Summary
Fat storage changes throughout life and affects body metabolism. Ageing impact on brown (BAT) and white adipose tissue (WAT) deserves attention, especially in females, because they are less prone to age‐induced weight gain. While in male mice the impact of ageing on adipose tissue remodelling is well characterized, the effects in female mice remain largely unclear. Thus, we investigated BAT and WAT remodelling during ageing in female C57BL/6 mice. At 3 months, body weight was 24 ± 0.3 g (mean±SD), and it increased from 6 to 9 months of age (+20%, P < 0.0001). Oral glucose tolerance test showed no disturbance of glucose metabolism. All WAT depots became heavier, and white adipocytes hypertrophied. The subcutaneous and visceral WAT had clusters of beige cells in younger mice, but they were progressively lost by ageing, indicating loss of WAT browning. Older mice had hypertrophied classic brown adipocytes that had larger cytoplasmic lipid droplets than younger mice. Pearson's correlation showed that WAT mass has a weak correlate with BAT mass, although white adipocyte diameter has a strong correlation with classic brown adipocyte size. In conclusion, our results indicate that female C57BL/6 mice have a progressive age‐dependent loss of subcutaneous and visceral WAT browning, and this process runs in parallel with BAT morphological changes towards a fat storer phenotype, independent of cycling or disturbances in glucose metabolism.
Keywords: ageing, female, metabolism, mice, obesity
The white adipose tissue (WAT) is not only important for fat storage but also for generating signals to inform the hypothalamus about body energy status (e.g. leptin). On the other hand, the brown adipose tissue (BAT) is important for fat burning, because it is the main mediator of adaptive thermogenesis (Rothwell 1989). Compared with WAT, BAT is more vascularized and has a dense adrenergic innervation, a great number of mitochondria and small cytoplasmic lipid droplets. Until recently, it was thought that BAT disappeared after birth in humans and played no role in adult physiology. However, this tissue was consistently found in adult humans extending from the anterior neck to the thorax, at perirenal, adrenal and paravertebral regions, as well as around great vessels such as the aorta and its main branches (Nedergaard et al. 2007). Beyond white and classic brown adipocytes, there is a third population referred to as brite (brown‐in‐white adipocytes) or beige adipocytes (Bartelt & Heeren 2013). These cells have brown‐like features, including the presence of multiple cytoplasmic lipid droplets and the expression of uncoupling protein 1 (UCP1). They are encountered in clusters within the WAT (Frontini & Cinti 2010) and their presence is associated with protection against diet‐induced obesity and metabolic diseases (Seale et al. 2011).
White adipose tissue mass increases with age and declines in the elderly. Fat is reallocated from subcutaneous into visceral depots, especially at the middle age, leading to increased waist circumference, which might increase 4.0 cm every 9 years in women (Visser et al. 2003; Hughes et al. 2004). These changes are related to an increased risk for type 2 diabetes, arterial hypertension, cancer, cognitive dysfunction and atherosclerosis, contributing to myocardial infarction and stroke (Guo et al. 1999; Lutz et al. 2008). In the elderly, there is a hypothesis that neck and upper chest BAT are replaced by WAT (Cypess et al. 2009), along with reduced BAT activity, a phenomenon that is related with decreased amount of body BAT depots (Au‐Yong et al. 2009). It is hypothesized that younger adults would have a phenotype characterized by normal weight and small white adipocyte, but after certain age, humans would present increasing obesity, large white adipocytes and absence of brown adipocytes (Zingaretti et al. 2009). However, the biological mechanisms regulating the maintenance and a possible decline of BAT mass during ageing have not been fully elucidated so far.
Brown adipose tissue development and biology have gained great attention in recent years due to its promising role against obesity and related diseases (Ohno et al. 2012). Its (re)discovery has stimulated the scientific interest on BAT regulation of body mass and metabolism, especially in obesity. As world life expectancy is increasing rapidly (WHO, 2015), it is important to study the impact of ageing on BAT biology. In face of this, our study was designed to provide the morphological characterization of WAT and BAT remodelling during ageing in female C57BL/6 mice.
Materials and methods
Ethical approval
The handling and experimental protocols were approved by the local ethics committee to Care and Use of Laboratory Animals (CEUA#446/14). The study was performed in accordance with the Animal Research Reporting in Vivo Experiments ARRIVE guidelines and the Guideline for the Care and Use of Laboratory Animals (US NIH Publication No 85‐23. Revised 1996; Kilkenny et al. 2010).
