Summary
Background
The lipoprotein(a) pathway is a causal factor in coronary heart disease. We used a genetic approach to distinguish the relevance of two distinct components of this pathway, apolipoprotein(a) isoform size and circulating lipoprotein(a) concentration, to coronary heart disease.
Methods
In this mendelian randomisation study, we measured lipoprotein(a) concentration and determined apolipoprotein(a) isoform size with a genetic method (kringle IV type 2 [KIV2] repeats in the LPA gene) and a serum-based electrophoretic assay in patients and controls (frequency matched for age and sex) from the Pakistan Risk of Myocardial Infarction Study (PROMIS). We calculated odds ratios (ORs) for myocardial infarction per 1-SD difference in either LPA KIV2 repeats or lipoprotein(a) concentration. In a genome-wide analysis of up to 17 503 participants in PROMIS, we identified genetic variants associated with either apolipoprotein(a) isoform size or lipoprotein(a) concentration. Using a mendelian randomisation study design and genetic data on 60 801 patients with coronary heart disease and 123 504 controls from the CARDIoGRAMplusC4D consortium, we calculated ORs for myocardial infarction with variants that produced similar differences in either apolipoprotein(a) isoform size in serum or lipoprotein(a) concentration. Finally, we compared phenotypic versus genotypic ORs to estimate whether apolipoprotein(a) isoform size, lipoprotein(a) concentration, or both were causally associated with coronary heart disease.
Findings
The PROMIS cohort included 9015 patients with acute myocardial infarction and 8629 matched controls. In participants for whom KIV2 repeat and lipoprotein(a) data were available, the OR for myocardial infarction was 0·93 (95% CI 0·90–0·97; p<0·0001) per 1-SD increment in LPA KIV2 repeats after adjustment for lipoprotein(a) concentration and conventional lipid concentrations. The OR for myocardial infarction was 1·10 (1·05–1·14; p<0·0001) per 1-SD increment in lipoprotein(a) concentration, after adjustment for LPA KIV2 repeats and conventional lipids. Genome-wide analysis identified rs2457564 as a variant associated with smaller apolipoprotein(a) isoform size, but not lipoprotein(a) concentration, and rs3777392 as a variant associated with lipoprotein(a) concentration, but not apolipoprotein(a) isoform size. In 60 801 patients with coronary heart disease and 123 504 controls, OR for myocardial infarction was 0·96 (0·94–0·98; p<0·0001) per 1-SD increment in apolipoprotein(a) protein isoform size in serum due to rs2457564, which was directionally concordant with the OR observed in PROMIS for a similar change. The OR for myocardial infarction was 1·27 (1·07–1·50; p=0·007) per 1-SD increment in lipoprotein(a) concentration due to rs3777392, which was directionally concordant with the OR observed for a similar change in PROMIS.
Interpretation
Human genetic data suggest that both smaller apolipoprotein(a) isoform size and increased lipoprotein(a) concentration are independent and causal risk factors for coronary heart disease. Lipoprotein(a)-lowering interventions could be preferentially effective in reducing the risk of coronary heart disease in individuals with smaller apolipoprotein(a) isoforms.
Funding
British Heart Foundation, US National Institutes of Health, Fogarty International Center, Wellcome Trust, UK Medical Research Council, UK National Institute for Health Research, and Pfizer.
Introduction
Epidemiological studies and human genetic analyses have suggested that the lipoprotein(a) pathway is causally associated with coronary heart disease and aortic stenosis.1, 2, 3, 4, 5 Lipoprotein(a) is an LDL-like particle synthesised by the liver that consists of an apolipoprotein B100 molecule covalently linked to a very large glycoprotein known as apolipoprotein(a).6, 7 The striking heterogeneity in the size of apolipoprotein(a) in the general population (eg, variation of up to ten times) is believed to be at least partly due to copy number variation in the LPA gene, which determines the number of repeats in the kringle IV type 2 (KIV2) protein domain present on apolipoprotein(a).7
Research in context.
Evidence before this study
We searched PubMed with the search terms “lipoprotein(a)”, “apolipoprotein(a) isoform protein size”, “coronary heart disease”, “mendelian randomisation”, and “oxidised phospholipids”, for observational or genetic epidemiological studies published in English before Jan 10, 2017, that had assessed lipoprotein(a) concentration, apolipoprotein(a) isoform size, measures of oxidised phospholipids, genome-wide genotypes, and clinical coronary heart disease outcomes in a common set of participants. Our search identified a few studies that had recorded combinations of some of the factors listed above. These studies reported results consistent with a causal role for the lipoprotein(a) pathway in coronary heart disease. However, we did not identify any studies that had concomitantly assessed all the relevant factors listed above in one population. Therefore, whether apolipoprotein(a) isoform size, soluble lipoprotein(a) concentration, or both, are causally associated with coronary heart disease is not known. Furthermore, whether oxidised phospholipids on apolipoprotein B100 are mediators of lipoprotein(a) pathways in coronary heart disease risk is unknown.
Added value of this study
The key distinctive feature of our study was its concomitant assessment of lipoprotein(a) concentration, apolipoprotein(a) isoform size (using complementary genotypic and phenotypic methods), oxidised phospholipids on apolipoprotein B-100, genome-wide genotypes, and confirmed first-onset myocardial infarction in over 15 000 patients and controls. We did integrative analyses by leveraging the principle of mendelian randomisation and supplemented our new data by accessing results from global genetics consortia of coronary heart disease and several cardiovascular traits.
Implications of all the available evidence
Our data have suggested three main findings. First, both smaller apolipoprotein(a) isoform size and elevated lipoprotein(a) concentration are independent and causal risk factors for coronary heart disease. Second, there are shallow and broadly continuous relationships (in opposite directions) of coronary heart disease risk with apolipoprotein(a) isoform size and with lipoprotein(a) concentration, even after these traits were adjusted for each other. Third, human genetic data support previous suggestions that oxidative damage could mediate the pathological effects of lipoprotein(a) on coronary heart disease. Our findings advance understanding of the causal relevance of the lipoprotein(a) pathway to coronary heart disease and suggest that interventions that lower lipoprotein(a) concentration could be more effective in reducing coronary heart disease risk in individuals who have smaller apolipoprotein(a) isoforms than individuals with larger isoforms.
