Abstract
It has been well established that some Armillaria species are symbionts of Polyporus umbellatus, However, little is known about the evolutionary history of P. umbellatus-Armillaria associations. In this research, we used an analysis based on the strength of the phylogenetic signal to investigate P. umbellatus-Armillaria associations in 57 sclerotial samples across 11 provinces of China. We isolated Armillaria strains from the invasion cavity inside the sclerotia of P. umbellatus and then phylogenetically analyzed these Armillaria isolates. We also tested the effect of P. umbellatus and Armillaria phylogenies on the P. umbellatus-Armillaria associations. We isolated forty-seven Armillaria strains from 26 P. umbellatus sclerotial samples. All Armillaria isolates were classified into the 5 phylogenetic lineages found in China except for one singleton. Among the 5 phylogenetic lineages, one lineage (lineage 8) was recognized by delimitation of an uncertain phylogenetic lineage in previous study. Results of simple Mantel test implied that phylogenetically related P. umbellatus populations tend to interact with phylogenetically related Armillaria species. Phylogenetic network analyses revealed that the interaction between P. umbellatus and Armillaria is significantly influenced by the phylogenetic relationships between the Armillaria species.
Introduction
Polyporus umbellatus (Pers.) Fries, belonging to Polyporaceae, is a widespread medicinal fungus which mainly distributed in China, Japan and other temperate regions of the Northern Hemisphere1. The dried sclerotia of P. umbellatus has been used as herbal medicine in China for more than 2000 years in China to cure edema and promote diuretic processes2. In recent years, a polysaccharide from P. umbellatus sclerotia was shown to promote anti-tumor and immunomodulating activities3, 4. At present, the supply of P. umbellatus for medicinal purposes is mainly dependent on wild collection. Increasing commercial demands and less effective protection have led to excessive harvests and a dramatic decline of wild P. umbellatus resources in China5.
Armillaria (Fr.) Staude (Physalacriaceae, Agaricales, Basidiomycota) is one of the most important of the macrofungi with world-wide distribution. Some species are important root rot pathogens of forest plants6, and some species exhibit high nutritional and medicinal value7.In early years, the taxonomy of Armillaria was established mainly via mating tests. At present, approximately 40 biological species have been reported with global range8. Among them, less than 30 species have been named, while the others are still called “biological species”. In China, 16 Chinese Biological species (CBS A to CBS P) of the Armillaria have been defined based on mating tests9, 10. However, due to the limits of mating tests, some ambiguous and confused biological species still need be further revised via modern molecular techniques, especially DNA-based analysis, e.g. rDNA ITS, IGS, β-tubulin, elongation factor-1 alpha (EF-1α), and combined multilocus sequence analysis. Coetzee et al.11 phylogenetically analyzed CBS, and elucidated four main phylogenetic clusters, i.e., A. ostoyae, A. gallica, A.tabescens, and A. mellea clusters. However, the relationship between the CBS and phylogenetic clades is still unclear and most of the CBS remain unnamed. Recently, Guo et al.12 revealed fifteen phylogenetic lineages of Armillaria from China, of which seven were newly discovered and two were recorded for the first time in China. Their work effectively established the link between the CBS and the phylogenetic lineages.
Some Armillaria species have been shown to be symbionts of P. umbellatus. It has been established that growth of P. umbellatus sclerotia is mainly dependent on Armillaria spp. to supply needed nutrition13. Based on this result, there have been attempts to produce P. umbellatus sclerotia in some provinces of China, via dual culture of small sclerotia of P. umbellatus with twigs or sticks which had been previously infected by rhizomorph of Armillaria spp.14. However, this kind of cultivation has experienced problems with both the quality of the sclerotia and production efficiency due to lack of information regarding the species and ecological characteristics of the Arimillaria used. Although Armillaia is an important factor that determines the efficiency and mass production of cultivated P. umbellatus, there have been few studies on the association of Armillaria species with P. umbellatus. In most of the books and articles related to P. umbellatus, the Armillaria were described as A. mellea or Armillaria spp. Kikuchi & Yamaji15 implied that Armillaria species which associated with P. umbellatus were some unidentified Armillaria biological species closely related to A. sinapina, A. calvescens, A. gallica, A. cepistipes, and A. nabsnona. However, due to their small sampling size (three P. umbellatus sclerotial samples from Japan and China, respectively), this finding requires further verification. In addition to the ambiguous Armillaria spp. with which P. umbellatus associates, P. umbellatus also exhibits high intraspecific diversification16. This raises questions of whether there is a phylogenetic signal in the mutual selection between P. umbellatus and Armillaria during the long-term evolutionary process, i.e. whether more closely related P. umbellatus populations tend to form symbiotic associations with more closely related Armillaria species.
