: 10.1093/carcin/9.6.925. [DOI] [PubMed] [Google Scholar]
- Madsen C. Squamous-cell carcinoma and oral, pharyngeal and nasal lesions caused by foreign bodies in feed. Cases from a long-term study in rats. Lab Anim. 1989 Jul;23(3):241–247. doi: 10.1258/002367789780810572. [DOI] [PubMed] [Google Scholar]
- Mirsalis J. C., Tyson C. K., Steinmetz K. L., Loh E. K., Hamilton C. M., Bakke J. P., Spalding J. W. Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following in vivo treatment: testing of 24 compounds. Environ Mol Mutagen. 1989;14(3):155–164. doi: 10.1002/em.2850140305. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P., Sesko A., Hill S., Shatos M. A., Doherty J., Petruska J., Adler K. B., Hemenway D., Mickey R. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., O'Neill J. P., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes. III. Longitudinal study of hprt gene structural alterations and T-cell clonal origins. Mutat Res. 1989 Dec;215(2):147–160. doi: 10.1016/0027-5107(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., O'Neill J. P., Sullivan L. M., Hunter T. C., Allegretta M., Chastenay B. F., Libbus B. L., Albertini R. J. Molecular analyses of in vivo hypoxanthine-guanine phosphoribosyltransferase mutations in human T-lymphocytes: II. Demonstration of a clonal amplification of hprt mutant T-lymphocytes in vivo. Environ Mol Mutagen. 1988;12(3):271–284. doi: 10.1002/em.2860120302. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Spradling A. C. Drosophila chorion gene amplification requires an upstream region regulating s18 transcription. Mol Cell Biol. 1986 Dec;6(12):4624–4633. doi: 10.1128/mcb.6.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence B. C., Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988 Jan;9(1):187–190. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T. L., Moran S., Kennan W., Glauert H. P., Maronpot R. R., Campbell H. A. A method to quantitate the relative initiating and promoting potencies of hepatocarcinogenic agents in their dose-response relationships to altered hepatic foci. Carcinogenesis. 1987 Oct;8(10):1491–1499. doi: 10.1093/carcin/8.10.1491. [DOI] [PubMed] [Google Scholar]
- Ramel C. Short-term testing--are we looking at wrong endpoints? Mutat Res. 1988 May-Aug;205(1-4):13–24. doi: 10.1016/0165-1218(88)90004-3. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Okamoto M., Sato C., Sugio K., Soejima J., Iwama T., Ikeuchi T., Tonomura A., Miyaki M., Sasazuki T. Loss of constitutional heterozygosity in colorectal tumors from patients with familial polyposis coli and those with nonpolyposis colorectal carcinoma. Cancer Res. 1989 Aug 15;49(16):4402–4406. [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D., Mehta R. D., Hastings P. J. Carcinogens induce intrachromosomal recombination in yeast. Carcinogenesis. 1989 Aug;10(8):1445–1455. doi: 10.1093/carcin/10.8.1445. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Gimeno C. J., Ames B. N. Urinary 8-hydroxy-2'-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke M. H., Stoker A. W., Bissell M. J. Evaluation of the cocarcinogenic effect of wounding in Rous sarcoma virus tumorigenesis. Cancer Res. 1989 Nov 15;49(22):6419–6424. [PubMed] [Google Scholar]
- Swenberg J. A., Richardson F. C., Boucheron J. A., Deal F. H., Belinsky S. A., Charbonneau M., Short B. G. High- to low-dose extrapolation: critical determinants involved in the dose response of carcinogenic substances. Environ Health Perspect. 1987 Dec;76:57–63. doi: 10.1289/ehp.877657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton A. Pre-existing, non-malignant disorders associated with increased cancer risk. J Environ Pathol Toxicol. 1980 Mar;3(4 Spec No):387–397. [PubMed] [Google Scholar]
- Tong C., Fazio M., Williams G. M. Cell cycle-specific mutagenesis at the hypoxanthine phosphoribosyltransferase locus in adult rat liver epithelial cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7377–7379. doi: 10.1073/pnas.77.12.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Grist S. A., Janatipour M., Morley A. A. Mutations in human lymphocytes commonly involve gene duplication and resemble those seen in cancer cels. Proc Natl Acad Sci U S A. 1988 May;85(9):3189–3192. doi: 10.1073/pnas.85.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyns A. J. Salt and gastrointestinal cancer. Nutr Cancer. 1988;11(4):229–232. doi: 10.1080/01635588809513992. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]
- Wilson V. L., Smith R. A., Ma S., Cutler R. G. Genomic 5-methyldeoxycytidine decreases with age. J Biol Chem. 1987 Jul 25;262(21):9948–9951. [PubMed] [Google Scholar]
- Wolff S., Afzal V., Wiencke J. K., Olivieri G., Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):39–47. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Tong M. J., Hwang B., Lee S. D., Hu M. M. Primary hepatocellular carcinoma and hepatitis B infection during childhood. Hepatology. 1987 Jan-Feb;7(1):46–48. doi: 10.1002/hep.1840070111. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990 Jul;11(7):1051–1058. doi: 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- Yang C. S., Newmark H. L. The role of micronutrient deficiency in carcinogenesis. Crit Rev Oncol Hematol. 1987;7(4):267–287. doi: 10.1016/s1040-8428(87)80002-1. [DOI] [PubMed] [Google Scholar]
- Yeh F. S., Mo C. C., Luo S., Henderson B. E., Tong M. J., Yu M. C. A serological case-control study of primary hepatocellular carcinoma in Guangxi, China. Cancer Res. 1985 Feb;45(2):872–873. [PubMed] [Google Scholar]
- Zeiger E. Carcinogenicity of mutagens: predictive capability of the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer Res. 1987 Mar 1;47(5):1287–1296. [PubMed] [Google Scholar]