Apolipoprotein(a) isoform size and lipoprotein(a) concentration are inversely correlated with each other, and have directionally opposite relationships with coronary heart disease risk.3 Findings from studies have generally showed lower relative risk for coronary heart disease in people with larger apolipoprotein(a) isoform size,3 and higher relative risk for coronary heart disease in people with higher lipoprotein(a) concentration.1, 3 Drugs have been developed to substantially reduce concentrations of lipoprotein(a).8, 9 However, whether apolipoprotein(a) isoform size, soluble lipoprotein(a) concentration, or both are causally relevant to coronary heart disease is unknown.
Using the principle of mendelian randomisation, we did a series of human genetic and biomarker analyses to investigate the causal effects of apolipoprotein(a) isoform size and lipoprotein(a) concentration on the risk of coronary heart disease. We also measured oxidised phospholipids on apolipoprotein B-100 (OxPL-apoB) to investigate previous suggestions10 of a potential role of OxPL-apoB in mediating the association of lipoprotein(a) pathway in coronary heart disease.
Methods
Study overview
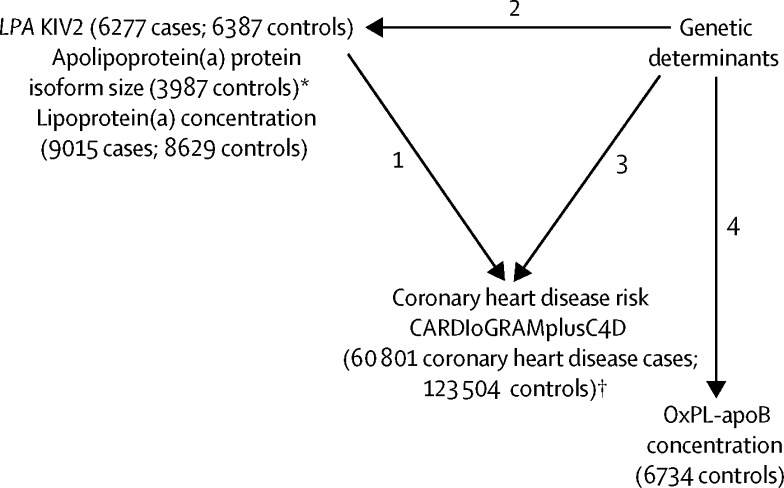
Our study had several inter-related components (figure 1). First, we calculated odds ratios (ORs) for myocardial infarction with measured values of LPA KIV2 repeats and with lipoprotein(a) concentration, adjusting them for each other. Second, to identify genetic variants exclusively associated with either apolipoprotein(a) protein isoform size or lipoprotein(a) concentration, we did genome-wide association analysis and investigated the likelihood of specificity of the genetic variants identified by analysing them in relation to established and emerging cardiovascular risk factors. Third, to test for causality, we compared ORs for coronary heart disease associated with either LPA KIV2 repeats or lipoprotein(a) concentration with similar differences produced by their trait-specific genetic variants. Fourth, we analysed soluble OxPL-apoB concentrations in relation to genome-wide genotypes, including those relevant to apolipoprotein(a) isoform size or lipoprotein(a) concentration, to investigate the relevance of OxPL-apoB in mediating the association of lipoprotein(a) pathway in coronary heart disease risk.
Figure 1.
Overview of analyses
Analysis 1: ORs for myocardial infarction with measured values of LPA KIV2 repeats and lipoprotein(a) concentration, adjusted for each other. Analysis 2: Genome-wide association analyses of apolipoprotein(a) protein isoform size and lipoprotein(a) concentration and identification of variants that are exclusively associated with either apolipoprotein(a) protein isoform size or lipoprotein(a) concentration. Analysis 3: Test for causality by comparison of ORs for coronary heart disease associated with either LPA KIV2 repeats or lipoprotein(a) concentration with equivalent differences produced by their trait-specific genetic variants. Analysis 4: Study of soluble OxPL-apoB concentrations in relation to genome-wide genotypes. KIV2=kringle IV type 2. OR=odd ratio. OxPL-apoB=oxidised phospholipid on apolipoprotein B100. *Data only used in genetic analyses. †Data used in mendelian randomisation analyses.
The PROMIS study was approved by the Institutional Review Board at the Center for Non-Communicable Diseases (Karachi, Pakistan).
Participants
We assayed LPA KIV2 repeats, apolipoprotein(a) protein isoform size, lipoprotein(a) concentration, and genome-wide genotypes in patients with acute myocardial infarction and frequency-matched controls in the Pakistan Risk of Myocardial Infarction Study (PROMIS),11 a case-control study of patients with confirmed first-onset acute myocardial infarction recruited from seven centres in five cities in Pakistan. Patients were eligible if they had a myocardial infarction and had characteristic symptoms of an event within 24 h of hospital admission, typical changes on electrocardiogram (ECG), and a positive troponin-I test. Controls were hospital visitors, concurrently identified in the same hospitals as patients with myocardial infarction, who did not have a self-reported history of cardiovascular disease or ECG changes consistent with a previous myocardial infarction. Controls were matched to myocardial infarction cases by sex and age (5-year bands). Non-fasting blood samples (with the time since last meal and time since onset of chest symptoms recorded) were taken from each participant and centrifuged within 45 min of venepuncture. Serum samples collected with serum separating tubes were stored at −80°C.
Based on sample availability, we selected a group of 9015 cases and 8629 controls for assay of lipoprotein(a) concentration and lipid-related markers. We had sufficient assay resources to measure LPA KIV2 repeats in 6277 cases and 6387 controls, OxPL-apoB in 8406 controls, and apolipoprotein(a) protein isoform size in 3987 controls. Data were available to the PROMIS investigators. Participants in PROMIS provided written informed consent.
