Abstract
Background:
Although hepatocellular carcinoma (HCC) usually develops in cirrhotic livers, a minority of cases occur in noncirrhotic livers (NCLs). We investigated etiology, clinicopathological features, and occult hepatitis B virus (HBV) infection (OBI) in patients with NCL HCC in an HBV-endemic area.
Methods:
A total of 710 patients who underwent resection or transplantation for HCC at the National Cancer Center (NCC), Korea, were enrolled. HCC and fibrosis stage were diagnosed pathologically.
Results:
A total of 178 patients (25%) did not have cirrhosis (NCL group). The main cause of HCC was HBV infection (77.2%), followed by cryptogenic disease (11.0%). The prevalence of NCL was 19.2%, 32.5%, 50.0%, and 48.7% among patients with HBV, hepatitis C virus (HCV), alcoholic, and cryptogenic disease, respectively (p < 0.05); corresponding nonfibrosis rates were 8.1%, 0%, 19.0%, and 24.3%, respectively. The NCL group was significantly older, with a larger tumor size, smaller tumor number, lower tumor stage, and more frequent non-HBV etiology. Among non-HBV HCC cases, 130 (80.2%) had antibodies against HBV core (HBc) and 55 (38.5%) had OBI. OBI-positive rates of 0%, 31.8%, and 52.6% were detected among HCV, alcoholic, and cryptogenic HCC cases, respectively. OBI did not correlate with advanced fibrosis. The NCL and liver cirrhosis (LC) groups did not differ in median overall survival.
Conclusion:
Regardless of etiology, a significant number of HCC patients, including half of nonviral cases, did not have LC. Half of cryptogenic HCC cases had OBI. This study promotes an understanding of fibrosis and OBI among patients with HCC in an HBV-endemic area.
Keywords: Hepatocellular carcinoma, noncirrhotic liver, occult hepatitis B virus infection
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent tumor type worldwide, and the second most common cause of cancer-related death.1 The reported risk factors for HCC vary, and include the main factors of hepatitis C virus (HCV) infection and alcoholic disease in Western countries2 and hepatitis B virus (HBV) infection in many Asian and African countries.3 Despite the initiation of a national universal vaccination program, a high prevalence of chronic HBV infection has been observed among adults in South Korea, which has the sixth highest incidence of HCC worldwide.1,4
Collectively, liver cirrhosis (LC) of any etiology is considered the main risk factor for HCC,5 and the diagnosis and management of HCC assume underlying LC.6 Nevertheless, a certain proportion (7–54%) of HCC cases arise in noncirrhotic livers (NCLs), with variability observed with regard to geographic area and liver disease etiology.5,7 Although HCC characteristics may differ according to the presence or absence of underlying cirrhosis, clinicopathological data of patients with NCL HCC are unfortunately scarce.
HBV, which integrates into the host genome, has a direct oncogenic effect on the liver, and could induce HCC in an NCL. Occult HBV infection (OBI), defined by the presence of HBV DNA in blood or liver tissue without detectable levels of HBV serum antigen (HBsAg) is a reportedly frequent occurrence in HBV-endemic areas such as East Asia. Although OBI also appears to have direct and indirect oncogenic effects, the prevalence of OBI has not been confirmed in non-HBV patients with HCC.8 In contrast, HCV has a far lower direct oncogenic potential than HBV, and consequently most HCV-related HCCs are known to occur against a background of advanced liver fibrosis or LC.6,9 Alcoholic liver disease is also remarkably less frequent among noncirrhotic HCC patients.7 Notably, cryptogenic cirrhosis is a common cause of liver-related morbidity in Western countries, and nonalcoholic fatty liver disease (NAFLD) is now recognized as the most common cause of cryptogenic cirrhosis.10 NAFLD or nonalcoholic steatohepatitis (NASH) might directly promote HCC independently of the presence of LC.11,12
Determination of the underlying LC status of a patient with HCC is an important step toward understanding hepatocarcinogenesis and making decisions regarding HCC surveillance, diagnosis, and management. Unfortunately, large volumes of data regarding HCC in the NCL are insufficient, and data concerning OBI in the noncirrhotic HCC are unknown. Accordingly, our study aimed to investigate etiology-based clinicopathological features of HCC patients with NCL, as well as the association of OBI with HCC in NCLs in a HBV-endemic area.
