Abstract
Folate deficiency has been linked to a wide range of disorders, including cancer, neural tube defects, and fetal growth restriction. Folate regulates cellular function mediated by its involvement in the synthesis of nucleotides, which are needed for DNA synthesis, and its function as a methyl donor, which is critical for DNA methylation. Here we review current data showing that folate sensing by mechanistic target of rapamycin (mTOR) constitutes a novel and distinct pathway by which folate modulates cell functions such as nutrient transport, protein synthesis, and mitochondrial respiration. The mTOR signaling pathway responds to growth factors and changes in nutrient availability to control cell growth, proliferation, and metabolism. mTOR exists in 2 complexes, mTOR complex (mTORC) 1 and mTORC2, which have distinct upstream regulators and downstream targets. Folate deficiency in pregnant mice caused a marked inhibition of mTORC1 and mTORC2 signaling in multiple maternal and fetal tissues, downregulation of placental amino acid transporters, and fetal growth restriction. In addition, folate deficiency in primary human trophoblast (PHT) cells resulted in inhibition of mTORC1 and mTORC2 signaling and decreased the activity of key amino acid transporters. Folate sensing by mTOR in PHT cells is independent of the accumulation of homocysteine and requires the proton-coupled folate transporter (PCFT; solute carrier 46A1). Furthermore, mTORC1 and mTORC2 regulate trophoblast folate uptake by modulating the cell surface expression of folate receptor α and the reduced folate carrier. These findings, which provide a novel link between folate availability and cell function, growth, and proliferation, may have broad biological significance given the critical role of folate in normal cell function and the multiple diseases that have been associated with decreased or excessive folate availability. Low maternal folate concentrations are linked to restricted fetal growth, and we propose that the underlying mechanisms involve trophoblast mTOR folate sensing resulting in inhibition of mTORC1 and mTORC2 and downregulation of placental amino acid transporters.
Keywords: folic acid, placenta, fetal growth, amino acid transporters, trophoblast
Introduction
Folate, formerly known as folacin, is an essential B vitamin that is highly present in green vegetables, legumes, egg yolk, liver, and citrus fruit. Folate dietary supplements typically contain folic acid, which is the synthetic oxidized monoglutamate form of the vitamin (1). For full biological activity, folate must be reduced to produce l-5-methyltetrahydrofolate (5-MTHF), which is the predominant form of folate that is taken up by cells and enters cellular metabolism (1).
Folate deficiency has been linked to a wide range of disorders, including neural tube defects (NTDs), fetal growth restriction, and cancer. Pregnant women have increased folate demands, and folate deficiency in the periconceptional period is associated with NTDs. Conversely, women who receive folate supplementation during the weeks around conception and in early pregnancy have lower risks of NTDs such as spina bifida and anencephaly, providing evidence for a critical role of folate for normal closure of the neural tube (2–4). Low maternal serum folate is also linked to a range of pregnancy complications associated with placental dysfunction, including preeclampsia, placenta abruption, preterm delivery, and fetal growth restriction (5), suggesting that folate is important in normal placental development and function (6, 7). In general agreement with this concept, folate has been reported to regulate trophoblast invasion, angiogenesis, and secretion of matrix metalloproteinases (8).
Folate deficiency or impaired folate metabolism has been associated with cancer in a range of tissues, including the cervix, breast, pancreas, and colon (9–12). A multitude of mechanisms may link low folate to carcinogenesis. For example, folate acts as a cofactor for enzymes involved in DNA biosynthesis, repair, and stability (7, 13, 14). Furthermore, folate is important for the conversion of methionine to S-adenosylmethionine (SAM), a universal donor of methyl groups for DNA methylation (13, 15), which is a critical regulator of gene silencing. Indeed, aberrations of DNA methylation are associated with cancer development (16).
