Abstract
Background
The objective of this study was to characterize the degree, pattern, lesion site, and temporal evolution of sudomotor dysfunction in multiple system atrophy (MSA) and to evaluate differences by parkinsonian (MSA-parkinsonism) and cerebellar (MSA-cerebellar) subtypes.
Methods
All cases of MSA evaluated at Mayo Clinic Rochester between 2005 and 2010 with postganglionic sudomotor testing and thermoregulatory sweat test were reviewed. Pattern and lesion site (preganglionic, postganglionic, or mixed) were determined based on thermoregulatory sweat test and postganglionic sudomotor testing.
Results
The majority of the 232 patients were MSA-parkinsonism (145, 63%). Initial postganglionic sudomotor testing was abnormal in 59%, whereas thermoregulatory sweat test was abnormal in 95% of all patients. MSA-parkinsonism patients were more likely to have an abnormal thermoregulatory sweat test compared with MSA-cerebellar (98% versus 90%, P = 0.006) and had a higher mean percentage of anhidrosis (57%) compared with MSA-cerebellar (48%; P = 0.033). Common anhidrosis patterns were regional (38%) and global (35%). The site of the lesion was preganglionic in 47% and mixed (preganglionic and postganglionic) in 41%. The increase in anhidrosis per year was 6.2% based on 70 repeat thermoregulatory sweat tests performed on 29 patients. The frequency of postganglionic sudomotor abnormalities increased over time.
Conclusions
Our findings suggest: (1) sudomotor dysfunction is almost invariably present in MSA and even more common and severe in MSA-parkinsonism than MSA-cerebellar; (2) a preganglionic pattern of sweat loss is common in MSA; however, pre- and post-ganglionic abnormalities may coexist; and (3) the increasing frequency of postganglionic sudomotor dysfunction over time suggests involvement of postganglionic fibers or sweat glands later in the disease course.
Keywords: multiple system atrophy, autonomic, α-synuclein, parkinsonism, ataxia
Multiple system atrophy (MSA) is a neurodegenerative disorder characterized by motor involvement of parkinsonism (MSA-P) and/or cerebellar ataxia (MSA-C) with autonomic failure,1 which may include sudomotor dysfunction.2,3 The degree of sweat loss in MSA is greater than in Parkinson’s disease and has traditionally been attributed to a preganglionic lesion.3,4 However, recent studies evaluating sweat gland nerve density suggest involvement of postganglionic fibers in MSA.5 Although hypo- or anhidrosis in MSA is well recognized, the degree, pattern, lesion site, and temporal evolution of sudomotor dysfunction in MSA has not been systematically evaluated in a large patient cohort.
The combination of autonomic function testing with thermoregulatory sweat testing (TST) and assessment of postganglionic sudomotor function (quantitative sudomotor axon reflex testing [QSART]) can determine the site of the lesion in the autonomic nervous system.6 The entire thermoregulatory pathway from the preoptic/anterior hypothalamus to the sweat gland is assessed using the TST, which allows for assessment of whole-body sweating, the pattern of abnormality, and quantification of percentage of anhidrosis.6,7 QSART assesses the integrity of the postganglionic sympathetic sudomotor axon and allows for quantification of the sweat response at selected sites.6 Therefore, an area of absent sweating on TST in an area with a normal QSART response would indicate a pre-ganglionic lesion. Conversely, a reduced or absent QSART indicates a postganglionic lesion.
In the present study, we sought to (1) characterize the degree and pattern of sweat loss on TST and QSART; (2) determine the site of the lesion; (3) examine the temporal evolution of sudomotor dysfunction in MSA; and (4) evaluate sudomotor differences by motor subtype of MSA.
Methods
This study was approved by the institutional review board of Mayo Clinic, Rochester, Minnesota.
Study Design
This was a retrospective review of all individuals evaluated at Mayo Clinic in Rochester, Minnesota, with the diagnosis of MSA between January 2005 and December 2010. Inclusion criteria included completion of both an initial autonomic reflex screen with QSART testing and a TST at diagnosis. Data from repeat TST and QSART evaluations were evaluated until December 2015.