Experimental design
Female C57BL/6 mice with 2 months of age were obtained from colonies maintained at the Federal Fluminense University and kept under standard conditions (12‐h light/dark cycles, 21 ± 2°C, humidity 60 ± 10% and air exhaustion cycle 15 min/h). Mice were randomly allocated into four groups according to age, and they were euthanized at three (n = 10), six (n = 10), nine (n = 11), or twelve (n = 11) months of age. Food and water were offered ad libitum, and body mass and food intake were measured at the week previous to euthanasia.
Glucose metabolism
Three days before euthanasia, food was removed at 8 a.m. and oral glucose tolerance test (OGTT) was performed after 6‐hour fast. Glucose 1.0 g/kg in sterile saline (0.9% NaCl) was administered by orogastric gavage. Blood was obtained by milking the tail after a little incision on its tip. Plasma glucose concentration was measured prior to glucose gavage and at 15, 30, 45, 60, 90 and 120 min after glucose gavage using a glucometer (One Touch Ultra, Johnson& Johnson, SP, Brazil). The area under the curve (AUC) was calculated using the trapezoid rule to assess glucose intolerance. On the day of euthanasia, 6‐hour fasted mice were deeply anesthetized with ketamine 100.0 mg/kg (Francotar®, Virbac, Brazil) and xylazine 10.0 mg/kg i.p. (Virbaxyl 2%®, Virbac, Brazil). An incision was made in the chest, and the heart was exposed for blood collection (right atrium). Blood was allowed to clot, and it was centrifuged (1500 g), and the serum was stored at −80°C for insulin assay, according to manufacturer's instructions (cat#EZRMI‐13K, Merck Millipore, Billerica, MA, EUA).
Liver triacylglycerol quantification
Approximately 50 mg of liver was weighed and homogenate in 1.0 ml of 100% isopropanol. The lysate was centrifuged at 2000 g for 15 min at 4°C. The supernatant was transferred into a new tube, and 2.0 μl was used for triacylglycerol assay, according to manufacturer's instructions (cat#K117, Bioclin, Belo Horizonte, MG, Brazil).
Fat harvesting
We focused on brown and white adipose tissue remodelling during ageing. Interscapular brown fat and white visceral (perigonadal and retroperitoneal) and subcutaneous (inguinal) fat pads were carefully dissected from both sides of the animal, weighted and then immersed in 4% phosphate‐buffered formalin pH 7.2 for 48 h. Samples of both contralateral fat pads were submitted to routine histological processing, embedded in paraplast, sectioned 3 μm thick and stained with haematoxylin and eosin. Brown‐to‐white fat ratio was calculated as [brown fat (mg)]/[perigonadal (mg) + retroperitoneal (mg) + inguinal fat (mg)]. Additionally, the uterus and ovary were harvested and weighed to verify whether mice were cycling.
Adipocyte morphometry
Digital images were obtained from histological sections using a Leica DMRBE microscope (Wetzlar, Germany) coupled to a video camera Kappa (Gleichen, Germany). Morphometry was performed in the Image‐Pro® Plus software v. 5.0 (Media Cybernetics, Silver Spring, MD, USA). In the interscapular BAT, eight non‐consecutive images were acquired to assess brown adipocyte diameter, lipid droplet diameter, and the percentage of tissue area occupied by lipid droplets. For lipid area, a selection tool was used to mark the pixels that represented lipid droplets. The selection was segmented in a new digital image in black and white, where the white colour represented the lipid droplets and the black colour represented the remaining tissue. Then, the area occupied by the white colour was quantified through the image histogram tool. In the three white fat depots studied, we assessed adipocyte diameter by measuring their smallest and largest diameters, as previously described (Fernandes‐Santos et al. 2009). For each analysis, we used six animals per group, four non‐consecutive images per animal, and randomly measured 10 adipocytes per image, totalizing 40 adipocytes per mice.
Statistics
Data are expressed as mean ± SD and tests to assess normality and homoscedasticity of variances were run. Comparison among groups was made by one‐way anova followed by a post hoc test of Tukey. Pearson's correlation and linear regression were performed for fat pad weight and morphometry data. Slopes provided by linear regression were compared. A P‐value of 0.05 was considered statistically significant (GraphPad® Prism software v. 6.0, La Jolla, CA, USA).
Ethical approval statement
Procedures have been approved by the Ethics Committee to Care and Use of Laboratory Animals of Universidade Federal Fluminense (CEUA #446/14).
