Supplemental Digital Content is available in the text
Keywords: Estrogen, Mesenchymal stem cells, Postmenopausal, Rat vagina
Abstract
Objective:
Reconstructive surgery for pelvic organ prolapse is plagued with high failure rates possibly due to impaired healing or regeneration of the vaginal wall. Here, we tested the hypothesis that postoperative administration of local estrogen, direct injection of mesenchymal stem cells (MSCs), or both lead to improved wound healing of the injured vagina in a menopausal rat model.
Methods:
Ovariectomized rats underwent surgical injury to the posterior vaginal wall and were randomized to treatment with placebo (n = 41), estrogen cream (n = 47), direct injection of MSCs (n = 39), or both (n = 43).
Results:
MSCs did not survive after injection and had no appreciable effects on healing of the vaginal wall. Acute postoperative administration of vaginal estrogen altered the response of the vaginal wall to injury with decreased stiffness, decreased collagen content, and decreased expression of transcripts for matrix components in the stromal compartment. Conversely, vaginal estrogen resulted in marked proliferation of the epithelial layer and increased expression of genes related to epithelial barrier function and protease inhibition. Transcripts for genes involved in chronic inflammation and adaptive immunity were also down-regulated in the estrogenized epithelium.
Conclusions:
Collectively, these data indicate that, in contrast to the reported positive effects of preoperative estrogen on the uninjured vagina, acute administration of postoperative vaginal estrogen has adverse effects on the early phase of healing of the stromal layer. In contrast, postoperative estrogen plays a positive role in healing of the vaginal epithelium after injury.
Pelvic organ prolapse (POP) is a common disorder in women in which the bladder, uterus, cervix, rectum, and vaginal wall herniate through the vaginal introitus. Women with POP suffer from urinary incontinence or retention, chronic pelvic pressure, fecal incontinence or obstruction, sexual dysfunction, social embarrassment, and isolation. The magnitude of this public health problem is enormous. Up to 11% of women have surgery for POP or urinary incontinence in their lifetime1 and more than 225,000 inpatient surgical procedures for POP are performed per year in the United States. Regrettably, of 400,000 operations performed for incontinence and prolapse per year, 116,000 are repeat operations (ie, 29%).1,2
It is difficult to understand the high failure rate of reconstructive surgery for POP because the underlying pathogenesis is unknown. It has been postulated, however, that impaired wound healing may lead to recurrence of prolapse after pelvic reconstructive surgery, and that estrogen may serve as an adjunct to surgical repair.3-6 Both the lower female genital and urinary tracts arise from the primitive urogenital sinus in which estrogen receptors (α and β) are expressed during development and in adult tissues of the vagina, urethra, bladder, and surrounding pelvic floor musculature.7-9 Local intravaginal estrogen in postmenopausal women by any delivery system (creams, tablets, or estrogen-containing rings) is known to decrease vaginal pH, increase blood flow, improve tissue compliance, and promote vaginal cell maturity.10 Vaginal wound closure, scar contraction, and biomechanical recovery were shown to be significantly impaired in ovariectomized rabbits relative to estrogenized sham-operated controls.3 In ovariectomized guinea pigs, preoperative systemic estrogen (with continued use postoperatively) resulted in significant growth, increased smooth muscle, improved vaginal thickness, increased distensibility without compromise of maximal force at failure, and increased total and cross-linked collagen that was refractory to the lathyrogenic effects of β-aminoproprionitrile.4 In ovariectomized rats, vaginal estrogen (in doses used in the current study) was shown to be superior to systemic estrogen in terms of increasing vaginal epithelial thickness, distensibility, and total and cross-linked collagen content in the uninjured vagina.5
In addition to estrogen, stem cell therapy has been proposed to facilitate repair after injury to the pelvic floor.11-14 In animal models of stress urinary incontinence, periurethral injection of bone marrow-derived MSCs restores the external urethral sphincter and improves urodynamic indices of incontinence.15,16 Direct injection of MSCs also improves healing of the external anal sphincter.17 Here, we used ovariectomized rats as a menopausal animal model to study the effects of postoperative vaginal estrogen cream and direct injection of mesenchymal stem cells (MSCs) on the biomechanical properties, collagen content, histomorphology and gene expression of the surgically injured vaginal wall. Effects were quantified in both vaginal stroma (defined as lamina propria and muscularis) and epithelium.
METHODS
All animals were handled and euthanized in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center at Dallas. A total of 170 virginal female Sprague Dawley rats (Charles River Laboratories) at 12 weeks of age were housed in IACUC-approved facilities under a 12-L:12-D cycle at 22°C.
Treatment groups
All animals underwent bilateral ovariectomy through 1-cm flank incisions. After 2 weeks, using a dissecting microscope, vaginal injury was created by a posterior vaginal wall incision from the introitus to the cervix through epithelium and stroma. Thereafter, using a list randomizer (www.random.org), animals were assigned into four treatment groups of either estrogen (conjugated equine estrogen, CEE) cream (Pfizer Pharmaceuticals) or placebo cream and either injection of MSCs or placebo phosphate buffered saline (PBS). Application of CEE or placebo cream began on the day of injury, whereas injection of MSCs or PBS injection (placebo) occurred 3 to 4 days after injury. The time point for stem cell injection was chosen to increase survival of the stem cells because histologic comparisons revealed that blood accumulation and acute inflammatory reaction improved substantially by 4 days. The four treatment groups were (1) placebo cream-placebo injection, (2) CEE cream-placebo injection, (3) placebo cream-MSCs injection, and (4) CEE cream-MSCs injection. The dose of estrogen cream was 625 ng CEE per dose delivered in 100 mg of cream with daily vaginal applications for 2 weeks and three applications per week thereafter. The dose equates to 2.5 μg/kg/d (similar to the 2.2-4.4 μg/kg/d in women). Placebo groups received vaginal applications in 100 mg doses on the same schedule. Green fluorescent protein (GFP)-labeled MSCs (4 million cells in 100 μL) were injected into the subepithelial space of the injured posterior vaginal wall using a syringe and 19-ga needle. Treatment groups were euthanized 1 or 3 weeks after injury. We reasoned that 1 week represented an early time point in the healing process and healing would be near completion by 3 weeks as we reported in the external anal sphincter.17
Tissue processing
After euthanasia, the abdominal cavity was opened, the pubic symphysis disarticulated, and uterine horns, bladder, cervix, and vagina dissected down to the perineal skin. Using microsurgical instruments and a dissection microscope, the perineal skin was removed and the bladder and urethra separated from the anterior vaginal wall. Uterine horns and cervix were removed from the vagina. For animals randomized for histologic analysis, the vagina was embedded with optimal cutting temperature (OCT) compound and frozen in an −80°C freezer. Before freezing, dilute India ink dye (10% in PBS) was injected in the midline of the posterior vaginal wall to aid in orientation when viewing cross-sectional slides. A ring of vagina was collected from the distal half of the vagina for biomechanical testing. The remaining distal vagina was opened longitudinally and epithelium over the wound scraped off with a scalpel and frozen in liquid N2. Equal portions of the remaining injured vaginal stroma (lamina propria and muscularis) were snap-frozen in liquid N2 and stored at −80 °C.