To develop a better understanding of the evolutionary history of P. umbellatus and Armillaria associations, we collected 57 sclerotial samples of P. umbellatus from 11 provinces in China, and we successfully isolated 47 Armillaria strains. The aim of this paper is to elucidate: (1) the Armillaria species that associate with P. umbellatus, (2) whether closely related P. umbellatus populations associate with closely related Armillaria species, and (3) whether the phylogenetic signal significantly drives the interaction between P. umbellatus and Armillaria?
Results
Armillaria species associated with P. umbellatus
In this study, we obtained a total of 47 Armillaria isolates with which P. umbellatus associated. The ITS, β-tubulin, EF-1α and three-locus matrices, derived from ML and BRC analyses yielded similar topologies. The three-locus matrix phylogenetic tree generated from ML and BRC analyses is shown in Fig. 1. The phylogenetic trees generated from ML analyses of ITS, β-tubulin, and EF-1α matrices are shown in Supplementary Information, Figure S1. Among the four matrices, ITS phylogeny (Supplementary Information, Figure S1C) and three-locus phylogeny (Fig. 1) present the lowest and the best branch resolution and support, respectively. Only a few branches were supported by bootstrap and posterior probabilities for the ITS phylogeny. The best branch resolution and support was obtained for the tree generated from three-locus phylogeny. From the three-locus phylogeny, Guo et al.12 revealed that there were at least 15 phylogenetic lineages of Armillaria in China. Our results support the 15 phylogenetic lineages. We further delimit an uncertain phylogenetic lineage that had been identified in a previous study, i.e. lineage 8. Lineage 8 is composed of two members, M20 (generated in this research) and a reported Chinese biological species (HKAS86607, CBS J), and was strongly supported by ML-BP (90%) and BRC-PP (0.97) in the three-locus phylogenetic tree. Lineage 8 was also strongly supported by ML-BP (99%) in the β-tubulin phylogenetic tree (Supplementary Information, Figure S1A).
Figure 1.

Phylogenetic tree generated from the three-locus (ITS, EF1-α and β-tubulin) data set. The blue labels on the nodes of the phylogram indicate phylogenetic lineages recognized by Guo et al.12. The red labels on the nodes of the phylogram indicate the new lineage (lineage 8) recognized in this research. The pink line represents the singleton. The values of the bootstrap frequencies of ML (BP > 70%) and posterior probability (PP > 0.90) are shown above the nodes. Armillaria isolates generated from this research are presented as M followed by a number.
The Armillaria isolates associated with P. umbellatus showed a high diversity and belonged to five independent phylogenetic lineages, including lineage 6, lineage 4, lineage 8, lineage 1, and A. cepistipes. Twenty-three Armillaria isolates, i.e., almost half of the total isolates, belonged to lineage 6. Thirteen isolates belonged to A. cepistipes. Eight isolates belonged to lineage 4. Lineage 1 and lineage 8 each include 1 isolate, respectively. Relative abundances of phylogenetic lineages of Armillaria isolates are shown in Fig. 2. One singleton (M3) showed relatively long branches compared with its sister group (Fig. 1). At present, the M3 strain is considered to be genetically divergent from its sisters.
Figure 2.

Relative abundance of the phylogenetic lineages of the forty seven Armillaria isolates.
Some isolates present evident geographic characteristics. Isolates from Shanxi, Gansu, Henan and Hebei provinces were all identified with lineage 6. Isolates from Northeast China, such as Jilin and Heilongjiang, belonged to A. cepistipes, except for isolate M47, which belonged to lineage 6. Isolates from Southwest China (Yunnan and Tibet) belonged to lineage 4. Among the 11 provinces sampled for P. umbellatus collected from, members of lineage 6 were found in 7 provinces (Shanxi, Shannxi, Gansu, Henan, Hebei, Sichuan and Jilin).
Phylogenetic network analyses of P. umbellatus-Armillaria associations
When we examined the phylogenetic distance of the Armillaria strains associated with each of the P. umbellatus samples, the simple Mantel test showed that the phylogenetic distance of P. umbellatus and Armillaria strains were positively and significantly correlated (r = 0.4787, p < 0.01). This means that phylogenetically related P. umbellatus populations tend to interact with Armillaria species that are closely related.