Results
Ageing leads to body mass gain, and it is not related to food intake or menopause in female C57BL/6 mice
Body mass remained stable from 3 to 6 months of age. Nine‐and 12‐month‐old female C57BL/6 mice had an increase in body mass of 19.5% and 15.3%, respectively, compared to 3‐month‐old mice (P < 0.0001). Food intake remained unchanged among groups; thus, body weight gain was not due to increased food intake (Table 1). To assess whether mice were or not on menopause, we weighed the uterus and ovary, and showed that there was no difference between the groups (Table 1). Additionally, we assessed the relative ratio of nucleated epithelial cells, cornified squamous epithelial cells and leucocytes present in vaginal smears daily the week before euthanasia to identify murine estrous stages (data not shown) (McLean et al. 2012). This procedure confirmed that mice were still cycling; thus, body mass gain was not due to menopause.
Table 1.
Biometry and glucose metabolism
| Parameter | 3 months | 6 months | 9 months | 12 months | P |
|---|---|---|---|---|---|
| Body mass (g) | 24.1 ± 0.27 | 24.7 ± 0.48 | 28.8 ± 0.68*,† | 27.8 ± 1.03*,† | <0.0001 |
| Uterus (mg) | 0.101 ± 0.014 | 0.081 ± 0.008 | 0.090 ± 0.018 | 0.087 ± 0.015 | N.S. |
| Ovary (mg) | 0.019 ± 0.002 | 0.021 ± 0.001 | 0.019 ± 0.003 | 0.024 ± 0.003 | N.S. |
| Food intake (g) | 3.62 ± 0.09 | 3.87 ± 0.29 | 3.69 ± 0.14 | 3.49 ± 0.06 | N.S. |
| Glucose (mg/dl) | 117.8 ± 3.96 | 114.9 ± 6.13 | 117.0 ± 4.01 | 104.1 ± 10.17 | N.S. |
| OGTT (AUC) | 20,075 ± 684.9 | 17,714 ± 975.5 | 21,204 ± 837.7 | 19,016 ± 1,253 | N.S. |
| Insulin (ng/ml) | 0.41 ± 0.04 | 0.46 ± 0.05 | 0.82 ± 0.24 | 0.73 ± 0.15 | N.S. |
| Liver (g) | 1.06 ± 0.03 | 1.07 ± 0.03 | 1.11 ± 0.04 | 1.04 ± 0.03 | N.S. |
| Hepatic triglycerides (mg/dl/mg) | 2.21 ± 0.24 | 3.40 ± 0.24 | 4.30 ± 0.64* | 4.31 ± 0.66* | 0.0055 |
Date are shown as mean ± SEM. OGTT, oral glucose tolerance test; AUC, area under curve; P, one‐way significance anova; N.S., non‐significant.
Letters indicate statistical difference [*] vs. 3‐month and [†] vs. 6‐month groups (post hoc test of Tukey).
Ageing did not change glucose metabolism, but increased hepatic fat in female C57BL/6 mice
Blood glucose and glucose intolerance, the later assessed by the oral glucose tolerance test, remained unchanged during ageing (Table 1). Serum insulin showed an apparent trend towards increase in 9‐and 12‐month‐old mice, but this was not significant. Liver weight did not differ among groups, but it had an increase in fat storage as triacylglycerol in mice by 9‐and 12‐month‐old (+95% compared to 3‐month‐old mice, P = 0.0055, Table 1).
Ageing‐induced body mass gain is associated with increased WAT mass, but not BAT mass
We studied three white fat depots, two of them visceral depots (perigonadal and retroperitoneal) and one subcutaneous fat depot (inguinal). All depots behaved similarly regardless of their location. Six‐month‐old mice already presented increased perigonadal and inguinal fat mass (+127% and 72% compared to 3‐month‐old mice, respectively, Figure 1a,c). We also found an increased fat mass in 9‐and 12‐month‐old mice compared to both 3‐and 6‐month groups. Perigonadal, retroperitoneal and inguinal fat pads were 789%, 158% and 793% greater in 12‐month‐old mice, respectively, compared to 3‐month‐old mice (Figure 1a–c).
Figure 1.
