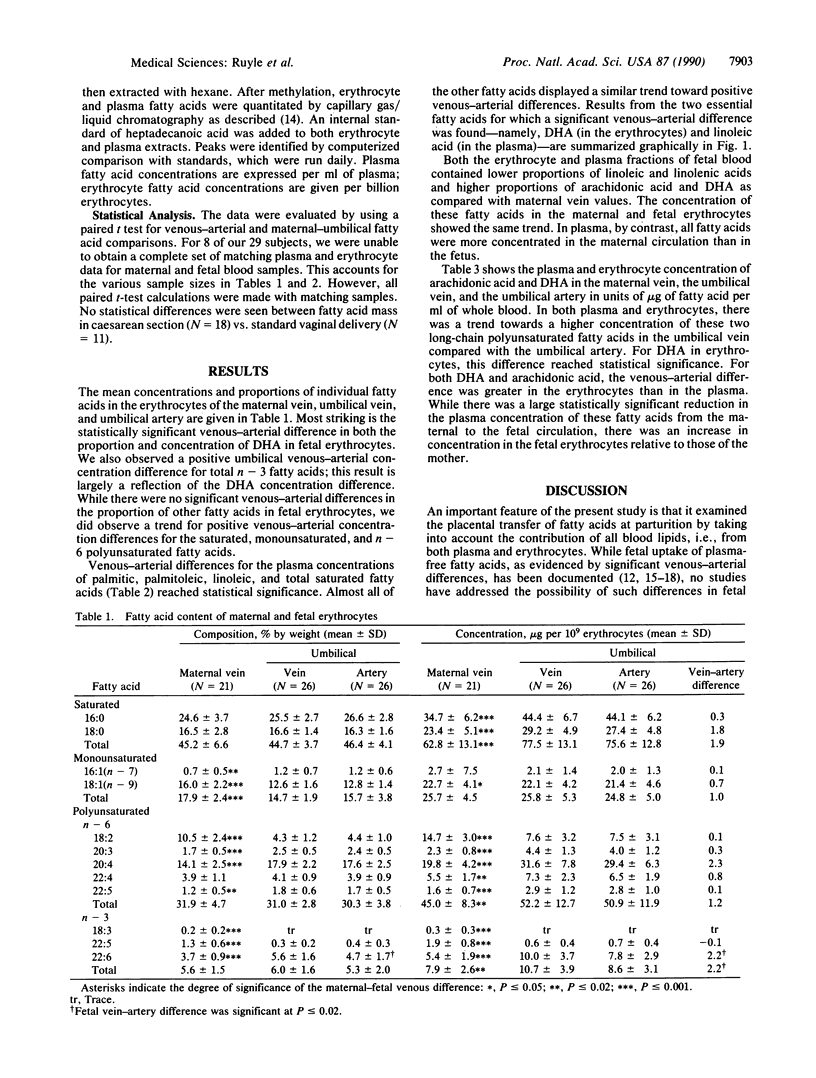
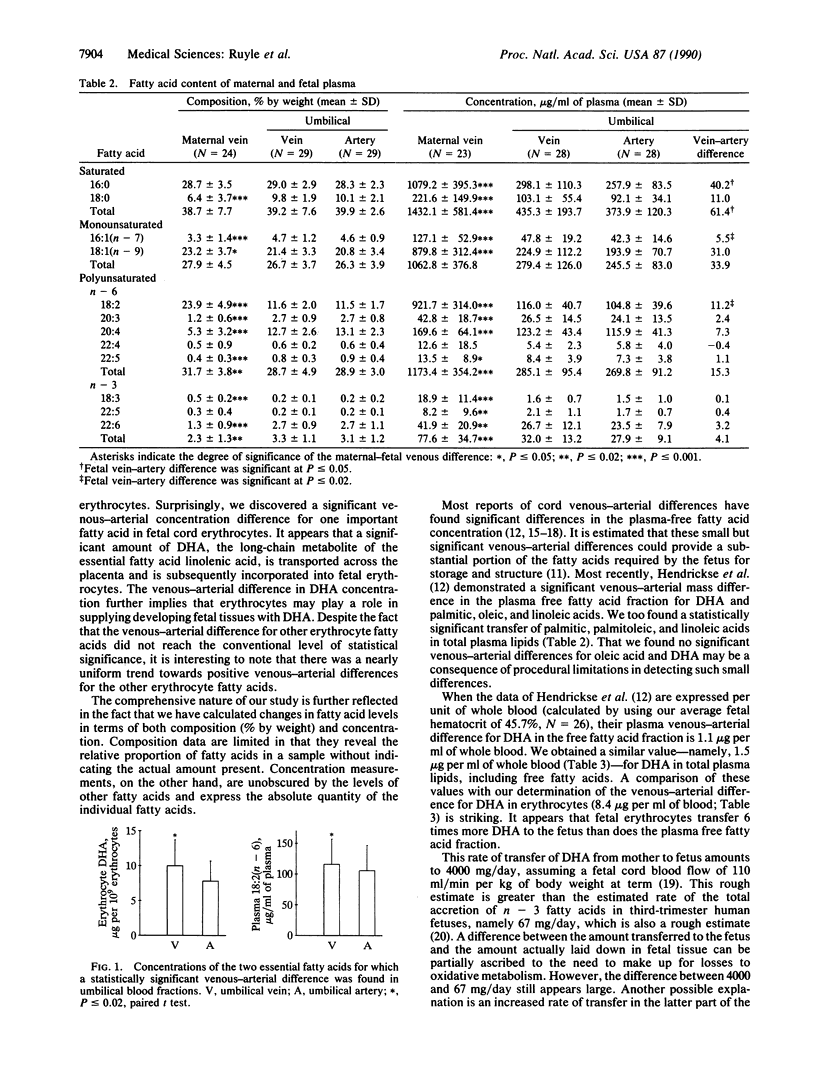
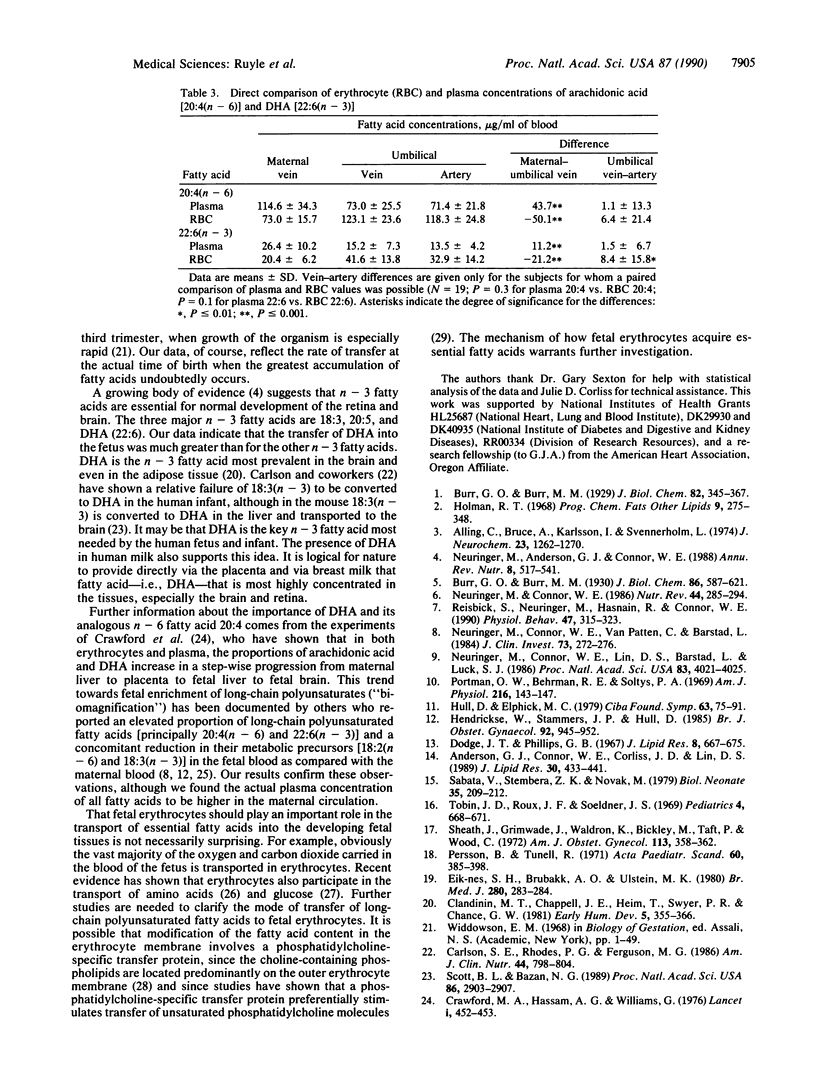
Abstract
Docosahexaenoic acid [22:6(n-3); 22:6(4,-7,10,13,16,19) (DHA)] is required in quantity by the developing nervous system of the fetus. This need could be met through synthesis of DHA from linolenic acid in the fetus or through placental transfer of DHA directly. To study the placental transfer of n-3 fatty acids, we obtained umbilical and maternal blood samples from 26 healthy women and infants at parturition and measured the fatty acid composition and content of both plasma and erythrocytes. A striking finding was a considerable venous-arterial difference for DHA in the umbilical erythrocytes as a proportion of total fatty acids and in absolute concentration. This difference of 2.2 micrograms per billion erythrocytes was 6 times larger than the difference in fetal plasma, when the plasma and erythrocyte concentrations were normalized to whole blood. Most other erythrocyte fatty acids showed a similar trend. In umbilical plasma, significant venous-arterial differences were found for 16:0, 16:1, 18:2, and total saturated fatty acids. There was a similar trend for most other plasma fatty acids. Compared with maternal blood, fetal plasma and erythrocytes had higher levels of 20:4 and DHA and lower levels of 18:2 and 18:3(n - 3) fatty acids as a proportion of total fatty acids. These results suggest that erythrocytes play a major role in the necessary transport of the essential fatty acid DHA into the fetus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alling C., Bruce A., Karlsson I., Svennerholm L. The effect of different dietary levels of essential fatty acids on lipids of rat cerebrum during maturation. J Neurochem. 1974 Dec;23(6):1263–1270. doi: 10.1111/j.1471-4159.1974.tb12226.x. [DOI] [PubMed] [Google Scholar]