Materials and methods
Patients and classification of HCC according to cause
A total of 710 patients who underwent resection (87.9%, n = 624) or transplantation (12.1%, n = 86) as an initial treatment for HCC were enrolled from among a cohort of 2876 patients with newly diagnosed HCC who were treated at the National Cancer Center (NCC) Hospital, Korea, between January 2000 and December 2009. Patients were enrolled prospectively, and relevant clinical and tumor characteristic data were extracted retrospectively from medical records. Patients were followed until December 2014. This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization-Good Clinical Practice. The study protocol was approved by the Institutional Review Board at the NCC, Korea [ClinicalTrials.gov identifier: NCC2016-0075]. All study participants provided their written informed consent for the study.
HCC was diagnosed according to the guidelines of the Korea Liver Cancer Study Group (KLCSG)-NCC, Korea.4 A radiological examination and pathologic evaluation of surgically resected tissue or extracted liver (transplantation) were used to assess tumor stage according to the modified Union for International Cancer Control (mUICC) staging system.4,13 The Child–Pugh score was used to classify cases according to liver dysfunction severity. HCC and LC were histologically diagnosed by three pathologists and confirmed by a senior pathologist (EKH). The five-point METAVIR system scale was used to stage fibrosis; this scale ranges from no fibrosis (F0) to complete cirrhosis (F4).14,15
HBV or HCV infection was considered the cause of HCC (HBV HCC or HCV HCC) if a patient had a positive HBsAg or anti-HCV serologic test result, regardless of the alcohol consumption history. Serum HBsAg and anti-HCV levels were determined using Architect HBsAg QT (Abbott Laboratories, USA) and Advia Centour XP chemiluminescent immunoassay systems and Advia Centaur HCV assays (Siemens, Germany).
Alcoholic HCC was diagnosed if a patient had negative HBsAg and anti-HCV test results and a history of chronic alcohol abuse; >4 drinks per day in men and 2 drinks per day in women on 5 or more days per week over 5 years. The latter parameter was determined through a review of the patient’s medical records, including questionnaires. HCC patients with negative HBsAg and anti-HCV test results and no history of chronic alcohol abuse were classified as having cryptogenic HCC.16 We additionally defined hyperglycemia as a fasting glucose level >126 mg/dl or a previous diagnosis of diabetes mellitus (DM).
Detection of OBI
We defined OBI as a positive HBV DNA test of liver tissue resected from HBsAg-negative and anti-HBV core (HBc) positive patients.17 Real-time polymerase chain reaction was used to detect HBV DNA from formalin-fixed, paraffin-embedded (FFPE) surgical specimens. Quantification of HBV DNA from the FFPE liver tissues was performed as follows: two 10-µm sections were cut from the FFPE liver tissue and deparaffinized. DNA was extracted using a Maxwell 16 FFPE DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The DNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and 2.1 µg of extracted DNA was subsequently used to detect HBV DNA. HBV DNA levels were quantified using the Abbott RealTime HBV assay (Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer’s instructions.
Statistical analysis
Categorical variables were compared using the Chi-square test or Fisher’s exact test. The Cochran–Armitage test and nonparametric trend test were used for trend analysis, and ordered logistic regression was used to evaluate relative differences in fibrosis stage, with HBV patients set as the referent category. The Kaplan–Meier method was used to calculate median survival durations. STATA, version 12.0 (Stata Corp, College Station, TX, USA) was used for all statistical analyses, and a p-value <0.05 was considered statistically significant.
Result
Clinicopathological characteristics: NCL group versus LC group
A total of 710 patients who underwent surgery (resection 624, transplantation 86) as an initial treatment for HCC were analyzed in this study. The median age was 54 years, and 83.1% of patients were male. In addition, 86% of patients had mUICC stage I or II disease, and 96.1% had Child–Pugh class A liver function. The main HCC etiology was HBV infection (77.2%), followed by cryptogenic disease (11.0%), alcoholic disease (6.2%), and HCV infection (5.6%). The proportion of each subgroup (resection or transplantation, respectively) by etiology was as follows: HBV (76%, 86%), cryptogenic (12.2%, 2.3%), alcoholic (6.7%, 2.3%), and HCV (5.1%, 9.3%).