Biomechanical testing
Viscoelastic properties of individual vaginal rings were quantified as previously described.5,18 Briefly, tissue rings were suspended between two stainless steel wire mounts, attached to a steel rod apparatus with a calibrated mechanical drive and to a force transducer. All tissues were kept in a physiologic salt solution (NaCl 120.5 mmol/L, KCl 4.8 mmol/L, MgSO4 1.2 mmol/L, NaH2PO4 1.2 mmol/L, NaHCO3 20.4 mmol/L, CaCl2 1.6 mmol/L, d-glucose 10 mmol/L, pyruvate 1 mmol/L, and pH of 7.4) in water baths at 37 °C with 95% O2 and 5% CO2. Each vaginal ring was equilibrated to slack length (ring diameter at resting tone) and serially stretched in 1 mm increments at 30-second intervals until plateau of force generation failure (ring breakage). Wet weights of vaginal rings were determined after testing. Stress (kPa) was calculated as maximum force per unit area and plotted against strain (change in length divided by slack length). The average slack length of rings suspended between the foot and force transducer was 8.74 ± 0.75 mm with weights of 17.6 ± 1.78 mg for placebo-treated animals; 8.54 ± 0.53 mm and 22.6 ± 3.3 mg for estrogen-treated animals; 8.52 ± 0.48 mm and 17.0 ± 1.8 mg for MSC; and 7.93 ± 0.54 mm and 23.9 ± 3 mg for MSC + E. Stiffness was calculated from the slope of the linear portion of the curve.
Histomorphology
Vaginal tissues embedded in OCT and frozen at −80 °C were cross-sectioned and stained with hematoxylin and eosin. Images of these cross-sections were captured using a Nikon E1600 microscope and Nikon NIS Elements AR software. The mean of 15 measurements was taken from each compartment of each specimen. Measurements were recorded in micrometers of the vaginal epithelium at the injury site (vaginal lumen to basement membrane), the vaginal muscularis (basement membrane to edge of muscular bundles), and total vaginal thickness (vaginal lumen to edge of muscular bundles). An outline of the area of the injury site (μm2) was traced using the Nikon NIS Elements AR software. This measurement was conducted blindly by two observers, and each data point represents mean of two observers. Interobserver variability was less than 15%.
Hydroxyproline assays
Collagen solubility measurements were used as an index of collagen structure within the tissue.19 Weights of vaginal tissues were determined before and after lyophilization. Lyophilized tissue was homogenized with 1 M NaOH with protease inhibitors (PIs—pancreas extract 0.02 mg/mL, pronase 0.005 mg/mL, and thermolysin 0.0005 mg/mL) at 4 °C for 24 hours, centrifuged, and the supernatant was saved as fraction A (newly synthesized, noncross-linked collagen fraction). The remaining insoluble residue was washed with water along with PIs and extracted with 0.5 M acetic acid along with PIs at 4 °C for 24 hours with gentle rotation. Samples were centrifuged, and the supernatant saved as fraction B (denatured mature collagen). The remaining tissue pellet was saved as fraction C (mature, cross-linked collagen). Collagen content in fractions A, B, and C were determined by measurement of hydroxyproline content by the chloramine-T method19 after hydrolysis in 6 M HCl overnight at 100 °C. Hydroxyproline values were converted to collagen (×7.6) and normalized to tissue wet weight. Total collagen was calculated as A + B + C and percentage of fraction C reported as percentage of total collagen.
Real-time quantitative polymerase chain reaction
Quantitative polymerase chain reaction (PCR) was used to determine the relative levels of mRNAs in vaginal tissues. Tissues were pulverized and homogenized in Trizol reagent (Invitrogen, Eugene, OR). RNA was isolated according to the manufacturer's protocol. Complementary DNA synthesis was carried out with 2 μg of total RNA in a reaction volume of 20 μL. Each reaction contained 10 mM dithiothreitol, 0.5 mM deoxynucleotide triphosphates, 0.015 μg/μL random primers, 40 units RNase inhibitor (Invitrogen), and 200 units reverse transcriptase (Invitrogen). PCR reactions were carried out in the ABI Prism 7000 sequence-detection system (Applied Biosystems; ABI, Foster City, CA). The reverse transcription product from 50 ng RNA was used as the template, and reaction volumes (30 μL) contained Master Mix (ABI). Primer concentrations were 900 nM. Cycling conditions were 2 minutes at 50°C, followed by 10 minutes at 95°C, then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Primer sequences for amplifications were chosen using published cDNA sequences and the primer express program (ABI). Primers were chosen such that the resulting amplicons would cross an exon junction thereby eliminating any possible false-positive signs from genomic DNA contamination. TaqMan primers available from ABI were used for amplicon detection (see Supplementary Digital Content 1, http://links.lww.com/MENO/A216 for sequences of primers used for quantitative polymerase chain reaction [qPCR]). Gene expression was normalized to expression of the housekeeping genes cyclophilin A and hypoxanthine-phosphoribosyltransferase (HPRT). All primer sets were tested to ensure that efficiency of amplification over a wide range of template concentrations was equivalent to the housekeeping genes and all assays included no template controls. A pre-programmed dissociation protocol was used after amplification to ensure all samples exhibited a single amplicon. Levels of mRNA were determined using the ddCt method (ABI) and were expressed relative to an external calibrator (Control) on each plate.
Mesenchymal stem cell culture
Rat bone marrow-derived mesenchymal stromal cells (ScienCell Research Laboratories, #R7500, Carlsbad, CA) were cultured in MSC media (ScienCell Research Laboratories, #7501). To maintain the early progenitor phenotype, cells were maintained at preconfluence at all times. Twenty-four hours before injection, cells were treated with an adenoviral construct of GFP (100 multiplicity of infection/cell).
Immunohistochemistry
Vaginal tissue samples embedded in OCT (Sakura Finetek, Torrence, CA) were cooled and stored at −80°C until sectioning. Cryosections were prepared at 8 μm thickness on a Leica CM3050S cryostat (Leica Microsystems, Richmond, IL) according to standard procedures. Rabbit anti-sera used for GFP immunolabeling was obtained from Invitrogen (RRID AB_10073917). After slides were thawed under laminar flow, cryosections were fixed in formaldehyde vapor. Sections were then washed free of OCT in PBS and permeabilized with 0.3% Triton. Triton surfactant was removed by PBS washes and sections were blocked against secondary antibody host-serum affinity by incubation with 3% normal goat serum. Serial-sections were then subjected to either primary antibody (1:250) or PBS (no-primary-antibody substitution-control) and incubated overnight at 4 °C. Immunofluorescence detection of bound primary was conducted the following day according to previously described methods.20,21 Thereafter, cell nuclei were counterstained with Hoechst 33342 (Invitrogen/Molecular Probes) and sections overlaid with Vectashield fluorescence mounting medium (Vector Labs, Burlingame, CA). Evaluation and imaging were conducted on a Nikon Eclipse E600 epifluorescence photomicroscope equipped with a Nikon DS-Fi2 CCD camera. Below saturation 2560 × 1920 pixel grayscale images were collected for GFP and nuclear counterstain fluorescence at the boundary of the injection site. Images were overlaid and pseudocolored for maximum contrast (GFP, green; nuclei, red) in Adobe Photoshop. Immunohistochemistry was conducted in triplicate at each time point with virtually identical results. Specificity of GFP-immunostaining is illustrated in Supplemental Digital Content 2, http://links.lww.com/MENO/A217 in which sections of the injured vaginal wall 3 hours after injection of GFP-labeled MSCs were developed with primary antibody for GFP or PBS.
Statistical analysis
A two-way analysis of variance (ANOVA) and nonparametric (Kruskal-Wallis) testing was performed, as appropriate, for multiple group comparisons. Posttesting between groups was performed using Holm-Sidak method for normally distributed data and Dunn tests for nonparametric distributions with uninjured/untreated animals as the control. P < 0.05 determined significance.