- Anderson G. J., Connor W. E., Corliss J. D., Lin D. S. Rapid modulation of the n-3 docosahexaenoic acid levels in the brain and retina of the newly hatched chick. J Lipid Res. 1989 Mar;30(3):433–441. [PubMed] [Google Scholar]
- Carlson S. E., Rhodes P. G., Ferguson M. G. Docosahexaenoic acid status of preterm infants at birth and following feeding with human milk or formula. Am J Clin Nutr. 1986 Dec;44(6):798–804. doi: 10.1093/ajcn/44.6.798. [DOI] [PubMed] [Google Scholar]
- Clandinin M. T., Chappell J. E., Heim T., Swyer P. R., Chance G. W. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum Dev. 1981 Sep;5(4):355–366. doi: 10.1016/0378-3782(81)90016-5. [DOI] [PubMed] [Google Scholar]
- Crawford M. A., Hassam A. G., Williams G. Essential fatty acids and fetal brain growth. Lancet. 1976 Feb 28;1(7957):452–453. doi: 10.1016/s0140-6736(76)91476-8. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J Lipid Res. 1967 Nov;8(6):667–675. [PubMed] [Google Scholar]
- Friedman Z., Danon A., Lamberth E. L., Jr, Mann W. J. Cord blood fatty acid composition in infants and in their mothers during the third trimester. J Pediatr. 1978 Mar;92(3):461–466. doi: 10.1016/s0022-3476(78)80450-8. [DOI] [PubMed] [Google Scholar]
- Hendrickse W., Stammers J. P., Hull D. The transfer of free fatty acids across the human placenta. Br J Obstet Gynaecol. 1985 Sep;92(9):945–952. doi: 10.1111/j.1471-0528.1985.tb03075.x. [DOI] [PubMed] [Google Scholar]