Although the link between folate deficiency and carcinogenesis is accepted, some studies have also suggested that a high folate intake promotes the progression of an already established cancer; however, the underlying molecular mechanism remains elusive (9, 17, 18). Folate excess may promote cancer proliferation by providing an abundance of building blocks for DNA synthesis, which is required for rapid cell division (19–21). In addition, it is believed that hypermethylation of specific CpG islands with subsequent transcriptional silencing of tumor suppressor genes contributes to cancer development (15, 22, 23), and it is therefore possible that increased folate availability promotes cancer by causing increased methylation and decreased expression of these genes (19). However, the link between increased folate availability and cancer progression remains contested because other studies have been unable to confirm this association (24, 25).
Although the critical role of folate availability in neural tube closure, fetal growth, and carcinogenesis is well recognized, the underlying molecular mechanisms are not fully understood. The current review discusses new evidence that shows that folate sensing by mechanistic target of rapamycin (mTOR) signaling constitutes a novel and distinct pathway by which folate modulates cellular nutrient transport, protein synthesis, and mitochondrial respiration (Figure 1). This review particularly emphasizes folate sensing by the mTOR signaling pathway in trophoblasts as a mechanistic link between maternal folate concentrations and fetal growth.
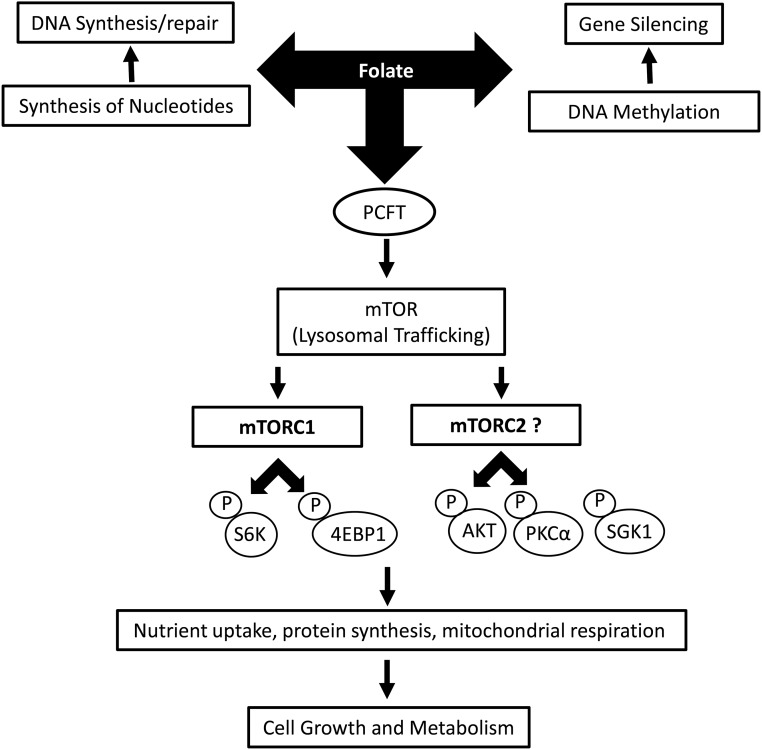
FIGURE 1.
Mechanisms linking folate to cellular function. Folate regulates cellular functions mediated by its well-established involvement in the synthesis of nucleotides, which are needed for DNA synthesis and repair, and its function as a methyl donor, critical for DNA methylation. We propose that folate sensing by mTOR constitutes a novel and distinct pathway by which folate modulates cell functions such as nutrient transport, protein synthesis, and mitochondrial respiration. Folate sensing by mTOR appears to involve both mTORC1 and mTORC2 and requires the folate transporter PCFT. The downstream cellular response to mTORC2 folate sensing in the lysosome is unknown. AKT, protein kinase B; mTOR, mechanistic target of rapamycin; mTORC, mechanistic target of rapamycincomplex; P, phosphorylated protein; PCFT, proton-coupled folate transporter; PKCα, protein kinase C α; SGK1, serum- and glucocorticoid-regulated kinase; S6K, p70 S6 kinase 1; 4EBP1, eukaryotic initiation factor 4E binding protein-1.