RESULTS
Effect of vaginal estrogen, MSCs, or both on biomechanical properties of the vaginal wall
To determine whether vaginal estrogen or MSCs altered the biomechanical properties of the injured vagina, stress-strain relationships were conducted on rings of vaginal tissue obtained from the site of injury from ovariectomized animals treated with placebo, vaginal estrogen, MSCs, or estrogen + MSCs for 1 or 3 weeks after surgery (Fig. 1). Results were compared with ovariectomized rats without injury. In placebo-treated animals after injury, small increases in strain resulted in marked increases in stress generation indicative of a stiff, nondistensible tissue. In contrast, in vaginal tissues of two treatment groups that received estrogen cream (estrogen or estrogen + MSCs), large increases in strain led to relatively small increases in stress. The MSC treatment group demonstrated a modest decrease in stress, but this effect was less than that with estrogen treatment. In agreement with prior studies,12 the uninjured vaginal wall from ovariectomized rats exhibited poor distensibility and maintained strength with considerable stress generation at maximal length (Fig. 1B, C). After injury, stiffness decreased in all treatment groups at 1 week after injury, which did not change at 3 weeks. Interestingly, decreased stiffness was more profound in animals treated with postoperative vaginal estrogen regardless of MSCs. Maximal stress of the vaginal wall was maintained in injured animals treated with vehicle or MSCs even at 1 week after injury (Fig. 1C). In contrast, maximal stress of the vaginal wall was decreased modestly in animals treated with vaginal estrogen 1 week after injury increasing to normal levels after 3 weeks. The results indicate that the injured vaginal wall is characterized by decreased stiffness and maintenance of vaginal strength both at early (1 wk) and late (3 wk) time points after surgical injury. Vaginal estrogen resulted in more profound decreases in stiffness with slightly compromised vaginal strength at the early time point. Overall, direct injection of MSCs into the vaginal wound alone or with estrogen did not alter the biomechanical properties of the injured vaginal wall.
FIG. 1.
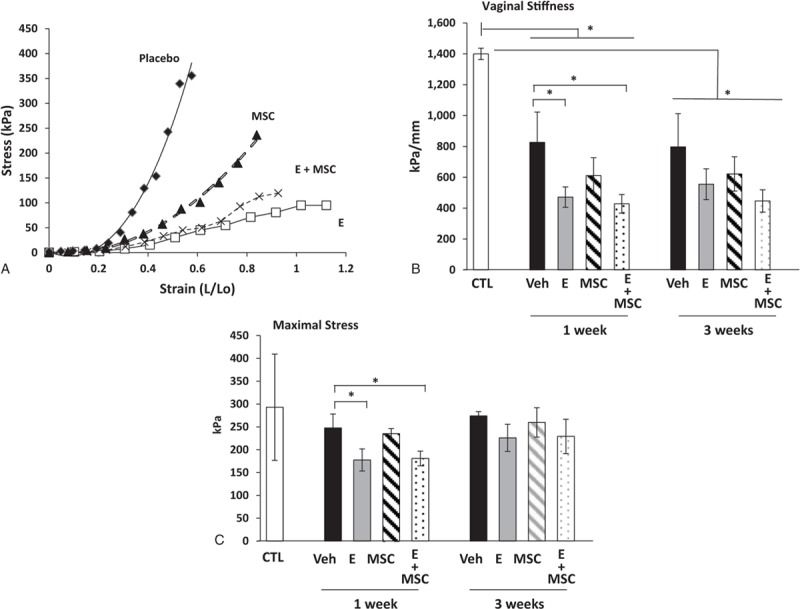
Biomechanical properties of the injured vaginal wall. Stress-strain relationships (A) of vaginal tissues from ovariectomized rats treated with placebo cream/placebo injection (Placebo, diamond, n = 8), estrogen cream/placebo injection (E, open square, n = 9), placebo cream/mesenchymal stem cell (MSCs) injection (MSC, triangle, n = 9), and estrogen cream + MSC injection (E + MSC, X, n = 9). Quantification of vaginal stiffness (B) and maximum vaginal stress (C) are presented as mean ± SEM. ∗Two-way analysis of variance. CTL, control; Veh, vehicle.
Effect of estrogen and MSCs on collagen content of the vagina
Collagen content of the vaginal wall was quantified using hydroxyproline assays. Fractions of tissue homogenates representing newly synthesized or denatured collagen were compared with those representing mature cross-linked collagen. Total collagen content of the vaginal wall decreased 60% to 73% after injury (Fig. 2A). Furthermore, the percentage of cross-linked collagen decreased from 90% to ∼10% regardless of treatment group. In contrast to reports indicating estrogen-induced increases in collagen content of the vaginal wall with systemic preoperative estrogen,4 loss of vaginal collagen after injury was more severe with postoperative vaginal estrogen (Fig. 2A). This decrease in total collagen content with postoperative estrogen was primarily due to decreases in the newly synthesized collagen fraction rather than a decline in cross-linked collagen (Fig. 2B, C). Interestingly, collagen content did not return to baseline 3 weeks after injury in any treatment group. Furthermore, production of newly synthesized collagen was ongoing at 3 weeks with little change in cross-linked fractions.
FIG. 2.
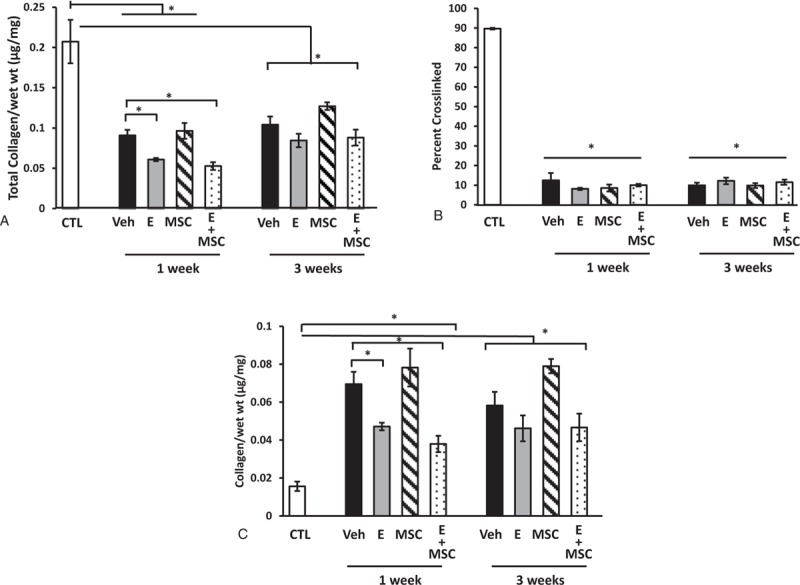
Effect of vaginal estrogen and mesenchymal stem cells (MSCs) on collagen content of the injured vaginal wall. (A) Total collagen content per milligram wet weight of vaginal stromal compartment collected from the site of injury 1 or 3 weeks after injury. Data represent injured vaginal stroma from ovariectomized rats treated with placebo cream/placebo injection (Placebo, solid bar), estrogen cream/placebo injection (E, gray bar, n = 9), placebo cream/MSC injection (MSC, lined bar), and estrogen cream + MSC injection (E + MSC, dotted bar). (B) Percentage of cross-linked mature collagen, and (C) newly synthesized immature collagen per milligram wet weight. Data represent mean ± SEM of 9-12 rats in each group. ∗Two-way analysis of variance (P < 0.05). CTL, control; Veh; vehicle.