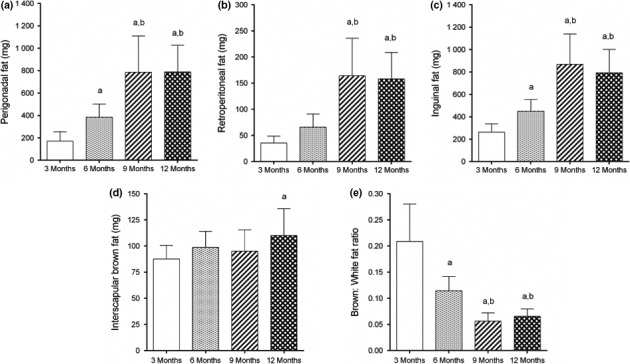
White visceral (a,b) and subcutaneous (c) fat depots increase in size from 3 to 12 months of age in female C57Bl/6 mice. (d) Interscapular brown fat mass remains unchanged, with a significant increase at 12 months. (e) Ageing decreases brown‐to‐white fat ratio. N = 10–11 mice/group, mean ± SD, one‐way analysis of variance, post hoc test of Tukey, P < 0.05, [a] vs. 3 months, [b] vs. 6 months.
We also studied the interscapular brown adipose tissue. BAT mass remained unchanged during ageing, increasing only at 12 months by 26% (P < 0.01, Figure 1d). Interestingly, despite no significant change in BAT mass at 6 months, the brown‐to‐white fat ratio diminished by 45% in this group compared to 3‐month‐old mice (P < 0.0001, Figure 1e). The smallest ratio was found in 9‐and 12‐month‐old mice (‐73% and ‐69%, respectively, compared to 3‐month‐old mice, P < 0.0001, Figure 1e).
Body mass had a strong correlation with perigonadal, retroperitoneal and inguinal fat mass, and the slope of linear regression was 100.2 ± 9.58, 22.4 ± 1.81 and 86.7 ± 9.33 respectively (Figure 2a). However, the interscapular BAT mass showed no correlation with body mass (r = 0.41, P < 0.009, slope 2.43 ± 0.88, Figure 2a). Slopes were significantly different from one another (P < 0.0001). We further investigated whether there was an association between BAT mass and WAT mass. We found that perigonadal, retroperitoneal and inguinal fat mass have a weak correlation with interscapular fat mass (Figure 2b). Slopes provided by linear regression were significantly different (P = 0.0068, Figure 2b), as follows: 8.3 ± 1.98 (perigonadal fat), 1.8 ± 0.42 (retroperitoneal fat) and 7.5 ± 1.79 (inguinal fat).
Figure 2.
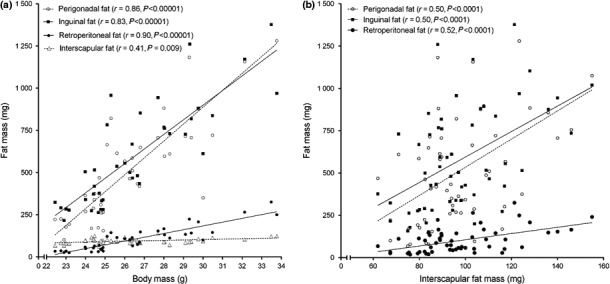
(a) Ageing‐induced body mass gain is strongly correlated with white visceral (perigonadal/retroperitoneal) and subcutaneous (inguinal) fat mass, but not interscapular BAT mass. (b) Interscapular BAT mass has a weak correlation with white fat mass. N = 10–11 mice/group, Pearson's correlation.
Female C57BL/6 mice exhibit loss of browning of WAT depots and adipocyte hypertrophy during ageing
Clusters of beige adipocytes were found within WAT depots of all 3‐month‐old female C57BL/6 mice studied (Figure 3a–d, perigonadal fat). These clusters were still found at 6 months, although less frequently. At 9 and 12 months, beige cells were barely seen or in most cases absent, showing that ageing leads to the loss of WAT browning. Morphological changes of perigonadal adipocyte size are illustrated in Figure 3e–h. Adipocytes from 3‐and 6‐month‐old mice did not differ in size, but all WAT depots studied showed adipocyte hypertrophy in mice aged 9 and 12 months (Figure 3i–k). Additionally, adipocytes from retroperitoneal and inguinal fat pads had a similar diameter in 9‐and 12‐month‐old mice (Figure 3j,k). On the other hand, adipocytes from perigonadal fat were larger in 12‐month‐old mice compared to 9 months (+54%, P < 0.001, Figure 3g–i). Photomicrographs of retroperitoneal and inguinal adipocytes are found in supplementary information (Figure S1).
Figure 3.