To further understand the phylogenetic influence on the P. umbellatus-Armillaria associations, we incorporated the identity of the interacting taxa in the network (Fig. 3) and measured a moderate but significant phylogenetic signal on the Armillaria phylogeny, both when considering the ML phylogeny (d A = 0.3522; 95% CI 0.1496–0.5327) and when considering the BRC phylogeny (d A = 0.2109; 95% CI 0.0508–0.3574). The phylogenetic signal of the P. umbellatus was close to zero and not significant: for the ML tree, d P < 0.0001 (95% CI 0–0.0100), and for BRC tree, d P < 0.0001 (95% CI 0–0.0047). The overall strength of the phylogenetic signal for the linear model fitted to the actual data (MSEd = 0.1282 and MSEd = 0.1382 for the ML and BRC tree sets, respectively) was closer to that found under the assumption of no phylogenetic covariances (MSEstar = 0.0829) than for the assumption of maximum phylogenetic signal (MSEb = 0.4655 and MSEb = 0.6035) for the ML and BRC tree sets, respectively). These results suggest that only phylogenetic relationships among the Armillaria species and not among the P. umbellatus impose structure on the interaction matrix. However, the overall phylogenetic signal is weak.
Figure 3.

Network of P. umbellatus-Armillaria interactions. Lines represent pairwise interactions. Maximum clade credibility trees from Bayesian relaxed clock analyses are shown for P. umbellatus and Armillaria isolates. Branch support values above the branches show maximum likelihood nonparametric bootstrap percentages. Support values below branches are posterior probabilities. PGT, P. umbellatus genotype; AGT, Armillaria genotype.
Discussion
A subset of species in the Armillaria genus are important plant pathogens and can cause serious root diseases in diverse trees and woody plants. Interestingly, some species of Armillaria are also well known as symbionts of Gastrodia elata Bl. (a myco-heterotrophic orchid used in traditional Chinese herbal medicine)17 and P. umbellatus. Taxonomic classification of Armillaria is complicated by high intraspecific diversification and the lack of sexual stages in many species. Despite early mating tests18 as well as studies that have utilized recently developed DNA data analysis19, 20 and mutilocus approaches21, there are still some ambiguous groups and unnamed biological species. In China, 16 CBS of Armillaria were identified by mating tests. However, due to the limits of mating tests, these CBS still need further verification via by modern molecular approaches. Coetzee et al.11 elucidated the four main phylogenetic groups of Armillaria in China. Subsequently, Guo et al.12 effectively established the link between the CBS and the phylogenetic lineages and identified at least 15 phylogenetic lineages in China. These works gradually clarified the taxonomy of Armillaria in China.
P. umbellatus sclerotial growths require the Armillaria rhizomorph to supply nutrition. To date, l little is known about the exact Armillaria species associated with P. umbellatus. In this study, we determined that all the Armillaria isolates belong to the 4 phylogneetic lineages recognized by Guo et al.12, except for two isolates, M20 and M3. We then further delimited an uncertain phylogenetic lineage found in a previous study (lineage 8), which was composed of one isolate (M20) generated in this research and another singleton (HKAS86607 CBS J)12 supported by high ML and RBC bootstrap. Ultimately, only one singleton (M3) did not belong to any lineages. Its classification requires further study. Lineage 6 included almost half of the total number of isolates. This lineage was defined as a new phylogenetic lineage in Guo et al.12. This lineage is represented in most of the samples from China previously considered as A. gallica, which was strongly divergent from European A. gallica. However, we also found evident divergence in lineage 6 which formed two subgroups in the β-tubulin phylogeny (Supplementary Information, Figure S1A). In contrast, ML bootstrap for this lineage was low in the EF-1α phylogeny (<70%) (Supplementary Information, Figure S1B). Twelve isolates in this study together with samples previously considered as A. gallica formed one subgroup, while ten isolates in this research with A. gallica (HKAS85517) and a CBS B (HKA86573) formed another subgroup. A probable reason for this result is that A. gallica has clear intraspecific differentiation and may be in the process of allopatric speciation22.
From this research, we have shown that P. umbellatus associates with diverse Armillaria partners. Previously published articles named the Armillaria species with which P. umbellatus associated as A. mellea or Armillaria spp. However, there has been no evidence to date to suggest that A. mellea is the fungal partner of P. umbellatus. Additionally, the Armillaria species used in the cultivation of P. umbellatus have not been identified. This study also found that the Armillaria isolates present certain geographic characteristics. For example, Armillaria isolates from Northeast China are mainly A. cepistipes, while isolates from Southwest China mainly belong to lineage 4. Although present results may not totally reflect the true Armillaria communities in some provinces due to small sample sizes, it is clear that different Armillaria isolates must be selected when used in cultivation of P. umbellatus in different regions. Inappropriate Armillaria isolates may lead to unstable yield and production efficiency.