Effect of estrogen and MSCs on the histomorphology of the vagina
Histologic sections of the vaginal wall at the site of injury from ovariectomized rats treated with placebo cream, estrogen, placebo injection, or MSCs are represented in Figure 3. In placebo-treated animals, the 1-week injury site was characterized by thickening of the epithelial layer, absence of the basement membrane, and a localized injury reaction comprised of infiltrated immune cells and destruction of the stroma underlying the injured epithelium (Fig. 3A). In animals treated with postoperative estrogen, the epithelial layer was increased even more dramatically than estrogen-induced thickening of the uninjured vagina (Fig. 3A). In contrast to the defective basement membrane of placebo animals, the basement membrane was intact in animals receiving estrogen. The injury reaction with estrogen, however, was large with extensive remodeling of the underlying and adjacent stroma. Histomorphology of MSCs alone was similar to placebo, and estrogen results were similar with or without MSCs (Fig. 3). Three weeks after injury, epithelium appeared intact after placebo or MSC treatment with marked, but incomplete, recovery of the underlying stroma. In contrast, the epithelium of estrogen-treated rats remained thickened and the underlying stroma did not recover as in placebo or MSC-treated groups. Stromal and epithelial thickness and area of the localized reaction to injury were quantified (Fig. 3 lower panel). Thickness of the posterior stromal layer was increased significantly in all animals 1 week after injury with estrogen groups exhibiting a magnified response compared with placebo or MSCs (more than twofold vs 1.6-fold, Fig. 3i). This early amplified response was accompanied by reduced stromal thickness at 3 weeks for estrogen and E + MSC treatment. Quantification of epithelial thickness over the injury site revealed remarkable increases in epithelium over the injury at 1 week, which was even more dramatic with estrogen treatment (5.5-fold to sixfold) compared with placebo or MSCs (fourfold). At 3 weeks, the epithelium returned toward baseline, but was persistently thicker in groups receiving estrogen. The results suggest that, in the acute postoperative period, estrogen has the most dramatic effect on the injured epithelium rather than the stroma. Furthermore, estrogen-induced modest increases in epithelial thickness of the uninjured anterior vaginal wall at both 1 and 3 week time points (see Supplementary Digital Content 3, http://links.lww.com/MENO/A218 in which epithelial thickness was quantified in ovariectomized rats treated with placebo cream + placebo injection vs estrogen cream + placebo injection 1 and 3 weeks after injury). The area of localized response to injury (ie, site of increased cell density and immune cell infiltration) was calculated by image analysis software (Fig. 3iii). As shown in Figure 3, surgical injury to the vaginal wall resulted in a localized injury reaction at 1 week. The injury reaction was quantified by computing the area of this response (Fig. 3iii). Estrogen amplified the early injury response relative to placebo or MSCs, which resolved substantially by 3 weeks despite incomplete regeneration of the stroma (Fig. 3). Taken together, histomorphologic analyses revealed that local estrogen treatment of the injured vaginal wall results in a massive injury response characterized by increases in both stromal and epithelial thickness and a greater inflammatory response compared to placebo or MSCs.
FIG. 3.
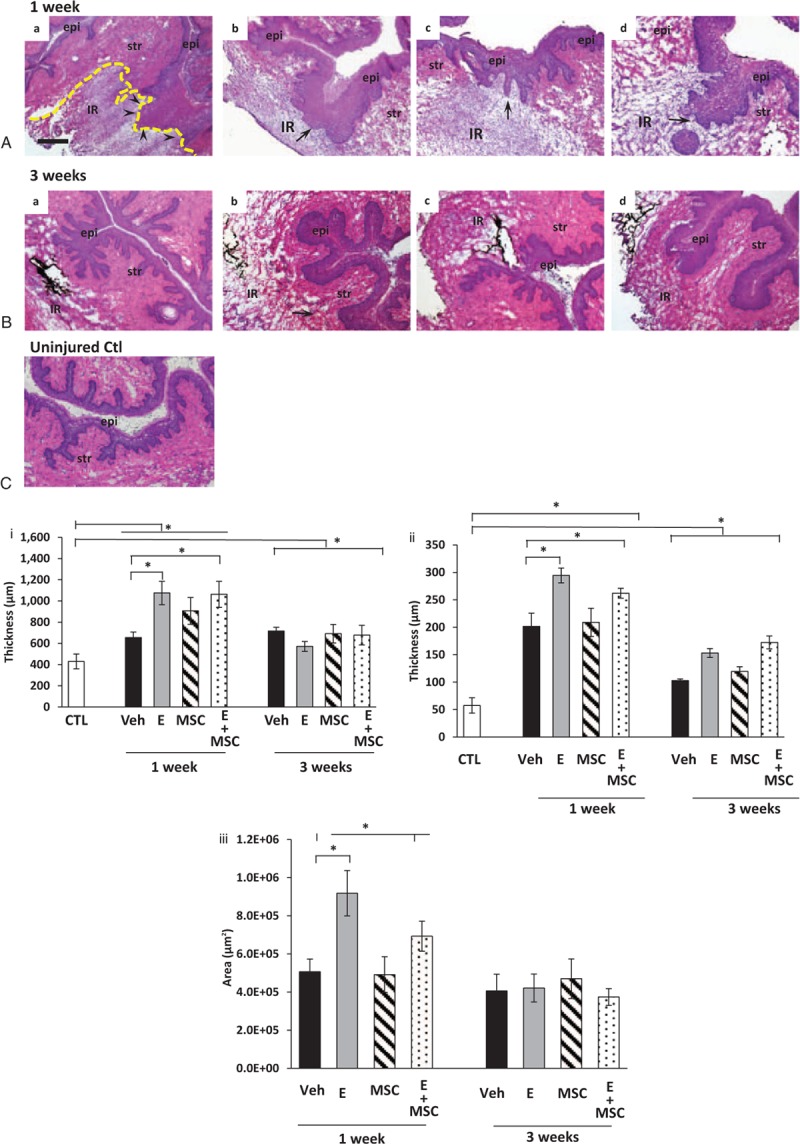
Histologic analysis of injury sites from ovariectomized rats treated with placebo, estrogen, MSCs, or MSCs + E at 1 (A) or 3 (B) weeks. epi, epithelium, str, stroma; IR, injury reaction. Arrows denote basement membrane at site of injury. Arrowheads in Aa indicate disruption of the basement membrane. (C) Uninjured control. Sections were stained with hematoxylin and eosin and captured at the same magnification (bar = 400 μm). Dark staining indicates incorporation of India ink at injury site. Dashed line indicates area of injury reaction. Lower panel (i), thickness of posterior vaginal stromal compartment or epithelium, (ii) at the injury site (μm) from ovariectomized rats treated with placebo cream/placebo injection (Placebo, solid bar), estrogen cream/placebo injection (E, gray bar, n = 9), placebo cream/mesenchymal stem cell injection (MSC, lined bar), or estrogen cream + MSC (E + MSC, dotted bar) 1 and 3 weeks after injury. Results were compared with uninjured untreated control ovariectomized rats (CTL). (iii) Area of injury reaction (μm2). Data represent mean ± SEM of 9-10 animals in each treatment group except uninjured controls (n = 3). ∗Two-way analysis of variance (P < 0.05). Veh, vehicle; CTL, control.