All patients were categorized as probable or possible MSA based on consensus criteria8 and classified by clinical phenotype of MSA-P or MSA-C based on predominant symptom complex and examination findings at initial evaluation. Sweating symptoms, orthostatic intolerance symptoms, and bladder symptoms of frequency/urgency, incontinence, and catheterization were obtained from the clinical history, whereas orthostatic and bladder symptoms were also obtained from standardized patient-completed symptom questionnaires. Bladder symptoms, urinary catheterization, and presence of orthostatic hypotension (defined as systolic blood pressure drop greater than 30 mm Hg or diastolic greater than 15 mm Hg at 5 minutes of head-up tilt) were associated with severity of QSART abnormalities and percentage of anhidrosis on TST. Comorbidities of diabetes mellitus and peripheral neuropathy were reviewed based on diagnostic codes, and neuropathy diagnosis severity was graded using electromyography (when available).
Postganglionic Sudomotor and Thermoregulatory Sweat Testing
Patients were instructed to discontinue medications that could interfere with sweating function for 48 hours prior to autonomic testing. Postganglionic sudomotor testing was performed in a highly standardized fashion as previously described using QSART at the following sites: forearm, proximal leg, distal leg, and foot.6 Sites were considered abnormal if sweat volume was less than 5% of controls or if there was a relative reduction in sweat volume at the foot site, defined as less than one-third of the adjacent proximal values. For patients with repeat autonomic testing later than 2010, Q-Sweat (WR Medical Electronics Co., Maplewood, MN) was used, and sites were considered abnormal if volume was less than 5% of Q-Sweat controls or a relative reduction of sweat volume at the foot site, defined as above. Composite autonomic severity scores were used to quantify the degree of sudomotor dysfunction based on QSART. A score of 0 represents no impairment, whereas the maximum score of 3 represents severe sudomotor dysfunction.9
TST was performed as previously described as a modification of Guttmann’s quinizarin sweat test.10 A participant lies supine, unclothed with exposed body surface covered with the indicator powder mixture.7,11 The body is warmed to 38 °C, and change in color of the indicator signifies sweating. Sweat distribution is documented using digital photography, and digital images are processed using a pixel counter to derive cumulative values for the area, and percentage of anterior body surface anhidrosis is derived. The TST is a standardized clinical procedure at Mayo Clinic.
Sweat Distribution Patterns
Patterns of anhidrosis on TST were defined as follows: length dependent, affecting fingers, legs below the knees, and/or lower abdomen with area of anhidrosis less than 25%; focal, isolated to a dermatome or peripheral nerve territory; segmental, involvement of a few adjacent roots or plexus; regional: large areas but less than 80% that may or may not be contiguous; global anhidrosis, greater than or equal to 80% of body surface affected; mixed: various combinations of length dependent, focal, segmental, or regional patterns. Patterns of reduced sweat volumes on QSART were defined as follows: length dependent, isolated to foot site or a gradient involving foot and leg sites; focal, isolated to a single site; global, all sites affected; mixed, abnormal but not conforming to other patterns.
Site of the Lesion
Lesions sites were classified as preganglionic, post-ganglionic, or mixed based on TST and QSART results. An area of reduced or absent sweating on TST in the context of a normal QSART response was determined to be a preganglionic lesion. Conversely, a reduced or absent QSART was considered a postganglionic lesion when corresponding to a normal or anhidrotic area on TST. The lesion site was deemed mixed when there was anhidrosis on TST with diminished (but not absent) corresponding QSART volumes, or if there were both postganglionic and preganglionic patterns at different QSART sites in an individual patient.
Evolution of Sudomotor Findings
Patients with multiple TST evaluations were evaluated for progression of anhidrosis. Repeat QSART studies were evaluated for the presence of abnormalities.
