Abstract
The objective of this study is to determine how a hibernating mammal avoids the formation of blood clots under periods of low blood flow. A microfluidic vascular injury model was performed to differentiate the effects of temperature and shear rate on platelet adhesion to collagen. Human and ground squirrel whole blood was incubated at 15 or 37°C and then passed through a microfluidic chamber over a 250 μm strip of type I fibrillar collagen at that temperature and shear rates of 50 s−1 or 300 s−1 to simulate torpid and aroused conditions respectively. At 15°C, both human and ground squirrel platelets showed a 90–95% decrease in accumulation on collagen independent of shear rate. At 37°C, human platelet accumulation reduced by 50% at 50 s−1 compared to 300 s−1, while ground squirrel platelet accumulation dropped by 80%. When compared to platelets from non-hibernating animals, platelets from animals collected after arousal from torpor showed a 60% decrease in binding at 37°C and 300 s−1, but a 2.5-fold increase in binding at 15°C and 50 s−1. vWF binding in platelets from hibernating ground squirrels were decreased by 50% relative to non-hibernating platelets. The source of the plasma that platelets were stored in did not affect the results indicating that the decreased vWF binding was a property of the platelets. Upon chilling, ground squirrel platelets increase microtubule assembly leading to the formation of long rods. This shape change is concurrent with sequestration of platelets in the liver and not the spleen. In conclusion, it appears that ground squirrel platelets are sequestered in the liver during torpor, have reduced binding capacity for plasma vWF, and lower accumulation on collagen at low shear rates and after storage at cold temperatures, while still being activated by external agonists. These adaptations would protect the animals from spontaneous thrombus formation during torpor but allow them to restore normal platelet function upon arousal.
Keywords: von Willebrand factor, hypothermia, fibrinogen, collagen, tubulin
INTRODUCTION
Blood clotting or hemostasis is a critical process in animals with a closed circulatory system. In primary hemostasis, circulating anucleated cells called platelets are activated, causing them to adhere to the vessel wall and aggregate with each other (Figure 1). Platelet adherence to the vessel wall is mediated by surface receptor glycoprotein Ibα (GPIbα) binding to von Willebrand Factor (vWF) which in turn binds to collagen exposed at sites of vascular injury (Weiss, Sussman et al. 1977, Lenting, Casari et al. 2012). Under high shear stress, vWF becomes elongated and is more likely to bind to platelets (Fuchs, Budde et al. 2010), but this is blocked at temperatures below 17°C (Hewlett, Zupancic et al. 2011). Platelets also respond to shear stress, with GPIbα receptors clustering in lipid rafts and increasing binding to vWF (Gitz, Koopman et al. 2013). Platelets aggregate with each other through binding of fibrinogen and other ligands to glycoprotein IIb/IIIa (GPIIb/IIIa) receptors (Marguerie, Plow et al. 1979). This binding is stimulated by various platelet agonists including thrombin and ADP (Zucker and Nachmias 1985).
Figure 1.
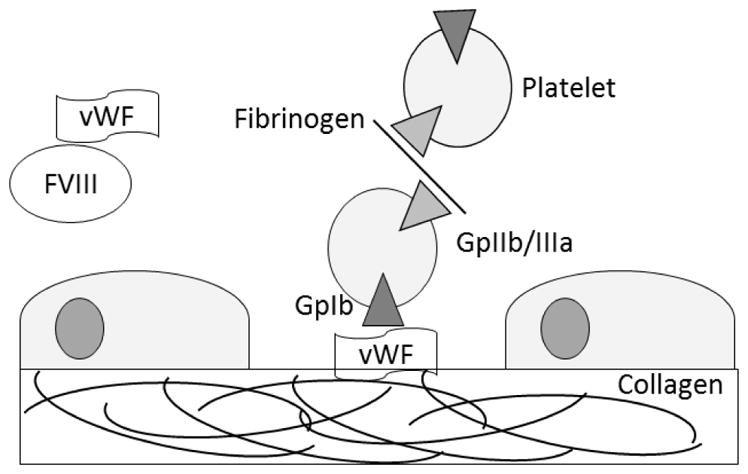
Adhesion and aggregation of platelets. Platelets adhere to a damaged vessel wall through binding of their glycoprotein Ib (GpIbα) receptor to von Willebrand Factor (vWF) which in turn binds to exposed collagen. Platelet aggregation occurs when two platelets bind to a single molecule of fibrinogen through their glycoprotein IIb/IIIa receptors (GpIIb/IIIa). Glycosylated proteins on the surface of the platelets also contain sialic acid caps, and the removal of these lead to clearance through Ashwell Morell receptors on hepatocytes.
When most mammalian platelets are chilled they undergo both structural and functional changes termed cold storage lesions (Michelson, MacGregor et al. 1994, Vostal and Mondoro 1997, Hoffmeister, Falet et al. 2001, Thon, Schubert et al. 2008, Springer, Miller et al. 2009, Van Poucke, Stevens et al. 2014). For example, platelet aggregation, vWF and fibrinogen binding, and P-selectin expression are enhanced under hypothermic temperatures (Michelson, MacGregor et al. 1994, Berger, Hartwell et al. 1998, Faraday and Rosenfeld 1998, Engelfriet, Reesink et al. 2000, Frelinger Iii, Furman et al. 2003, Xavier, White et al. 2007, Hogberg, Erlinge et al. 2009, Scharbert, Kalb et al. 2010, Gitz, Koopman et al. 2013). Platelets have a dynamic circumferential microtubule ring (Patel-Hett, Richardson et al. 2008) and this depolymerizes in the cold, causing platelets to change from a discoid shape to a sphere (White and Krivit 1967, White and Rao 1998, Italiano, Bergmeier et al. 2003). When platelets from mice are chilled, the GPIbα subunit of the vWF receptor clusters and is recognized by complement receptor type 3 (CR3) on Kupfer cells, and terminal sialic acid residues on glycoproteins are cleaved creating a ligand for Ashwell Morell receptors on hepatocytes. Both events contribute to platelet phagocytosis, clearance, and apoptosis (Hoffmeister, Felbinger et al. 2003, Rumjantseva, Grewal et al. 2009, van der Wal, Du et al. 2010). Because of this units of human platelets cannot be stored in the cold and can only be stored for 3–4 days at 22°C, increasing the risk of bacterial contamination and resulting in half of all units being discarded (Engelfriet, Reesink et al. 2000, Jacobs, Palavecino et al. 2001). The characteristics of cold storage lesions are observed in several mammalian species, but do not appear to occur in mammalian hibernators (Cooper, Richters et al. 2012, de Vrij, Vogelaar et al. 2014).