mTOR Signaling
mTOR is an evolutionarily conserved serine/threonine protein kinase that senses nutrient availability, resulting in the regulation of several cell functions including cell growth, proliferation, survival, and metabolism (26–28). It consists of 2 protein complexes with distinct physiologic functions, mTOR complex (mTORC) 1 and mTORC2. Both complexes contain the proteins mTOR, mammalian lethal with Sec13 protein 8 (mLST8), and DEP-domain–containing mTOR-interacting protein (Deptor). In addition, mTOR associates with regulatory-associated protein of mTOR (Raptor) and proline-rich protein kinase B substrate 40 kDa (PRAS40) to form mTORC1 and with rapamycin-insensitive companion of mTOR (Rictor), mammalian stress-activated protein kinase interacting protein (mSIN1), and protein observed with Rictor-1 (Protor-1) to form mTORC2. Deptor negatively regulates both mTORC1 and mTORC2 activity (29). The 2 mTOR complexes differ in both their upstream regulators and downstream targets.
mTORC1 regulates cell metabolism by promoting mitochondrial biogenesis and the synthesis of proteins, lipids, and nucleotides and by inhibiting autophagy (26). mTORC1 is regulated by growth factor signaling and senses the cellular concentrations of energy, oxygen, and nutrients, in particular amino acids. Tuberous sclerosis complex 1 and 2 (TSC1/2) is an upstream regulator of mTOR activity via inhibition of Ras homolog enriched in brain (Rheb) (26). Downstream effectors of mTORC1 are the 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) and the eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1). Rapamycin is a specific inhibitor of mTORC1, and rapamycin analogs have been introduced clinically as a treatment for various disorders, in particular certain cancers. mTORC2 is largely rapamycin insensitive and regulates the actin cytoskeleton through Rho GTPases (30) and cellular metabolism. Downstream targets of mTORC2 are protein kinase B (AKT), protein kinase C (PKC), and serum- and glucocorticoid-regulated protein kinase 1 (SGK1) (31).
Recently, it was reported that mTORC1 induces purine synthesis through the control of the mitochondrial tetrahydrofolate cycle (32) and that mTOR promotes the generation of SAM (33), a key intermediate in one-carbon metabolism. However, previous information on folate regulating mTORC1 and mTORC 2 activity is limited. Folic acid has been shown to promote muscle cell differentiation via activation of the AKT pathway, phosphorylation of mTOR, and downstream activation of p70S6K1 and 4E-BP1 (34).
mTORC1 and mTORC2 Function as Folate Sensors In Vivo
Rosario et al. (35) established an experimental model of folate deficiency in which female mice were fed a folate-free diet for 6 wk, which decreased their serum folate concentrations by 60%. Subsequently, mice were mated and studied at the end of pregnancy. Folate deficiency resulted in a pronounced inhibition of mTORC1 and mTORC2 signaling in maternal skeletal muscle, heart, and liver and fetal liver and heart. These findings are consistent with the possibility that both mTOR complexes are markedly inhibited in a wide range of tissues by decreased maternal folate availability.
Maternal folate deficiency was associated with decreased fetal weights at embryonic day 18.5 (35), recapitulating the clinical observations of an association between low maternal folate concentrations and fetal growth restriction (35, 36). Folate deficiency inhibited phosphorylation of mTOR at serine 2448 and the downstream mTOR targets p70S6K1, S6 ribosomal protein, and 4E-BP1 (mTORC1 targets) and PKCα, SGK1, and AKT (mTORC2 targets) in placental homogenates. Importantly, whereas phosphorylation of placental AKT at serine 473 (a functional readout of mTORC2 signaling) was markedly decreased in response to maternal folate deficiency, Thr-308 AKT phosphorylation [the target for insulin/insulin-like growth factor I (IGF-I) signaling] was unchanged, suggesting that the effect of folate deficiency was specific to the mTOR signaling pathway.
Placental mTORC1 and mTORC2 inhibition in folate-deficient dams was associated with decreased protein expression and activity of the amino acids transporters solute carrier (SLC) 38A2 [also known as sodium coupled neutral amino acid transporter 2 (SNAT2), an isoform of the system A amino acid transporter] and SLC7A5 [also known as large neutral amino acid transporter 1 (LAT1), a system L amino acid transporter] in isolated trophoblast apical plasma membranes (35).