Statistical Analysis
Statistical analyses were performed using the statistical software SPSS, version 21, with statistical significance set at P <0.05. Summary statistics were presented as mean and standard deviation for continuous data or frequency and percentage for categorical data. Comparisons between MSA-P and MSA-C for categorical data were performed using chi-square testing or Fisher’s exact test, when appropriate. Continuous variables were compared between MSA-P and MSA-C using the Student t test for normative data or the Mann-Whitney U test for skewed data. The false discovery rate was used to test for multiple comparisons.
Results
Demographics and Clinical Characteristics
A total of 232 patients with the diagnosis of MSA had both a QSART and TST at first presentation to our institution. Of those patients, 210 (91%) met criteria for probable MSA during clinical evaluation. Demographics and clinical features are listed in Table 1. Although orthostatic intolerance and bladder symptoms were common complaints (75% and 84%, respectively), sweating symptoms were infrequent, occurring in only 16% of patients. Reduced sweating was the most common sweating complaint; however, a few patients reported increased sweating, suggesting compensatory hyperhidrosis. The prevalence of diabetes was 1.3% and of peripheral neuropathy was 5%, with the majority of those cases (78%) graded as mild based on electromyography. There was no difference between MSA-P or MSA-C for the presence of diabetes (P = 0.880) or peripheral neuropathy (P = 0.761).
TABLE 1.
Patient demographics and clinical features
| Overall | MSA-P | MSA-C | Pa | |
|---|---|---|---|---|
| Number (%) | 232 (100) | 145 (62.5) | 87 (37.5) | - |
| Sex | 0.585a | |||
| Male | 128 (55.2) | 78 (53.8) | 50 (57.5) | |
| Female | 104 (44.8) | 67 (46.2) | 37 (42.5) | |
| Age of onset | 60.0 (10.1) | 61.5 (10.2) | 57.5 (9.4) | 0.0025 |
| Autonomic Symptoms | ||||
| Reduced sweating | 25 (11) | 19 (13) | 6 (7) | 0.140 |
| Increased sweating | 12 (5) | 6 (4) | 6 (7) | 0.358 |
| Heat intolerance | 10 (4) | 7 (5) | 3 (3) | 0.617 |
| Orthostatic intolerance | 173 (75) | 114 (79) | 59 (68) | 0.067 |
| Bladder symptoms | 194 (84) | 120 (83) | 74 (85) | 0.647 |
| Urinary catheterization | 33 (14) | 22 (15) | 11 (13) | 0.591 |
Values displayed as frequency (percent) for categorical variables and mean (standard deviation) for continuous variables.
Between MSA-P and MSA-C.
MSA, multiple system atrophy; MSA-C, multiple system atrophy-cerebellar; MSA-P, multiple system atrophy-parkinsonism
The median time from initial symptom onset to TST was 3.39 years (interquartile range, 2.04–5.09 years). The median time from initial symptom onset to QSART was 3.36 years (interquartile range, 2.04–5.06 years). The median duration between the QSART and TST was 1 day (interquartile range, 1–3 days). There was no difference between the time from symptom onset to QSART or TST between MSA-P and MSA-C (P = 0.9308). The median time from initial symptom onset to TST in those with autopsy confirmation of MSA was 3.08 years (interquartile range, 1.46–6.63 years), with no difference between those without autopsy confirmation (P = 0.5968).