Within our cohort, 532 (75%) and 178 (25%) patients did (LC group) and did not (NCL group) have LC, respectively, according to a review of surgical pathology. Etiologically, 19.2%, 32.5%, 50.0%, and 48.7% of patients with HBV, HCV, alcoholic, and cryptogenic HCC, respectively, did not have cirrhosis. The NCL group was significantly older, had a larger tumor size, lower tumor number, and lower mUICC stage, and was more likely to exhibit a non-HBV etiology (p < 0.05; Table 1).
Table 1.
Baseline characteristics of patients.
| Characteristics | NCL (n = 178, 25%) | LC (n = 532, 75%) | p-value* |
|---|---|---|---|
| Age | |||
| ⩽50 years | 45 (25.3) | 189 (35.5) | 0.012 |
| >50 years | 133 (74.7) | 343 (64.5) | |
| Sex | |||
| Male | 154 (86.5) | 436 (81.7) | 0.160 |
| Female | 24 (13.5) | 96 (18.3) | |
| Etiology | |||
| HBV | 105 (59.0) | 443 (83.3) | <0.001 |
| HCV | 13 (7.3) | 27 (5.1) | |
| Alcoholic | 22 (12.4) | 22 (4.1) | |
| Cryptogenic | 38 (21.3) | 40 (7.5) | |
| Non-HBV | |||
| Anti-HBc (+) | 60 (82.2) | 70 (78.7) | 0.573 |
| Anti-HBc (+), OBI (+) | 27 (45.0) | 28 (40.0) | 0.456 |
| Hyperglycemia | 27 (15.2) | 84 (15.8) | 0.843 |
| Child–Pugh class | |||
| A | 171 (96.1) | 495 (93.6) | 0.200 |
| B | 7 (1.3) | 30 (5.6) | |
| C | 0 (9.0) | 7 (1.3) | |
| Mean BMI | 23.6 | 23.3 | 0.591b |
| Number of tumors | |||
| 1 | 143 (80.3) | 363 (68.2) | 0.01a |
| 2–3 | 22 (16.1) | 115 (21.6) | |
| ⩾4 | 13 (7.3) | 54 (10.2) | |
| Tumor size (cm) | |||
| ⩽2 | 16 (9.0) | 115 (21.6) | <0.01a |
| 2–5 | 64 (36.0) | 293 (55.1) | |
| 5–10 | 57 (32.0) | 97 (18.2) | |
| >10 | 41 (23.0) | 27 (5.1) | |
| PV invasion | 10 (5.6) | 24 (4.5) | 0.553 |
| mUICC stage I | 11 (6.2) | 59 (11.1) | 0.001 |
| II | 142 (79.8) | 350 (65.8) | |
| III | 17 (10.1) | 112 (21.1) | |
| IV | 7 (3.9) | 11 (2.1) | |
| AFP (ng/ml) | |||
| ⩽20 | 92 (51.7) | 208 (39.1) | |
| 20–400 | 21 (11.8) | 150 (28.2) | 0.242a |
| >400 | 65 (36.5) | 174 (32.7) |
Chi-square test; aCochrane–Armitage test for trend; bStudent’s t-test.
AFP, alpha-fetoprotein; BMI, body mass index; HBc, HBV core; HBV, hepatitis B virus; HCV, hepatitis C virus; LC, liver cirrhosis; mUICC, modified Union for International Cancer Control; NCL, noncirrhotic livers; OBI, occult HBV infection; PV, portal vein.
Characteristics of the NCL group according to etiology
The prevalence of NCL was statistically higher among patients with non-HBV HCC versus those with HBV HCC (p < 0.05; Tables 1 and 2). Among the 548 patients with HBV HCC, the LC group was more likely to present with stage I disease at the initial diagnosis (Table 2).
Table 2.
Characteristics of the NCL and LC groups according to etiologies.