Hibernating mammals routinely sustain body temperatures below 10°C for weeks at a time. Hibernation can be broken into two phases, prolonged periods of torpor with decreased body temperature and heart rate, and brief 12–24 hour periods of interbout arousal (IBA) with a rapid restoration of euthermia and heart rate (Carey, Andrews et al. 2003). During the torpor phase of hibernation, 13-lined ground squirrels (Ictidomys tridecemlineatus), hereafter referred to as ground squirrels, reduce their body temperatures from 35–38°C to 4–8°C, heart rates from 200–300 to 3–5 beats/min, and respiration from 100–200 to 4–6 breaths/min(Lechler and Penick 1963, Reddick, Poole et al. 1973, Zatzman 1984). Under these conditions of low flow, hibernating mammals could be at increased risk of forming stasis clots or deep vein thrombi. Stasis is thought to promote thrombosis by (i) causing local hypoxia leading the endothelial cell activation and subsequent recruitment of platelet, leukocytes, and microparticles to the vessel wall via adhesive ligands (P- and E-selectin, vWF) and (ii) in the absence of dilution by flow, coagulation products can accumulate leading to fibrin formation (Mackman and Davis 2011, Rana and Neeves 2016). The hibernators will have some protection because their lower body temperatures decrease both primary and secondary hemostasis by reducing the activity of the enzymes and receptors involved in both processes (Van Poucke, Stevens et al. 2014). However, hibernators have also adapted to this extreme decrease in blood flow by reversibly decreasing platelet, monocyte, neutrophil, vWF, FVIII, and FIX levels (Suomalainen and Lehto 1952, Svihla, Bowman et al. 1952, Svihla, Bowman et al. 1952, Svihla, Bowman et al. 1953, Smith, Lewis et al. 1954, Lechler and Penick 1963, Pivorun and Sinnamon 1981, Cooper, Richters et al. 2012, de Vrij, Vogelaar et al. 2014, Cooper, Sell et al. 2016).
During torpor, ground squirrel platelets go through reorganization of their circumferential microtubule ring to form long rods and circulating platelet levels drop by 90%, returning to normal levels within two hours post arousal (Reddick, Poole et al. 1973, Reznik, Reznik-Schuller et al. 1975, Cooper, Richters et al. 2012, de Vrij, Vogelaar et al. 2014). This rapid release is not consistent with new synthesis of platelets, but rather release of platelets that had been sequestered (Cooper, Richters et al. 2012). Initial models proposed that the rod shape may cause platelets to become trapped in the spleen (Reddick, Poole et al. 1973), however splenectomy in Syrian hamsters (Mesocricetus auratus) did not affect sequestration during hibernation (de Vrij, Vogelaar et al. 2014). New platelet synthesis by megakaryocytes in the bone marrow of hibernating ground squirrels lags 24–48 hours behind arousal, indicating that platelet synthesis is also decreased during hibernation (Cooper, Richters et al. 2012). However, most proteins known to be involved in platelet function did not show differential expression in the bone marrow of hibernating squirrels including the surface glycoprotein receptors (GPIBA, GPIBB, GPIIB, GPIIIB) and proteins found in secretory granules (vWF, P selectin) (Cooper, Sell et al. 2016).
Ground squirrels are adapted to spending several months each year with a body temperature of 4–8°C, and their platelets are resistant to the clearance seen in non-hibernating mammals (Cooper, Richters et al. 2012). In this study we measured binding of vWF and fibrinogen to platelets and platelet adhesion to collagen at different temperatures and shear rates to determine if the decrease in blood clotting during hibernation is a property of the squirrel’s platelets, plasma, body temperature, or blood flow rate. In addition, the kinetics of the temperature dependent microtubule rearrangements and location of platelets during torpor were examined.
MATERIALS AND METHODS
Animals
Ground squirrels were born in captivity and housed at the University of Wisconsin-La Crosse following protocols approved by the institutional IACUC. Non-hibernating animals were housed individually in rooms with a Wisconsin photoperiod (9 hours in December gradually increasing to 15.5 hours in June and then decreasing again). Animals were implanted with a temperature transponder (IPTT-300, BioMedic Data Systems) and body temperatures monitored with a hand held reader. In October when an animal’s body temperature dropped to 25°C (ambient) they were moved into a 4°C hibernaculum. Blood and organs were collected from hibernating animals in the torpid state in January and February at a body temperature of 9.8±2.1°C. Blood samples were collected from anesthetized animals 2 hours post-arousal in March (36.4±0.8°C), and from non-hibernators in June-July (35.5±2.6°C).
Platelet isolation
Blood was collected in 1/9th volume of acid citrate dextrose (ACD) anticoagulant from the tail arteries of summer non-hibernating or winter IBA ground squirrels while under anesthesia with isoflurane (1.5–5%). Blood cell counts were performed using a HemaVet HV950 (Drew Scientific, Waterbury, CT). Human blood was collected in ACD from volunteers by venipuncture. Differential centrifugation was performed at 25°C with a brake to isolate platelets. Blood was centrifuged at 200 x g for 8 minutes, and the platelet rich plasma (PRP), white blood cells, and a limited number of red blood cells were isolated and centrifuged at 100 x g for 6 minutes. Prostaglandin E1 (PGE1, final concentration of 6.0 μM) was added to the PRP to prevent platelet activation and centrifuged at 800 x g for 5 minutes. Plasma aliquots were stored at −20°C for incubations, and the platelet pellet was resuspended in 200 μl of Tyrodes-HEPES buffer (12 mM NaHC03, 138 mM NaCI, 5.5 mM glucose, 2.9 mM KCI, 10 mM HEPES, pH 7.4). Platelets were counted on a hemacytometer and resuspended at 1 × 109 platelets/ml in Tyrodes.
To measure the kinetics of platelet shape change, PRP was incubated at 37°C for two hours and then placed in an ice bath and aliquots removed at time points, fixed with 5% glutaraldehyde, and centrifuged onto polylysine coated slides at 2,100 x g for 4 min in a Cytofuge2 (StatSpin, Norwood, MA), before Wright staining and counting the percentage of platelets with a rod shape. To determine if the shape changes required tubulin polymerization or depolymerization, platelets were pre-incubated for two hours at 37°C and then incubated at 4°C or 37°C for two hours and fixed on slides as described. Some aliquots were treated with 1μM taxol (Life Technologies, Carlsbad CA) or 250nM nocodazole (Thermo Fisher) for 20 minutes at 37°C prior to incubation at 4°C or 37°C. Platelets were visualized with anti-β-tubulin antibodies (Life Technologies, Eugene, OR) and a secondary Alexa Fluor 488 goat anti-mouse antibody (Life Technologies) as described previously (Cooper, Richters et al. 2012) and the percentage of platelets in a rod or ring formation counted.