To investigate whether single nucleotide polymorphisms for either apolipoprotein(a) protein isoform size or lipoprotein(a) concentration were relevant to coronary heart disease and cardiovascular traits, we accessed results from the largest available genetic consortia of relevant outcomes and traits: CARDIoGRAMplusC4D consortium (60 801 coronary heart disease cases, 123 504 controls12), Global BPgen Consortium (134 433 participants with blood pressure values13), Global Lipids Genetics Consortium (188 577 participants with conventional lipid fractions14), Genetic Investigation of ANthropometric Traits (183 727 participants with anthropometric traits15), and Meta-Analyses of Glucose and Insulin-related traits Consortium (46 368 participants with glycaemic traits16).
Procedures
Technicians were not aware of the phenotypic status of the participants' samples. We measured apolipoprotein(a) isoform size with complementary genotypic and phenotypic methods. We genotyped LPA KIV2 repeats in PROMIS participants with a validated quantitative real-time polymerase chain reaction (qPCR) assay that measured copy number variation repeats in exon 4 of the LPA gene.4 We did a multiplex qPCR with probes for the KIV2 sequence with albumin as a control gene. Primers specific for the KIV2 sequence were used to amplify 106 base pairs of exon 4 of the LPA coding sequence. All reactions were carried out in 96-well plates with each plate containing nine calibration controls with a known number of KIV2 repeats. The LPA KIV2 repeat for each sample was established by calculating the difference in cycle thresholds (ΔCT) between the KIV2 sequence and albumin probes. We assessed serum apolipoprotein(a) protein isoforms in a randomly selected subset of participants with electrophoresis and immunoblotting.17 Apolipoprotein(a) isoform size was reported as the size of the major isoform with the highest density. The size of the major apolipoprotein(a) isoform measured with this method is directly proportional to the number of KIV2 domains of the major isoform.17
We measured lipoprotein(a) concentration using methods insensitive to apolipoprotein(a) isoform size heterogeneity in two laboratories. We used a highly sensitive immunoturbidimetric assay (Denka Seiken, San Jose, CA, USA) in all PROMIS participants.18 We also assessed lipoprotein(a) concentration in a subset of participants with an in-house ELISA that used a monoclonal antibody against apolipoprotein(a), does not recognise the KIV2 domain and, hence, is independent of apolipoprotein(a) isoform size.
We measured OxPL-apoB using chemiluminescent ELISA as described previously.19 Briefly, monoclonal antibody MB47 was added to microtiter well plates to bind a saturating amount of apolipoprotein B100, plasma was added (1:50 dilution), and oxidised phospholipids detected with biotinylated murine monoclonal antibody E06. This measurement represents the immunoreactivity of monoclonal antibody E06 to OxPL-apoB containing-lipoproteins and is not a direct chemical measure of oxidised phospholipids in plasma. The coefficients of variation for lipoprotein(a) concentration, apolipoprotein(a) protein size, LPA KIV2 repeats, and OxPL-apoB concentration were all less than 5%. We also measured several additional soluble analytes (appendix pp 2–3).
We did genome-wide genotyping in 17 503 participants (9013 cases of myocardial infarction and 8490 controls from PROMIS) using high-density gene arrays (Illumina Human 650K and Omni express 770K; Illumina, San Diego, CA, USA). SNPs with departure from Hardy-Weinberg equilibrium (p<0·0005), call rate of less than 95%, or minor allele frequency of less than 1% were excluded from the analyses. Participants were omitted from the analysis if there was evidence of cryptic relatedness, inconsistency of self-reported and genetically defined sex, or if more than 5% of genotype data were missing. Measured genome-wide genotypes were imputed using the global 1000 Genomes reference panel (version 3),20 yielding information on roughly 9 million variants.
Statistical analysis
To calculate risk of myocardial infarction for soluble lipoprotein(a) concentration or LPA KIV2 repeats, analysis was restricted to participants with complete data. We log-transformed lipoprotein(a) concentrations. Analysis of the cross-sectional correlates of lipoprotein(a) traits was restricted to controls in PROMIS. We characterised associations of log-lipoprotein(a) concentrations or LPA KIV2 repeats with other risk factors using linear regression analyses adjusted for age and sex and calculated partial correlation coefficients adjusted for age and sex. From each fitted regression model, we calculated overall adjusted mean values for log-lipoprotein(a) concentrations or KIV2 repeats within quintiles of continuous markers, or within each category for categorical variables as described previously.1 We calculated ORs for myocardial infarction per 1-SD differences in LPA KIV2 repeats or log-lipoprotein(a) concentration using unconditional logistic regression analyses, progressively adjusting for age, sex, self-reported ethnicity, recruitment centre, history of diabetes, waist-to-hip ratio, hypertension, and tobacco smoking. We adjusted ORs with LPA KIV2 repeats for lipoprotein(a) concentration and vice versa. To characterise shapes of associations with myocardial infarction, ORs were calculated using quintiles or other predefined categories of exposure variables; corresponding 95% CIs were estimated as described previously.1 Effect modification was evaluated by fitting a multiplicative term to the model.
To identify genetic determinants of apolipoprotein(a) protein isoform size or lipoprotein(a) concentration in healthy controls in PROMIS, we used linear regression additive models adjusted for age, sex, and ten principal components. To control for population stratification, we used measured genome-wide variants to calculate principal components. The predominant apolipoprotein(a) isoform size was used in the genetic analyses. We implemented step-wise conditional analyses to identify SNPs independently associated with apolipoprotein(a) isoform size or lipoprotein(a) concentration. Each independent SNP was assessed for associations with a panel of soluble analytes (appendix pp 2–3). Our mendelian randomisation analyses involved both single-variant and multiple-variant approaches.21 Pleiotropy was declared at a nominal p<0·01. Linkage disequilibrium (LD) plots were visualised using directly genotyped SNPs in PROMIS. Analyses were done with Stata (version 10), R (version 3.3.2), HAPLOVIEW (version 4.2), IMPUTE-2 (version 2), and SNPTEST-2 (version 2).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. DS had access to all the data in the study and DS, DJR, and JD had final responsibility for the decision to submit for publication.