It is now well established that mTORC1 is recruited to the lysosomal surface in response to amino acids, and it is believed that mTORC1 amino acid sensing occurs at the outer lysosomal surface (37). Interestingly, Rosario and coworkers (35, 38) used a proximity ligation assay and confocal microscopy to provide evidence to suggest that mTOR trafficking to the lysosomes is required for mTOR folate sensing in cultured primary human trophoblast (PHT) cells and in the mouse placenta in vivo. Thus, it is possible that folate sensing by mTORC1 requires translocation of mTOR to the lysosomal surface, thereby sharing some common basic mechanisms with mTOR amino acid sensing. However, the question of which complexes (mTORC1, mTORC2, or both) were involved in mTOR folate sensing was not specifically addressed. Whereas mTORC1 trafficking to the lysosome in amino acid sensing is well established, a lysosomal localization of mTORC2 was only recently reported (39). Thus, mTORC2 trafficking to the lysosome in mTOR folate sensing remains speculative (Figure 1).
In support of their findings in mice, Rosario et al. (35) also reported that maternal folate serum concentrations were positively correlated with placental mTORC1 signaling, amino acid transporter expression and activity, and fetal weights in a group of baboons fed a control or calorie-restricted diet. To explore the clinical relevance of the findings in mice and nonhuman primates, the relation between maternal serum folate and placental microvillous plasma membrane system L activity was studied in term placentas from a cohort of healthy women undergoing cesarean delivery at term. Indeed, maternal folate serum concentrations were also positively correlated with placental mTORC1 and mTORC2 signaling, amino acid transporter activity, and birth weights in humans (35, 38). To our knowledge, mTOR folate sensing regulating placental nutrient transport constitutes a novel specific molecular link between maternal folate availability and fetal growth.
mTORC1 and mTORC2 Function as Folate Sensors in Human Primary Trophoblast Cells
In the human placenta, the syncytiotrophoblast cell layer represents the interface between the maternal and fetal circulations and plays a critical role in mediating nutrient transport to the fetus (40). To investigate the mechanistic link between folate and mTOR signaling, Rosario et al. (38) used PHT cells isolated from term placentas. These cells form syncytial islands in culture, which is regarded as a relevant model for the in vivo syncytiotrophoblast.
Consistent with the in vivo mouse model of maternal folate deficiency, culturing PHT cells in folate-deficient media caused a marked inhibition of both mTORC1 and mTORC2 signaling, as evidenced by decreased phosphorylation of p70S6K1, S6 ribosomal protein, 4E-BP1, and AKT (Ser-473). As in the previous in vivo studies, AKT phosphorylation at Thr-308 was unaffected by folate deficiency, suggesting the insulin/IGF-I signaling pathway does not respond to changes in folate availability. Folate deficiency also resulted in the downregulation of Raptor and Rictor protein expression in PHT cells. By using a proximity ligation assay and confocal microscopy, Rosario et al. (38) showed that the mTOR protein colocalized with lysosomes in cells cultured in normal folate concentrations but not in folate-deficient cells, suggesting that folate regulates the subcellular localization of mTOR in PHT cells.
Because folate is required for the metabolic conversion of homocysteine to methionine, folate deficiency results in the accumulation of homocysteine (41). It is therefore possible that the inhibition of mTOR signaling in response to folate deficiency is mediated by the accumulation of homocysteine. However, culturing PHT cells in various concentrations of homocysteine had no effect on mTOR signaling, suggesting that mTORC1 and mTORC2 are modulated directly by low folate concentrations rather than indirectly by high homocysteine (38). In addition, the proton-coupled folate transporter (PCFT) was shown to be required for mTORC1 and mTORC2 folate sensing (38). Although PCFT is critical in mediating intestinal folate uptake (42), PCFT is unlikely to mediate cellular uptake of folate in most cells, including trophoblast cells, because of the lack of inwardly directed proton gradient in vivo. However, PCFT is localized in lysosomes where the transporter mediates the efflux of folate from the acidic lysosome interior to the cytoplasm (43). Therefore, it was proposed that PCFT may function as the actual folate sensor, linking folate concentrations to mTOR signaling at the surface of the lysosome (Figure 2).