(a–h) Remodelling of white fat depots by ageing. (a–h) Photomicrographs of the perigonadal fat of female C57Bl/6 mice with 3 (a,e), 6 (b,f), 9 (c,g) and 12 (d,h) months of age (H&E stain). Clusters of beige cells (a,b arrows) are often seen within this depot in 3‐month‐old mice (a), some are still seen at 6 months (b), but barely found or absent at 9‐(c) and 12‐month‐old mice (d). It indicates ageing‐induced loss of browning of the perigonadal fat depot of female C57Bl/6 mice.(e) Closer view showing a beige adipocyte (circle) that displays morphological features of brown adipocytes, such as central nucleus and numerous cytoplasmic lipid droplets. Adipocyte hypertrophy induced by the ageing process is evidenced by images (e–h). (i–k), All white fat depots studied undergone adipocyte hypertrophy by ageing, especially after 9 months, regardless of their body location. N = 6 mice/group, mean ± SD, one‐way analysis of variance, post hoc test of Tukey, P < 0.05, [a] vs. 3 months, [b] vs. 6 months, [c] vs. 9 months.
Brown adipose tissue undergoes an adverse remodelling in aged female C57BL/6 mice
Morphological changes of BAT were already noticed in mice aged 6 months. Brown adipocytes were hypertrophied at 6 months (16.6 ± 0.93 μm vs. 14.5 ± 1.29 μm, P < 0.05) and even larger at 9 and 12 months (+44% and 40% compared to 3 months, P < 0.0001, Figure 4a–e). Cytoplasmic lipid droplets were also hypertrophied at 6 months and had no additional increment in size after this age (Figure 4f). Lipid droplets measured 3.30 ± 0.35 μm in 3‐month‐old mice and increased 52%, 60% and 62%, respectively, in 6‐, 9‐and 12‐month‐old mice (P < 0.0001, Figure 4a–d and f). As a consequence of lipid droplet remodelling, the area of BAT occupied by lipids increased (Figure 4g). Lipids occupied 22.9 ± 6.69% of BAT, and lipid area increased 98%, 121% and 140% in mice aged 6, 9 and 12 months respectively (P < 0.0001, Figure 4g). Unlike lipid droplet diameter, lipid area had a further increase by 21% from 9 to 12 months (P < 0.05, Figure 4g).
Figure 4.
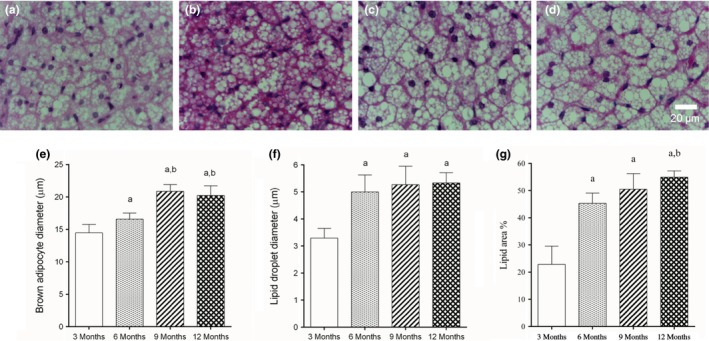
(a–d) Photomicrographs showing BAT morphological remodelling during ageing in female C57Bl/6 mice aged 3, 6, 9 and 12 months, respectively (H&E stain). (e) Brown adipocytes hypertrophied with increasing ages, similar to white adipocytes. (f) Cytoplasmic lipid droplets were increased in size in mice aged six months, but no further increase was observed. (g) Tissue area occupied by lipid droplets was quantified. There was an important increase in size of this droplets in mice aged 6 months, and a further increase was noticed at 12 months. N = 6 mice/group, mean ± SD, one‐way analysis of variance, post hoc test of Tukey, P < 0.05, [a] vs. 3 months, [b] vs. 6 months.
We further investigated whether a change in white adipocyte size would be related to a change in brown adipocyte size as well. Pearson's correlation showed a strong positive correlation between brown adipocyte diameter and white adipocyte diameter, as shown in Figure 5. The strongest correlation was found between BAT adipocytes and retroperitoneal white adipocytes (r = 0.84, P < 0.00001). Slopes provided by linear regression were 4.7 ± 1.01 (perigonadal fat), 6.7 ± 1.01 (retroperitoneal fat) and 2.7 ± 0.54 (inguinal fat), and they were statistically different (P = 0.009).
Figure 5.