Results
Baseline characteristics of the participants in PROMIS are summarised in the table. As would be expected, LPA KIV2 repeats were inversely associated with lipoprotein(a) concentration and OxPL-apoB in the control participants (appendix p 4). LPA KIV2 repeats and apolipoprotein(a) protein isoform size were significantly, but imperfectly, correlated (r=0·33, p<0·0001; appendix p 5) in the control group. Apolipoprotein(a) protein isoform size was more strongly related to lipoprotein(a) concentration and OxPL-apoB than were LPA KIV2 repeats in the control participants (appendix pp 4, 6). However, neither apolipoprotein(a) protein isoform size nor LPA KIV2 repeats were strongly associated with the other cardiovascular traits we studied (appendix pp 4, 6). Lipoprotein(a) concentration was positively associated with LDL cholesterol, HDL cholesterol, and OxPL-apoB, and, inversely correlated with triglycerides (appendix p 7). As would be expected, there was a high degree of correlation (r=0·92, p<0·0001; appendix p 8) between lipoprotein(a) concentrations measured using two different assay methods, yielding similar genetic and other cross-sectional correlates for each method (appendix p 9).
Table.
Participant characteristics in PROMIS
|
Controls |
Patients |
||||
|---|---|---|---|---|---|
| n/N (%) analysed | Mean (SD) or n (%) | n/N (%) analysed | Mean (SD) or n (%) | ||
| LPA KIV2 repeats (ΔCT)* | 6387/8629 (74%) | 3·17 (0·49) | 6277/9015 (70%) | 3·14 (0·49) | |
| Lipoprotein(a) concentration (nmol/L)† | 8629/8629 (100%) | 41·01 (48·60) | 9015/9015 (100%) | 48·91 (53·91) | |
| Apolipoprotein(a) protein isoform size (KIV2 repeats) | 3987/8629 (46%) | 23·95 (5·64) | ·· | ·· | |
| Demographic and anthropometric markers | |||||
| Age (years) | 8629/8629 (100%) | 54 (9) | 9015/9015 (100%) | 54 (11) | |
| Men | 8629/8629 (100%) | 6817 (79%) | 9015/9015 (100%) | 7482 (83%) | |
| Waist-to-hip ratio | 8629/8629 (100%) | 0·96 (0·20) | 9015/9015 (100%) | 0·97 (0·06) | |
| Lipid-related markers | |||||
| Total cholesterol concentration (mg/dL) | 8629/8629 (100%) | 181 (52) | 9015/9015 (100%) | 195 (52) | |
| LDL-cholesterol concentration (mg/dL) | 8629/8629 (100%) | 110 (39) | 9015/9015 (100%) | 127 (44) | |
| HDL-cholesterol concentration (mg/dL) | 8629/8629 (100%) | 35 (10) | 9015/9015 (100%) | 35 (10) | |
| Triglyceride concentration (mg/dL)‡ | 8629/8629 (100%) | 183·16 (0·54) | 9015/9015 (100%) | 169·01 (0·56) | |
| OxPL-apoB concentration (nM) | 6734/8629 (78%) | 7·3 (3·3) | ·· | ·· | |
| Medical history, blood pressure, and tobacco use | |||||
| History of type 2 diabetes | 8629/8629 (100%) | 1210 (14%) | 9015/9015 (100%) | 1801 (20%) | |
| History of hypertension | 8629/8629 (100%) | 2416 (28%) | 9015/9015 (100%) | 2881 (32%) | |
| Systolic blood pressure (mm Hg) | 8629/8629 (100%) | 128 (10) | 9015/9015 (100%) | 130 (23) | |
| Current tobacco users | 8629/8629 (100%) | 2591 (30%) | 9015/9015 (100%) | 4415 (49%) | |
KIV2=kringle IV type 2. OxPL-apoB= oxidised phospholipids on apolipoprotein B100.
LPA KIV2 repeat for each sample was established by calculating the difference in cycle thresholds (ΔCT) between the KIV2 sequence and albumin (control gene) probes.
Eight participants were found to have a lipoprotein(a) value of 0 and were omitted from the analyses.
Geometric mean.
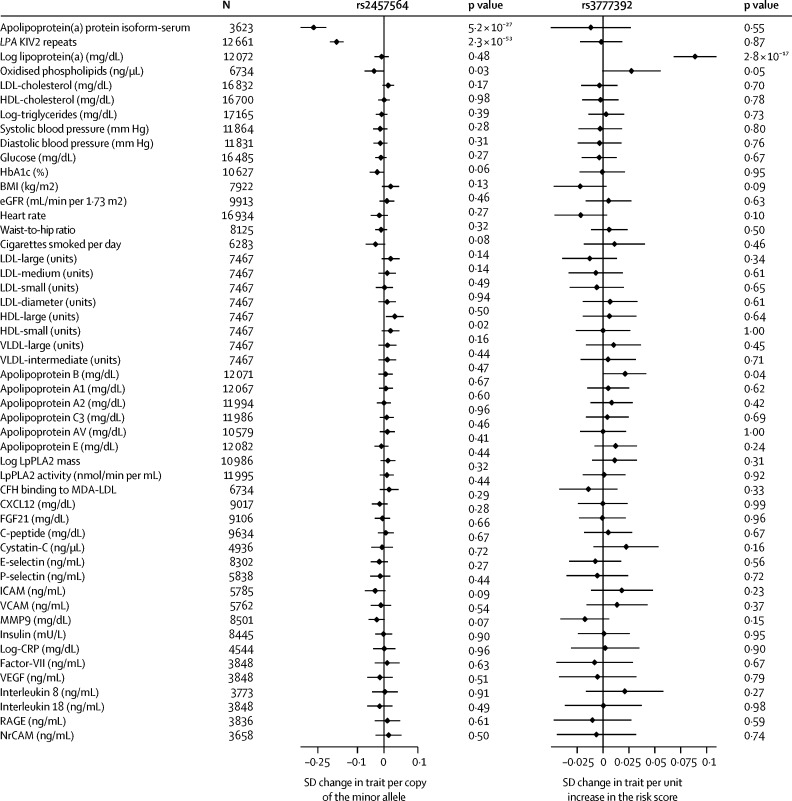
We found two SNPs, rs10455872 and rs3798220, which were previously reported as a genetic score for lipoprotein(a) concentration,2 to be associated with apolipoprotein(a) isoform size and LPA KIV2 repeat number (appendix p 10). By contrast, our objective was to identify genetic variants exclusively associated with either apolipoprotein(a) isoform size or log-lipoprotein(a) concentration, but not both. Our genome-wide association analyses identified 232 SNPs associated with apolipoprotein(a) protein isoform size at p<5 × 10−8 (genome-wide significance threshold; appendix p 11), including eight SNPs shown to be independent from each other in step-wise conditional analyses (appendix p 12). Only rs2457564 was not related to lipoprotein(a) concentration or the other traits we measured (figure 2). Each copy of the minor allele at rs2457564 (minor allele frequency: 0·41) decreased apolipoprotein(a) protein isoform size by 0·24 SD units (p=5·20 × 10−27). Allelic variation at rs2457564 was associated with LPA KIV2 repeat number (β=0·15 SD units; p=2·3 × 10−53; figure 2). The minor allele frequency of rs2457564 in PROMIS was similar to the minor allele frequencies observed for other global ethnicities (0·45) in the 1000 Genomes project.