Degree and Pattern of Sweat Loss
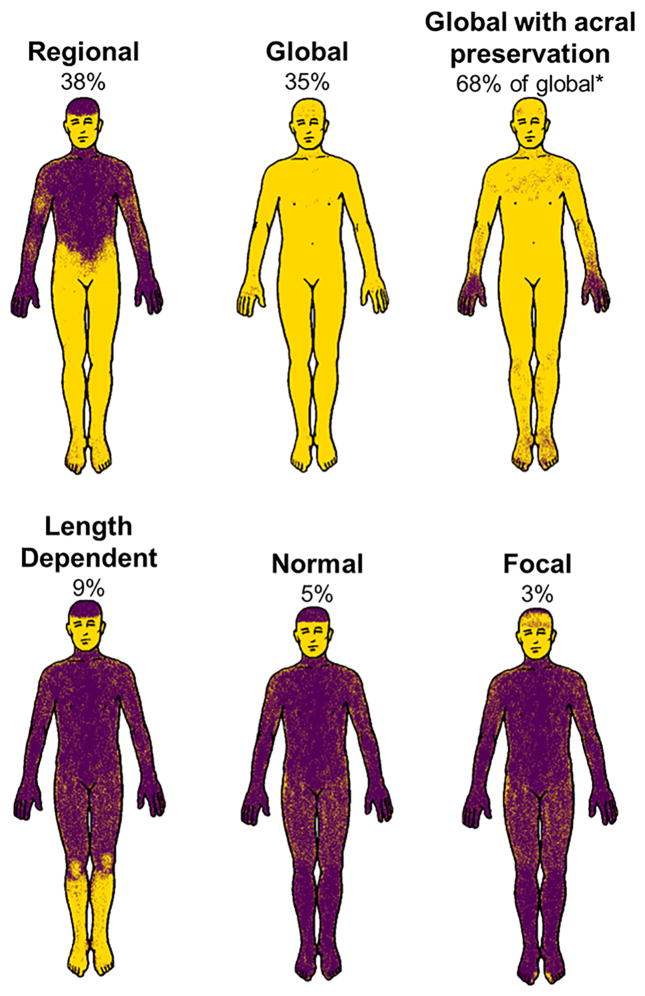
The initial QSART was abnormal in 59% of patients, whereas the initial TST was abnormal in 95%, with 64% demonstrating more than 40% anhidrosis (Table 2). The most common TST patterns were regional and global, which tended to be in an ascending pattern with the lower extremities more greatly affected, whereas the majority of patients with global anhidrosis had acral preservation of sweating (Fig. 1). Length dependent reduction in sweat volume was the most common pattern observed on QSART (Table 2). Although there was no difference in QSART pattern between subtypes, MSA-P patients were more likely to have an abnormal TST and had a greater anhidrosis percentage compared with MSA-C patients (Table 2). MSA-P and MSA-C patients otherwise demonstrated similar patterns of sweat loss on TST.
TABLE 2.
Sudomotor results in MSA patients
| Overall | MSA-P | MSA-C | P | |
|---|---|---|---|---|
| Number (%) | 232 (100) | 145 (62.5) | 87 (37.5) | — |
| Abnormal QSART | 136 (58.6) | 89 (61.4) | 47 (54.0) | 0.271a |
| QSART Distribution | 0.348a | |||
| Normal | 96 (41.4) | 56 (38.6) | 40 (46.0) | |
| Focal | 21 (9.1) | 11 (7.6) | 10 (11.5) | |
| Distal | 64 (27.6) | 44 (30.3) | 20 (23.0) | |
| Mixed | 30 (12.9) | 18 (12.4) | 12 (13.8) | |
| Global | 21 (9.1) | 16 (11.0) | 5 (5.7) | |
| Abnormal TST | 220 (94.8) | 142 (97.9) | 78 (89.7) | 0.006a |
| TST Pattern | 0.013a | |||
| Normal | 12 (5.2) | 3 (2.1) | 9 (10.3) | 0.035c |
| Focal | 7 (3.0) | 5 (3.5) | 2 (2.3) | 0.617c |
| Distal | 20 (8.6) | 10 (6.9) | 10 (11.5) | 0.315c |
| Regional | 88 (37.9) | 51 (35.2) | 37 (42.5) | 0.315c |
| Mixed | 24 (10.4) | 19 (13.1) | 5 (5.8) | 0.150c |
| Global | 81 (34.9) | 57 (39.3) | 24 (27.6) | 0.150c |
| TST anhidrosis (%) | 53.7 (34.8) | 57.2 (34.3) | 47.9 (35.0) | 0.033b |
| 95% CI | 49.2–58.2 | 51.5–62.8 | 40.4–55.3 | |
| TST anhidrosis >40% | 148 (63.8) | 97 (66.9) | 51 (58.6) | 0.204a |
| Site of lesion | 0.0822 | |||
| Normal | 13 (5.6) | 5 (3.5) | 8 (9.2) | |
| Preganglionic | 110 (47.4) | 68 (46.9) | 42 (48.3) | |
| Postganglionic | 13 (5.6) | 5 (3.5) | 8 (9.2) | |
| Mixed | 96 (41.4) | 67 (46.2) | 29 (33.3) |
Values displayed are frequency (percent) for categorical variables and mean (standard deviation) for continuous variables.
