Abstract
Background
Malaria control has not been routinely informed by the assessment of subnational variation in malaria deaths. We combined data from the Malaria Atlas Project and the Global Burden of Disease Study to estimate malaria mortality across sub-Saharan Africa on a grid of 5 km2 from 1990 through 2015.
Methods
We estimated malaria mortality using a spatiotemporal modeling framework of geolocated data (i.e., with known latitude and longitude) on the clinical incidence of malaria, coverage of antimalarial drug treatment, case fatality rate, and population distribution according to age.
Results
Across sub-Saharan Africa during the past 15 years, we estimated that there was an overall decrease of 57% (95% uncertainty interval, 46 to 65) in the rate of malaria deaths, from 12.5 (95% uncertainty interval, 8.3 to 17.0) per 10,000 population in 2000 to 5.4 (95% uncertainty interval, 3.4 to 7.9) in 2015. This led to an overall decrease of 37% (95% uncertainty interval, 36 to 39) in the number of malaria deaths annually, from 1,007,000 (95% uncertainty interval, 666,000 to 1,376,000) to 631,000 (95% uncertainty interval, 394,000 to 914,000). The share of malaria deaths among children younger than 5 years of age ranged from more than 80% at a rate of death of more than 25 per 10,000 to less than 40% at rates below 1 per 10,000. Areas with high malaria mortality (>10 per 10,000) and low coverage (<50%) of insecticide-treated bed nets and antimalarial drugs included much of Nigeria, Angola, and Cameroon and parts of the Central African Republic, Congo, Guinea, and Equatorial Guinea.
Conclusions
We estimated that there was an overall decrease of 57% in the rate of death from malaria across sub-Saharan Africa over the past 15 years and identified several countries in which high rates of death were associated with low coverage of antimalarial treatment and prevention programs. (Funded by the Bill and Melinda Gates Foundation and others.)
Measuring the burden of malaria according to age and geographic area and over time is important for malaria-control programs and health care providers for planning, implementing, monitoring, and evaluating control and elimination efforts.1,2 The Malaria Atlas Project has produced high-spatial-resolution (5-km2) estimates of the prevalence of malaria infection (parasite rate) and clinical incidence rates in sub-Saharan Africa from 2000 through 2015.3 In parallel, the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), also known as the Global Burden of Disease Study, has produced national-level estimates of morbidity and mortality from malaria on an annual basis since 1990.4,5
To date, no high-spatial-resolution estimates of malaria mortality have been available. Understanding the spatial and temporal distribution of malaria deaths is important for measuring the effect of investments in malaria control, for which development assistance totaled $2.3 billion in 2015.6,7 Enhanced geographic data on disease burden will also better target populations that are disproportionately affected by malaria and in whom future prevention and treatment interventions have the potential for greatest benefit.
We integrated the work of the Malaria Atlas Project and the GBD 2015 to produce estimates of age-specific and sex-specific malaria mortality in sub-Saharan Africa for each 5-km2 grid cell and for individual years from 1990 through 2015.
Methods
Overview
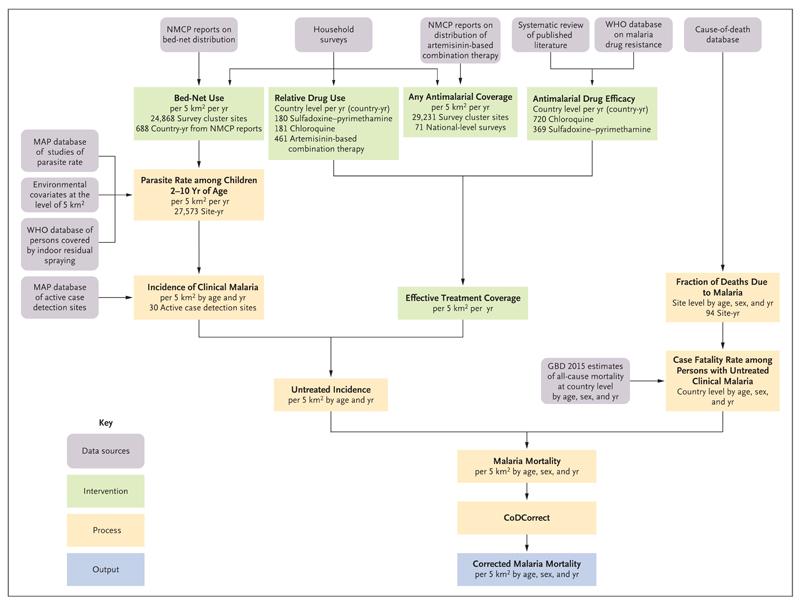
We estimated malaria mortality by combining estimates of the following variables within a geospatial modeling framework: the clinical incidence of malaria at the level of a 5-km2 grid, according to age and year; effective antimalarial drug treatment of clinical malaria cases at the level of a 5-km2 grid, according to year; and the case fatality rate among untreated persons with malaria. Fig. 1 summarizes the estimation procedure.