The evolution of traits involved in ecological interactions such as predator-prey, host-parasite, and plant-pollinator interactions, are likely to be shaped by the phylogenetic history of both parties. In the P. umbellatus-Armillaria interactions, the phylogenetic distance of P. umbellatus and Armillaria strains were positively and significantly correlated. This means that phylogenetically related P. umbellatus populations tend to interact with a closely related Armillaria species. However, the P. umbellatus phylogeny does not show a significant phylogenetic signal on the interaction with their associated Armillaria species, but the Armillaria exhibits a significant phylogenetic signal on the interaction. Such asymmetric patterns have also been reported in other systems, e.g. orchid mycorrhizal symbiosis23, 24 and ectomycorrhizal symbiosis25. Additionally, within tropical and parasitic networks, interaction conservatism is often stronger for resources than for consumers. This means that related prey species tend to share more consumers than related consumers share prey species26–30. In the P. umbellatus-Armillaria interaction, sclerotia of P. umellatus digested the penetrated rhizomorph of Armillaria to meet their nutrition demands. However, the Armillaria are not dependent on P. umbellatus for their reproduction and dispersal and can survive as either saprophytes or parasites. Their distribution is independent of P. umbellatus. Armillaria species are a major component of the mycobiota of many forest ecosystems, however, the origin and diversification of this genus is complicated and poorly known. Some of the Armillaria species present considerable intraspecific genetic differentiation, and are in the process of allopatric speciation22, 31, 32. In addition to the intraspecific genetic diversity of Armillaria species, P. umbellatus also contains levels of intraspecific genetic diversity16. It is unlikely that Armillaria species have evolved substantially in response to the P. umbellatus, which may explain the asymmetric relationship of P. umbellatus-Armillaria associations.
Methods
Collection of P. umbellatus sclerotial samples
We collected 57 wild sclerotial samples of P. umbellatus from the following eleven provinces of China: Heilongjiang, Jilin, Shanxi, Shaanxi, Henan, Yunnan, Gansu, Sichuan, Tibet, Guizhou and Hebei. (Fig. 4). Details regarding the samples are shown in Supplementary Information, Table S1. For each sample, at least 12 sclerotia from different individuals growing 30–50 m apart were chosen. In total, more than 684 individual sclerotia were collected. Once the fresh sclerotia were collected, they were delivered to the lab within two to three days for further processing. Some sclerotia from each sample were also numbered and allowed to air-dry at room temperature. These sclerotia were then deposited in the herbarium of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences.
Figure 4.

Map of China showing successful sampling sites of P. umbellatus. Red circles: the P. umbellatus samples from which Armillaria were isolated; Blue circles: the P. umbellatus samples from which Armillaria were not successfully isolated. The map was generated using ArcGIS 9.3 (ESRI, Redlands, CA, USA; http://www.esri.com).
Isolation of Armillaria
The sclerotia of P. umbellatus were washed thoroughly in running tap water for 10 min and were surface sterilized via submerion in 75% ethanol for 1 min, a solution of 3.5% (v/v) Chlorox for 2 min and 75% ethanol for 30 s. The surface sterilized sclerotia were washed with sterile distilled water three times and blotted with sterile absorbent paper. Ten individual sclerotia from each sample were used for Armillaria isolation. In order to obtain the Armillaria isolates which actually infected sclerotia of P. umbellatus, we only isolated the Armillaria from the infection cavity inside the sclerotia. To accomplish this, the surface sterilized sclerotia were bisected from the evident penetration site of the Armillaria rhizomorph on the sclerotial surface. Once the sclerotia were cut from the correct site, a cavity where Armillaria had colonized can be seen (Fig. 5).
Figure 5.