MSA-C, multiple system atrophy-cerebellar; MSA-P, multiple system atrophy-parkinsonism; QSART, quantitative sudomotor axon reflex testing; TST, thermo-regulatory sweat test.
Based on chi-Square test with level of significance set to 0.05.
Based on Mann-Whitney test with level of significance set to 0.05.
Test for multiple comparisons using False Discovery Rate.
FIG. 1.
Anhidrosis patterns with the thermoregulatory sweat test. Representative patterns of anhidrosis are shown with the percentage of patients with each pattern listed. Mixed pattern accounted for 10% (not shown) and represents a combination of regional, length-dependent, and focal distributions. *Global anhidrosis with acral preservation accounted for 68% of all global cases (24% of all cases).
Thirty-four patients eventually had autopsy-proven MSA. Of those patients, the mean percentage anhidrosis on first TST was 69% (standard deviation, 29%). Two of these patients had percentage anhidrosis of 5% or less.
Site of the Lesion
The site of the lesion was deemed preganglionic in 47% and mixed (preganglionic and postganglionic) in 41%. The site of the lesion was similar in MSA-P and MSA-C patients (Table 2).
Evolution of Anhidrosis
Of 29 patients who had more than 1 TST during the course of the disease, 22 were MSA-P. The mean increase in percentage anhidrosis per year was 6.2% (standard deviation, 14.6; 95% confidence interval, 0.6%–11.8%); there was no significant difference between MSA-P and MSA-C (P = 0.161). Results from repeat TST and postganglionic sudomotor axon testing are shown in Table 3. The frequency of abnormal QSART increased from 29% at the initial testing to 60% at the third testing. The number of patients with an abnormal result increased, as did the mean percentage anhidrosis on TST with repeat testing.
TABLE 3.
Evolution of anhidrosis on repeat thermoregulatory sweat test and postganglionic sudomotor axon reflex testing
| Initial evaluation | Second evaluation | Third evaluation | |
|---|---|---|---|
| Evolution of TST | |||
| Number with repeat TST | 29 | 29 | 12 |
| Time from symptom onset to evaluation, years | 3.1 (2.0–5.7) | 5.2 (3.4–7.4) | 6.5 (4.8–8.5) |
| Abnormal TST | 23 (79) | 28 (97) | 12 (100) |
| Mean percentage anhidrosis (standard deviation) | 23.0 (32.2) | 31.2 (27.6) | 47.5 (35.4) |
| Evolution of postganglionic sudomotor axon reflex testing | |||
| Number with repeat QSART | 24 | 24 | 10 |
| Time from symptom onset to evaluation, years | 3.6 (1.5–5.8) | 5.4 (3.5–7.0) | 6.7 (4.5–8.1) |
| Abnormal postganglionic sudomotor axon reflex testing | 7 (29) | 10 (42) | 6 (60) |
Values displayed are frequency (percent) for categorical variables and median (interquartile range) for continuous variables unless otherwise stated. QSART, quantitative sudomotor axon reflex test; TST, thermoregulatory sweat test.