Figure 2.
Association of variants specifically associated with apolipoprotein(a) isoform size and lipoprotein(a) concentration with various factors in PROMIS
Bars are 95% CIs. LPA=lipoprotein(a) gene. KIV2=kringle IV type 2. eGFR=estimated glomerular filtration rate. LpPLA2=lipoprotein-associated phospholipase A2. CFH=complement factor H. MDA-LDL=malondialdehyde-modified LDL. CXCL12=C-X-C motif chemokine 12. FGF21=fibroblast growth factor 21. ICAM=intercellular adhesion molecule 1. VCAM=vascular cell adhesion molecule. MMP9=matrix metallopeptidase 9. CRP=C-reactive protein. VEGF=vascular endothelial growth factor. RAGE=receptor for advanced glycation end products. NrCAM=neural cell adhesion molecule.
We identified 324 SNPs associated with log-lipoprotein(a) concentration at p<5 × 10−8 (appendix p 13), including 25 SNPs shown to be independent from the others in step-wise conditional analyses (appendix p 14). rs3777392 (minor allele frequency 0·09) located in the first LD block of LPA was associated with log-lipoprotein(a) concentration, but not with apolipoprotein(a) protein isoform size nor other cardiovascular and other traits we measured (figure 2).
The minor allele frequency of rs2457564 in PROMIS was similar to the minor allele frequencies observed for other global ethnicities (0·06) in the 1000 Genomes project. The two variants rs2457564 and rs3777392 were found to be in low LD (r2=0·05). rs2457564 or rs3777392 were not convincingly associated with cardiometabolic risk factors analysed in previously published consortia based analyses (appendix p 15).
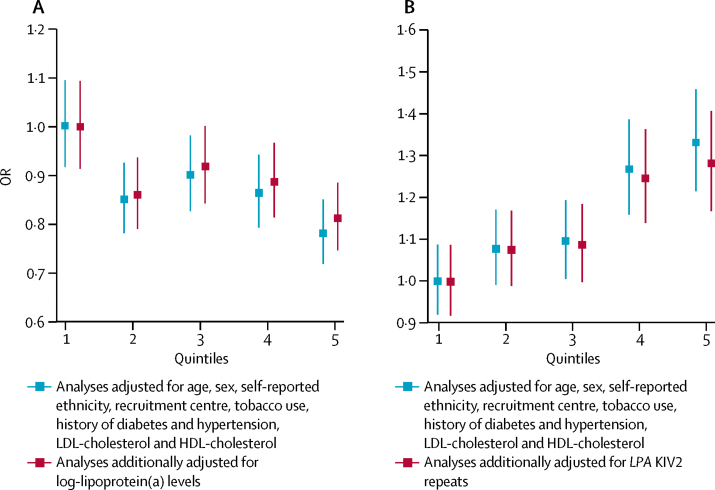
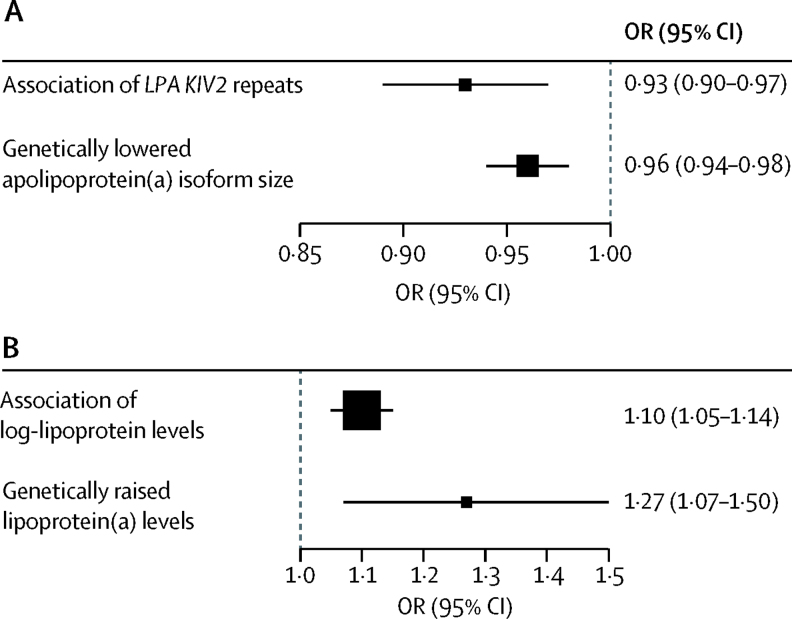
In PROMIS, associations of myocardial infarction risk with measured values of LPA KIV2 repeats and lipoprotein(a) concentration were weak and broadly continuous (figure 3). After adjustment for lipoprotein(a) concentration and several other cardiovascular risk factors, the OR for myocardial infarction was 0·93 (95% CI 0·90–0·97; p<0·0001) per 1-SD increment in LPA KIV2 repeats. This finding was directionally concordant with the OR in CARDIoGRAMplusC4D for coronary heart disease of 0·96 (0·94–0·98; p<0·0001) per 1-SD increment in apolipoprotein(a) isoform size due to rs2457564 (figure 4), and with the OR for coronary heart disease of 0·98 (0·98–0·99; p<0·0001; appendix p 12) with a genetic score consisting of the eight independent LPA KIV2 variants.