- Hull D., Elphick M. C. Evidence for fatty acid transfer across the human placenta. 1978 Mar 30-Apr 1Ciba Found Symp. (63):75–91. doi: 10.1002/9780470720462.ch5. [DOI] [PubMed] [Google Scholar]
- Jacquez J. A. Red blood cell as glucose carrier: significance for placental and cerebral glucose transfer. Am J Physiol. 1984 Mar;246(3 Pt 2):R289–R298. doi: 10.1152/ajpregu.1984.246.3.R289. [DOI] [PubMed] [Google Scholar]
- Kuypers F. A., Andriesse X., Child P., Roelofsen B., Op den Kamp J. A., van Deenen L. L. The rate of uptake and efflux of phosphatidylcholine from human erythrocytes depends on the fatty acyl composition of the exchanging species. Biochim Biophys Acta. 1986 May 9;857(1):75–84. doi: 10.1016/0005-2736(86)90100-8. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Anderson G. J., Connor W. E. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu Rev Nutr. 1988;8:517–541. doi: 10.1146/annurev.nu.08.070188.002505. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Connor W. E., Lin D. S., Barstad L., Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuringer M., Connor W. E., Van Petten C., Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984 Jan;73(1):272–276. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuringer M., Connor W. E. n-3 fatty acids in the brain and retina: evidence for their essentiality. Nutr Rev. 1986 Sep;44(9):285–294. doi: 10.1111/j.1753-4887.1986.tb07660.x. [DOI] [PubMed] [Google Scholar]