Fibrinogen Binding
Fresh platelets were isolated from non-hibernating ground squirrels and allowed rest for 30 minutes at room temperature to reduce isolation-induced platelet activation. PRP samples were stored for two days at either 4°C or room temperature (20–25°C). Platelets were diluted to 2 × 108/ml in Tyrodes and centrifuged at 300 x g at 25°C to remove plasma proteins, and resuspended in Tyrodes at 2 × 108/ml. Washed platelets were mixed with 0.5 μg/μl Alexa-488 fibrinogen (Invitrogen) and then activated with 50 μM ADP, 200 μM thrombin receptor activation peptide (TRAP), or 200 μM protease activated receptor 4 peptide (PAR4) for 5 minutes in the dark at 37 C. Samples were diluted in 1 mL PBS and fixed with 4% paraformaldehyde before counting on a flow cytometer. Mean fluorescence values were analyzed by two-way ANOVA for the human and squirrel TRAP results and three-way ANOVA for the three agonist treated squirrel samples.
vWF binding
Changes in vWF binding during hibernation could be due to effects of temperature, changes in ground squirrel platelets, or be due to a 10-fold decrease in vWF relative to normothermic ground squirrels (Cooper, Sell et al. 2016). To isolate these three variables, a triple-cross over experiment was designed in which platelets from non-hibernating and IBA ground squirrels were incubated with shaking in plasma from both non-hibernating and IBA animals at 4°C or 37°C for 48 hours. Platelets were pelleted and resuspended twice in Tyrodes buffer as described for the fibrinogen binding assay to remove unbound proteins. 1 x 106 platelets were diluted in an equal volume of 2X sample buffer containing a protease inhibitor cocktail and separated on a 6% acrylamide gel. Proteins were transferred onto a PVDF membrane, blocked, and then probed with a 1:1000 dilution of rabbit anti-human vWF antibody (Dako). The blots were then stripped and probed with a 1:1000 dilution of mouse anti-actin antibody (BD biosciences) as a loading control. After each primary antibody incubation, a 1:10,000 dilution of goat anti-rabbit secondary antibody linked to horseradish peroxidase was added, followed by a chemiluminescent substrate (Thermo Scientific) and exposed to film. Quantification was done by scanning the film, using Image J to normalize mean pixels to the actin loading control, followed by a student t-test to analyze the results.
Microfluidics
Blood was collected in PPACK (40 μM) and low-molecular weight heparin (5 U/mL) and incubated at 37°C for 10–15 min, followed by platelet labeling for 5 min with DiOC6 (3,3′-Dihexyloxacarbocyanin iodide, 0.5 μg/mL). PDMS microfluidic devices each containing three channels 500 μm wide and 50 μm high were attached by vacuum to a microscope slide containing a 250 μm wide strip of type I fibrillar collagen as described (Neeves and Diamond 2008, Lehmann, Wallbank et al. 2015). The channels were washed and blocked with HEPES buffered saline (HBS; 7.5 mM CaCl2, 3.75 mM MgCl2, 1 U/mL heparin) containing 5% denatured bovine serum albumin. Flow rates were adjusted using a syringe pump (PHD2000, Harvard Apparatus) at 0.7 and 3.5 μl/min to produce shear rates of 50 sec−1 and 300 sec−1 respectively. The blood samples were incubated at 15°C or 35°C for 30 minutes and passed over the collagen strip for 5 minutes on a microscope stage heated or cooled to the same temperature, followed by HBS for one minute and then HBS containing 0.2% gluteraldehyde for 5 minutes. The microfluidic device was removed from the slide, the slide was fixed for 30 minutes in 2% gluteraldehyde, and a coverslip added with anti-fade mounting media. The platelet volume bound to each collagen strip was analyzed by confocal microscopy.
Splenectomy and platelet localization
In July, 10 ground squirrels received a splenectomy and 10 a sham surgery while under anesthesia with isoflurane (1.5–5%). Pre and post-surgery blood samples were collected a week before and after the surgery respectively. Two months after the surgery a blood sample was taken during entry into hibernation when the animal’s body temperature reached ambient temperature (25°C). The ground squirrels were then moved to a hibernaculum maintained at 4°C, and additional samples were taken during torpor by treating the animal’s tail with lidocaine, pricking the tail and collecting a blood smear. An IBA sample was collected in animals that had spontaneously aroused during the winter. Blood smears from torpid animals were counted manually while those from alert animals were also counted on a Hemavet. In situ platelet localization was performed on frozen 5 μm liver sections prepared at −30°C in a cryotome and fixed in cold (4°C) acetone for 5 minutes. Endogenous peroxidase activity was inhibited with 0.3% H2O2 and 0.1% NaN3 for 30 minutes, and blocked with 10% goat serum/PBS for 30 minutes. Tissue sections were incubated with a 1:200 dilution of polyclonal rabbit anti-GPIbα antibody (Aviva Systems Biology) followed by a horseradish peroxidase coupled secondary HRP-goat anti-rabbit IgG antibody (Thermo Scientific). A negative control with just secondary antibody was performed to ensure the secondary antibody was not cross-reacting with liver antigens and that endogenous peroxidase activity was blocked. Aminoethylcarbazole (AEC) was used as a substrate and the tissue sections counterstained with Mayer’s hematoxylin. Platelets were counted in Image J, adjusted to platelets per μm2 and compared by t-test.
RESULTS
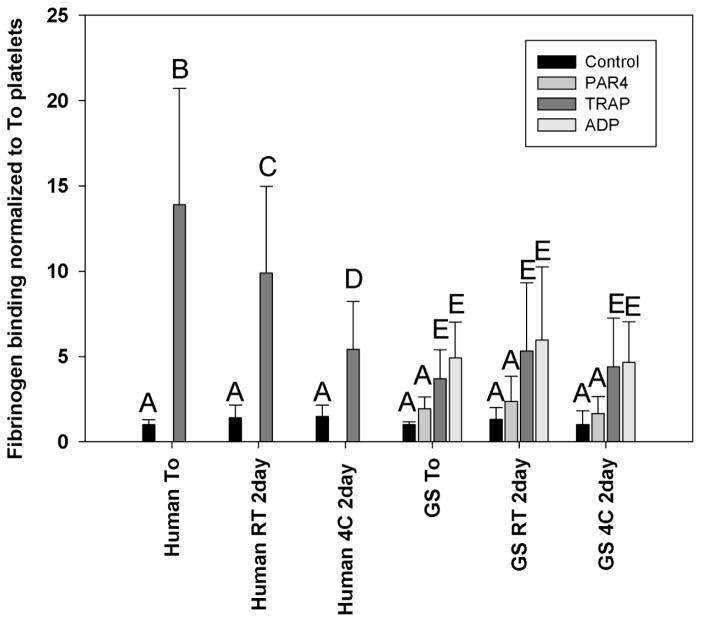
Increased fibrinogen binding to GpIIb/IIIa is a measure of platelet activation by various agonists. In fresh human platelets, TRAP caused a 10.8-fold increase in fibrinogen binding when normalized to unstimulated platelets (Figure 2). After 2 days of storage at room temperature or 4°C, normalized fibrinogen binding decreased to 62% and 32% of the fresh platelets respectively (ANOVA, p<0.01). It was unclear which agonists might stimulate ground squirrel platelets, so three different agonists were used. ADP produced a significant increase in fibrinogen binding to freshly collected ground squirrel platelets with a 4.2-fold increase, followed by TRAP with a 3.7-fold increase (ANOVA, p<0.01). PAR4 activating peptide showed a 1.7-fold increase in fibrinogen binding which was not significantly higher than controls by ANOVA. After two days of storage at room temperature or 4°C, there was no significant loss in platelet fibrinogen binding with any of the agonists (ANOVA p=0.195) (Figure 2). Human samples showed a significant interaction between storage conditions and agonist stimulation (ANOVA, P<0.001) while ground squirrel samples did not (ANOVA, P=0.968).
Figure 2.