Effect of estrogen on expression of matricellular and growth factor genes in the vaginal stroma
In an effort to understand responses of the injured vaginal wall, we quantified gene expression of key matrix genes in the stromal compartment. First, we used expression of ERα and progesterone receptor (PR) as positive controls for estrogen action (Fig. 4). Interestingly, ERα was down-regulated in the injured vaginal wall compared with uninjured controls, and local estrogen treatment amplified injury-induced down-regulation of ERα in the stroma at both 1 and 3 weeks after injury (Fig. 4A). Like ERα, PR was also down-regulated in the injured vaginal wall in animals treated with vehicle or MSCs. As expected, estrogen rescued injury-induced down-regulation of PR and increased PR mRNA.
FIG. 4.
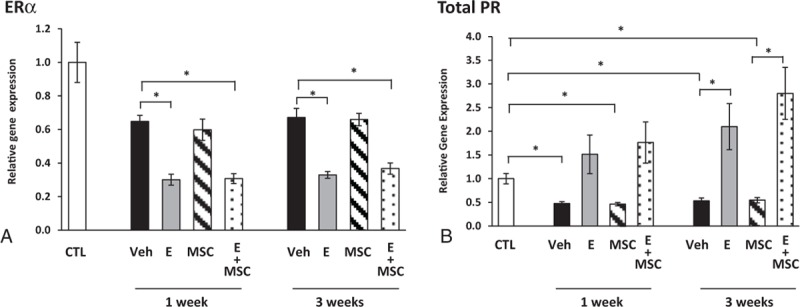
Effect of vaginal estrogen and mesenchymal stem cells (MSCs) on gene expression of ERα (A) or progesterone receptor (PR) (B) in the vaginal stroma before or after injury. Relative gene expression was determined by quantitative PCR in vaginal stroma from ovariectomized rats before injury (CTL) or 1 and 3 weeks after injury treated with placebo cream/placebo injection (Placebo, solid bar), estrogen cream/placebo injection (E, gray bar, n = 9), placebo cream/MSC injection (MSC, lined bar), or estrogen cream + MSC (E + MSC, dotted bar). Data represent mean ± SEM of 7-9 animals in each group. ∗P < 0.05 compared with uninjured controls, analysis of variance with Dunn posthoc testing. ERα, estrogen receptor alpha; CTL, control; Veh, vehicle.
These results, together with the lack of any effect of MSCs on biomechanical properties, collagen content, or histomorphology led us to focus singularly on the effects of estrogen, not MSCs, on genes involved in collagen and elastin synthesis (Fig. 5). Injury induced 2.8-fold increases in tropoelastin mRNA at 1 week after injury. Vaginal injury resulted in increased expression of both Col1a1 (sixfold) and Col3a1 (4.5-fold) after 1 week with a return to baseline by 3 weeks (Fig. 5B, C). Interestingly, estrogen mitigated early injury-induced increases in both collagen types 1 and 3 mRNA and late injury-induced up-regulation of tropoelastin. These results are consistent with estrogen-induced decreases in newly synthesized collagen after injury (Fig. 2). Injury also induced fivefold increases in mRNA encoding the major collagen cross-linking enzyme (LOX) at 1 week returning to twofold increases at 3 weeks. Postoperative estrogen blunted late responses in LOX gene expression significantly (Fig. 5D). Taken together, results indicate that postoperative treatment with estrogen has an inhibitory effect on collagen synthesis and tropoelastin mRNA.
FIG. 5.
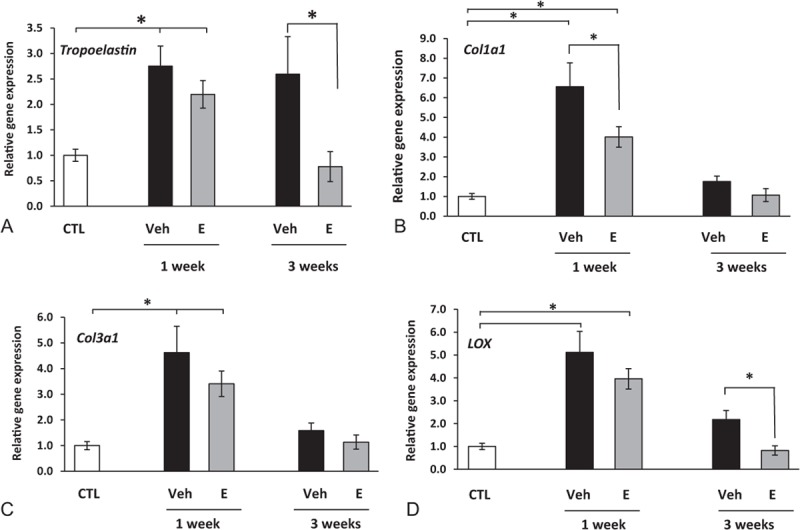
Effect of vaginal estrogen on gene expression of tropoelastin (A), Col1a1 (B), Col3a1 (C), or lysyl oxidase (LOX, D) in vaginal stroma before or after injury. Relative gene expression was determined by qPCR. Data represent mean ± SEM of 7-9 animals in each group. ∗P < 0.05 compared with uninjured controls, analysis of variance. qPCR, quantitative polymerase chain reaction; Col1a1, collagen type 1 a1; Col3a1, collagen type 3 a1; CTL, control; SEM, standard error of the mean; Veh, vehicle.
Transforming growth factor β1 (TGFβ1) is known to increase collagen synthesis in MSCs and regulate expression of LOX.22 To determine whether postoperative local estrogen treatment altered expression of TGFβ1, mRNA levels of TGFβ1 and other growth factors were quantified in the stromal compartment of the vaginal wall before or after injury (Table 1). Although not altered 1 week after injury, estrogen decreased TGFβ1 gene expression at 3 weeks. Epidermal growth factor (EGF), transforming growth factor α (TGFα), and insulin-like growth factor 1 (IGF1) were induced with injury after 1 and 3 weeks. Estrogen had no impact on EGF, amplified injury-induced increases in TGFα, and suppressed injury-induced increases in IGF1, especially at 3 weeks. FGF2 and FGF7 were also suppressed by vaginal estrogen. VEGFa, on the other hand, was not affected by injury or estrogen. The pro-inflammatory gene IL12b (expressed by activated macrophages to sustain Th1 cell development) was induced twofold with injury. Estrogen decreased injury-induced expression of this gene significantly, especially at 3 weeks. The anti-inflammatory gene IL10 was induced more than fourfold with injury and estrogen demonstrated a more profound suppression of IL10 compared with IL12b. This estrogen-induced loss of anti-inflammatory genes may be mechanistically involved in the magnified injury response of the stroma to injury (Fig. 3). Overall, results indicate that the vaginal stroma responds to surgical injury by immune cell infiltration, induction of genes involved in collagen and elastin synthesis, increased expression of numerous growth factors, and immunomodulators such as IL-12b and IL-10. Postoperative estrogen impacts the injured stroma by decreasing IGF1 and TGFβ1, decreasing collagen content, and decreasing the anti-inflammatory gene IL-10. Although some effects of estrogen may be positive (eg, mitigation of injury-induced expression of IL-12b), overall, our data indicate that postoperative estrogen exhibits a net deleterious effect on early wound healing of the vaginal stroma.
TABLE 1.