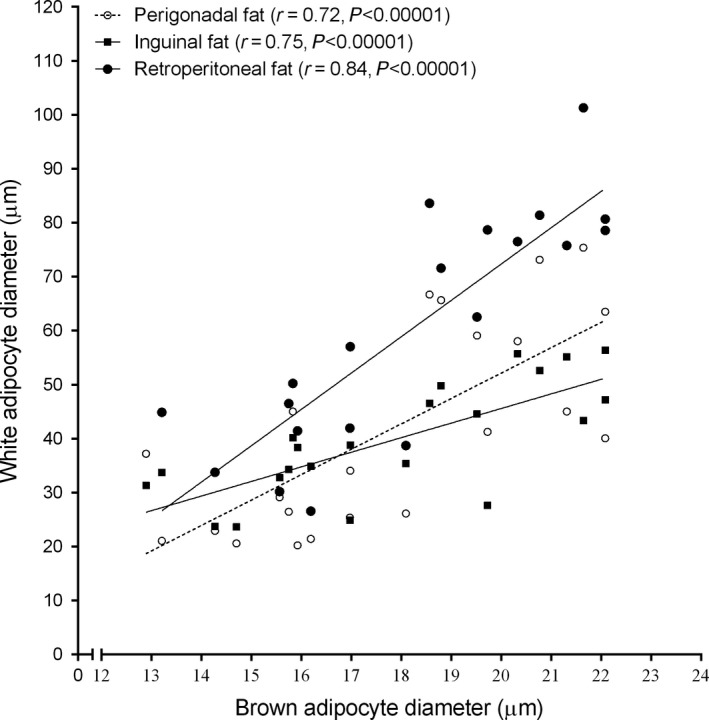
Pearson's correlation showed that ageing‐induced brown adipocyte hypertrophy has a strong correlation with white adipocyte hypertrophy, regardless of WAT location. The strongest correlation of brown adipocyte diameter was seen with retroperitoneal white adipocytes. N = 10–11 mice/group.
Discussion
The present work highlights some important features of WAT and BAT remodelling during ageing in female mice, contributing to a better understanding on this field. We found that there is a progressive age‐dependent loss of WAT browning, and this process runs in parallel with BAT morphological changes. Both changes were guided towards a fat storer phenotype. Interestingly, body fat gain and remodelling were not due to increased food intake or female cycling. Also, not only the subcutaneous WAT consisted of beige adipocytes in younger mice, but also the perigonadal and retroperitoneal WAT, showed these changes. A curious fact was that BAT remodelling started at 6 months, but BAT mass was unaltered until 12 months.
White adipocyte size contributes to fat pad enlargement, and it is a risk factor for metabolic complications. Our previous study in young male C57BL/6 mice reported that insulin resistance increases as adipocytes become larger (Fernandes‐Santos et al. 2009). However, the present data in female mice indicate a lack of correlation between adipocyte size and glucose metabolism. An possible explanation for this sexual dimorphism is that young female C57BL/6 mice and their adipocytes from perigonadal and subcutaneous WAT are more insulin‐sensitive and have increased lipogenic capacity (Macotela et al. 2009). These features are due to an increased insulin‐stimulated Akt and ERK activation, as well as increased expression of genes that regulate glucose and lipid metabolism, such as glucose transporter 1 (GLUT1), glucose transporter 4 (GLUT4), fatty acid synthase (FAS) and acetyl‐CoA carboxylase (ACC) (Macotela et al. 2009). A recent study showed that treatment of male C57BL/6 mice with oestradiol prevents high fat diet‐induced increases in adipose tissue mass as well as disturbance of glucose–insulin homeostasis (Dakin et al. 2015).
Unlike males (Rogers & Smith 2012), our data on young female mice show WAT browning of perigonadal and retroperitoneal fat depots as well. The subcutaneous WAT has beige adipocytes until 6 months, some of these cells are still seen at 9 months, and at 12 months, they are barely detected. The retroperitoneal and perigonadal depots are fully beige at 3 months, and the progression of WAT whitening resembles the subcutaneous depot. In humans, a reduced brown phenotype of the abdominal subcutaneous WAT is associated with reduced insulin sensitivity (Yang et al. 2003), but we found no correlation between WAT loss of browning and glucose metabolism, although it has been shown before for male mice (Rogers & Smith 2012). A consensus regarding the mechanism underlying WAT loss of browning during ageing does not exist. A reduced local sensitivity to sympathetic tone, but not sympathetic input itself, is one of the explanations proposed by Graja (Graja & Schulz 2014). Agreeing with it, a lower expression of UCP1 and β3‐adrenergic receptors, and high levels of the enzyme monoamine oxidase A (MAO‐A) that degrades endogenous catecholamine are found in the subcutaneous WAT of senescent male mice (Rogers & Smith 2012). However, we could not find similar reports on senescent female mice.