Figure 1. Main Components of the Modeling Framework for Estimating Malaria Mortality.
The use of insecticide-treated bed nets was defined as the proportion of people sleeping under such a net during the previous night. Relative drug use was defined as the relative proportion of patients with malaria who were treated with chloroquine, sulfadoxine–pyrimethamine, or artemisinin-based combination therapy (the values in the figure indicate the number of country-years of data per drug). Any antimalarial coverage was defined as the fraction of all patients who were treated with any antimalarial drug. Antimalarial drug efficacy was defined as the fraction of the time that treatment was effective; artemisinin-based combination therapy was assumed to be 100% efficacious (the values in the figure indicate the number of country-years of data per drug). Incidence of clinical malaria was defined as cases per person per year, according to age and year at the level of a 5-km2 grid. Effective treatment coverage was defined as the fraction of malaria cases that were effectively treated. Untreated incidence was defined as the rate of untreated or ineffectively treated cases of malaria. CoDCorrect is the Global Burden of Disease Study (GBD) algorithm that standardizes estimates of cause-specific mortality (including from malaria) to match estimates of all-cause mortality. MAP denotes Malaria Atlas Project, NMCP National Malaria Control Program, and WHO World Health Organization.
We used simulation analysis to determine the uncertainty level in the final estimate of malaria mortality on the basis of the uncertainty levels of each previous estimation step. We elaborate on the estimation of these variables below; further details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Prevalence of Malaria
The Malaria Atlas Project has previously assembled large databases of geolocated data on the malaria parasite rate, intervention coverage, and environmental covariates to estimate age-standardized infection prevalence within each 5-km2 grid cell across Africa from 2000 through 2015.3 Here, we included a subnational estimate of the effective treatment rate (as determined by levels of community access to, and efficacy of, antimalarial drugs and described in further detail below and in the Supplementary Appendix) and extended our estimates of the parasite rate back to 1990. The relationship between the parasite rate and the age-specific incidence of clinical malaria has been defined previously.8 We fitted an ensemble of independently developed microsimulation models9–11 to a data set of co-observed community-level malaria parasite rates and clinical incidence from 30 sites across Africa,12 with the resulting modeled relationships stratified according to age group, level of seasonality, and past and present transmission levels to capture population exposure history. The use of a multimodel ensemble allowed conceptual as well as statistical uncertainty to be propagated into the final uncertainty boundaries around the predicted relationships. We used this model, along with high-resolution gridded population maps,13 to generate 5-km2 spatiotemporal estimates of the number of clinical malaria cases in each grid cell for each age group and year.
Effective Antimalarial Drug Treatment
We used a spatiotemporal model of geolocated survey data for antimalarial drug use in children with fever to estimate the year-specific coverage of any antimalarial drug at the level of a 5-km2 grid. The country-specific and year-specific treatment efficacy of chloroquine and sulfadoxine–pyrimethamine was estimated with a spatiotemporal model of in vivo efficacy studies that were identified from the scientific literature and the World Health Organization (WHO) database of antimalarial drug resistance.14 For sub-Saharan Africa, we assumed no drug resistance for artemisinin-based combination therapy.15 We estimated the relative use of chloroquine, sulfadoxine–pyrimethamine, and artemisinin-based combination therapy in a given country in a given year using a spatiotemporal model of survey data for antimalarial drug use in children with fever, which was supplemented by program data for the supply of artemisinin-based combination therapy. The combination of these data provided an estimate of effective antimalarial drug coverage, which reflected the overall drug treatment rate; the relative use of chloroquine, sulfadoxine–pyrimethamine, and artemisinin-based combination therapy; and the corresponding year-specific and country-specific drug efficacy. These estimates were applied to the estimates of clinical malaria incidence to determine the number of untreated or ineffectively treated cases for each grid cell.
Malaria Case Fatality Rate
The GBD systematically compiles and analyzes cause-of-death data from all available sources (vital statistics, verbal autopsy, registries, and surveillance, census, and survey data).16 For sub-Saharan Africa, cause-of-death data are primarily from verbal autopsy. The GBD list of causes uses categorical attribution of deaths to a single underlying cause, according to the principles outlined in the International Statistical Classification of Diseases and Related Health Problems (ICD).16 We estimated case fatality and death rates on the basis of this principle — that is, we did not include increased mortality from other causes associated with the presence of malaria (indirect effects of malaria on mortality from other causes).16,17 For each study data point (Table S4 in the Supplementary Appendix), after data processing for comparability and geolocating each study by latitude and longitude, we calculated the malaria cause fraction (i.e., the number of deaths attributable to malaria divided by all deaths in each study) and multiplied this number by the estimated national rate of death from any cause from the GBD to determine the rate of death from malaria. The malaria mortality for each data point was divided by the corresponding age, sex, year, and geolocated incidence of untreated or ineffectively treated malaria to determine the case fatality rate among untreated persons with malaria.