Relative gene expression in vaginal stroma in ovariectomized, injured rats treated with vaginal estrogen or placebo
| Uninjured | 1 week | 3 week | |||||
| Gene | Controls | Placebo | Estrogen | P | Placebo | Estrogen | P |
| TGFβ1 | 1.0 ± 0.13 | 1.42 ± 0.08a | 1.01 ± 0.06 | 0.001 | 1.15 ± 0.02b,c | 0.58 ± 0.02b,d,e | <0.001 |
| Growth Factors | |||||||
| EGF | 1.0 ± 0.09 | 3.92 ± 0.52a | 3.71 ± 0.47a | <0.001 | 3.17 ± 0.32d | 3.27 ± 0.54b | <0.001 |
| TGFα | 1.0 ± 0.09 | 1.63 ± 0.16a | 2.93 ± 0.25f | <0.001 | 1.90 ± 0.22b,g | 3.09 ± 0.27b,d | <0.001 |
| IGF1 | 1.0 ± 0.07 | 3.53 ± 0.58a | 2.37 ± 0.24a,f | <0.001 | 3.20 ± 0.15b,d | 0.92 ± 0.12b,e | <0.001 |
| FGF2 | 1.0 ± 0.11 | 0.96 ± 0.12 | 0.50 ± 0.05a | <0.001 | 1.53 ± 0.12b,c | 0.43 ± 0.07b,d | <0.001 |
| FGF7 | 1.0 ± 0.11 | 0.50 ± 0.05a | 0.30 ± 0.03a,f | <0.001 | 0.82 ± 0.04c,g | 0.24 ± 0.03b,g | <0.001 |
| VEGFa | 1.0 ± 0.08 | 0.84 ± 0.09 | 0.86 ± 0.06 | 0.24 | 0.87 ± 0.07 | 0.76 ± 0.09 | 0.12 |
| Inflammatory modulators | |||||||
| IL12b | 1.0 ± 0.21 | 2.17 ± 0.33a | 1.45 ± 0.15a,f | 0.005 | 2.75 ± 0.57d,f | 0.61 ± 0.08d,e | <0.001 |
| IL10 | 1.0 ± 0.15 | 4.26 ± 0.69a | 1.74 ± 0.61f | <0.001 | 3.24 ± 0.94b | 0.36 ± 0.05d,b,e | <0.001 |
TGFβ1, transforming growth factor β1; EGF, epidermal growth factor; TGFα, transforming growth factor α; IGF1, insulin-like growth factor 1; FGF2, fibroblast growth factor-2; FGF7, fibroblast growth factor-7; IL12b, interleukin 12b; IL10, interleukin 10.
One- and 3-week time points reference the time sacrificed after surgical injury to the posterior vaginal wall. Data represent mean ± SEM with 7-9 animals per treatment group.
P values represent analysis of variance performed to compare uninjured controls, placebo after injury, and estrogen after injury at either 1- or 3-week time points. Superscript letters “a-b” indicate significant differences between groups by analysis of variance testing.
cDenotes significance noted with P < 0.05 between placebo at 1 week and 3 weeks.
dIndicate significant differences between groups by analysis of variance testing.
eDenotes significance (P < 0.05) between estrogen at 1 week and 3 weeks.
Superscript letters “f-g” indicate significant differences between groups by analysis of variance testing.
Effect of estrogen on expression of immunomodulator and growth factor genes in vaginal epithelium
In contrast with vaginal stroma, estrogen appeared to exhibit positive effects on healing of the vaginal epithelial layer (Fig. 3). To investigate potential molecular events that may underpin these effects, relative gene expression of epithelial growth factors, the basement membrane protein laminin, tight junction proteins, and immunomodulators were quantified before and after vaginal injury ± postoperative estrogen (Table 2). RNA quality was poor and quantity was insufficient in epithelium scraped from the injured vagina at 1 week. Thus, gene expression studies were limited to epithelium 3 weeks after injury. Whereas the epithelial growth factor TGFα was down-regulated 3 week after injury, local estrogen prevented down-regulation of TGFα. Gene expression of EGF and TGFβ were unchanged, and the keratinocyte growth factors FGF-2 and -7 were not expressed in vagina epithelial cells. Epithelial IGF-1 gene expression, on the other hand, was decreased by estrogen. Estrogen increased expression of the basement membrane protein laminin B3. In the early stages of wound healing, skin epithelial cells become activated and release inflammatory molecules such as IL-1 and IL-8, which are linked to innate immune responses and neutrophil recruitment. Interestingly, although estrogen did not affect expression of CXCL1 or IL-1β, molecules linked to adaptive immune responses, such as, CCL28, were suppressed by vaginal estrogen after injury. In contrast, mRNA levels of occludin, a protein important in tight junction stability, barrier function, and regulation of mucosal inflammation, was decreased after injury, and vaginal estrogen prevented injury-induced loss of this transcript suggesting increased barrier function after injury, and, together with decreased CCL28, less inflammation. Claudin-1 gene expression was not changed. Secretory leukocyte protease inhibitor (SLPI) was also increased threefold in epithelium from estrogen-treated animals. Like vaginal stroma, ERα mRNA was down-regulated in the epithelium after injury and down-regulated further with postoperative estrogen.
TABLE 2.
Relative gene expression in vaginal epithelium of ovariectomized injured rats treated with vaginal estrogen or placebo
| Uninjured | Injured | Injured | ||
| Gene | Control | Placebo | Estrogen | Pa |
| Growth factors | ||||
| TGFα | 1.00 ± 0.03b | 0.40 ± 0.07b,c | 1.07 ± 0.12c | <0.001 |
| EGF | 1.00 ± 0.02 | 1.20 ± 0.20 | 1.68 ± 0.43 | 0.433 |
| IGF-1 | 1.00 ± 0.13b | 0.97 ± 0.18c | 0.41 ± 0.10b,c | 0.004 |
| TGFβ1 | 1.00 ± 0.01b,c | 0.31 ± 0.03b | 0.23 ± 0.06c | <0.001 |
| VEGFa | 1.00 ± 0.11b | 0.49 ± 0.03b,c | 2.5 ± 0.61c | 0.001 |
| Basement membrane | ||||
| Laminin β3 | 1.00 ± 0.10b | 0.89 ± 0.06c | 2.32 ± 0.14b,c | 0.002 |
| Inflammatory modulators | ||||
| CXCL1 | 1.00 ± 0.12 | 0.87 ± 0.26 | 4.97 ± 1.75 | 0.183 |
| IL1β | 1.00 ± 0.32 | 0.33 ± 0.01 | 0.85 ± 0.25 | 0.303 |
| E-Cadherin | 1.00 ± 0.03b,c | 0.30 ± 0.08b | 0.40 ± 0.11c | <0.001 |
| Adaptive immune responses | ||||
| CCL20 | 1.00 ± 0.17 | 1.07 ± 0.30 | 0.35 ± 0.06 | 0.188 |
| CCL28 | 1.00 ± 0.06b,c | 0.53 ± 0.05c,d | 0.26 ± 0.07b,d | <0.001 |
| Tight junctions, barrier function | ||||
| OCLN | 1.00 ± 0.05b | 0.60 ± 0.04b,c | 1.21 ± 0.15c | 0.002 |
| CLDN1 | 1.00 ± 0.10 | 0.70 ± 0.14 | 0.83 ± 0.06 | 0.161 |
| Protease inhibitor | ||||
| SLPI | 1.00 ± 0.15b | 1.84 ± 0.28 | 8.64 ± 2.51b | 0.001 |
| Hormone receptor | ||||
| ERa | 1.00 ± 0.04b,c | 0.44 ± 0.06b | 0.29 ± 0.05c | <0.001 |
TGFβ1, transforming growth factor β1; EGF, epidermal growth factor; TGFα, transforming growth factor α; IGF-1, insulin-like growth factor 1; SLPI, secretory leukocyte protease inhibitor; OCLN, occludin, CCL20; chemokine, CC motif, ligand 20 (macrophage inflammatory protein -3alpha); CCL28, chemokine, CC motif, ligand 28; IL1b, interleukin 1 beta; CXCL1, chemokine, CXC motif, ligand 1 (GRO1); IL10, interleukin 10; IL12b, interleukin 12b; VEGFa, vascular endothelial growth factor alpha; FGF7, fibroblast growth factor-7; FGF2, fibroblast growth factor-2.