| Characteristics n (%) |
HBV NCL (n = 105) LC (n = 443) |
HCV NCL (n = 13) LC (n = 27) |
Alcoholic NCL (n = 22) LC (n = 22) |
Cryptogenic NCL (n = 38) LC (n = 40) |
||||
|---|---|---|---|---|---|---|---|---|
| Age >50 years | 67 (63.8) | 262 (59.1) | 13 (100) | 27 (100) | 22 (100) | 18 (81.8) | 31 (81.6) | 36 (90.0) |
| Sex, male | 91 (86.7) | 262 (81.7) | 12 (92.3) | 21 (77.8) | 22 (100) | 22 (100) | 29 (76.3) | 31 (77.5) |
| Anti-HBc (+) | 9 (69.2) | 17 (63.0) | 21 (95.5) | 19 (86.4) | 30 (78.9) | 34 (85.0) | ||
| Anti-HBc (+) & OBI (+) | 0 | 0 | 9 (40.4) | 5 (22.7) | 18 (47.4) | 23 (57.5) | ||
| Hyperglycemia | 13 (12.4) | 58 (13.1) | 1 (7.7) | 7 (25.9) | 8 (36.4) | 7 (31.8) | 5 (13.2) | 12 (30.0) |
| Child–Pugh class | ||||||||
| A | 101 (96.1) | 412 (93.0) | 13 (100) | 25 (92.6) | 21 (95.5) | 20 (90.9) | 36 (94.7) | 38 (95.0) |
| B | 4 (3.8) | 26 (5.9) | 0 (0) | 2 (7.4) | 1 (4.5) | 0 (0) | 2 (5.3) | 2 (5.0) |
| C | 0 (0) | 5 (1.1) | 0 (0) | 0 (0) | 0 (0) | 2 (9.1) | 0 (0) | 0 (0) |
| Mean BMI | 23.5 | 23.4 | 21.5 | 24.0** | 23.5 | 23.4 | 23.3 | 23.8 |
| mUICC stage I | 7 (6.7) | 52 (11.7) | 1 (7.7) | 1 (3.7) | 1 (4.5) | 2 (9.1) | 2 (5.3) | 4 (10.0) |
| II | 79 (75.2) | 292 (65.9) | 11 (84.6) | 17 (63.0) | 19 (86.4) | 14 (63.6) | 33 (86.8) | 27 (67.5) |
| III | 14 (13.3) | 90 (20.3) | 1 (7.7) | 8 (29.6) | 2 (9.1) | 6 (27.3) | 1 (2.6) | 8 (20.0) |
| IV | 5 (4.8) | 9 (2.0) | 0 (0) | 1 (3.7) | 0 (0) | 0 (0) | 2 (5.3) | 1 (2.5) |
p-value: BMI, Student’s t-test/others: Chi-square test.
Statistically significant (p-value 0.002).
BMI, body mass index; HBc, hepatitis B virus core; HBV, hepatitis B virus; LC, liver cirrhosis; mUICC, modified International Union for Cancer Control; NCL, noncirrhotic liver; OBI, occult HBV infection.
Among the 40 patients with HCV HCC, 13 (32.5%) did not have cirrhosis. Here, the NCL and LC groups did not differ significantly, with the exceptions of body mass index (BMI); specifically, the LC group had a higher BMI (p = 0.002). Although 9 (69.2%) and 17 (63.0%) of patients in the NCL and LC groups, respectively, were anti-HBc positive, HBV DNA was not detected in the liver tissues of patients with HCV HCC.
Among the 44 patients with alcoholic HCC and 78 with cryptogenic HCC, 22 (50.0%) and 38 (48.7%) were classified as NCL, respectively. Among patients with these etiologies, the NCL and LC groups did not differ significantly with respect to comparison variables. Anti-HBc positivity was observed in 90.9% of alcoholic HCC and 82.1% of cryptogenic HCC cases, and HBV DNA was detected in the liver tissues from 31.8% of alcoholic HCC and 52.6% of cryptogenic HCC cases. Notably, no statistical differences in HBV DNA positivity were observed according to the presence of cirrhosis (Table 2).
In the NCL group, HBV HCC patients were significantly younger and had higher serum alpha-fetoprotein (AFP) levels relative to those with non-HBV etiologies (p < 0.05; Supplement 1). Alcoholic HCC patients were more likely to present with co-morbid hyperglycemia. Among the non-HBV etiologies, alcoholic and cryptogenic patients were significantly more likely to exhibit liver HBV DNA positivity (OBI) when compared with patients with HCV HCC (p = 0.005; Supplement 1).