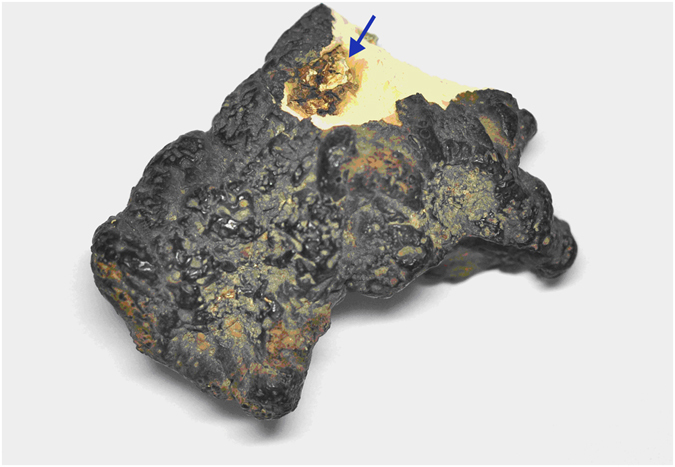
Sclerotia of P. umbellatus bisected from the penetration site of Armillaira rhizomorph on the sclerotial surface. The arrow shows a cavity beneath the surface which contains the penetrated Armillaira rhizomorph.
The residual Armillaria rhizomorph in the cavity was extracted and placed on potato dextrose agar (PDA) medium plates amended with streptomycin to suppress the growth of bacteria. As the Armillaria rhizomorph in the cavity were in different digestion stage, only the newly penetrated and undigested rhizomorph could be selected. This resulted in a low successful isolation rate of Armillaria strain. The selected rhizomorph of Armillaria were extracted and cultured. Plates were incubated at 23 °C in the dark. The growing tips of the Armillaria rhizomorph were transferred to new plates. All isolates were numbered and kept for further identification.
DNA extraction
An Armillaria rhizomorph was extracted out from each plate using sterile forceps. The rhizomorph surface attached to the media were removed carefully using a dissecting needle. For the P. umbellatus sclerotial DNA extraction, the sclerotia were then cut in half and 100 mg of medullar tissue was removed. Both sclerotia and rhizomorph samples were ground with a mortar and pestle in liquid nitrogen. Genomic DNA was extracted using the E.Z.N.A. TM Fungal DNA kit (Omega) following the manufacturer’s instructions.
PCR amplication and sequencing
The ITS, β-tubulin and elongation factor-1 alpha (EF-1α) have been used to infer phylogenetic relationships for various species of Armillaria 33–35. In this study of Armillaria fungal strains, we amplified the ITS1-5.8 S rDNA-ITS2 using the universal primer pair ITS1 and ITS436, the EF-1α using pair EF595F/EF1160R37, and the β-tubulin using primer pair TubF/TubR34. For P. umbellatus, we amplified the ITS1-5.8 S rDNA-ITS2 using the universal primer pair ITS1 and ITS436. PCR amplification was performed in a 25 μL reaction volume containing approximately 20 ng of DNA, 1 μL of each primer, and 12.5 μL of PCR master mix (Aidlab Biotech Co., Ltd., Beijing, China). All PCR reactions were carried out on a BIO-RAD T100 Thermal Cycler under the following reaction conditions. ITS followed predenaturation at 95 °C for 10 min, followed by 36 cycles of denaturation at 94 °C for 15 s, annealing at 53 °C for 30 s, and elongation at 72 °C for 2 s. A final elongation at 72 °C for 7 min was included after the cycles. β-tubulin followed predenaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 40 s, annealing at 53 °C for 40 s, and elongation at 72 °C for 90 s. EF-1α followed predenaturation at 95 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 15 s, annealing at 56 °C for 30 s, and elongation at 72 °C for 30 s. A final elongation at 72 °C for 30 s was included after the cycles. The PCR products were separated on a 1% (w/v) agarose gel and the bands were visualized under UV illumination. For most of the samples, PCR amplification yielded a single strong band. PCR products that could not be sequenced successfully were cloned into a Trans 5α vector (TransGen Beijing, China) and then sequenced with universal primers M13F /M13R. The contiguous sequences were assembled with SeqMan (DNASTAR Inc., USA). The sequences for ITS, β-tubulin and EF-1α of Armillaria isolates and ITS sequences of P. umbellatus were deposited in GenBank (accession numbers KY389147 - KY389193, KY389267 - KY389313, KY389220 - KY389266, and KY389194 - KY389219).
Sequence alignment and phylogenetic analyses
In order to understand the phylogenetic relationship between Armillaria isolates generated in this research and the phylogenetic lineages of Armillaria in China recognized by Guo et al.12, four matrices were compiled in this research, i.e. ITS, β-tubulin, EF-1α and three-locus matrices. We downloaded the sequences of ITS, β-tubulin and EF-1α published by Guo et al.12 from GenBank. All ITS, β-tubulin and EF-1α, and reference sequences were aligned with Clustal X version 2.038, respectively, and ambiguous regions in both sides of each region were excluded.