Platelet fibrinogen binding. Platelets were isolated from non-hibernating squirrels and incubated with Alexa 488 fluorescently labeled fibrinogen. Some samples were activated with different agonists including ADP, TRAP, and PAR4 activating peptide for 5 minutes. Samples were then fixed with paraformaldehyde, analyzed on a flow cytometer, and normalized to matched unstimulated platelet fibrinogen binding. N=5 samples run in triplicate and analyzed by ANOVA, bars with different letters were significantly different, p<0.05.
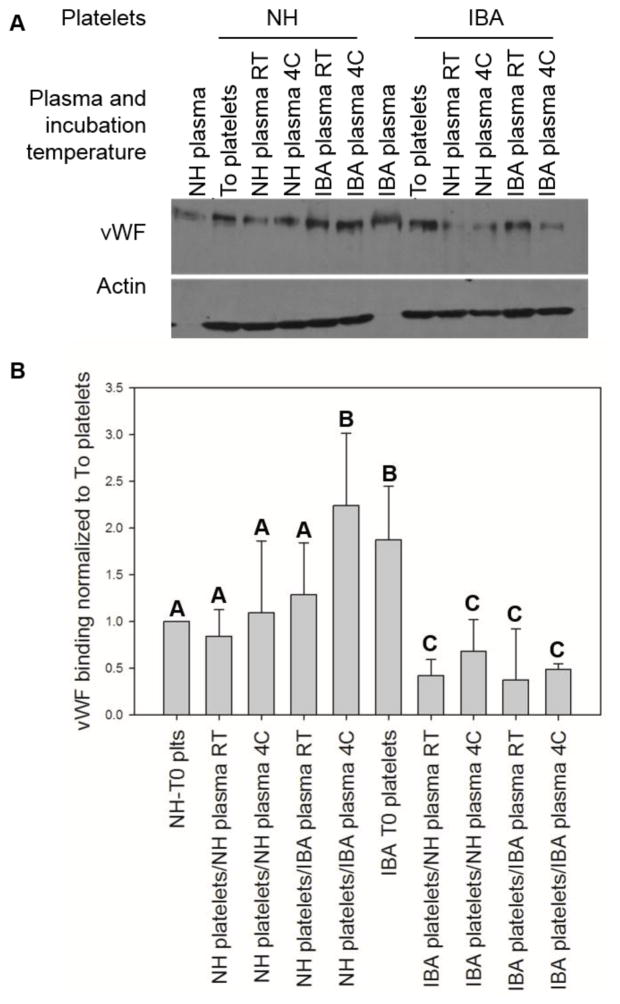
Glycoprotein GpIbα is a sub-unit of the vWF receptor on platelets, and binds other ligands including thrombospondin, P-selectin, αMβ2 (Mac-1), thrombin, Factor XI, Factor XII and kininogen (Berndt and Andrews 2011). Non-hibernating ground squirrels have 24% the plasma level of vWF as humans, and this drops an additional 10-fold during torpor (Cooper, Sell et al. 2016). Human platelets have been shown to bind increased vWF in the cold (Rumjantseva, Grewal et al. 2009). Because of the variation in vWF levels, effect of temperature on binding, and potential differences in platelets isolated from hibernating and non-hibernating ground squirrels, all three variables were changed in a triple-cross over experiment. vWF binding to non-hibernating fresh (T0) platelets was used to normalize results to the amount of vWF bound under normothermic conditions in vivo (Figure 3a). Non-hibernating ground squirrel platelet binding of vWF was not influenced significantly by storage for two days with the exception of a two-fold increase when stored in IBA plasma at 4°C. Platelets from IBA animals that had been stored in the cold for weeks in situ bound 2–4 fold less vWF than platelets from normothermic animals, regardless of the PRP storage temperature or source of plasma they were stored in for two days (Figure 3b).
Figure 3.
Effects of temperature and plasma on platelet binding. Platelets were isolated from humans, non-hibernating ground squirrels (NH), and those in an interbout arousal (IBA). Different aliquots of the washed platelets were then incubated for two days in either plasma from the non-hibernating or IBA squirrels for two days at 4°C or room temperature. Platelets were washed, and immunoblotted for vWF, size bar is 50μm on the large images and 10μm on the insets (panel A). Band densities were quantified and normalized to the To platelets (panel B). N=5 samples analyzed by student t-test, bars with different letters were significantly different, p<0.05.
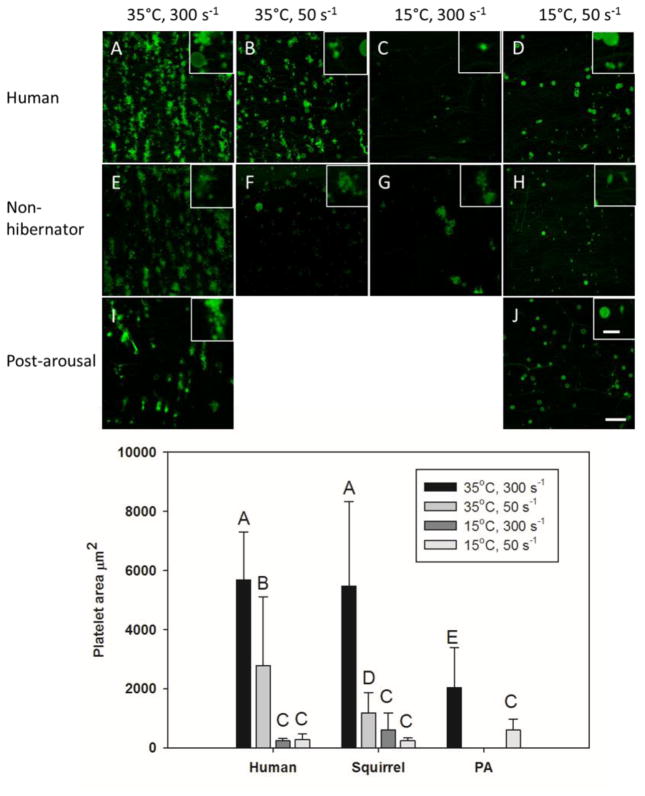
In circulation, vWF will bind to collagen exposed by endothelial cell damage. This binding is increased by high shear stress as heart rate increases. To differentiate the effects of temperature, shear rate, and the source of the platelets on their ability to bind to collagen, a microfluidic flow assay was performed. Human and ground squirrel whole blood was incubated at 15°C or 35°C and then passed through a microfluidic chamber over a 250 μm strip of type I fibrillar collagen at shear rates of 50 s−1 or 300 s−1 to simulate torpid and aroused conditions respectively (Figure 4a). At 15°C, both human and ground squirrel platelets showed a 90–95% decrease in accumulation on collagen under either shear rate compared to 35°C. At 35°C, a decrease in shear rate from 300 s−1 to 50 s−1 reduced human platelet binding by 50%, while ground squirrel platelet binding dropped by 80%. When compared to platelets from non-hibernating animals, platelets from animals collected after arousal from torpor showed a 60% decrease in binding at 35°C and 300 s−1, but a 2.5-fold increase in binding at 15°C and 50 s−1 (Figure 4b). Half of ground squirrel platelets bound to collagen at 15°C showed a ring conformation and half a rod conformation, while human platelets were all round. At 35°C all ground squirrel and human platelets were in a ring conformation.
Figure 4.