Figure 3.
Mutually adjusted association of LPA KIV2 repeats and lipoprotein(a) concentration with coronary heart disease in PROMIS
Bars are 95% CIs. (A) Association of LPA KIV2 repeats with coronary heart disease risk. (B) Association of lipoprotein(a) with coronary heart disease risk. KIV2=kringle IV type 2. OR=odds ratio.
Figure 4.
Phenotypic and genotypic assessment of apolipoprotein(a) isoform size and lipoprotein(a) concentration in coronary heart disease
(A) OR for coronary heart disease per 1-SD increment in LPA KIV2 repeats. (B) OR for coronary heart disease per 1-SD increment in lipoprotein(a) concentration. Phenotypic associations were computed in PROMIS and genetic associations were computed using data from the CARDIoGRAMplusC4D consortium. KIV2=kringle IV type 2. OR=odds ratio.
After adjustment for LPA KIV2 repeats and several other cardiovascular risk factors, the OR in PROMIS for myocardial infarction was 1·10 (95% CI 1·05–1·14; p<0·0001) per 1-SD increment in lipoprotein(a) concentration (appendix p 16). This finding was directionally concordant with results from CARDIoGRAMplusC4D; the OR for coronary heart disease of 1·27 (1·07–1·50; p=0·007) per 1-SD higher log-lipoprotein(a) concentration due to rs3777392 (figure 4), and with the OR for coronary heart disease of 1·12 (1·10–1·14; p<0·0001) with a genetic score comprising the 25 independent lipoprotein(a) variants. Associations of lipoprotein(a) concentration with coronary heart disease risk in PROMIS were similar across groups defined by different mean LPA KIV2 repeats (pinteraction=0·1; appendix p 17).
Among the randomly selected subset of 6734 healthy controls who had genome-wide genotypes and OxPL-apoB data from PROMIS, we observed genome-wide significant associations of rs10455872, rs3798220, and other LPA gene variants with circulating OxPL-apoB (eg, p=4 × 10−13 for rs7770628, the lead variant in LPA; appendix p 18), but not variants at any other loci across the genome. The association of rs7770628 with OxPL-apoB was not significant after adjustment for LPA KIV2 repeats (or for log-lipoprotein[a] concentrations; figure 5), and the associations of OxPL-apoB with genetic variation at the LPA locus were no longer significant after analyses were conditioned on LPA KIV2 or log lipoprotein(a) concentration (appendix pp 18–19).
Figure 5.
Association of rs7770628 with OxPL-apoB after adjustment for KIV2 repeats and log-lipoprotein(a) concentration in PROMIS
Bars are 95% CIs. KIV2=kringle IV type 2. OxPL-apoB=oxidised phospholipid on apolipoprotein B100.
Discussion
Our large-scale integrative human genetic and biomarker study aimed to investigate the causal components of the lipoprotein(a) pathway that could increase coronary heart disease risk. Our mendelian randomisation analyses suggest that both small apolipoprotein(a) isoform size and increased lipoprotein(a) concentration are independent and causal risk factors for coronary heart disease. Both apolipoprotein(a) isoform size and lipoprotein(a) concentration were associated with coronary heart disease risk, independent of conventional lipids. We found weak and broadly continuous relations (in opposite directions) between coronary heart disease risk and apolipoprotein(a) isoform size and lipoprotein(a) concentration, even after adjustment for each other. Furthermore, in a genome-wide analysis of OxPL-apoB concentration, we found that LPA variants are strongly associated with OxPL-apoB concentration, supporting previous suggestions that oxidative damage could mediate the pathological effects of lipoprotein(a) in coronary heart disease.10 Collectively, these findings advance understanding of the role of the lipoprotein(a) pathway in coronary heart disease and might have implications for the prevention of coronary heart disease.
Previous observational studies could not distinguish the effects of apolipoprotein(a) isoform size from lipoprotein(a) concentration on coronary heart disease because they did not measure both traits in the same participants in sufficiently large numbers. A previous gene-centric study found that two SNPs (rs3798220 and rs10455872), which together explain about one-third of the variation in lipoprotein(a) concentration, were robustly associated with coronary heart disease.2 However, because the SNPs were both located in the LPA gene and showed associations with both apolipoprotein(a) isoform size and lipoprotein(a) concentration, it was not possible to conclude which component of the lipoprotein(a) pathway was causally associated with coronary heart disease. Similar considerations applied to a mendelian randomisation study4 that used another genetic tool, the LPA apo(a) isoform size-determining copy-number variant. By contrast, in our study we were able to distinguish the causal effects of apolipoprotein(a) isoform size from lipoprotein(a) concentration on coronary heart disease because we identified genetic variants associated with one of these traits but not with both.