The best-fitted substitution model for each matrix was determined via jModelTest 239 based on the Akaike Information Criterion (AIC). TN93+G+I and T92+G were selected as the best models for the three-locus and ITS matrices, respectively. K2+G was selected as the best model for the EF1-α and the β-tubulin matrix, respectively. Maximum likelihood (ML) bootstrap analyses were conducted for the four matrices. ML phylogeny was constructed with RAxML 7.0.440. Clade support was estimated with RAxML via nonparametric bootstrap analysis on 1000 pseudo-replicate data sets. In addition to the ML trees, we constructed ultrametric trees with a BRC analysis using BEAST 1.5.441. The uncorrelated lognormal clock model42 was selected and a pro forma calibration point was enforced: the root height was fixed at 1.0. Posterior distributions of parameters were approximated using two independent Markov chain Monte Carlo analyses of 2.0 × 107generations followed by a discarded burn-in of 2.0 × 106 generations (10%).
Data analyses
In order to test whether phylogenetic relatedness of P. umbellatus samples correlates with phylogenetic relatedness of Armillaria species, a simple Mantel test implemented in ZT 1.143 was used to compare phylogenetic distance matrices of P. umbellatus with phylogenetic distance matrices of associated Armillaria strains. The phylogenetic distance for both P. umbellatus and Armillaria strains was calculated using the ‘distance’ option in Geneious 8.1.6 (http://www.geneious.com) based on the highest likelihood tree from the ML analysis. The simple Mantel test was run with 10000 randomizations.
Besides the phylogenetic relatedness of P. umbellatus-Armillaria associations, we further evaluated the strength of phylogenetic signal of the two phylogenies on the P. umbellatus–Armillaria interactions via using a linear model approach that fits the phylogenetic variance–covariance matrix to the plant–fungi interaction matrix26. ITS sequences of both P. umbellatus and Armillaria isolates were used. Prior to the analysis, we first analyzed the pairwise distances of all the P. umbellatus samples and Armillaria isolates, respectively, we then treated the pairwise distances equal to zero as one genotype and the pairwise distances >0 as different genotypes. Finally, the 26 P. umbellatus samples were classed into 11 genotypes, and the 47 Armillaria isolates were classed into 35 genotypes. We then generated a P. umbellatus-Armillaria interaction matrix composed of 0/1 (present/absent) data. Because measurements of phylogenetic signal are based on evolutionary rates (branch lengths) estimated by phylogenetic inference, we examined phylogenetic signal on ML trees, where branch lengths are estimated without a molecular clock assumption and represent genetic distance, and Bayesian relaxed clock (BRC) trees, where branch lengths are estimated under a relaxed molecular clock assumption and represent time. The ITS sequences of 11 P.umbellatus genotypes and 35 Armillaria genotypes were aligned with Clustal X version 2.038, respectively. K2+G and T92+G models of evolution were identified as the best-fit model for the P. umbellatus and Armillaria data sets, respectively, using AIC implemented in jModelTest 239.
We applied the phyogenetic bipartite linear model of Ives and Gofray26. The structure of the association matrix is decomposed into a phylogenetically corrected mean association strength and a vector of residuals depending on the phylogenies via an estimated general least square (EGLS) analysis. The reference evolution model used to calculate the phylogenetic structure is the Ornstein–Uhlenbeck (OU) process, which can incorporate stabilizing selection44. We calculated the independent phylogenetic signals of the P. umbellatus (d P) and Armillaria (d A) phylogenies on the interaction matrix (association present/absent) and the strength of the signal of both phylogenies combined (MSEd). MSEd was compared with MSE values for a model that assumes no phylogenetic structure (MSEstar) and a Brownian evolution model (MSEb). The model minimizing the mean squared error was considered the best fit. Bipartite linear models were performed using the pblm function in the picante R package45 and were carried out on the ML and BRC results for P. umbellatus–Armillaria phylogeny sets. Statistical significance of the d values was estimated by calculating 95% bootstrap confidence intervals on 100 replicates.
Data availability
All data generated during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This research was financially supported by the National Natural Sciences Foundation of China (No. 31572180), CAMS Initiative for Innovative Medicine (No.2016-I2M-2-002) and Special research fund for the central level public welfare research institute (YZ-1307). We also thank the anonymous reviewers for the useful comments on this manuscript.