We modeled the logit of the case fatality rate in a mixed-effects regression with the log rate of death from any cause and sex as fixed effects and the site of the study as a random effect. Data in the regression analysis were weighted according to sample size. We predicted malaria mortality according to age, sex, and year by applying the estimated case fatality rate to the estimate of untreated incidence for each 5-km2 grid cell. To ensure that the sum of cause-specific mortality was equivalent to all-cause mortality, we used the CoDCorrect algorithm to rescale the sum of national-level cause-specific estimates to equal national-level all-cause mortality.16
Data Access and Sharing
This study complied with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER; http://gather-statement.org/). Researchers using GATHER agree to disclose definitions of health indicators, provide access to statistical source codes, and make data and analyses publicly available. GATHER is meant to aid in the replication of results and in collaboration. In keeping with GATHER, all data citations, code, analyses, and results associated with this study are available on either the Institute for Health Metrics and Evaluation website (http://ghdx.healthdata.org/gbd-2015) with respect to the analysis of mortality or the Malaria Atlas Project website (www.map.ox.ac.uk/gather-compliance/) with respect to the analysis of infection prevalence and clinical incidence.
Results
National and Continental-Scale Trends in Malaria Mortality
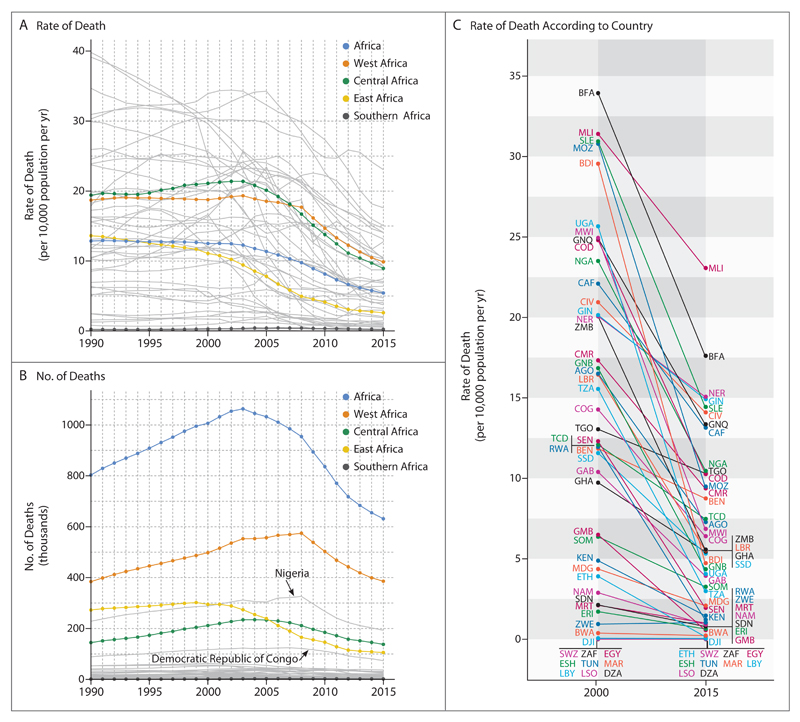
From 1990 through 2000 in sub-Saharan Africa, despite declines in mortality from many other causes, the estimated malaria death rate remained largely flat in West Africa and rose in Central Africa (Fig. 2A), events that were driven primarily by resistance to chloroquine.19 A comparison among the regions of Africa after 2000 shows a continent-wide decline in estimated death rates, although the decrease began earlier and more rapidly in East Africa before decelerating in recent years. In both Central and West Africa, declines were modest until 2005, but then the rates decreased rapidly and remain on a steeper downward trajectory than in East Africa.
Figure 2. Estimated Changes in Plasmodium falciparum Mortality in Sub-Saharan Africa.