- Persson B., Tunell R. Influence of environmental temperature and acidosis on lipid mobilization in the human infant during the first two hours after birth. Acta Paediatr Scand. 1971 Jul;60(4):385–398. doi: 10.1111/j.1651-2227.1971.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Behrman R. E., Soltys P. Transfer of free fatty acids across the primate placenta. Am J Physiol. 1969 Jan;216(1):143–147. doi: 10.1152/ajplegacy.1969.216.1.143. [DOI] [PubMed] [Google Scholar]
- Reisbick S., Neuringer M., Hasnain R., Connor W. E. Polydipsia in rhesus monkeys deficient in omega-3 fatty acids. Physiol Behav. 1990 Feb;47(2):315–323. doi: 10.1016/0031-9384(90)90149-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. Amino acid transport in human red blood cells. Acta Psychiatr Scand Suppl. 1988;345:25–28. doi: 10.1111/j.1600-0447.1988.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Scott B. L., Bazan N. G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheath J., Grimwade J., Waldron K., Bickley M., Taft P., Wood C. Arteriovenous nonesterified fatty acids and glycerol differences in the umbilical cord at term and their relationship to fetal metabolism. Am J Obstet Gynecol. 1972 Jun 1;113(3):358–362. doi: 10.1016/0002-9378(72)90684-9. [DOI] [PubMed] [Google Scholar]
- Tobin J. D., Roux J. F., Soeldner J. S. Human fetal insulin response after acute maternal glucose administration during labor. Pediatrics. 1969 Nov;44(5):668–671. [PubMed] [Google Scholar]
- Zimmermann T., Winkler L., Möller U., Schubert H., Goetze E. Synthesis of arachidonic acid in the human placenta in vitro. Biol Neonate. 1979;35(3-4):209–212. doi: 10.1159/000241174. [DOI] [PubMed] [Google Scholar]
- van Deenen L. L. Structural organization and dynamics of phospholipids in red cell membranes. Prog Clin Biol Res. 1979;30:451–456. [PubMed] [Google Scholar]