OBI in non-HBV HCC patients
The HBV DNA positivity (sensitivity) rate of liver tissues from 89 HBV patients in the NCL group was 93.0%; accordingly, these tissues were used as positive controls. A total of 130 (80.2%) of 162 patients with non-HBV HCC expressed anti-HBc antibodies; among them, 55 patients (38.5%) harbored HBV DNA (17-13588 IU/ml) in their liver tissue (Supplement 2). Technical failures in HBV DNA collection were experienced for 5 of the 130 anti-HBc positive cases (Tables 1 and 2). The anti-HBc positivity and liver HBV DNA positivity (OBI) rates of non-HBV cases were 82.2% and 45.0% in the NCL group, and 78.7% and 40.0% in the LC group, respectively (p > 0.05; Table 1). Among patients with HCV HCC, although 69.2% and 63.0% of patients in the NCL and LC groups, respectively, were anti-HBc positive, no cases had OBI. Among alcoholic HCC patients, 95.5% and 86.4% of patients in the NCL and LC groups, respectively, were anti-HBc positive. The corresponding liver HBV DNA detection rates were 40.4% and 22.7%, respectively (p > 0.05). Among cryptogenic HCC patients, 78.9% and 85.0% of those in the NCL and LC groups, respectively, were anti-HBc positive. The corresponding liver HBV DNA detection rates were 47.4% and 57.5%, respectively (p > 0.05; Table 2).
Fibrosis staging in the NCL group
For METAVIR fibrosis staging, 170 available specimens from 178 NCL cases were subjected to pathologic reappraisal; surgical specimens from the remaining 8 cases were missing or uninformative. No fibrosis was identified in 8.1%, 0%, 19.0%, and 24.3% of HBV, HCV, alcoholic and cryptogenic liver tissues, respectively. The fibrosis stage was significantly lower among nonviral (alcoholic and cryptogenic) patients when compared with those with HBV (odds ratio: 0.24, p = 0.01; odds ratio: 0.34, p = 0.04, respectively). Although patients with HCV tended to have a lower fibrosis stage when compared with those with HBV, this difference was not statistically significant (Table 3).
Table 3.
METAVIR fibrosis stages among patients without LC.
| Etiology | Fibrosis stage |
Ordered logistic regression |
|||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | Odds ratio | p-value | 95% CI | |
| HBV n = 99 (%) |
8 (8.1) | 13 (13.1) | 37 (37.4) | 41 (41.4) | 1.00 | ||
| HCV n = 13 (%) |
0 (0) | 3 (23.1) | 7 (53.8) | 3 (23.1) | 0.68 | 0.447 | 0.26–1.83 |
| Alcoholic n = 21 (%) |
4 (19.0) | 8 (38.1) | 7 (33.3) | 2 (9.5) | 0.24 | 0.001 | 0.10–0.55 |
| Cryptogenic n = 37 (%) | 9 (24.3) | 10 (29.0) | 7 (18.9) | 11 (29.7) | 0.34 | 0.004 | 0.16–0.70 |
CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; LC, liver cirrhosis.
Additionally, the relationship between the presence of HBV DNA in liver tissues (OBI) and fibrosis stage was evaluated in patients with anti-HBc positive, non-HBV NCL. However, the presence of HBV DNA in the liver (OBI) was not statistically associated with the METAVIR stage in this patient subset (Table 4).
Table 4.
Association between the presence of OBI and advanced fibrosis stage in non-HBV NCL group with anti-HBc positive.
| Etiology | HBV DNA+ in liver tissue (OBI) | METAVIR stage |
p-value* | |
|---|---|---|---|---|
| F0–F2 no (%) | F3 no (%) | |||
| HCV n = 9 (%) |
negative positive |
6 (66.7) 0 (0) |
3 (33.3) 0 (0) |
– |
| Alcoholic n = 20 (%) |
negative positive |
10 (90.9) 8 (88.9) |
1 (9.1) 1 (11.1) |
0.711 |
| Cryptogenic n = 29 (%) |
negative positive |
8 (72.7) 11 (61.1) |
3 (27.3) 7 (38.9) |
0.411 |
Chi-square test.
HBV, hepatitis B virus; HCV, hepatitis C virus; NCL, noncirrhotic liver; OBI, occult HBV infection.
Overall survival
The median follow-up duration for all patients was 130.1 months. The median survival durations were 100.8 months in the NCL group and 121.3 months in the LC group, and this difference was not statistically significant (p > 0.05; Figure 1). Furthermore, no significant differences in overall survival were observed according to etiology and the presence of OBI.
Figure 1.