Our study identified several variants at the LPA locus that were associated with OxPL-apoB concentration. We also showed that these genetic associations were dependent on apolipoprotein(a) isoform size or lipoprotein(a) concentration, suggesting that oxidised phospholipids, apolipoprotein(a) isoform size, and lipoprotein(a) concentration are causally linked and located on the same pathway. Oxidised phospholipids are immunogenic, accumulate in atherosclerotic lesions, and mediate plaque destabilisation.22 Previous studies have reported that the oxidised phospholipids with lipoprotein(a) might modulate trafficking of monocytes during atherogenesis and might also mediate pro-inflammatory activation of monocytes.23 Lipoprotein(a) and its associated oxidised phospholipids have been proposed to have multiple atherogenic effects, including proliferation of vascular smooth muscle cells and foam-cell formation, which promote release of pro-inflammatory mediators (eg, interleukin 8) and anti-fibrinolytic effects.22 Oxidised phospholipids and lipoprotein(a) could also mediate macrophage apoptosis by signalling through the CD36–Toll-like receptor 2 pathway, generating reactive oxygen species dependent on NADPH oxidase.22 In view of our findings, elucidation of the exact mechanisms by which the lipoprotein(a) pathway exerts vascular effects by involving oxidised phospholipids should be a priority.
Our findings might have implications for emerging therapies that target lipoprotein(a) pathways, which, to date, have been hampered by poor understanding of the mechanisms by which this lipoprotein is formed and cleared.24 The LDL receptor does not seem to have a major role in lipoprotein(a) clearance, which could explain why statins are generally ineffective in the reduction of lipoprotein(a) concentration.25 However, mipomersen, which lowers LDL by inhibiting apolipoprotein B100 synthesis, lowers lipoprotein(a) concentration by about 25%.26 Monoclonal antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) also lower lipoprotein(a) concentration by 20–35%,27 although the mechanism is unknown. Similarly, inhibitors of cholesteryl ester transfer protein, which have not yet been shown to reduce the risk of cardiovascular disease, can lower lipoprotein(a) concentration by 30–40%.28 By contrast, trials of apolipoprotein(a)-specific antisense oligonucleotide have reported up to 90% mean reductions in lipoprotein(a) concentration (and a proportional decrease in associated oxidised phospholipids) through inhibition of hepatic apolipoprotein(a) synthesis.8, 9 Because these reductions in lipoprotein(a) were achieved in individuals with both low and high baseline concentrations of lipoprotein(a) and were not accompanied by substantial changes in concentrations of other lipoproteins, such new agents could help test whether specific and substantial reductions in lipoprotein(a) concentration prevent cardiovascular disease outcomes. Our results imply that therapies that lower lipoprotein(a) concentrations might have greatest clinical effect in patients with small apolipoprotein(a) isoforms and high lipoprotein(a) concentration.
Our study has several strengths. It was unique in its combination of statistical power, detailed characterisation of the lipoprotein(a) pathway, and use of multiple assays to enhance validity. For example, in one group of participants, we assayed apolipoprotein(a) isoform size (with two different, but complementary, methods), lipoprotein(a) concentration (with two similar methods in two different laboratories), oxidised phospholipids, several established and emerging cardiovascular risk factors, and genome-wide genotypes. Specifically, we made use of the advantages of both genotypic and phenotypic methods to measure apolipoprotein(a) isoform size; the genotypic methods afforded the scalability necessary for a genetic epidemiological study and the phenotypic methods ensured a high degree of biological specificity. Consistent with a previous study,29 we observed a modest correlation between the genotypic and phenotypic methods we used to measure apolipoprotein(a) isoform size, reflecting the inherent strengths and limitations of each approach. For example, whereas the genetic method (qPCR) cannot estimate the number of KIV2 repeats in an allele-specific manner, the phenotypic method (immunoblotting) provided information for each expressed allele. Conversely, the phenotypic method cannot differentiate between an individual who is a carrier of the null allele compared with individuals homozygous for an active allele.29 The frequency of the null allele has been reported to be 3% in a white European population.30
Our study also had some limitations. Patients in PROMIS, who tended to have early-onset myocardial infarction in urban Pakistan, could be unrepresentative of patients with coronary heart disease in other populations. However, we found that the results from genetic analysis of coronary heart disease in almost 18 000 participants in PROMIS were broadly similar to those observed in over 180 000 participants in the global CARDIoGRAMplusC4D consortium, which included patients with coronary heart disease who were mostly of white European ancestry with diverse clinical characteristics. We could not directly investigate whether associations of LPA with lipoprotein(a) traits varied by ethnicity because the relevant lipoprotein(a) traits were not available from CARDIoGRAMplusC4D. PROMIS was not sufficiently powered to study phenotypically assessed apolipoprotein(a) isoform size directly in relation to myocardial infarction because, due to reasons of cost and feasibility, apolipoprotein(a) isoform size was measured in only about one-fifth of PROMIS participants. For analyses that required extraction of data from external genetic consortia, we could not adjust associations between genotypes and coronary heart disease risk for potential mediators, nor analyse instrumental variables. In principle, the few genetic variants we found to be associated with apolipoprotein(a) isoform size or lipoprotein(a) concentration, but not both, could somehow be unrepresentative of lipoprotein(a) biology, but there is, as yet, no direct evidence to support or refute this theoretical possibility.
We conclude that human genetic data suggest that both small apolipoprotein(a) isoform size and high lipoprotein(a) concentration are independent and causal risk factors for coronary heart disease. Interventions that lower lipoprotein(a) concentration could be more effective in reducing coronary heart disease risk in individuals who have small apolipoprotein(a) isoforms.
Contributors
Declaration of interests
Acknowledgments
Acknowledgments
The genetic and biomarker assays in this study (consumables and labour) were supported by grants from the British Heart Foundation (PG/09/035/27378), US National Institutes of Health (RC2HL101834 and RC1TW008485), Fogarty International Center (RC1TW008485), the Wellcome Trust (084711/Z/08/Z), and Pfizer, as well as by underpinning support from the UK Medical Research Council (MR/L003120/1), British Heart Foundation (RG/13/13/30194), and National Institute for Health Research Cambridge Biomedical Research Centre. We thank Stephen Burgess for helpful comments on earlier drafts of this report; and Moazzam Zaidi, Maria Samuel, Madiha Ishaq, Kevin Trinidad, Muhammad Waqar Khan, Muhammad Razaq Khan, Abdul Ghafoor, Mir Alam, Muhammad Riazuddin, Muhammad Irshad Javed, Abdul Ghaffar, Tanveer Baig Mirza, Muhammad Shahid, Jabir Furqan, Muhammad Iqbal Abbasi, Tanveer Abbas, Rana Zulfiqar, Muhammad Wajid, Irfan Ali, Muhammad Ikhlaq, Danish Sheikh, and Muhammad Imran for helpful contributions to the fieldwork and biomarker measurements in PROMIS.