Effects of temperature and shear rate on platelet binding to collagen. Whole blood samples from humans, non-hibernating ground squirrels, and those in an IBA were incubated at 15°C or 35°C for 30 minutes. Platelets were labeled with DiOC6, and perfused over a 250μm strip of type I collagen for 5 minutes at 15°C or 35°C and shear stresses of 50 s−1 or 300 s−1 followed by fixative (panel A). Platelets were then fixed and quantified on a confocal microscope. N=5 samples analyzed by student t-test, bars with different letters were significantly different, p<0.05.
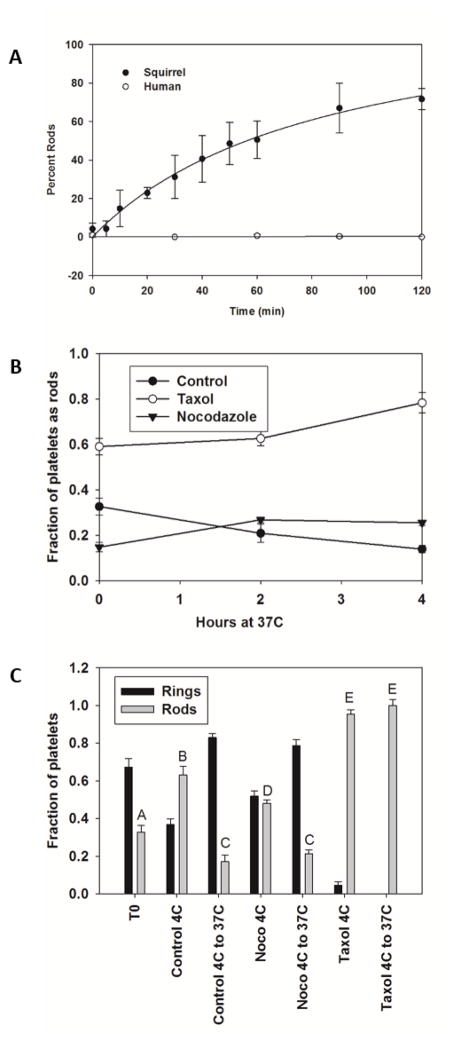
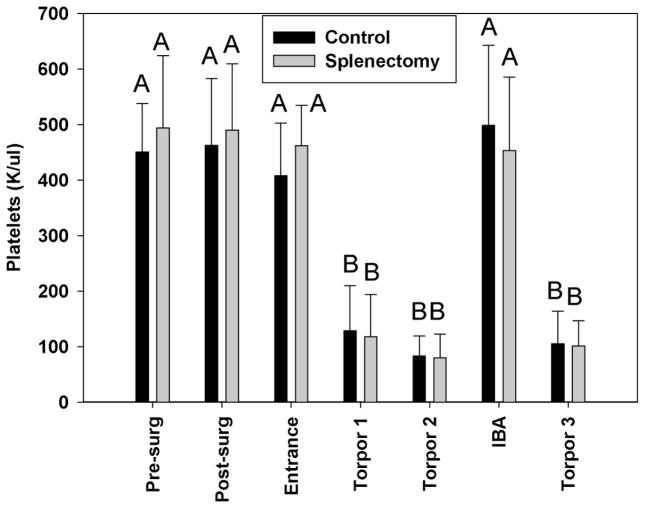
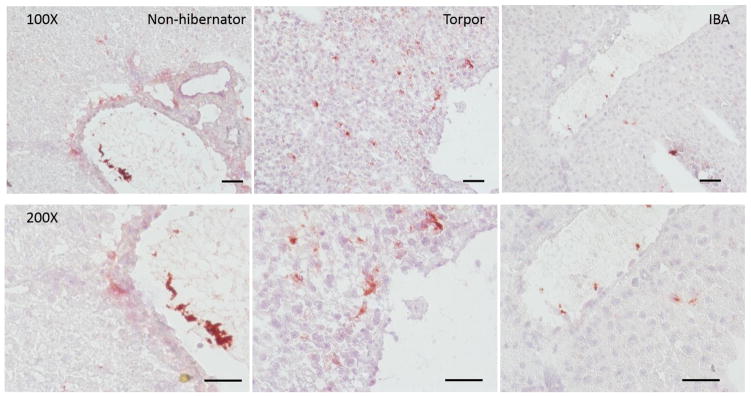
A dramatic conformational change in chilled ground squirrel platelets occurs upon warming or chilling, with a half time of 60 min (Figure 5A). Rod formation appears to be dependent on polymerization, as blocking depolymerization with taxol (Xiao, Verdier-Pinard et al. 2006) at 37°C leads to increased rod formation and prevents rods that form at 4°C from returning to rings when the platelets are rewarmed to 37°C (Figure 5B). In contrast, blocking polymerization with nocodazole (Vasquez, Howell et al. 1997) reduced rod formation (Figure 5C). One proposed function of this shape change is to sequester platelets in the spleen (Reddick, Poole et al. 1973). In splenectomized or sham operated ground squirrels there was no significant difference in the sequestration of platelets during torpor, or their release during an IBA (Figure 6). In contrast, significantly more GPIbα staining of platelets was observed in extrasinusoidal regions of the liver in torpid animals (80.0±38.1/mm2) compared with sinusoidal staining in non-hibernating and IBA animals (13.6±7.0/mm2 and 21.8±16.7/mm2 respectively, t-test p<0.05) (Figure 7).
Figure 5.
Platelet microtubule shape changes at 4°C. Panel A: Ground squirrel platelet rich plasma was incubated at 37°C for 2 hours and then place on ice. At each time point, aliquots were removed, centrifuged onto polylysine coated microscope slides, fixed and the percentage of platelets in a rod shape counted. The cold storage curve fit best to a hyperbolic curve, F= 0.312*x/(14.67+x). N=6 samples run in duplicate and time points at 30, 60, 90 and 120 minutes compared by student t-test. Panel B: Taxol treated platelets showed increased rod formation even at 37°C, while no changes were observed in control or nocodazole treated platelets. Panel C: Taxol treated platelets form rods and will not revert to rings upon rewarming to 37°C unlike control or nocodazole treated platelets. N=6 samples run in duplicate and analyzed by student t-test, bars with different letters were significantly different, p<0.05.
Figure 6.
Effects of splenectomy on platelet levels during hibernation. Ground squirrels received either a splenectomy or sham surgery during the summer. Blood samples were collected pre-and post-surgery samples, upon entrance into hibernation and during an IBA, and platelets counted on a Hemavet. During torpor, blood smears were collected without waking the animal and counted manually. N= 10 animals, analyzed by student t-test, bars with different letters were significantly different, p<0.05.
Figure 7.
Platelets are observed in extrasinusoidal regions of liver during torpor. Frozen sections of liver were prepared from non-hibernating, torpid, and IBA animals. The sections were incubated with rabbit anti-GpIbα and goat anti-rabbit HRP antibodies followed by the chromogenic substrate AEC. Sections were then counterstained with hematoxylin, size bars are 100μm (n=3).