Shown are estimated time series from 1990 through 2015 of the rate of death (Panel A) and number of deaths (Panel B) from malaria for each country in which malaria is endemic (gray lines, with the two countries with the largest burden, Nigeria and Democratic Republic of Congo, labeled for deaths) and aggregated into West, Central, East, and Southern Africa regions. Also shown is the change in malaria mortality for each country between 2000 and 2015 (Panel C), with each country identified by its International Organization for Standardization (ISO) country code.18 AGO denotes Angola, BDI Burundi, BEN Benin, BFA Burkina Faso, BWA Botswana, CAF Central African Republic, CIV Ivory Coast, CMR Cameroon, COD Democratic Republic of Congo, COG Congo, DJI Djibouti, DZA Algeria, EGY Egypt, ERI Eritrea, ESH Western Sahara, ETH Ethiopia, GAB Gabon, GHA Ghana, GIN Guinea, GMB Gambia, GNB Guinea-Bissau, GNQ Equatorial Guinea, KEN Kenya, LBR Liberia, LBY Libya, LSO Lesotho, MAR Morocco, MDG Madagascar, MLI Mali, MOZ Mozambique, MRT Mauritania, MWI Malawi, NAM Namibia, NER Niger, NGA Nigeria, RWA Rwanda, SDN Sudan, SEN Senegal, SLE Sierra Leone, SOM Somalia, SSD South Sudan, SWZ Swaziland, TCD Chad, TGO Togo, TUN Tunisia, TZA Tanzania, UGA Uganda, ZAF South Africa, ZMB Zambia, and ZWE Zimbabwe.
Coupled with an expanding population at risk, the number of estimated malaria deaths in Africa rose substantially during the 1990s (Fig. 2B), peaking at an estimated 1,063,000 deaths (95% uncertainty interval, 742,000 to 1,403,000) continent-wide in 2003. The trends in total estimated deaths are dominated in West Africa by Nigeria (contributing 31% of all estimated malaria deaths in Africa in 2015) and in Central Africa by the Democratic Republic of Congo (which contributed 12% of all estimated malaria deaths in Africa in 2015).
The period since 2000 has seen remarkable reductions in the estimated rate of malaria deaths in almost all countries in Africa (Fig. 2C). We estimate an overall decrease of 57% (95% uncertainty interval, 46 to 65) in the malaria death rate over this period, from 12.5 deaths (95% uncertainty interval, 8.3 to 17.0) per 10,000 population per year in 2000 to 5.4 (95% uncertainty interval, 3.4 to 7.9) in 2015. Despite the growth in the population, this led to a net decrease of 37% (95% uncertainty interval, 36 to 39) in the estimated number of deaths annually, from 1,007,000 (95% uncertainty interval, 666,000 to 1,376,000) in 2000 to 631,000 (95% uncertainty interval, 394,000 to 914,000) in 2015. The four countries with the highest estimated rates of malaria deaths in 2000, all in West Africa, all had large declines in 2015: Burkina Faso (33.9 to 17.6 deaths per 10,000 per year), Mali (31.4 to 23.1), Sierra Leone (31.0 to 14.4), and Mozambique (30.9 to 9.5). The two countries with the highest malaria burden also saw large declines in rates: Nigeria (23.6 to 10.5) and Democratic Republic of Congo (24.8 to 10.3). Elsewhere, Uganda (25.6 to 4.1) and Burundi (29.6 to 4.7) also saw notable declines in rates per 10,000 per year.
Subnational Variation in 2015 Malaria Mortality
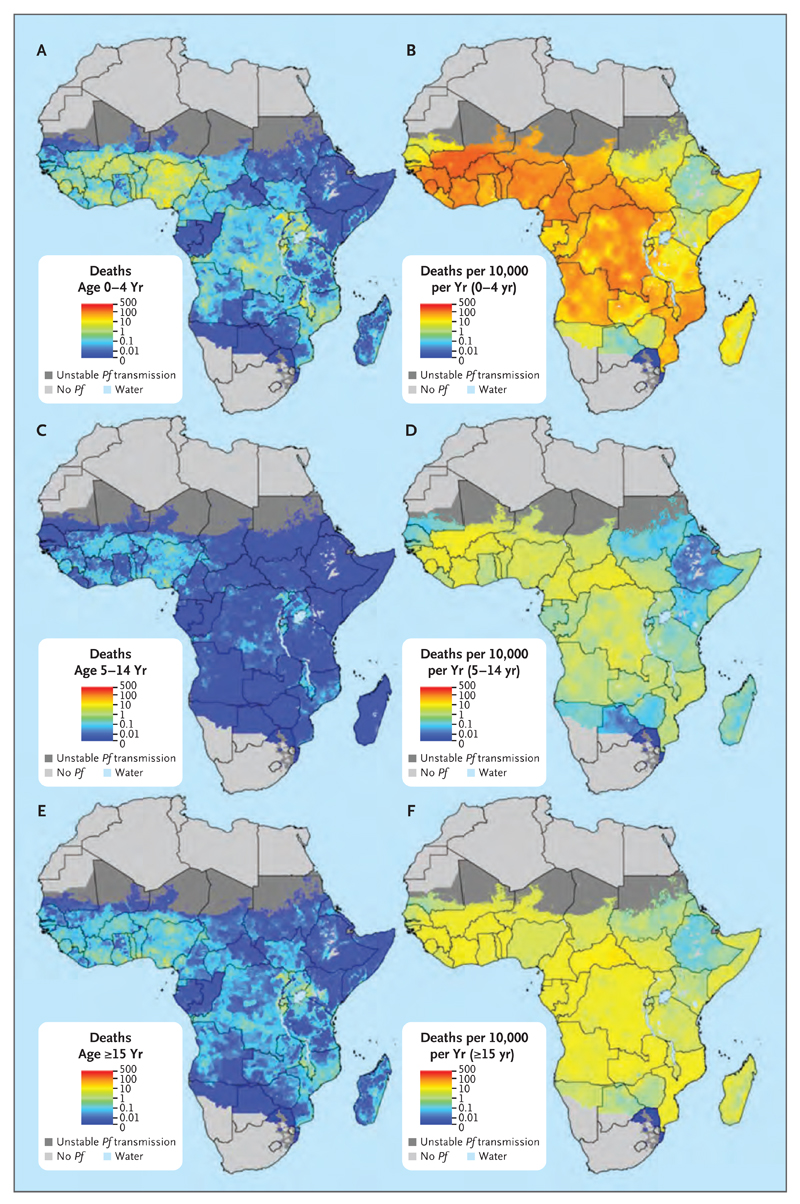