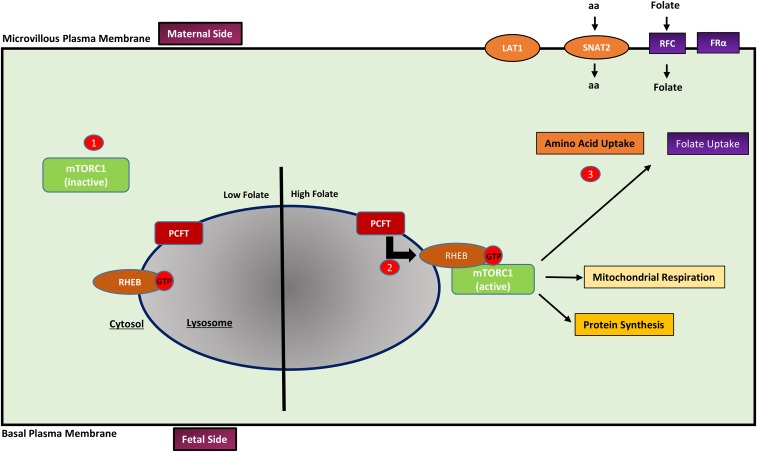
FIGURE 2.
A proposed model of mTORC1 folate sensing is shown. (1) When intracellular folate concentrations are low, mTORC1 is predominantly localized in the cytosol and inactive. (2) Increased intracellular folate concentrations are sensed by PCFT in the lysosome, which (by unknown mechanisms) promotes the trafficking of mTORC1 to the lysosome surface where it interacts with GTP-bound RHEB, initiating mTORC1 signaling. (3) Activation of mTORC1 stimulates mitochondrial respiration, protein synthesis, aa (LAT1 and SNAT2), and folate (RFC and FRα) transporters trafficking to the plasma membrane, which increases aa and folate uptake. aa, amino acid; FRα, folate receptor α; LAT1, large neutral amino acid transporter 1; mTORC1, mechanistic target of rapamycin complex 1; PCFT, proton-coupled folate transporter; RFC, reduced folate carrier; RHEB, Ras homolog enriched in brain; SNAT2, sodium-dependent neutral amino acid transporter 2.
mTORC1 and mTORC2 Are Positive Regulators of Folate Transporters
Cellular folate uptake is mediated by specific transport mechanisms, in particular the reduced folate carrier (RFC), folate receptor α (FR-α), and PCFT (44, 45). These transporters are all expressed and active in the human placenta and are believed to mediate the transfer of folate from the maternal circulation to the fetus (44, 46). RFC has high affinity for 5-MTHF and exchanges folate with organic anions (47). RFC is localized in the microvillous plasma membrane, facing the maternal circulation, and it has been shown to be involved in placental folate uptake in association with FR-α (48). FR-α mediates transport via internalization of the receptor-folate complex, and it is highly expressed in cancer cells (49). Deletion of RFC or FR-α in mice results in embryonic lethality (50, 51). PCFT has optimal activity in a low-pH environment (52) and has been characterized as the major folate transporter across the apical brush border membrane of the small intestine due to the acidic microenvironment of this region (42).
The activation of mTOR signaling stimulates cell growth, proliferation, and metabolism, which require an increased supply of nutrients such as amino acids. Interestingly, mTOR is not only an amino acid sensor but also functions as a positive regulator of cellular amino acid uptake (53–55), thereby directly linking amino acid availability, cellular amino acid uptake, and cell growth and proliferation. Given that cell growth and proliferation are also critically dependent on folate availability and that mTOR functions as a folate sensor, Rosario et al. (56) tested the hypothesis that mTOR regulates cellular folate uptake. By using small interfering RNA gene silencing approaches in PHT cells, it was shown that the inhibition of mTORC1 or mTORC2 markedly decreased folate uptake into PHT cells. In particular, it was shown that mTOR regulates folate transport by influencing the plasma membrane trafficking of RFC and FR-α, but not PCFT. In contrast to mTORC1 regulation of cellular amino acid uptake, mTOR regulation of folate transporter trafficking does not involve the ubiquitin ligase neuronal precursor cell-expressed, developmentally down-regulated protein 4-2 (56).