Author Contributions
X.X. organized the sampling, performed statistical data analysis and wrote the paper. M.J. preformed the molecular experiments, and cooperated in statistical data analysis. G.S. cooperated in experiment design and manuscript revision. All authors revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiaoke Xing and Jinxin Men contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04578-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoke Xing, Email: xkxing2009@hotmail.com.
Shunxing Guo, Email: sxguo@implad.ac.cn.
References
- 1.Xu, J. T. Medicinal Mycology in China. Beijing, Beijing Medical University and Peking Union medical University Union Press (1997).
- 2.Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW. Species diversity and utilization of medicinal mushrooms and fungi in China. Int J Med Mushrooms. 2009;11:287–302. doi: 10.1615/IntJMedMushr.v11.i3.80. [DOI] [Google Scholar]
- 3.Yang L, et al. The effect of Polyporus umbellatus polysaccharide on the immunosuppression property of culture supernatant of S180 cells. Chinese J Cell Mol Immunol. 2004;20:234–237. [PubMed] [Google Scholar]
- 4.Zeng X, et al. Effects of Polyporus umbellatus and Polyporus polysaccharide on the phagocytosis function and costimulatory molecules expression of peritoneal macrophages in rat bladder cancer. Chinese J Immunol. 2011;27:414–418. [Google Scholar]
- 5.Li SQ. Endangered Polyporus umbellatus resource needing protection and development an investigation from producing areas. Modern Chinese Medicine. 2008;10(6):43–44. [Google Scholar]
- 6.Wargo PM, Cgiii S. Armillaria root rot: the puzzle is being solved. Plant Dis. 1985;69:826–832. doi: 10.1094/PD-69-826. [DOI] [Google Scholar]
- 7.Hood, I. A., Redfern, D. B. & Kile G. A. Armillaria in planted hosts. Agriculture Handbook 122–149 (1991).
- 8.Mei J, Xing X, Guo S. Biological species and identification methods of the genus Armillaria (Agaricales, Basidiomycota): a review. Mycosystema. 2016;35(11):1281–1302. [Google Scholar]
- 9.Qin GF, Zhao J, Korhonen KA. Study on intersterility groups of Armillaria in China. Mycologia. 2007;99:430–441. doi: 10.1080/15572536.2007.11832568. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, et al. New biological species of Armillaria from China. Mycosystema. 2008;27:156–170. [Google Scholar]
- 11.Coetzee MPA, Wingfield BD, Zhao J, van Coller SJ, Wingfield MJ. Phylogenetic relationships among biological species of Armillaria from China. Mycoscience. 2015;56:530–541. doi: 10.1016/j.myc.2015.05.001. [DOI] [Google Scholar]
- 12.Guo T, Wang HC, Xue WQ, Zhao J, Yang ZL. Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic lineages in China, seven of which are associated with cultivated Gastrodia elata. Plos One. 2016;11:0154794. doi: 10.1371/journal.pone.0154794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S, Xu J. Nutrient source of sclerotia of Grifola umbellata and its relationship to Armillaria mellea. Acta Botanica Sinica. 1992;34(8):576–580. [Google Scholar]
- 14.Yao, L., Cheng, H.& Yang, Z. Guidelines for good agricultural practice of Chinese crude drugs (in Chinese). China Agricultural Press, Beijing, 1213–1222 (2006).