DISCUSSION
The reduction of both platelets and leukocytes during torpor was first reported over 50 years ago (Lechler and Penick 1963). However, the activity of these platelets has only been assayed at a general level (Cooper, Richters et al. 2012, de Vrij, Vogelaar et al. 2014). In this study we examined platelet binding to fibrinogen, vWF, and collagen. Fibrinogen binding can be used as a measure of platelet activation and GpIIb/IIIa activity. Ground squirrel platelet ability to bind fibrinogen after activation with agonists was more resistant to storage at room temperature or in the cold than were human platelets, showing no significant decrease after two days of storage at 4°C or 25°C. This is consistent with restored platelet activity measured by thromboelastography on whole blood samples taken from ground squirrels two hours post-arousal (Cooper, Richters et al. 2012). Similarly in humans, both thromboelastography and platelet aggregation were preserved in blood stored up to 21 days at 4°C compared with room temperature (Pidcoke, McFaul et al. 2013). Finally, in mice, binding of fluorescent-labeled fibrinogen increases at 34°C and 31°C after TRAP exposure (Lindenblatt, Menger et al. 2005). In these three species, cold storage does not appear to decrease the ability of platelets to aggregate and form stable clots, however, all of these assays are done under static conditions and may be different under flow. One caveat in this study was that ground squirrel platelet activation required higher levels of agonist than published for other species (Chung, Jurasz et al. 2002, Nylander, Mattsson et al. 2006, Duvernay, Young et al. 2013), this could be due in part to addition of PGE1 to samples before the 48 hour storage period.
GpIbα is a subunit of the vWF receptor, and its activity can be measured by vWF binding. Plasma vWF is made primarily by endothelial cells and is necessary to support hemostasis at high shear stresses, while platelet vWF can compensate partially for decreased plasma vWF (Kanaji, Fahs et al. 2012). This is consistent with ground squirrel plasma vWF decreasing in hibernation along with decreased endothelial cell derived lung vWF mRNA levels (Cooper, Sell et al. 2016), but no change in megakaryocyte derived bone marrow vWF mRNA (Cooper, Sell et al. 2016). vWF binding to human platelets increases with cold storage (Rumjantseva, Grewal et al. 2009), while long term cold storage decreases surface GpIbα (Reddoch, Pidcoke et al. 2014). By storing platelets from hibernating and non-hibernating ground squirrels in each plasma, differences in vWF binding could be attributed to a platelet or plasma effect. Platelets from non-hibernating ground squirrels bound roughly equal to or more vWF regardless of plasma type with only a slight increase in binding when stored at 4°C. Conversely, platelets from ground squirrels in an IBA bound 2–4 fold less vWF than the non-hibernating platelets, regardless of temperature or plasma type. These results suggest the level of vWF binding is influenced by the source of the platelets and not the plasma or temperature of storage. This could be due to either fewer GpIbα or other vWF receptors on the cell surface, decreased vWF binding affinity, or decreased signal transduction upon binding. Structural changes through tubulin repolymerization or the formation of lipid rafts could also affect vWF binding. The decrease in vWF binding could prevent platelet adhesion to collagen as seen in GpIbα−/− mice and patients with GpIbα deficiency in Bernard-Soulier syndrome (Berndt and Andrews 2011). One drawback of this assay and other clotting assays like thromboelastography is that they are static, while hemostasis occurs under flow. Another drawback of this assay is that it cannot distinguish between internal vWF in alpha granules and externally bound vWF, as a result direct comparisons in changes in binding were measured with the assumption that intracellular vWF did not change.
Platelet binding to vWF, and subsequently accumulation on collagen, is increased when shear stress stretches vWF, exposing A1 domain binding sites. At a temperature and shear rate simulating the conditions in a non-hibernating ground squirrel (35°C, 300 s−1) platelets from IBA animals that had been stored in situ at 4–8°C for weeks showed less accumulation on collagen than platelets from non-hibernating squirrels. Ground squirrel platelets also displayed a greater decrease in binding under low flow conditions than did human platelets. The binding of all platelets studied were decreased at 15°C, regardless of the shear rate, possibly because plasma vWF cannot form long cell-surface strings at temperatures below 17°C which would interfere with its ability to bind to platelets (Hewlett, Zupančič et al. 2011). While a body temperature of 15°C is not physiological for humans, this decrease in collagen or vWF binding could protect hibernating mammals from activation of primary hemostasis as they pass in and out of torpor. Ground squirrels can achieve body temperatures of 4–8°C, but we could only chill our microscope stage to 15°C. However, since platelet accumulation on collagen was significantly decreased at 15°C, no further decrease would be likely at 4°C. Viscosity is a strong function of temperature, typically with exponential or power law dependence. As a result, exposure of the A1 domain of vWF is reduced and GP1bα-vWF bonds are less likely to form at lower temperatures for the same shear rate because of a reduced shear stress (shear stress = viscosity x shear rate). This reduced force on vWF and platelet is potentially another antithrombotic effect of lowering body temperature. The hematocrit of blood could also affect viscosity, but it does not change in ground squirrels through their hibernal annual cycle (Hampton, Nelson et al. 2010, Cooper, Sell et al. 2016).
Platelets from hibernating ground squirrels go through dramatic shape changes upon chilling. This is due to rearrangement of a circumferential band of microtubules, resulting in long rod like projections. When this was first reported, one proposed function was mechanical sequestration in the spleen followed by the release of the platelets upon warming. During an IBA, ground squirrels will warm their bodies from 4°C to 37°C in two hours by metabolic activity of brown adipose tissue deposits, and can cool down again in about four hours as they go into torpor (Carey, Andrews et al. 2003). The kinetics of rod formation in platelets indicates that the conformational change could happen within this time frame. However, this study in ground squirrels confirms work done by de Vrij (de Vrij, Vogelaar et al. 2014) in hamsters, that the spleen is not the primary or sole location of platelet sequestration during torpor, as ground squirrels lacking a spleen could both sequester platelets during torpor and release them in an IBA. In non-hibernating and IBA animals platelet GPIbα staining is seen in the lumen of liver sinusoids, however during torpor the staining leaves the sinusoids and becomes diffuse throughout the liver. The staining distribution of GPIbα in the liver is consistent with platelet storage in the liver during torpor and is supported by observations in the hamster liver (de Vrij, personal communication). Liver sinusoids contain Kupffer cells which endocytose chilled platelets (Rumjantseva, Grewal et al. 2009) and stellate cells expressing ADAMTS-13 which degrades vWF (Vollmar and Menger 2009, Lenting, Casari et al. 2012), so sequestration out of the sinusoids may protect platelets from clearance by the liver. A drawback of this study was the use of frozen sections instead of paraffin embedded sections which would give better resolution of liver structure. However, given the dramatic differences between torpid and IBA or non-hibernating samples, we do not feel that the results would be different with more resolution. Unlike most mammalian tubulins, ground squirrel microtubules grow in the cold, a process stimulated by taxol and blocked by nocodazole. The effects of taxol were more complete than those of nocodazole, possible explanations could include that depolymerization is faster than polymerization, or that nocodazole is not as an effective inhibitor as taxol. This conformational change could be coincidental with sequestration, but possible physiological roles include the rods forming extensions that trap the platelets in the liver sinusoids or sterically block phagocytosis (Hoffmeister, Felbinger et al. 2003, Rumjantseva, Grewal et al. 2009). A lack of tubulin depolymerization in the cold could also maintain the overall structure of platelet surface receptors like GPIbα in chilled platelets preventing their clearance (Gitz, Koopman et al. 2013).