Conclusions and Speculations
We reviewed compelling evidence that implicates mTOR signaling as an important cellular folate sensor (35, 38, 56). mTOR folate sensing constitutes a novel link between folate availability and cell function, distinct from the established role of folate in DNA synthesis and methylation reactions (Figure 1). Given that mTOR is a key regulator of protein synthesis, mitochondrial respiration, metabolism, and cellular nutrient uptake, folate availability affects diverse aspects of cell biology far beyond what has previously been recognized.
PCFT is required for mTOR-folate sensing and may represent the actual folate sensor. Both in vivo (35) and in vitro (38) data suggest that mTOR is recruited to the lysosome in response to increased folate availability. This is reminiscent of the current model of the mechanisms underlying mTOR amino acid sensing, which requires the recruitment of mTORC1 to the outer lysosomal surface, mediated by Rag GTPase-dependent and independent mechanisms and involves the vacuolar H+-ATPase (37, 57–59). Although the molecular identity of the sensor linking amino acid concentrations to mTORC1 signaling has remained elusive, recent reports show that the lysosomal amino acid transporter SLC38A9 constitutes a key component of the mTOR amino acid sensing machinery (60–62). It is notable that SLC38A9 is an amino acid transporter mediating the efflux of amino acids from the lumen of the lysosome energized by the outwardly directed proton gradient. It is therefore tempting to speculate that PCFT, a proton-coupled folate transporter, plays a role in mTOR folate sensing similar to SLC38A9 for amino acid sensing. Further determination of the cellular localization of mTOR, PCFT, and other proteins involved in mTOR activation, such as Rag and Ragulator, is necessary to clarify the specific role of these proteins in the folate-mTOR signaling pathway.
mTOR folate sensing may have broad biological significance given the critical role of folate in normal cell function and the multiple diseases that have been associated to decreased or excessive folate availability. Low maternal folate concentrations are linked to fetal growth restriction, and the underlying mechanisms may involve trophoblast mTOR folate sensing. Specifically, we propose that placental mTOR is inhibited in response to low maternal folate concentrations, which result in a decrease in placental amino acid transport, protein synthesis, and mitochondrial respiration. Consequently, fetal nutrient availability is reduced, which results in restricted fetal growth (Figure 3). In proliferating cells, including cancer cells, PCFT and mTOR may modulate cell growth and proliferation in response to changes in folate availability.
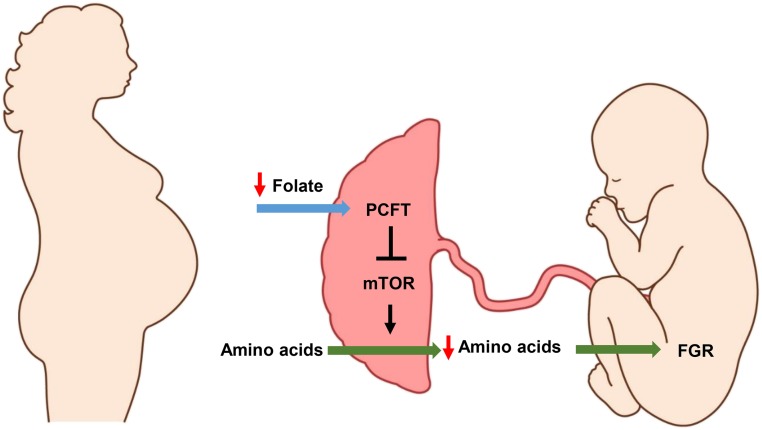
FIGURE 3.