Data represent mean ± SEM of 3 data points, each representing pools of 3 different rats except uninjured controls in which n = 4 representing pools of 3 different rats per pool. All data represent 3 weeks.
aP value represents analysis of variance with multiple comparisons. For those found to be statistically significant (P < 0.05), multiple comparisons were made with Dunn or Holm-Sidak method as appropriate. Matching superscripts “b-d” denote significance between groups.
MSCs did not alter wound healing of the vagina: mechanisms
The effects of MSCs on histology, collagen content, and biomechanics of the injured vagina were virtually identical to placebo. Furthermore, effects of MSCs + estrogen did not differ from those of estrogen alone. Thus, we used immunostaining with anti-GFP antibodies to determine the fate of these cells in the vaginal wall. Injured ovariectomized rats were sacrificed 3 hours after direct injection of MSCs (Fig. 6A). Interesting, although cells were injected in the subepithelial space of the injury site, GFP + MSCs aggregated in the epithelium with some MSCs also in the stromal compartment. One week after injury (ie, 3-4 days after MSC injection) scant MSCs were distributed in the stromal, but none visualized in the epithelium (Fig. 6B, arrows). By 3 weeks, GFP-tagged MSCs were absent.
FIG. 6.
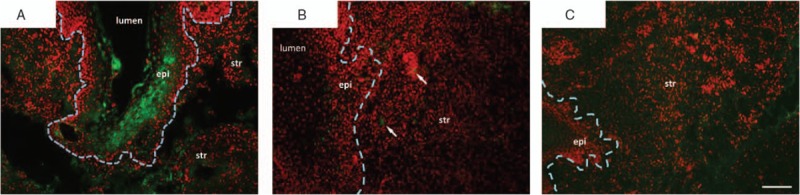
Immunostaining of green fluorescent protein (GFP)-labeled MSCs in vaginal wall of ovariectomized rats after injury. Ovariectomized rats underwent surgical injury of the posterior vaginal wall. Four days after injury, GFP-labeled mesenchymal stem cells (MSCs) were injected into the site of injury. Tissues were collected 3 hours (A), 3 days (1 week after injury) (B), or 17 days (3 weeks after injury) after injection (C). Sections were stained for GFP (green) and counterstained with Hoechst nuclear stain (red) and represent experiments conducted in duplicate. The border between epithelium and stroma is indicated by a dashed line. Arrows note MSCs in the 1-week group. epi, epithelium; str, stroma. Arrows denote basement membrane at site of injury. Bar = 100 μm.
DISCUSSION
The response to tissue injury requires synchronous interactions of immune cells, epithelial cells, stromal fibroblasts, and endothelial cells which unite to seal the epithelial defect, deposit new matrix, and generate a new blood supply. During healing, a provisional fibrin-fibronectin matrix acts as a scaffold for cell adhesion and migration. Extravasation of blood from damaged vessels also initiates an inflammatory response characterized by release of a host of chemotactic mediators, cytokines, and growth factors leading to recruitment of neutrophils and monocytes to the site of injury. Macrophages and neutrophils produce cytokines thereby amplifying the immune response by stimulating or activating other immune cells and also directly modulating repair mechanisms.23,24 EGF and IGF1 stimulate proliferation of epithelial cells to accomplish re-epithelialization.
The mechanistic role of estrogen in wound healing of estrogen-responsive tissues is not well-understood. No doubt, increased estradiol during the early follicular phase of the menstrual cycle facilitates healing of the endometrium and cessation of menstruation.25 There are also reports that macrophages may express estrogen receptors and improve healing of skin wounds in mice.26 Previously, we demonstrated that preoperative estrogen improved wound healing of ovariectomized guinea pigs.4 Furthermore, 6 weeks of preoperative vaginal estrogen resulted in increased collagen synthesis and content in the vaginal wall of menopausal women undergoing pelvic reconstructive surgery.6 Many pelvic surgeons do not prescribe preoperative estrogen due to the lack of a confirmed positive effect of vaginal estrogen on healing of the vaginal wall in women and delay of surgery to allow estrogen-induced collagen synthesis and epithelial growth. Here, our model was to test the idea that vaginal estrogen in the immediate postoperative time period may facilitate healing of the injured vaginal wall. The results suggest that immediate use of postoperative estrogen has unfavorable effects on the vaginal stroma but positive effects on the vaginal epithelium.
Postoperative estrogen adversely affects the stromal compartment
In this study, we found that acute postoperative vaginal estrogen after injury results in increased area of immune cell aggregate in response to injury accompanied by decreased expression of stromal IGF1 and TGFβ1, decreased collagen content, and decreased expression of the anti-inflammatory gene IL-10. The mechanisms underlying this adverse response are unclear. It is well-appreciated that estrogens have pleiotropic effects that are tissue- and gene-specific activating or repressing gene expression in response to environmental cues. Basic studies have demonstrated that estrogen treatment prevents apoptosis and necrosis of cardiac and endothelial cells and attenuates pathologic cardiac hypertrophy. Clinical investigations, however, have not shown these clear-cut benefits. In the case of endometriosis, estrogen stimulates growth, immune cell reactions, and tissue damage.27 Estrogens directly regulate the function of human uterine natural killer cells by increasing natural killer cell migration and secretion of chemokine (C-C motif) ligand 2 (CCL2).28 We suggest that estrogen induces stromal growth and matrix deposition in the uninjured vaginal wall, but, with injury, the immune cell aggregate decreases the number of ER + cells per area of injury and estrogen further down-regulates ER gene expression. Moreover, cytokine signaling in the wound likely redistributes nuclear factor kappa-light-chain-enhancer of activated B cells binding sites across the genome, which creates latent estrogen receptor alpha (ERα) binding sites that may underlie altered patterns of gene expression after injury.29 It should be emphasized that various estrogen preparations and route of delivery may result in different effects. Here, we used conjugated equine estrogens because it is the most commonly used vaginal preparation in women. Creams or rings containing 17β-estradiol may produce different findings.
Mesenchymal stem cells
Recently, investigations have focused on potential mechanisms whereby stem cells may alter the wound healing process to improve functional outcomes. Good results have been obtained in animal models using IV MSCs assuming that the cells homed to the site of injury. In most cases, however, functional improvement occurs despite minimal engraftment at the site of injury suggesting that MSCs may have paracrine effects by secreting factors to enhance regeneration without attachment.30-33 Previously, we demonstrated that healing of the injured external anal sphincter was enhanced by local injection of MSCs, but not IV administration.17 In agreement with the current study, stem cells were scarce in the injured sphincter at 48 hours and absent by 7 days. We postulated that the striking immune reaction of the anal sphincter to injury may have impaired stem cell survival. Thus, in the current study, we chose to inject MSCs 4 days after injury to allow the acute reaction to subside before treatment. However, MSCs did not survive with or without estrogen, and, in contrast to the anal sphincter, we found no improvement in collagen content, biomechanical properties, or wound healing. It is interesting that acute injection of MSCs resulted in homing to the injured epithelium within 3 hours. Epithelial accumulation of the cells may deter the paracrine or local effects on the stromal compartment and lead to early sloughing or loss of MSCs.