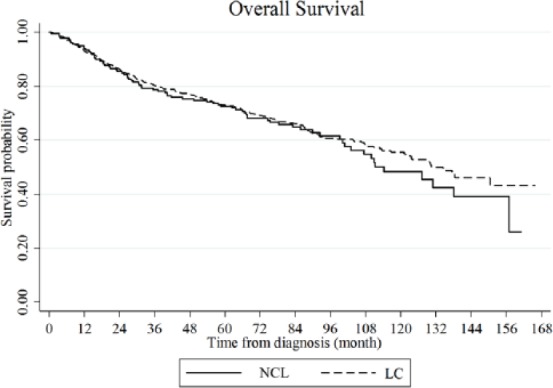
Overall survival.
The median survival durations were 100.8 months in the NCL group and 121.3 months in the LC group. This inter-group difference is not statistically significant p > 0.05).
LC, liver cirrhosis; NCL, noncirrhotic livers.
Discussion
Currently available evidence suggests that HCC usually arises in a liver with established cirrhosis.18 Accordingly, most practice guidelines for the diagnosis and management of HCC assume the presence of underlying LC in patients at high risk for HCC;4,6,13,19 Although HCC typically occurs in the context of hepatic cirrhosis, as many as 20% of HCC cases involve a NCL.7 Although this study features a constitutive selection bias toward surgically treated patients, the present study revealed that 178 patients with HCC (25%) did not have LC, supporting the concept that chronic liver diseases such as HBV or HCV infection, alcohol-related, and cryptogenic etiologies can lead to the development of HCC independently of cirrhosis.
Here, the prevalence of NCL was statistically higher among patients with non-HBV HCC (p < 0.05), with rates of 50.0% and 48.7% among cases of alcoholic and cryptogenic disease, respectively. In most series, current or previous HBV or HCV infection was more commonly observed in cirrhotic HCC patients, compared with noncirrhotic HCC patients.20 However, the present study observed a higher NCL prevalence rate (32.5%) among patients with HCV-related HCC.5,7 Similarly, a significant portion of HCV HCC cases also had NCL.21 This result was unexpected for what we understand for the knowledge of HCV-related HCC.
In HBV-endemic areas such as South Korea, the higher rate of NCL among patients with non-HBV etiologies could be attributed to OBI. It is presumed that patients with non-HBV etiologies were under a risk of HBV infection. Accordingly, we studied the presence of OBI and found that 40.4% of patients in the alcoholic NCL and 47.4% cryptogenic NCL groups had OBI. However, we did not observe a significant difference in OBI prevalence between the NCL and LC groups. (Table 2) In HBV-endemic areas, occult HBV monoinfection or coinfection with HCV have been reported to associate with HCC, and anti-HBc positivity is a marker of high HCC risk in patients with HCV cirrhosis.22 In the present study, anti-HBc positivity was observed in 69.2% and 63.0% of NCL and LC patients with HCV HCC, respectively; interestingly, however, no cases of OBI were detected. Although an inverse relationship in the replicative levels of HBV and HCV has been noted,23 the small number of patients with HCV HCC in this study (n = 40) might have been insufficient to determine the significance of OBI.
Among alcoholic and cryptogenic HCC patients, very high levels of anti-HBc positivity were observed in both the NCL and LC groups, which might suggest that previous HBV infection did not affect fibrotic progression. Interestingly, four (19.0%) alcoholic cases and nine (24.3%) cryptogenic cases presented with F0 disease rate. However, OBI was not found to correlate with fibrosis stage, although the small number of enrolled cases limited our ability to draw a solid conclusion. Recently, NAFLD and metabolic syndrome, which are potentially major causes of cryptogenic HCC, have been described as main risk factors for HCC in the absence of cirrhosis.12 In surgical specimens of this study, nonalcoholic steatohepatitis was very rare and we did not analyze it. The additional risk factor of heavy alcohol intake tends to be remarkably less frequent among noncirrhotic HCC patients.7 However, we did not precisely analyze the association between alcohol intake volume and fibrosis stage in this retrospective study.
Some variations in the prevalence of cirrhosis might be attributable to differences in the recruitment of HCC cases and types of diagnostic material. In the present study, all patients were treated with surgical resection or liver transplantation, and accordingly most had good liver function (Child–Pugh class A); this study showed no difference between the LC and the NCL groups in the overall survival (p > 0.05; Figure 1), therefore, this study does not reflect the entire population of patients with HCC. However, 80.8% of patients with HBV HCC presented with cirrhosis, in agreement with other reports,5,24 and therefore the selection bias might be negligible. Although this study features a constitutive selection bias toward surgically treated patients, the etiology-dependent difference in the cirrhosis prevalence and OBI presence in this study might facilitate an understanding of hepatocarcinogenesis.