A recent work in male mice showed that ageing is associated with BAT morphologic abnormalities and thermogenic dysfunction (Sellayah & Sikder 2014). Brown adipocytes are gradually enlarged in size, and glucose intolerance increases similarly. By the age of 12 months, mRNA expression of UCP1, lipoprotein lipase (LPL), PR domain containing 16 (PRDM16), peroxisome proliferator‐activated receptor gamma coactivator 1 alpha (PGC‐1α), mitochondrial transcription factor A (TFAM) and deiodinase 2 (DIO2) are diminished in BAT and, as a result, senescent mice have reduced cold tolerance (Sellayah & Sikder 2014). On the other hand, female rodents are better at maintaining their thermogenic capacity with increased age compared to male (McDonald & Horwitz 1999). Valle and co‐workers (Valle et al. 2008) showed in female rats but not males a decline in T3 with ageing, a hormone that promotes UCP1 synthesis and activity (Raasmaja 1990). They suggest that the ageing‐induced decline in T3 and 17β‐oestradiol in female rats may mediate, at least in part, the effect of gender on the functional decline in BAT during ageing. As our data showed a constant BAT mass as female mice got older, with a mild increase at 12 months, we might suggest that BAT activity is still somehow preserved. However, a molecular approach needs to be undertaken to confirm this hypothesis. Also, it would be interesting to evaluate BAT and WAT physiology in aged female mice during non‐shivering thermogenesis.
To date the molecular differences between genders in BAT biology during senescence has not been fully elucidated but some clues come from work undertaken with young male and female rodents. Fibroblast growth factor 21 (FGF21) acts in an autocrine/paracrine manner to increase UCP1 and PGC‐1α thus promoting BAT thermogenesis and WAT browning (Fisher et al. 2012). In WAT, BMPs regulate adipogenesis, but less is known about their function in BAT. BAT of female mice has higher basal expression of BMP8b and lower FGF21 expression than male mice, although cold exposure increases their expression in both genders (Grefhorst et al. 2015). Gonadectomy diminishes BAT BMP8b expression, whereas short‐(1 week) and long‐term (90 days) exposure to dihydrotestosterone modulates BAT UCP1, BMP8b and FGF21 in female mice (Grefhorst et al. 2015). Taken together, these data demonstrate an important contribution of sex‐specific hormones to BAT gene profile and thermogenesis capacity.
Studies about the ageing process are extremely important because there is a dramatic increase in life expectancy of world population (WHO, 2015). Also, women live longer than men all over the world (WHO, 2015), justifying studies aiming to elucidate gender differences in biological process. Further studies are required to elucidate why young female mice have high amounts of beige cells in WAT depots, and which mechanisms underlie their lost during ageing. Also, it is important to address whether older female mice (18‐24 months of age) would have further changes in WAT and BAT mass and morphology, as well as to compare them with male mice. Finally, researchers often focus solely on adipocyte size or the overall appearance of the adipose tissue, and the present methodology will serve as a tool for future investigations on BAT plasticity under different conditions.
In conclusion, female mice have a progressive age‐dependent loss of subcutaneous and visceral WAT browning, and this process runs in parallel with BAT morphological changes, towards a fat storer phenotype, independent of cycling or disturbances in glucose metabolism.
Conflict of interest
The authors declare no conflict of interest.
Funding source
This study was supported by a grant from the Brazilian agency FAPERJ (Rio de Janeiro Research Foundation, Procs. Nr. E‐26/111.358/2014) and PROPPI (Fluminense Federal University Research Foundation). Student's scholarship was provided by FAPERJ (Procs. Nr. E‐26/260.004/2015) and CNPq (Brazilian Nacional Research and Development Council).
Supporting information
Figure S1. Photomicrographs representing the morphological remodeling of retroperitoneal (A‐D) and inguinal (E‐H) fat depots (H&E stain). Mice with 3 (A, E), 6 (B, F), 9 (C, G) and 12 (D, H) months old are shown. Note the progressive hypertrophy of white adipocytes and loss of beige adipocytes (browning) with increasing ages.
Acknowledgements
Authors are thankful to Dilliane da Paixão Rodrigues Almeida for her technical assistance.