The results illustrate the complexities of designing stem cell therapies for injury. The timing of injection may have still been too early for improvement of wound healing. In studies by Sun et al,34 MSCs were injected 3 weeks after partial transection of the anal sphincter to improve functional measures.34 In an effort to attract and protect regenerative stem cells, other investigators have shown improvement using scaffolds, nanoparticles, hydrogels, or addition of bioactive molecules.35-37 Studies aimed to increase the survivability and effects of MSCs on regeneration of the vaginal wall after injury are needed.
LIMITATIONS
Although findings of this study indicate that postoperative use of estrogen impairs wound-healing events in the early phases after injury, the results should be interpreted with caution. Injury-induced matrix remodeling is a balance between synthesis and degradation. Accentuation of matrix degradation and immune cell infiltrate in the early phase may result in long-term regeneration of new matrix components that ultimately yield strength and better repair. In many studies, 3 to 4 weeks time points represent long-term healing in the rat.38,39 Our results indicate that newly synthesized collagen was not cross-linked and vaginal wound healing was clearly incomplete at 3 weeks. Thus, future studies will concentrate on long-term time points. Although this ovariectomized rat model system does not include the variability in estrogen responses among menopausal women as a function of age, estrogen metabolism, or body weight, the model provides insight into the potential effects of postoperative estrogen in the acute postoperative period.
CONCLUSIONS
Acute administration of vaginal estrogen after injury results in divergent effects on stromal and epithelial compartments. In the stroma, estrogen results in decreased collagen content and decreased expression of transcripts for matrix components. Conversely, estrogen resulted in increased epithelial thickness over the wound, increased expression of genes related to epithelial barrier function and increased expression of serine PIs that may protect the matrix from proteolytic degradation during wound healing. Collectively, these results suggest that, in contrast to the positive effects of estrogen on the uninjured vagina preoperatively, acute administration of vaginal estrogen may have adverse effects on the early phase of healing of the fibromuscular layer despite its positive effects on the epithelium.
Potential clinical value
This work in rats indicates that although preoperative estrogen before injury has a positive effect on vaginal wound healing, use of acute postoperative estrogen cream after reconstructive surgery may have deleterious effects.
Footnotes
Funding/support: This study was supported by grants numbers National Institutes of Health AG028048 and National Institutes of Health AG047290.
Financial disclosure/conflicts of interest: None reported.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.menopause.org).
REFERENCES
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997; 89:501–506. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JOL. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol 2005; 192:1488–1495. [DOI] [PubMed] [Google Scholar]
- 3.Abramov Y, Webb AR, Botros SM, Goldberg RP, Ameer GA, Sand PK. Effect of bilateral oophorectomy on wound healing of the rabbit vagina. Fertil Steril 2011; 95:1467–1470. [DOI] [PubMed] [Google Scholar]
- 4.Balgobin S, Montoya TI, Shi H, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod 2013; 89:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montoya TI, Maldonado PA, Acevedo JF, Word RA. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol Reprod 2015; 92:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahn DD, Good MM, Roshanravan SM, et al. Effects of preoperative local estrogen in postmenopausal women with prolapse: a randomized trial. J Clin Endocrinol Metab 2014; 99:3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int 2000; 86:32–38. [DOI] [PubMed] [Google Scholar]
- 8.Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower uninary tract. Am J Obstet Gynecol 1981; 141:817–820. [DOI] [PubMed] [Google Scholar]
- 9.Smith P. Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol-releasing vaginal ring. Acta Obstet Gynecol Scand Suppl 1993; 157:1–26. [PubMed] [Google Scholar]
- 10.Krause M, Wheeler TL, 2nd, Snyder TE, Richter HE. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg 2009; 15:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz M, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser M. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int 2012; 2012:612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich D, Edwards SL, Su K, et al. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A 2014; 20:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran C, Damaser MS. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol 2015; 7:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dissaranan C, Cruz MA, Kiedrowski MJ, et al. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant 2014; 23:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin M, Chen Y, Zhou Y, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther 2016; 7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corcos J, Loutochin O, Campeau L, et al. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn 2011; 30:447–455. [DOI] [PubMed] [Google Scholar]
- 17.Pathi SD, Acevedo JF, Keller PW, et al. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol 2012; 119:134–144. [DOI] [PubMed] [Google Scholar]
- 18.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol 2008; 198:590.e1–590.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol 1982; 82 pt A:33–64. [DOI] [PubMed] [Google Scholar]
- 20.Cianga P, Medesan C, Richardson JA, Ghetie V, Ward ES. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol 1999; 29:2515–2523. [DOI] [PubMed] [Google Scholar]
- 21.Borvak J, Richardson J, Medesan C, et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 1998; 10:1289–1298. [DOI] [PubMed] [Google Scholar]
- 22.Knippenberg M, Helder MN, Doulabi BZ, Bank RA, Wuisman PI, Klein-Nulend J. Differential effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of collagen-modifying enzymes in human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A 2009; 15:2213–2225. [DOI] [PubMed] [Google Scholar]
- 23.Hackam DJ, Ford HR. Cellular, biochemical, and clinical aspects of wound healing. Surg Infect (Larchmt) 2002; 3 suppl 1:S23–S35. [DOI] [PubMed] [Google Scholar]
- 24.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001; 69:513–521. [PubMed] [Google Scholar]
- 25.Salamonsen LA, Kovacs GT, Findlay JK. Current concepts of the mechanisms of menstruation. Baillieres Best Pract Res Clin Obstet Gynaecol 1999; 13:161–179. [DOI] [PubMed] [Google Scholar]
- 26.Ashcroft GS, Mills SJ, Lei K, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest 2003; 111:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013; 34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson DA, Greaves E, Critchley HO, Saunders PT. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum Reprod 2015; 30:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco HL, Nagari A, Kraus WL. TNFalpha signaling exposes latent estrogen receptor binding sites to alter the breast cancer cell transcriptome. Mol Cell 2015; 58:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 2007; 104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 2007; 292:F1626–F1635. [DOI] [PubMed] [Google Scholar]
- 33.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 2007; 354:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Yeh J, Xie Z, Kuang M, Damaser MS, Zutshi M. Electrical stimulation followed by mesenchymal stem cells improves anal sphincter anatomy and function in a rat model at a time remote from injury. Dis Colon Rectum 2016; 59:434–442. [DOI] [PubMed] [Google Scholar]
- 35.Li GY, Zhou F, Gong YQ, et al. Activation of VEGF and ERK1/2 and improvement of urethral function by adipose-derived stem cells in a rat stress urinary incontinence model. Urology 2012; 80:953.e1–953.e8. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Wang X, Sun X, Deng C, Atala A, Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013; 34:8617–8629. [DOI] [PubMed] [Google Scholar]
- 37.Zhao W, Zhang C, Jin C, et al. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol 2011; 59:155–163. [DOI] [PubMed] [Google Scholar]
- 38.Calvin M, Dyson M, Rymer J, Young SR. The effects of ovarian hormone deficiency on wound contraction in a rat model. Br J Obstet Gynaecol 1998; 105:223–227. [DOI] [PubMed] [Google Scholar]
- 39.Demirbilek S, Bernay F, Rizalar R, Baris S, Gurses N. Effects of estradiol and progesterone on the synthesis of collagen in corrosive esophageal burns in rats. J Pediatr Surg 1994; 29:1425–1428. [DOI] [PubMed] [Google Scholar]