Fig. 3 shows estimates of the number of malaria deaths (Panels A, C, and E) and death rates (Panels B, D, and F) in sub-Saharan Africa during 2015 for children 4 years of age or younger (Panels A and B), those 5 to 14 years of age (Panels C and D), and persons 15 years of age or older (Panels E and F).20 These estimates of malaria mortality rate are conditional on population being present in the corresponding 5-km2 grid cell. Both metrics display enormous heterogeneity across the continent and within individual countries, although such heterogeneity is more pronounced for the numbers of malaria deaths than for the rates. Notable are areas in which moderate-to-high estimated rates of death coincide with densely populated regions, leading to foci of a high estimated number of malaria deaths across, for example, much of Nigeria, southern and eastern Democratic Republic of Congo, Lake Victoria coastal regions, and parts of Malawi and Mozambique.
Figure 3. Estimated Number and Rate of Plasmodium falciparum Deaths in Sub-Saharan Africa in 2015.
Estimates are provided for each 5-km2 grid cell within the previously defined limits of stable Plasmodium falciparum (Pf) transmission.20 Shown are the estimated number of deaths (Panels A, C, and E) and deaths per 10,000 population per year (Panels B, D, and F) per grid cell for children 4 years of age or younger (Panels A and B), those 5 to 14 years of age (Panels C and D), and persons 15 years of age or older (Panels E and F).
Comparing the maps of the numbers and rates of malaria deaths reveals distinct geographic patterns that convey different but complementary information. Regions with large numbers of deaths may not have the highest rates of death but nevertheless represent the need for concentration of resources and commodity provision to reduce disease burden. Conversely, many regions with very high mortality rates are associated with only moderate or low population densities but present challenges for preventive and vector-control activities. These comparisons are often apparent within a single country. In Nigeria, for example, the most densely populated southern regions contribute the largest share of estimated deaths, despite having lower estimated death rates than the more rural, higher-transmission areas to the north.
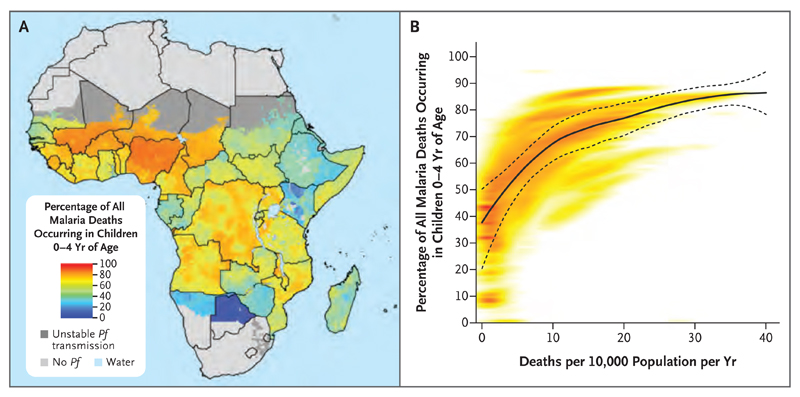
A comparison among age groups highlights the markedly higher vulnerability to death from malaria among children younger than 5 years of age, and this risk is reflected in both the highest rates and highest numbers of deaths across the continent in this age group. However, the share of estimated malaria deaths between children younger than 5 years of age and those 5 years of age or older varies considerably according to geographic region (Fig. 4A). This variation highlights the use of these estimates for informing malaria control and treatment strategies that are tailored to the demographic structure of local malaria burdens. Such efforts will become increasingly important as the malaria burden is further reduced and shifts from children toward adults (Fig. 4B). As transmission declined between 2000 and 2015, the share of annual malaria deaths in children 4 years of age or younger and in those 5 to 14 years of age decreased (from 78.7% to 73.5% and from 6.7% to 4.8%, respectively), and the share in persons 15 years of age or older increased (from 14.6% to 21.7%). An expanded set of mapped estimates according to age group and year is provided in Figs. S2 and S3 in the Supplementary Appendix.