Ground squirrel platelets had reduced adhesion to vWF and thus collagen under low flow and after storage at cold temperatures, while still being activated by external agonists. This should protect the animals from the formation of clots during torpor, but allow them to restore platelet function if injured upon arousal. The decreased binding under low flow could also protect an animal as it rapidly changes both blood flow and temperature going into and out of an IBA. It remains to be determined how platelets are retained in the liver without being activated or cleared by phagocytosis yet still retain activity after weeks in the cold.
Acknowledgments
We would like to thank Amy Cooper, for her care of the ground squirrels and surgical expertise. This work was supported by grants from the NIH (1R15HL093680) to S.C. and NSF CAREER (CBET-1351672), American Heart Association (14GRNT20410094), and the National Institutes of Health (R01HL120728, R21NS082933) to K.N. S.L. and C.L. received a UW-La Crosse Dean’s Distinguished Undergraduate Summer fellowship.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author Contributions
S.C. and K.N. conceived the ideas and designed the experiments. A.K. and M.Z. performed the platelet vWF and fibrinogen binding assays. S.L., K.D., T. Theisen, and M.L. performed microfluidics. M.G. and T. Tenpas performed immunohistochemistry. X.L., K.B. and S.H. measured microtubule kinetics. S.M. and C.L. performed splenectomy experiments.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- Berndt MC, Andrews RK. Bernard-Soulier syndrome. Haematologica. 2011;96:355–359. doi: 10.3324/haematol.2010.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chung AWY, Jurasz P, Hollenberg MD, Radomski MW. Mechanisms of action of proteinase-activated receptor agonists on human platelets. British Journal of Pharmacology. 2002;135:1123–1132. doi: 10.1038/sj.bjp.0704559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Sell S, Nelson L, Hawes J, Benrud JA, Kohlnhofer BM, Burmeister BR, Flood VH. Von Willebrand factor is reversibly decreased during torpor in 13-lined ground squirrels. J Comp Physiol B. 2016;186:131–139. doi: 10.1007/s00360-015-0941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, Richters KE, Melin TE, Liu ZJ, Hordyk PJ, Benrud RR, Geiser LR, Cash SE, Simon Shelley C, Howard DR, Ereth MH, Sola-Visner MC. The hibernating 13-lined ground squirrel as a model organism for potential cold storage of platelets. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1202–1208. doi: 10.1152/ajpregu.00018.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, Sell SS, Fahrenkrog M, Wilkinson K, Howard DR, Bergen H, Cruz E, Cash SE, Andrews MT, Hampton M. Effects of hibernation on bone marrow transcriptome in thirteen-lined ground squirrels. Physiol Genomicsphysiolgenomics. 2016 doi: 10.1152/physiolgenomics.00120.2015. 00120 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij EL, Vogelaar PC, Goris M, Houwertjes MC, Herwig A, Dugbartey GJ, Boerema AS, Strijkstra AM, Bouma HR, Henning RH. Platelet dynamics during natural and pharmacologically induced torpor and forced hypothermia. PLoS One. 2014;9:e93218. doi: 10.1371/journal.pone.0093218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay M, Young S, Gailani D, Schoenecker J, Hamm H. Protease-Activated Receptor (PAR) 1 and PAR4 Differentially Regulate Factor V Expression from Human Platelets. Molecular Pharmacology. 2013;83:781–792. doi: 10.1124/mol.112.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelfriet CP, Reesink HW, Blajchman MA, Muylle L, Kjeldsen-Kragh J, Kekomaki R, Yomtovian R, Hocker P, Stiegler G, Klein HG, Soldan K, Barbara J, Slopecki A, Robinson A, Seyfried H. Bacterial contamination of blood components. Vox Sang. 2000;78:59–67. doi: 10.1159/000031151. [DOI] [PubMed] [Google Scholar]
- Faraday MDN, Rosenfeld AMDB. In Vitro Hypothermia Enhances Platelet GPIIb-IIIa Activation and P-Selectin Expression Anesthesiology. 1998;88:1579–1585. doi: 10.1097/00000542-199806000-00022. [DOI] [PubMed] [Google Scholar]
- Frelinger AL, Iii, Furman MI, Barnard MR, Krueger LA, Dae MW, Michelson AD. Combined effects of mild hypothermia and glycoprotein IIb/IIIa antagonists on platelet–platelet and leukocyte–platelet aggregation. The American Journal of Cardiology. 2003;92:1099–1101. doi: 10.1016/j.amjcard.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Budde U, Schulz A, Kessler CM, Fisseau C, Kannicht C. Flow-based measurements of von Willebrand factor (VWF) function: binding to collagen and platelet adhesion under physiological shear rate. Thromb Res. 2010;125:239–245. doi: 10.1016/j.thromres.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Gitz E, Koopman CD, Giannas A, Koekman CA, van den Heuvel DJ, Deckmyn H, Akkerman JW, Gerritsen HC, Urbanus RT. Platelet interaction with von Willebrand factor is enhanced by shear-induced clustering of glycoprotein Ibalpha. Haematologica. 2013;98:1810–1818. doi: 10.3324/haematol.2013.087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton M, Nelson BT, Andrews MT. Circulation and metabolic rates in a natural hibernator: an integrative physiological model. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1478–1488. doi: 10.1152/ajpregu.00273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett L, Zupancic G, Mashanov G, Knipe L, Ogden D, Hannah MJ, Carter T. Temperature-dependence of Weibel-Palade body exocytosis and cell surface dispersal of von Willebrand factor and its propolypeptide. PLoS One. 2011;6:e27314. doi: 10.1371/journal.pone.0027314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett L, Zupančič G, Mashanov G, Knipe L, Ogden D, Hannah MJ, Carter T. Temperature-Dependence of Weibel-Palade Body Exocytosis and Cell Surface Dispersal of von Willebrand Factor and Its Propolypeptide. PLoS ONE. 2011;6:e27314. doi: 10.1371/journal.pone.0027314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister KM, Falet H, Toker A, Barkalow KL, Stossel TP, Hartwig JH. Mechanisms of cold-induced platelet actin assembly. J Biol Chem. 2001;276:24751–24759. doi: 10.1074/jbc.M011642200. [DOI] [PubMed] [Google Scholar]
- Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- Hogberg C, Erlinge D, Braun OO. Mild hypothermia does not attenuate platelet aggregation and may even increase ADP-stimulated platelet aggregation after clopidogrel treatment. Thromb J. 2009;7:2. doi: 10.1186/1477-9560-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano JE, Jr, Bergmeier W, Tiwari S, Falet H, Hartwig JH, Hoffmeister KM, Andre P, Wagner DD, Shivdasani RA. Mechanisms and implications of platelet discoid shape. Blood. 2003;101:4789–4796. doi: 10.1182/blood-2002-11-3491. [DOI] [PubMed] [Google Scholar]
- Jacobs MR, Palavecino E, Yomtovian R. Don’t bug me: the problem of bacterial contamination of blood components--challenges and solutions. Transfusion. 2001;41:1331–1334. doi: 10.1046/j.1537-2995.2001.41111331.x. [DOI] [PubMed] [Google Scholar]
- Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet versus endothelial VWF to platelet adhesion and hemostasis. Journal of thrombosis and haemostasis : JTH. 2012;10:1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler E, Penick GD. Blood clotting defect in hibernating ground squirrels (Citellus tridecemlineatus) Am J Physiol. 1963;205:985–988. doi: 10.1152/ajplegacy.1963.205.5.985. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Wallbank AM, Dennis KA, Wufsus AR, Davis KM, Rana K, Neeves KB. On-chip recalcification of citrated whole blood using a microfluidic herringbone mixer. Biomicrofluidics. 2015;9:064106. doi: 10.1063/1.4935863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. Journal of Thrombosis and Haemostasis. 2012;10:2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- Lindenblatt N, Menger MD, Klar E, Vollmar B. Sustained hypothermia accelerates microvascular thrombus formation in mice. Am J Physiol Heart Circ Physiol. 2005;289:H2680–2687. doi: 10.1152/ajpheart.00425.2005. [DOI] [PubMed] [Google Scholar]
- Mackman N, Davis GE. Blood coagulation and blood vessel development: is tissue factor the missing link? Arterioscler Thromb Vasc Biol. 2011;31:2364–2366. doi: 10.1161/ATVBAHA.111.236703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie GA, Plow EF, Edgington TS. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979;254:5357–5363. [PubMed] [Google Scholar]
- Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71:633–640. [PubMed] [Google Scholar]
- Neeves KB, Diamond SL. A membrane-based microfluidic device for controlling the flux of platelet agonists into flowing blood. Lab Chip. 2008;8:701–709. doi: 10.1039/b717824g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander S, Mattsson C, Lindahl TL. Characterisation of species differences in the platelet ADP and thrombin response. Thrombosis Research. 2006;117:543–549. doi: 10.1016/j.thromres.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Patel-Hett S, Richardson JL, Schulze H, Drabek K, Isaac NA, Hoffmeister K, Shivdasani RA, Bulinski JC, Galjart N, Hartwig JH, Italiano JE., Jr Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood. 2008;111:4605–4616. doi: 10.1182/blood-2007-10-118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC, Aden JK, Jones JA, Bryant RS, Scherer MR, Reddy HL, Goodrich RP, Cap AP. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53:137S–149S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivorun EB, Sinnamon WB. Blood coagulation studies in normothermic, hibernating, and aroused Spermophilus franklini. Cryobiology. 1981;18:515–520. doi: 10.1016/0011-2240(81)90212-1. [DOI] [PubMed] [Google Scholar]
- Rana K, Neeves KB. Blood flow and mass transfer regulation of coagulation. Blood Rev. 2016;30:357–368. doi: 10.1016/j.blre.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick RL, Poole BL, Penick GD. Thrombocytopenia of hibernation. Mechanism of induction and recovery. Lab Invest. 1973;28:270–278. [PubMed] [Google Scholar]
- Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4 °C and 22 °C. Shock (Augusta, Ga) 2014;41:54–61. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik G, Reznik-Schuller H, Emminger A, Mohr U. Comparative studies of blood from hibernating and nonhibernating European hamsters (Cricetus cricetus L) Lab Anim Sci. 1975;25:210–215. [PubMed] [Google Scholar]
- Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharbert G, Kalb ML, Essmeister R, Kozek-Langenecker SA. Mild and moderate hypothermia increases platelet aggregation induced by various agonists: a whole blood in vitro study. Platelets. 2010;21:44–48. doi: 10.3109/09537100903420269. [DOI] [PubMed] [Google Scholar]
- Smith DE, Lewis YS, Svihla G. Prolongation of clotting time in the dormant bat (Myotis lucifugus) Experientia. 1954;10:218. doi: 10.1007/BF02159280. [DOI] [PubMed] [Google Scholar]
- Springer DL, Miller JH, Spinelli SL, Pasa-Tolic L, Purvine SO, Daly DS, Zangar RC, Jin S, Blumberg N, Francis CW, Taubman MB, Casey AE, Wittlin SD, Phipps RP. Platelet proteome changes associated with diabetes and during platelet storage for transfusion. J Proteome Res. 2009;8:2261–2272. doi: 10.1021/pr800885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen P, Lehto E. Prolongation of clotting time in hibernation. Experientia. 1952;8:65. doi: 10.1007/BF02139024. [DOI] [PubMed] [Google Scholar]
- Svihla A, Bowman H, Pearson R. Prolongation of blood clotting time in the dormant hamster. Science. 1952;115:272. doi: 10.1126/science.115.2984.272. [DOI] [PubMed] [Google Scholar]
- Svihla A, Bowman H, Ritenour R. Relation of prothrombin to the prolongation of clotting time in aestivating ground squirrels. Science. 1952;115:306–307. doi: 10.1126/science.115.2986.306. [DOI] [PubMed] [Google Scholar]
- Svihla A, Bowman H, Ritenour R. Stimuli and their effects on awakening of dormant ground squirrels. Am J Physiol. 1953;172:681–683. doi: 10.1152/ajplegacy.1953.172.3.681. [DOI] [PubMed] [Google Scholar]
- Thon JN, Schubert P, Devine DV. Platelet storage lesion: a new understanding from a proteomic perspective. Transfus Med Rev. 2008;22:268–279. doi: 10.1016/j.tmrv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- van der Wal DE, Du VX, Lo KS, Rasmussen JT, Verhoef S, Akkerman JW. Platelet apoptosis by cold-induced glycoprotein Ibalpha clustering. J Thromb Haemost. 2010;8:2554–2562. doi: 10.1111/j.1538-7836.2010.04043.x. [DOI] [PubMed] [Google Scholar]
- Van Poucke S, Stevens K, Marcus AE, Lance M. Hypothermia: effects on platelet function and hemostasis. Thromb J. 2014;12:31. doi: 10.1186/s12959-014-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Molecular Biology of the Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- Vostal JG, Mondoro TH. Liquid cold storage of platelets: a revitalized possible alternative for limiting bacterial contamination of platelet products. Transfus Med Rev. 1997;11:286–295. [PubMed] [Google Scholar]
- Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest. 1977;60:390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967;30:625–635. [PubMed] [Google Scholar]
- White JG, Rao GH. Microtubule coils versus the surface membrane cytoskeleton in maintenance and restoration of platelet discoid shape. Am J Pathol. 1998;152:597–609. [PMC free article] [PubMed] [Google Scholar]
- Xavier RG, White AE, Fox SC, Wilcox RG, Heptinstall S. Enhanced platelet aggregation and activation under conditions of hypothermia. Thromb Haemost. 2007;98:1266–1275. doi: 10.1160/th07-03-0189. [DOI] [PubMed] [Google Scholar]
- Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by Taxol. Proceedings of the National Academy of Sciences. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatzman ML. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology. 1984;21:593–614. doi: 10.1016/0011-2240(84)90220-7. [DOI] [PubMed] [Google Scholar]
- Zucker MB, Nachmias VT. Platelet activation. Arteriosclerosis. 1985;5:2–18. doi: 10.1161/01.atv.5.1.2. [DOI] [PubMed] [Google Scholar]