References
- Au‐Yong I.T., Thorn N., Ganatra R., Perkins A.C. & Symonds M.E. (2009) Brown adipose tissue and seasonal variation in humans. Diabetes 58, 2583–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A., & Heeren J. (2013) Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36. [DOI] [PubMed] [Google Scholar]
- Cypess A.M., Lehman S., Williams G. et al (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin R.S., Walker B.R., Seckl J.R., Hadoke P.W. & Drake A.J. (2015) Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int. J. Obes. (Lond) 39, 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes‐Santos C., Carneiro R.E., de Souza Mendonca L., Aguila M.B. & Mandarim‐de‐Lacerda C.A. (2009) Pan‐PPAR agonist beneficial effects in overweight mice fed a high‐fat high‐sucrose diet. Nutrition 25, 818–827. [DOI] [PubMed] [Google Scholar]
- Fisher F.M., Kleiner S., Douris N. et al (2012) FGF21 regulates PGC‐1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A. & Cinti S. (2010) Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 11, 253–256. [DOI] [PubMed] [Google Scholar]
- Graja A. & Schulz T.J. (2014) Mechanisms of aging‐related impairment of brown adipocyte development and function. Gerontology 61, 211–217. [DOI] [PubMed] [Google Scholar]
- Grefhorst A., van den Beukel J.C., van Houten E.L., Steenbergen J., Visser J.A. & Themmen A.P. (2015) Estrogens increase expression of bone morphogenetic protein 8b in brown adipose tissue of mice. Biol. Sex Differ. 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S.S., Zeller C., Chumlea W.C. & Siervogel R.M. (1999) Aging, body composition, and lifestyle: the fels longitudinal study. Am. J. Clin. Nutr. 70, 405–411. [DOI] [PubMed] [Google Scholar]
- Hughes V.A., Roubenoff R., Wood M., Frontera W.R., Evans W.J. & Fiatarone Singh M.A. (2004) Anthropometric assessment of 10‐y changes in body composition in the elderly. Am. J. Clin. Nutr. 80, 475–482. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W.J., Cuthill I.C., Emerson M. & Altman D.G. (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W., Sanderson W. & Scherbov S. (2008) The coming acceleration of global population ageing. Nature 451, 716–719. [DOI] [PubMed] [Google Scholar]
- Macotela Y., Boucher J., Tran T.T. & Kahn C.R. (2009) Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58, 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R.B. & Horwitz B.A. (1999) Brown adipose tissue thermogenesis during aging and senescence. J. Bioenerg. Biomembr. 31, 507–516. [DOI] [PubMed] [Google Scholar]
- McLean A.C., Valenzuela N., Fai S. & Bennett S.A. (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. e4389, doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Bengtsson T. & Cannon B. (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452. [DOI] [PubMed] [Google Scholar]
- Ohno H., Shinoda K., Spiegelman B.M. & Kajimura S. (2012) PPARγ agonists induce a white‐to‐brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasmaja A. (1990) Alpha 1‐and beta‐adrenergic receptors in brown adipose tissue and the adrenergic regulation of thyroxine 5′‐deiodinase. Acta Physiol. Scand. Suppl. 590, 1–61. [PubMed] [Google Scholar]
- Rogers N.H. & Smith R.G. (2012) Brown‐to‐white transition in subcutaneous fat: linking aging and disease. Aging (Albany NY) 4, 728–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N.J. (1989) Central control of brown adipose tissue. Proc. Nutr. Soc. 48, 197–206. [DOI] [PubMed] [Google Scholar]
- Seale P., Conroe H.M., Estall J. et al (2011) Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D. & Sikder D. (2014) Orexin restores aging‐related brown adipose tissue dysfunction in male mice. Endocrinology 155, 485–501. [DOI] [PubMed] [Google Scholar]
- Valle A., Santandreu F.M., García‐Palmer F.J., Roca P. & Oliver J. (2008) The serum levels of 17beta‐estradiol, progesterone and triiodothyronine correlate with brown adipose tissue thermogenic parameters during aging. Cell. Physiol. Biochem. 22, 337–346. [DOI] [PubMed] [Google Scholar]
- Visser M., Pahor M., Tylavsky F. et al (2003) One‐and two‐year change in body composition as measured by DXA in a population‐based cohort of older men and women. J. Appl. Physiol. 94, 2368–2374. [DOI] [PubMed] [Google Scholar]
- WHO (2015) WHO | Life expectancy. WHO, http://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/. [Google Scholar]
- Yang X., Enerback S. & Smith U. (2003) Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes. Res. 11, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Zingaretti M.C., Crosta F., Vitali A. et al (2009) The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Photomicrographs representing the morphological remodeling of retroperitoneal (A‐D) and inguinal (E‐H) fat depots (H&E stain). Mice with 3 (A, E), 6 (B, F), 9 (C, G) and 12 (D, H) months old are shown. Note the progressive hypertrophy of white adipocytes and loss of beige adipocytes (browning) with increasing ages.