- 15.Kikuchi G, Yamaji H. Identification of Armillaria, species associated with Polyporus umbellatus, using ITS sequences of nuclear ribosomal DNA. Mycoscience. 2010;51(5):366–372. doi: 10.1007/S10267-010-0053-8. [DOI] [Google Scholar]
- 16.Xing X, Ma X, Hart MM, Wang A, Guo S. Genetic Diversity and Evolution of Chinese Traditional Medicinal Fungus, Polyporus umbellatus, (Polyporales, Basidiomycota) Plos One. 2013;8:58807. doi: 10.1371/journal.pone.0058807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha JY, Igarashi T. Armillaria species associated with Gastrodia elata in Japan. Eur J Forest Path. 1995;25:319–326. doi: 10.1111/j.1439-0329.1995.tb01347.x. [DOI] [Google Scholar]
- 18.Korhonen K. Interfertility and clonal size in the Armillaria mellea complex. Karstenia. 1978;18:31–42. [Google Scholar]
- 19.Taylor JW, et al. Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- 20.Coetzee MPA, et al. Identification of Armillaria isolates from Bhutan based on DNA sequence comparisons. Plant Path. 2005;54:36–45. doi: 10.1111/j.1365-3059.2005.01110.x. [DOI] [Google Scholar]
- 21.Tsykun T, Rigling D, Prospero S. A new multilocus approach for a reliable DNA-based identification of Armillaria species. Mycologia. 2013;105:1059–1076. doi: 10.3852/12-209. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, et al. Genetic diversity of Armillaria gallica isolates from China and Europe revealed with ISSR analysis. Biodiversity. Science. 2012;20:224–230. [Google Scholar]
- 23.Jacquemyn H, et al. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus orchis (Orchidaceae) New Phytol. 2011;192:518–28. doi: 10.1111/j.1469-8137.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 24.Xing X, Ma X, Mei J, Chen Y, Guo S. Phylogenetic constrains on mycorrhizal specificity in eight Dendrobium (Orchidiaceae) species. Science China Life Sciences. 2017 doi: 10.1007/s11427-017-9020-1. [DOI] [PubMed] [Google Scholar]
- 25.Leho T, Marit M, Ishida TA, Mohammad B. Phylogenetic relationships among host plants explain differences in fungal species richness and community composition in ectomycorrhizal symbiosis. New Phytol. 2013;199:822–831. doi: 10.1111/nph.12328. [DOI] [PubMed] [Google Scholar]
- 26.Ives AR, Godfray HCJ. Phylogenetic analysis of trophic associations. Am Natural. 2006;168:1–14. doi: 10.1086/505157. [DOI] [PubMed] [Google Scholar]
- 27.Bersier LF, Kehrli P. The signature of phylogenetic constraints on food–web structure. Ecol Complex. 2008;5:132–139. doi: 10.1016/j.ecocom.2007.06.013. [DOI] [Google Scholar]
- 28.Kawakita A, Okamoto T, Goto R, Kato M. Mutualism favours higher host specificity than does antagonism in plant–herbivore interaction. P Roy Soc B. 2010;277:2765–2774. doi: 10.1098/rspb.2010.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cagnolo L, Salvo A, Valladares G. Network topology: patterns and mechanisms in plant–herbivore and host–parasitoid food webs. J Anim Ecol. 2011;80:342–351. doi: 10.1111/j.1365-2656.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 30.Krasnov BR, et al. Phylogenetic signal in module composition and species connectivity in compartmentalized host–parasite networks. Am Nat. 2012;179:501–511. doi: 10.1086/664612. [DOI] [PubMed] [Google Scholar]
- 31.Qin GF, Hantula J. Genetic diversity and molecular identification of northern hemisphere species of Armillaria gallica. Scientia Silvae Sinicae. 2001;37:61–68. [Google Scholar]
- 32.Baumgartner K, Travadon R, Bruhn J, Bergemann SE. Contrasting patterns of genetic diversity and population structure of Armillaria mellea sensu stricto in the eastern and western United States. Phytopathology. 2010;100:708–718. doi: 10.1094/PHYTO-100-7-0708. [DOI] [PubMed] [Google Scholar]
- 33.Schulze S, Bahnweg G, Möller EM. & HS, Jr. Identification of the genus Armillaria by specific amplification of an rDNA-ITS fragment and evaluation of genetic variation within A. ostoyae by rDNA-RFLP and RAPD analysis. Forest Path. 2007;27(4):225–239. doi: 10.1111/j.1439-0329.1997.tb00865.x. [DOI] [Google Scholar]
- 34.Wang, H. C. Systematic studies on Armillaria from China: University of Chinese Academy of Sciences (2007).
- 35.Hasegawa E, Ota Y, Hattori T, Kikuchi T. Sequence-based identification of Japanese Armillaria species using the elongation factor-1 alpha gene. Mycologia. 2010;102(4):898–910. doi: 10.3852/09-238. [DOI] [PubMed] [Google Scholar]
- 36.White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. eds. RCR protocols: a guide to methods and applications. New York, USA Academic Press (1990).
- 37.Wendland J, Kothe E. Isolation of tef1 encoding translation elongation factor EF1 [alpha] from the homobasidiomycete Schizophyllum commune. Mycol Res. 1997;101:798–802. doi: 10.1017/S0953756296003450. [DOI] [Google Scholar]
- 38.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 39.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 41.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolut Biol. 2007;7:e214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biology. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnet E, Van de Peer Y. ZT: a software tool for simple and partial Mantel tests. J Stat Softw. 2002;7:1–12. doi: 10.18637/jss.v007.i10. [DOI] [Google Scholar]
- 44.Blomberg P, Garland T, Jr., Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 45.Kembel SW, Cowan PD, Helmus MR, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article (and its Supplementary Information files).