Figure 4. Age Pattern of Malaria Mortality in 2015.
Panel A shows the estimated percentage of all malaria deaths in 2015 that occurred in children 4 years of age or younger in each 5-km2 pixel in sub-Saharan Africa. Panel B shows the relationship between estimated all-age death rates per pixel (x axis) and the estimated percentage of all malaria deaths in children 4 years of age or younger (y axis). Each pixel is plotted with the density of pixels indicated by the color shading (with highest density in red). The solid and dashed lines indicate the mean and 95% confidence intervals, respectively, of a locally estimated scatterplot-smoothed (loess) regression used here to visualize the central tendency of this relationship.
Targeting Malaria-Control Strategies
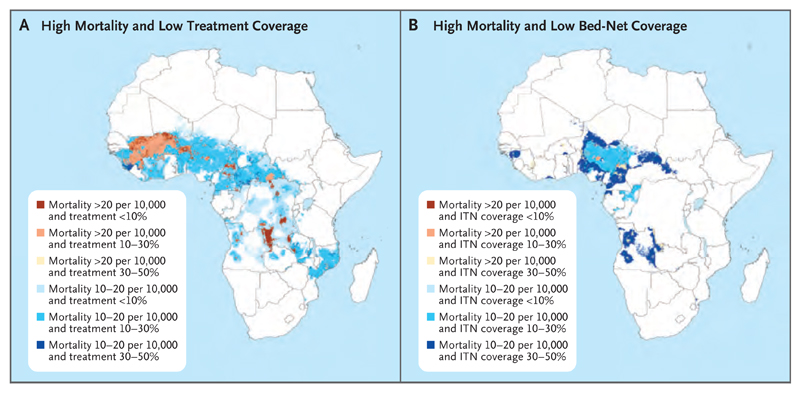
Despite illustrating the progress in reducing the burden of malaria, our maps also reveal large swaths of Africa where estimated malaria mortality remains high and the coverage of prevention and treatment remains low, which shows the potential to save hundreds of thousands more lives by further scaling up malaria control. Efforts to localize malaria control currently do not use detailed geographic descriptions of case rates. Fig. 5 shows the regions of highest estimated risk of death from malaria and low coverage of drug treatment and insecticide-treated bed nets in 2015. For drug treatment, areas with the highest estimated mortality (>20 malaria deaths per 10,000) and low antimalarial drug coverage (<30%) are concentrated in the southern half of Mali; the border areas between Mali, Burkina Faso, Niger, Benin, and Nigeria; west Guinea; southeast Democratic Republic of Congo; and various hotspots in Niger, Cameroon, Central African Republic, Angola, and Ivory Coast (Fig. 5A). Areas with lower estimated malaria mortality (10 to 20 malaria deaths per 10,000) and low drug coverage (<30%) encompass much of West Africa, Central African Republic, and Democratic Republic of Congo, as well as parts of Angola, Mozambique, and Zambia. For insecticide-treated bed nets, coverage is relatively higher, as expected; however, areas with the highest estimated mortality (>20 malaria deaths per 10,000) and low coverage of insecticide-treated bed nets (<50% of people at risk sleeping under an insecticide-treated bed net) remain in focal areas throughout West and Central Africa, notably in Guinea, Burkina Faso, Niger, Nigeria, Cameroon, and Angola21 (Fig. 5B). Figure 5 also highlights areas with high estimated malaria mortality and low coverage of both insecticide-treated bed nets and antimalarial drugs — namely, Nigeria, Angola, and Cameroon and parts of Central African Republic, Congo, Guinea, and Equatorial Guinea.
Figure 5. Identifying Regions with High Mortality and Low Malaria-Control Coverage.
Shown are areas with high mortality and low coverage of antimalarial treatment (Panel A) and areas with high mortality and low coverage of insecticide-treated bed nets (ITNs) (Panel B). Both maps show data for 2015. Data on bed nets were obtained from earlier geospatial mapping.