Correlation of Sudomotor Findings With Autonomic Dysfunction
The severity of QSART abnormalities was not associated with orthostatic hypotension during the head-up tilt test (P = 0.700), bladder symptoms (P = 0.212), or urinary catheterization (P = 0.208). However, the mean percentage of anhidrosis on TST was greater in those with orthostatic hypotension on the head-up tilt test (59.2; standard deviation, 34.1) compared with those without orthostatic hypotension (46.5; standard deviation, 34.6; P = 0.006). There was no difference in the mean percentage of anhidrosis in MSA patients with bladder symptoms (P = 0.272) or those requiring urinary catheterization (P = 0.138).
Discussion
This study provides a systematic characterization of sudomotor dysfunction in MSA based on a large patient cohort evaluated at a single institution with standardized autonomic testing. Our findings suggest that sudomotor dysfunction is almost invariably present in MSA and more commonly seen in MSA-P than in MSA-C. Although a preganglionic pattern of sweat loss is most common in MSA, this is not absolute, and there is an increasing frequency of postganglionic sudomotor dysfunction over time, which may suggest involvement of postganglionic fibers or sweat glands later in the disease course.
The mean percent anhidrosis on TST was more than 50% on first evaluation, and the pattern tended to be ascending, meaning that often lower extremities were more greatly affected than upper extremities, which has been seen in smaller studies.12 However, even though most MSA patients have large areas of anhidrosis that increase with time, this observation is not invariable. A few autopsy-confirmed patients had only a small percentage of anhidrosis despite neuropathological evidence of MSA. Therefore, although involvement of sweating is useful in differentiating MSA from other neurodegenerative diseases, relatively normal sudomotor function, although unusual, does not exclude the diagnosis of MSA.
Evaluating TST patterns confirmed our previous clinical observation that MSA is often characterized by widespread anhidrosis with sparing of distal extremities.13 This interesting phenomenon of global anhidrosis with acral preservation was seen in most patients with widespread anhidrosis. Preserved acral sweating in the presence of widespread anhidrosis may be related to the “cold hands sign” of MSA, in which patients report cold hands and feet, often with color changes.14 Further work has demonstrated that the skin vasomotor response to local heating is intact in MSA patients, with the cold hand sign suggesting preserved sympathetic tone to the hands.15 Intact sympathetic tone may manifest as vasoconstriction but also as sweating. Preserved acral sweating and cold hands may therefore relate to selective preservation or possibly uninhibited sympathetic innervation or dysregulation of sympathetic activity to the hands. Mechanistically, selective preservation of sweating to the hands may relate to different pathways involved in acral sweating, such as emotional sweating pathways, and may be preserved in the neurodegenerative process in MSA. Another mechanistic possibility for preserved acral sweating may relate to sweat gland density. The sweat response may be lost globally, but areas with a greater population of sweat glands may still be active and compensate for loss in other areas. We have not seen this pattern of anhidrosis in other neurodegenerative disorders, but future work will need to systematically assess the specificity of this finding for MSA.
The site of the lesion demonstrated by TST and QSART was more likely to be preganglionic in MSA patients, which is expected based on our current neuropathological and pathophysiologic understanding of MSA and similar to previous studies.3,4,16 The finding corresponds to the areas of neurodegeneration in MSA that affect thermoregulatory regions in the brain stem projecting to the spinal cord.17,18 We also found that a greater degree of anhidrosis was associated with the presence of orthostatic hypotension, reflecting at least some degree of concordance of neuronal loss in central sudomotor and baroreflex pathways. However, in addition to preganglionic sudomotor deficits, there was also evidence for postganglionic sudomotor dysfunction. This has been increasingly recognized and has been attributed to loss of preganglionic input leading to transsynaptic changes as the disease progresses.5,16,19 However, recent studies using skin biopsy and immunohistochemistry have shown that in some patients with long duration of disease, there are skin nerve fiber abnormalities,20 and phosphorylated α-synuclein has been demonstrated in dermal nerve fibers, suggesting that peripheral α-synuclein burden may contribute to postganglionic sudomotor impairment.21 These reports are in concordance with our results showing an increase in abnormal postganglionic sudomotor function with longer disease duration. This area has become an area of particular interest, as there is increasing effort to use skin biopsy and epidermal nerve fiber pathology to discriminate MSA from Parkinson’s disease.22
Our finding of predominant preganglionic sudomotor lesions in MSA relate to studies using myocardial scintigraphy with [123I]-meta-iodobenzylguanidine (MIBG) for the assessment of parkinsonism. Initial studies suggested preservation of myocardial MIBG uptake in MSA, consistent with a preganglionic lesion.23,24 However, subsequent evaluation has demonstrated that approximately a third of MSA patients have evidence of abnormal MIBG uptake, suggesting postganglionic sympathetic involvement.25 The proposed mechanism for these findings in MSA is similar to the suspected mechanism for postganglionic sudomotor involvement: secondary transsynaptic loss of postganglionic neurons as well as α-synuclein pathology in postganglionic neurons.25 In contrast to our findings, however, loss of myocardial MIBG uptake in MSA patients was not related to disease duration, which suggests that alternative mechanism may be contributing to postganglionic sympathetic neuronal loss dependent on location and/or function.