Patients at high risk for developing HCC, including those with cirrhosis of any etiology, should be enrolled in a surveillance program, whereas those with noncirrhotic NAFLD and alcoholic liver disease are not recommended for surveillance.19 In this study, half of the patients with alcoholic and cryptogenic HCC did not have cirrhosis, and may therefore not have been targeted for HCC surveillance according to the existing guidelines. Accordingly, the observation of significant larger tumor sizes in the NCL group (Table 1) might not be coincidental. In contrast, more patients with HBV HCC and cirrhosis were diagnosed at stage I (Table 2), which may be a result of intensive surveillance.
In conclusion, regardless of etiology, a significant number of patients with HBV, HCV, alcoholic and cryptogenic HCC did not have cirrhosis; in particular, half of all nonviral cases had an NCL. In addition, half of the cryptogenic HCC cases had OBI; however, the NCL and LC groups did not differ significantly in terms of OBI prevalence, and OBI did not statistically correlate with advanced fibrosis.
Supplementary Material
Footnotes
Funding: This study was supported by a grant from the National Cancer Center, Korea (#1510520).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Chang Woo Shim, Center for Liver Cancer, National Cancer Center, Korea.
Joong-Won Park, Center for Liver Cancer, National Cancer Center, 323 Ilsan-ro, Ilsan dong-gu, Goyang, Gyeonggi 411-769, South Korea.
So Hee Kim, Biometric Research Branch, National Cancer Center, Korea.
Jin Sook Kim, Liver and Pancreatobiliary Cancer Branch, National Cancer Center, Korea.
Bo Hyun Kim, Center for Liver Cancer, National Cancer Center, Korea.
Sung Hoon Kim, Center for Liver Cancer, National Cancer Center, Korea.
Eun Kyung Hong, Center for Liver Cancer, National Cancer Center, Korea.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015; 35: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Fact Sheet: hepatitis B, www.who.int/mediacentre/factsheets/fs204/en/ (2015, accessed 25 July 2016).
- 4. Korean Liver Cancer Study Group (KLCSG) and National Cancer Center, Korea (NCC). 2014. KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015; 9: 267–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127: S35–S50. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver (EASL) and European Organisation for Research and Treatment of Cancer (EORTC). EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- 7. Trevisani F, Frigerio M, Santi V, et al. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis 2010; 42: 341–347. [DOI] [PubMed] [Google Scholar]
- 8. Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014; 21: 153–162. [DOI] [PubMed] [Google Scholar]
- 9. Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol 2015; 21: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. J Am Med Assoc 2003; 289: 3000–3004. [DOI] [PubMed] [Google Scholar]
- 11. Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis 2012; 32: 30–38. [DOI] [PubMed] [Google Scholar]
- 12. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016; 14: 124–131. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011; 29: 339–364. [DOI] [PubMed] [Google Scholar]
- 14. Group FMCS. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20: 15–20. [PubMed] [Google Scholar]
- 15. Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. J Hepatol 2003; 38: 223–229. [DOI] [PubMed] [Google Scholar]
- 16. Kwak HW, Park JW, Koh YH, et al. Clinical characteristics of patients with cryptogenic hepatocellular carcinoma in a hepatitis B virus-endemic area. Liver Cancer 2016; 5: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008; 49: 652–657. [DOI] [PubMed] [Google Scholar]
- 18. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–430. [DOI] [PubMed] [Google Scholar]
- 19. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trevisani F, D’Intino PE, Caraceni P, et al. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer 1995; 75: 2220–2232. [DOI] [PubMed] [Google Scholar]
- 21. Alkofer B, Lepennec V, Chiche L. Hepatocellular cancer in the non-cirrhotic liver. J Visc Surg 2011; 148: 3–11. [DOI] [PubMed] [Google Scholar]
- 22. Ikeda K, Marusawa H, Osaki Y, et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med 2007; 146: 649–656. [DOI] [PubMed] [Google Scholar]
- 23. Alberti A, Pontisso P, Chemello L, et al. The interaction between hepatitis B virus and hepatitis C viurs in acute and chronic liver disease. J Hepatol 1995; 22(Suppl. 1): 38–41. [PubMed] [Google Scholar]
- 24. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