Placental mTOR folate sensing may link low maternal folate to FGR. We propose that the PCFT senses low maternal folate concentrations and inhibits placental mTOR, which results in a decrease in placental amino acid transport, protein synthesis, and mitochondrial respiration. As a consequence, fetal nutrient availability is reduced, resulting in FGR. FGR, fetal growth restriction; mTOR, mechanistic target of rapamycin; PCFT, proton-coupled folate transporter.
Acknowledgments
The authors’ responsibilities were as follows—ES, FJR, TLP, and TJ: wrote the manuscript; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: AKT, protein kinase B; FR-α, folate receptor α mTOR, mechanistic target of rapamycin; mTORC, mechanistic target of rapamycin complex; NTD, neural tube defect; PCFT, proton-coupled folate transporter; PHT, primary human trophoblast; RFC, reduced folate carrier; Rictor, rapamycin-insensitive companion of mTOR; SLC, solute carrier; SNAT, sodium-dependent neutral amino acid transporter; 4E-BP1, eukaryotic initiation factor 4E binding protein 1.
References
- 1.Caudill MA. Folate bioavailability: implications for establishing dietary recommendations and optimizing status. Am J Clin Nutr 2010;91:1455S–60S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr 2007;85:598S–603S. [DOI] [PubMed] [Google Scholar]
- 3.Laurence KM. Recurrence risk of neural tube defects. J Med Genet 1981;18:322–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurence KM, Campbell H. Trial of folate treatment to prevent recurrence of neural tube defect. Br Med J (Clin Res Ed) 1981;282:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol 2011;4:52–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Padmanabhan N, Watson ED. Lessons from the one-carbon metabolism: passing it along to the next generation. Reprod Biomed Online 2013;27:637–43. [DOI] [PubMed] [Google Scholar]
- 7.Padmanabhan N, Jia D, Geary-Joo C, Wu X, Ferguson-Smith AC, Fung E, Bieda MC, Snyder FF, Gravel RA, Cross JC, et al. . Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 2013;155:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PJ, Bulmer JN, Innes BA, Broughton Pipkin F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol Reprod 2011;84:1148–53. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr 2007;86:271–3. [DOI] [PubMed] [Google Scholar]
- 10.Hao M, Zhao W, Zhang L, Wang H, Yang X. Low folate levels are associated with methylation-mediated transcriptional repression of miR-203 and miR-375 during cervical carcinogenesis. Oncol Lett 2016;11:3863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohan TE, Jain MG, Howe GR, Miller AB. Dietary folate consumption and breast cancer risk. J Natl Cancer Inst 2000;92:266–9. [DOI] [PubMed] [Google Scholar]
- 12.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem 1999;10:66–88. [DOI] [PubMed] [Google Scholar]
- 13.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 1999;55:578–92. [DOI] [PubMed] [Google Scholar]
- 14.Arinze IJ. Facilitating understanding of the purine nucleotide cycle and the one-carbon pool. Part I: The purine nucleotide cycle. Biochem Mol Biol Educ 2005;33:165–8. [DOI] [PubMed] [Google Scholar]
- 15.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 2012;3:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet 2010;70:27–56. [DOI] [PubMed] [Google Scholar]
- 17.Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Klemp M. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open 2012;2:e000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor CM, Atkinson C, Penfold C, Bhattacharya S, Campbell D, Davey Smith G, Leary S, Ness A. Folic acid in pregnancy and mortality from cancer and cardiovascular disease: further follow-up of the Aberdeen folic acid supplementation trial. J Epidemiol Community Health 2015;69:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulrich CM. Folate and cancer prevention—where to next? Counterpoint. Cancer Epidemiol Biomarkers Prev 2008;17:2226–30. [DOI] [PubMed] [Google Scholar]
- 20.Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol 2008;108:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gates SB, Mendelsohn LG, Shackelford KA, Habeck LL, Kursar JD, Gossett LS, Worzalla JF, Shih C, Grindey GB. Characterization of folate receptor from normal and neoplastic murine tissue: influence of dietary folate on folate receptor expression. Clin Cancer Res 1996;2:1135–41. [PubMed] [Google Scholar]
- 22.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998;72:141–96. [PubMed] [Google Scholar]
- 23.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet 1999;21:163–7. [DOI] [PubMed] [Google Scholar]
- 24.Qin T, Du M, Du H, Shu Y, Wang M, Zhu L. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci Rep 2015;5:12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev 2006;15:189–93. [DOI] [PubMed] [Google Scholar]