Our study also detected differences regarding sweating function in the subtypes of MSA. Namely, sudomotor dysfunction was worse in MSA-P patients than in MSA-C patients, manifest as a greater percentage of patients with an abnormal TST and a higher mean percentage of anhidrosis in MSA-P patients. One possible explanation for this finding might be that the age of onset was slightly later in our MSA-P patients compared with MSA-C patients, which corresponds to previous studies in MSA.26,27 However, one would not expect this small degree of age difference to account for this difference. Instead, we suspect that this finding is more likely explained by the MSA motor subtypes having a different pattern of autonomic involvement.
The main limitations of this study are its retrospective nature and that only a small percentage of patients were autopsy confirmed. However, those with autopsy-confirmed disease had findings similar to the entire cohort, and we have previously reported on a series of biopsy-confirmed patients with MSA and the high congruence with the premortem clinical diagnosis at our institution.28 Another limitation is that the mode of postganglionic sudomotor testing changed in 2010 from an institutionally developed sudorometer to the commercial Q-Sweat system, which negated the ability to follow sweat volumes over time in individual patients. Nevertheless, the techniques used for both methods are highly similar, utilizing the same principle of axon-reflex-mediated sweat response quantification induced by iontophoresis of acetylcholine, and established normative values for the respective methods that were utilized.
Our study provides a systematic multimodality evaluation of sudomotor dysfunction in a large group of patients with MSA. We conclude that severe sudomotor deficits are present in the majority of patients with MSA, which are commonly consistent with a preganglionic etiology. Although almost invariably present in both motor subtypes, sudomotor deficits were more common and severe in MSA-P than in MSA-C. The finding of increasing frequency of postganglionic sudomotor dysfunction with advancing disease has implications in understanding disease pathogenesis and for the development of disease biomarkers for MSA.
Acknowledgments
Funding agencies: This publication was supported by NIH (R01NS 92625, NS44233, R01 FD004789, U54NS065736, K23NS075141), Mayo CCaTS (UL1TR000135), and Cure MSA Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Relevant conflicts of interest/financial disclosures: The authors report no disclosures.