Discussion
Because data on malaria mortality remain challenging to define and collect, such data are relatively sparse in sub-Saharan Africa. Here, we have developed an approach that leverages data on the prevalence of malaria and clinical incidence (and relationships between these metrics) to generate more detailed subnational estimates of malaria mortality in sub-Saharan Africa and how rates of death have changed before and during the recent era of malaria-control efforts.
The declines in estimated malaria mortality across the continent support the value of the recent global push to control the disease. The ability to audit in detail where control efforts have and have not yielded declines can also identify case studies to further inform efforts needed to reach the new Sustainable Development Goal target to end the epidemic of malaria by 2030.22 At the same time, the historical trend in estimated malaria mortality in the 1990s, which was driven by chloroquine resistance,23 serves as a stark warning. Existing gains and future effects could be reversed if artemisinin resistance24 is not managed appropriately. Furthermore, given the central role of insecticide-treated bed nets in reducing the burden of malaria during the past decade, pyrethroid resistance25 will also need to be managed carefully.
Our estimates further highlight that the residual burden of deaths from malaria remains intolerably large and that the scope for enhanced control is substantial.21 The examples provided here of identifying high-risk areas that have low coverage of interventions form the basis for much more sophisticated targeting of prevention and treatment efforts. This potential for increased efficiency and effectiveness of programmatic efforts is critical in a climate of plateauing development assistance for health.6 With the generation of estimates at a level of 5 km2, targeting could be carried out at the level of individual health facility catchments or villages or aggregated to any desired level at district, province, national, or international levels.
The provision of age-specific maps facilitates targeting not just according to geographic area but also according to demographic strata. The variable age pattern in the share of estimated malaria deaths — ranging from more than 80% of deaths at death rates of more than 25 per 10,000 to less than 40% at levels below 1 per 10,000 among children younger than 5 years of age — also highlights the importance of malaria-control programs that can adapt to an older target population as malaria control is scaled up and disease burden reduced. This changing age profile of burden will probably affect clinical management such as drug treatment.26,27
As for previous rounds of annual estimates,17 our estimates of total malaria mortality across the continent in 2015 are somewhat higher than those presented by the WHO28 for that year, although we estimated similar proportional declines in both rates and numbers of deaths during the 2000–2015 period. Both estimation processes incorporate the same underlying spatiotemporal malaria parasite rate and clinical-incidence predictions, but differences stem from the mortality data that are included and the modeling.3
Our analysis allows detailed subnational depiction of malaria mortality in Africa, but it is important to recognize a number of limitations. First, sparse data with a reliance on verbal autopsy remain the norm for causes of death in sub-Saharan Africa. Our estimates depend on the accuracy of verbal autopsy, which is notably lower than medical certification of causes of death.29 Increased precision of future estimates may be realized by integrating hospital-based data on malaria case fatality rates. Second, our estimates quantify malaria mortality according to ICD rules; future efforts could include rates of death from other causes that are increased by malaria infection. Third, we relied on national-level rates of death from any cause (with the exception of Kenya) to determine the case fatality rate. No systematic analysis of subnational variation in all-cause mortality rates according to age, sex, and time is currently available. This assumption will bias our estimates if there is a correlation between the fraction of malaria deaths observed in the study data and subnational variation in all-cause mortality. Finally, the time resolution of our estimates is limited to calendar years. Strong seasonal patterns exist for malaria burden as well as the coverage of interventions. Producing monthly estimates would better assist decision makers in timely planning, implementation, and monitoring of malaria control.
Substantial progress has been made in reducing the burden of malaria, but a large unfinished agenda remains. The risk of death from malaria is a complicated function of environmental, demographic, and programmatic factors that can result in highly localized patterns of risk. Data on the geographic and temporal distribution of the risk of death from malaria and its associated factors are important in the planning, implementing, and refining of control strategies to reach the goal of eradicating malaria.