- 26.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009;122:3589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol 2013;203:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017;169:361–71. [DOI] [PubMed] [Google Scholar]
- 29.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009;137:873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004;6:1122–8. [DOI] [PubMed] [Google Scholar]
- 31.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Sahra I, Hoxhaj G, Ricoult SJ, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016;351:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kottakis F, Nicolay BN, Roumane A, Karnik R, Gu H, Nagle JM, Boukhali M, Hayward MC, Li YY, Chen T, et al. . LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature 2016;539:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang SY, Kang YJ, Sung B, Kim M, Kim DH, Lee Y, Yoo MA, Kim CM, Chung HY, Kim ND. Folic acid promotes the myogenic differentiation of C2C12 murine myoblasts through the Akt signaling pathway. Int J Mol Med 2015;36:1073–80. [DOI] [PubMed] [Google Scholar]
- 35.Rosario FJ, Nathanielsz P, Powell TL, Jansson T. Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction. Sci Rep. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–55. [DOI] [PubMed] [Google Scholar]
- 37.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosario FJ, Powell TL, Jansson T. mTOR folate sensing links folate availability to trophoblast cell function. J Physiol 2017. Apr 4 (Epub ahead of print; DOI: 10.1113/JP272424). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arias E, Koga H, Diaz A, Mocholi E, Patel B, Cuervo AM. Lysosomal mTORC2/PHLPP1/Akt regulate chaperone-mediated autophagy. Mol Cell 2015;59:270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 2012;33:e23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMullin MF, Young PB, Bailie KE, Savage GA, Lappin TR, White R. Homocysteine and methylmalonic acid as indicators of folate and vitamin B12 deficiency in pregnancy. Clin Lab Haematol 2001;23:161–5. [DOI] [PubMed] [Google Scholar]
- 42.Zhao R, Goldman ID. The proton-coupled folate transporter: physiological and pharmacological roles. Curr Opin Pharmacol 2013;13:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 2009;284:4267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad PD, Ramamoorthy S, Moe AJ, Smith CH, Leibach FH, Ganapathy V. Selective expression of the high-affinity isoform of the folate receptor (FR-alpha) in the human placental syncytiotrophoblast and choriocarcinoma cells. Biochim Biophys Acta 1994;1223:71–5. [DOI] [PubMed] [Google Scholar]
- 45.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr 1999;19:91–122. [DOI] [PubMed] [Google Scholar]
- 46.Henriques C, Trugo NM. Partial characterization of folate uptake in microvillous membrane vesicles isolated from human placenta. Braz J Med Biol Res 1996;29:1583–91. [PubMed] [Google Scholar]
- 47.Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, Smith SB. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda S, Hasui S, Yamamoto C, Yoshioka C, Kobayashi M, Itagaki S, Hirano T, Iseki K. Placental folate transport during pregnancy. Biosci Biotechnol Biochem 2008;72:2277–84. [DOI] [PubMed] [Google Scholar]
- 49.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev 2004;56:1085–97. [DOI] [PubMed] [Google Scholar]
- 50.Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet 1999;23:228–32. [DOI] [PubMed] [Google Scholar]
- 51.Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, Edelmann W, Goldman ID. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem 2001;276:10224–8. [DOI] [PubMed] [Google Scholar]
- 52.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006;127:917–28. [DOI] [PubMed] [Google Scholar]
- 53.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 2007;582:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 2009;297:C723–31. [DOI] [PubMed] [Google Scholar]
- 55.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol 2013;591:609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosario FJ, Powell TL, Jansson T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-alpha and the RFC. Sci Rep 2016;6:31705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008;320:1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008;10:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism: differential regulation of mTORC1 by leucine and glutamine. Science 2015;347:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 2015;517:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al. . Metabolism: lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015;347:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, et al. . SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015;519:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]