References
- 1.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015;372:249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 2.Parikh SM, Diedrich A, Biaggioni I, Robertson D. The nature of the autonomic dysfunction in multiple system atrophy. J Neurol Sci. 2002;200:1–10. doi: 10.1016/s0022-510x(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 3.Lipp A, Sandroni P, Ahlskog JE, et al. Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Arch Neurol. 2009;66:742–750. doi: 10.1001/archneurol.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donadio V, Nolano M, Elam M, et al. Anhidrosis in multiple system atrophy: a preganglionic sudomotor dysfunction? Mov Disord. 2008;23:885–888. doi: 10.1002/mds.21972. [DOI] [PubMed] [Google Scholar]
- 5.Provitera V, Nolano M, Caporaso G, et al. Postganglionic sudomotor denervation in patients with multiple system atrophy. Neurology. 2014;82:2223–2229. doi: 10.1212/WNL.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 6.Low PA. Evaluation of sudomotor function. Clin Neurophysiol. 2004;115:1506–1513. doi: 10.1016/j.clinph.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 10.Guttmann L. The management of the quinizarin sweat test. Overseas Postgrad Med J. 1947;1:69–82. [PubMed] [Google Scholar]
- 11.Low PA, Walsh JC, Huang CY, McLeod JG. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study Brain. 1975;98:341–356. doi: 10.1093/brain/98.3.341. [DOI] [PubMed] [Google Scholar]
- 12.Kihara M, Sugenoya J, Takahashi A. The assessment of sudomotor dysfunction in multiple system atrophy. Clin Auton Res. 1991;1:297–302. doi: 10.1007/BF01819835. [DOI] [PubMed] [Google Scholar]
- 13.Coon EA, Fealey RD, Benarroch EE, Sandroni P, Low PA, Singer W. Purple hands in multiple system atrophy: global anhidrosis with preserved acral sweating. Neurology. 2016;86(24):2314. doi: 10.1212/WNL.0000000000002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein C, Brown R, Wenning G, Quinn N. The “cold hands sign” in multiple system atrophy. Mov Disord. 1997;12:514–518. doi: 10.1002/mds.870120407. [DOI] [PubMed] [Google Scholar]
- 15.Pietzarka K, Reimann M, Schmidt C, et al. The cold hand sign in multiple system atrophy: skin perfusion revisited. J Neural Transm (Vienna) 2010;117:475–479. doi: 10.1007/s00702-010-0375-x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J, Low P, Fealey R, Sheps S, Jiang NS. Somatic and autonomic function in progressive autonomic failure and multiple system atrophy. Ann Neurol. 1987;22:692–699. doi: 10.1002/ana.410220604. [DOI] [PubMed] [Google Scholar]
- 17.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Involvement of medullary serotonergic groups in multiple system atrophy. Ann Neurol. 2004;55:418–422. doi: 10.1002/ana.20021. [DOI] [PubMed] [Google Scholar]
- 18.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Differential involvement of the periaqueductal gray in multiple system atrophy. Auton Neurosci. 2010;158:111–117. doi: 10.1016/j.autneu.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baser SM, Meer J, Polinsky RJ, Hallett M. Sudomotor function in autonomic failure. Neurology. 1991;41:1564–1566. doi: 10.1212/wnl.41.10.1564. [DOI] [PubMed] [Google Scholar]
- 20.Donadio V, Cortelli P, Elam M, et al. Autonomic innervation in multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2010;81:1327–1335. doi: 10.1136/jnnp.2009.198135. [DOI] [PubMed] [Google Scholar]
- 21.Doppler K, Weis J, Karl K, et al. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord. 2015;30:1688–1692. doi: 10.1002/mds.26293. [DOI] [PubMed] [Google Scholar]
- 22.Haga R, Sugimoto K, Nishijima H, et al. Clinical utility of skin biopsy in differentiating between Parkinson’s Disease and multiple system atrophy. Parkinsons Dis. 2015;2015:167038. doi: 10.1155/2015/167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshita M. Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci. 1998;155:60–67. doi: 10.1016/s0022-510x(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 24.Druschky A, Hilz MJ, Platsch G, et al. Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci. 2000;175:3–12. doi: 10.1016/s0022-510x(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 25.Nagayama H, Ueda M, Yamazaki M, Nishiyama Y, Hamamoto M, Katayama Y. Abnormal cardiac [(123)I]-meta-iodobenzylguanidine uptake in multiple system atrophy. Mov Disord. 2010;25:1744–1747. doi: 10.1002/mds.23338. [DOI] [PubMed] [Google Scholar]
- 26.Coon EASD, Suarez MD, Mandrekar JN, et al. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain. 2015;138(Pt 12):3623–3631. doi: 10.1093/brain/awv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14:710–719. doi: 10.1016/S1474-4422(15)00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iodice V, Lipp A, Ahlskog JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry. 2012;83:453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]