Supplementary Material
Acknowledgments
Supported by a grant from the Bill and Melinda Gates Foundation (OPP1132415). Dr. Gething is a Career Development Fellow (K00669X) who is jointly funded by the U.K. Medical Research Council (MRC) and the U.K. Department for International Development (DFID) under the MRC/DFID Concordat agreement, also part of the Second European and Developing Countries Clinical Trials Partnership Program supported by the European Union, and receives support from the Bill and Melinda Gates Foundation (OPP1068048 and OPP1106023). These grants also support Drs. Weiss, Bisanzio, Bhatt, Cameron, and Battle, Ms. Dalrymple, and Ms. Rozier.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Peter W. Gething, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Daniel C. Casey, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Daniel J. Weiss, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Donal Bisanzio, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Samir Bhatt, Department of Infectious Disease Epidemiology, Imperial College London, London, United Kingdom
Ewan Cameron, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Katherine E. Battle, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Ursula Dalrymple, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Jennifer Rozier, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Puja C. Rao, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Michael J. Kutz, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Ryan M. Barber, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Chantal Huynh, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Katya A. Shackelford, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Matthew M. Coates, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Grant Nguyen, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Maya S. Fraser, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Rachel Kulikoff, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Haidong Wang, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Mohsen Naghavi, Institute for Health Metrics and Evaluation, University of Washington, Seattle
David L. Smith, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Christopher J.L. Murray, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Simon I. Hay, Institute for Health Metrics and Evaluation, University of Washington, Seattle
Stephen S. Lim, Institute for Health Metrics and Evaluation, University of Washington, Seattle
References
- 1.Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015. Jun, ( http://www.who.int/malaria/publications/atoz/9789241564991/en/) [Google Scholar]
- 2.Malaria No More. From aspiration to action ( http://www.endmalaria2040.org/)
- 3.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJL, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieleman JL, Schneider MT, Haaken-stad A, et al. Development assistance for health: past trends, associations, and the future of international financial flows for health. Lancet. 2016;387:2536–44. doi: 10.1016/S0140-6736(16)30168-4. [DOI] [PubMed] [Google Scholar]
- 7.Gething PW, Battle KE, Bhatt S, et al. Declining malaria in Africa: improving the measurement of progress. Malar J. 2014;13:39. doi: 10.1186/1475-2875-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron E, Battle KE, Bhatt S, et al. Defining the relationship between infection prevalence and clinical incidence of Plasmodium falciparum malaria. Nat Commun. 2015;6:8170. doi: 10.1038/ncomms9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun. 2014;5:3136. doi: 10.1038/ncomms4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith T, Killeen GF, Maire N, et al. Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: overview. Am J Trop Med Hyg. 2006;75(2 Suppl):1–10. doi: 10.4269/ajtmh.2006.75.2_suppl.0750001. [DOI] [PubMed] [Google Scholar]
- 11.Eckhoff P. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 2013;88:817–27. doi: 10.4269/ajtmh.12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battle KE, Guerra CA, Golding N, et al. Global database of matched Plasmodium falciparum and P. vivax incidence and prevalence records from 1985-2013. Sci Data. 2015;2:150012. doi: 10.1038/sdata.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WorldPop. What is WorldPop? ( http://www.worldpop.org.uk/)
- 14.WHO global antimalarial drug efficacy database. Geneva: World Health Organization; 2015. Oct 2, ( http://www.who.int/malaria/areas/drug_resistance/drug_efficacy_database/en/) [Google Scholar]
- 15.Artemisinin and artemisinin-based combination therapy resistance. Geneva: World Health Organization; 2016. Apr, ( http://apps.who.int/iris/bitstream/10665/208820/1/WHO_HTM_GMP_2016.5_eng.pdf?ua=1) [Google Scholar]
- 16.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanks GD, Hay SI, Bradley DJ. Malaria’s indirect contribution to all-cause mortality in the Andaman Islands during the colonial era. Lancet Infect Dis. 2008;8:564–70. doi: 10.1016/S1473-3099(08)70130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Country codes — ISO 3166. Geneva: International Organization for Standardization; ( http://www.iso.org/iso/country_codes) [Google Scholar]
- 19.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–7. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 20.Guerra CA, Gikandi PW, Tatem AJ, et al. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5(2):e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt S, Weiss DJ, Mappin B, et al. Coverage and system efficiencies of insecticide-treated nets in Africa from 2000 to 2017. Elife. 2015;4 doi: 10.7554/eLife.09672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United Nations sustainable development goals. goal 3: ensure healthy lives and promote well-being for all at all ages. New York: United Nations; ( http://www.un.org/sustainabledevelopment/health/) [Google Scholar]
- 23.O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–55. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 24.Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trape J-F, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–32. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 26.Dondorp AM, Lee SJ, Faiz MA, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–7. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 27.Olliaro P. Mortality associated with severe Plasmodium falciparum malaria increases with age. Clin Infect Dis. 2008;47:158–60. doi: 10.1086/589288. [DOI] [PubMed] [Google Scholar]
- 28.World malaria report 2015. Geneva: World Health Organization; 2015. Dec, ( http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/) [Google Scholar]
- 29.Murray CJ, Lozano R, Flaxman AD, et al. Using verbal autopsy to measure causes of death: the comparative performance of existing methods. BMC Med. 2014;12:5. doi: 10.1186/1741-7015-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.