Acknowledgments
DS and JD conceived and designed the study and drafted the report, with key input from DJR. DS, PCH, and WZ did the statistical analyses. The research groups of SM, JLW, ST, and DJR did the soluble biomarker assays. All other authors played key roles in study design, recruitment of participants, or generation of data. All authors provided critical input into revised versions of the report.
DS has received grants from the National Heart, Lung, and Blood Institute, Fogarty International, Pfizer, Regeneron, Eli Lilly, and Genentech. ST is an employee of Ionis Pharmaceuticals and is co-inventor of patents on oxidation-specific antibodies licensed via University of California San Diego. SM has received personal fees from Denka Seiken and MedTest Dx; and has a patent issued on the methods and materials for the immunoassay of apolipoprotein(a) and lipoprotein(a). JLW has received personal fees from Ionis Pharmaceuticals, Cymabay, Intercept, and Prometheus; and has patents and disclosures on use of oxidation-specific antibodies held by University of California San Diego, with royalties paid. JD reports grants from UK Medical Research Council, British Heart Foundation, UK National Institute of Health Research, European Commission Framework Programme; personal fees and non-financial support from Merck Sharp & Dohme UK Atherosclerosis, Novartis Cardiovascular & Metabolic Advisory Board, Pfizer Population Research Advisory Panel, Sanofi Advisory Board; and grants from British Heart Foundation, BUPA Foundation, diaDexus, European Research Council, European Union, Evelyn Trust, Fogarty International Centre, GlaxoSmithKline, Merck, National Heart, Lung, and Blood Institute, National Institute of Health Research, National Institute of Neurological Disorders and Stroke, NHS Blood and Transplant, Novartis, Pfizer, UK Medical Research Council, Wellcome Trust, and UK Biobank, unrelated to the present work. All other authors declare no competing interests.
Contributor Information
Danish Saleheen, Email: saleheen@mail.med.upenn.edu.
John Danesh, Email: jd292@medschl.cam.ac.uk.
Supplementary Material
References
- 1.Emerging Risk Factors Collaboration Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke R, Peden JF, Hopewell JC. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 3.Erqou S, Thompson A, Di AE. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 5.Thanassoulis G, Campbell CY, Owens DS. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anuurad E, Boffa MB, Koschinsky ML, Berglund L. Lipoprotein(a): a unique risk factor for cardiovascular disease. Clin Lab Med. 2006;26:751–772. doi: 10.1016/j.cll.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsimikas S, Viney NJ, Hughes SG. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 9.Viney NJ, van Capelleveen JC, Geary RS. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 10.Leibundgut G, Scipione C, Yin H. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleheen D, Zaidi M, Rasheed A. The Pakistan Risk of Myocardial Infarction Study: a resource for the study of genetic, lifestyle and other determinants of myocardial infarction in South Asia. Eur J Epidemiol. 2009;24:329–338. doi: 10.1007/s10654-009-9334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikpay M, Goel A, Won HH. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wain LV, Verwoert GC, O'Reilly PF. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willer CJ, Schmidt EM, Sengupta S. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt SI, Gustafsson S, Magi R. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45:501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott RA, Lagou V, Welch RP. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clin Chem. 1996;42:436–439. [PubMed] [Google Scholar]
- 18.Marcovina SM, Albers JJ, Scanu AM. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 19.Byun YS, Lee JH, Arsenault BJ. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J Am Coll Cardiol. 2015;65:1286–1295. doi: 10.1016/j.jacc.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abecasis GR, Altshuler D, Auton A. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer TM, Lawlor DA, Harbord RM. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–242. doi: 10.1177/0962280210394459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seimon TA, Nadolski MJ, Liao X. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fefer P, Tsimikas S, Segev A. The role of oxidized phospholipids, lipoprotein (a) and biomarkers of oxidized lipoproteins in chronically occluded coronary arteries in sudden cardiac death and following successful percutaneous revascularization. Cardiovasc Revasc Med. 2012;13:11–19. doi: 10.1016/j.carrev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Snyder ML, Hay RV, Whitington PF, Scanu AM, Fless GM. Binding and degradation of lipoprotein(a) and LDL by primary cultures of human hepatocytes. Comparison with cultured human monocyte-macrophages and fibroblasts. Arterioscler Thromb. 1994;14:770–779. doi: 10.1161/01.atv.14.5.770. [DOI] [PubMed] [Google Scholar]
- 25.Haffner S, Orchard T, Stein E, Schmidt D, LaBelle P. Effect of simvastatin on Lp(a) concentrations. Clin Cardiol. 1995;18:261–267. doi: 10.1002/clc.4960180507. [DOI] [PubMed] [Google Scholar]
- 26.Visser ME, Wagener G, Baker BF. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2012;33:1142–1149. doi: 10.1093/eurheartj/ehs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raal FJ, Giugliano RP, Sabatine MS. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls SJ, Ruotolo G, Brewer HB. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol. 2016;10:519–527. doi: 10.1016/j.jacl.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Lanktree MB, Rajakumar C, Brunt JH, Koschinsky ML, Connelly PW, Hegele RA. Determination of lipoprotein(a) kringle repeat number from genomic DNA: copy number variation genotyping using qPCR. J Lipid Res. 2009;50:768–772. doi: 10.1194/jlr.D800050-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyriakou T, Seedorf U, Goel A. A common LPA null allele associates with lower lipoprotein(a) levels and coronary artery disease risk. Arterioscler Thromb Vasc Biol. 2014;34:2095–2099. doi: 10.1161/ATVBAHA.114.303462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.