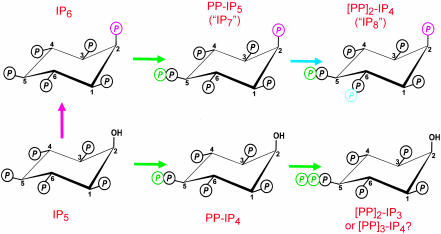
The pervasive inositol phosphate signaling family includes a specialized subgroup with “high-energy” pyrophosphate groups that turn over rapidly (Fig. 1 and refs. 1 and 2). Some of the enzymes directing these reactions have yet to be cloned (see Fig. 1 legend). Therefore, proposed roles of inositol pyrophosphates ([PP]x-IPy) in vivo have inevitably depended on observing phenotypes when the entire subgroup is either eliminated or overproduced. Such experiments have led to conclusions that inositol pyrophosphates act as a group to regulate apoptosis, vesicle trafficking, yeast vacuole biogenesis, transcription, chemotaxis, and DNA repair (see ref. 3 for access to the literature). This tendency to treat these pyrophosphates as all acting in a functionally similar manner has not helped us understand the molecular basis by which they apparently regulate so many biological processes. Now, two groups working independently [Sol Snyder's laboratory at Johns Hopkins as described in this issue of PNAS (4) and John York's team at Duke (5)] have used yeast genetics to obtain evidence that inositol pyrophosphates regulate yet another fundamental process, in this case telomere length. However, an important new feature of these studies is that specific inositol pyrophosphates, namely, those with a hydroxyl group at the 2-position (Fig. 1), are held responsible for this biological activity. This departure from the standpoint that inositol pyrophosphates are a functionally redundant family offers opportunities to develop a molecular understanding of these specific events.
Fig. 1.
This figure offers guidance for coping with a complicated nomenclature and uncertain isomeric assignments. Let us begin with what is known: the synthesis of inositol pyrophosphates can be attributed to three classes of enzymes, namely, the IP5 kinase (purple arrow; also known as ipk1), the versatile IP6 kinases (green arrows), and the PP-IP5 kinase (blue arrows) that forms [PP]2-IP4 (but note: IP7 and IP8 are frequently used colloquisms for PP-IP5 and [PP]2-IP4). We also know that the mammalian PP-IP5 isomer has the diphosphate group attached to the 5-carbon (17). In mammals, three isoforms of IP6 kinases synthesize PP-IP5 (13, 18). Only one homologue, Kcs1, has been cloned from Saccharomyces cerevisiae (18). All IP6 kinases also phosphorylate IP5 to PP-IP4 (12). Not shown is the hydrolysis of the diester phosphates by specific phosphohydrolases (19). So, what don't we know? First, PP-IP4 is itself further phosphorylated by the IP6 kinase, and the product is unclear. It was originally proposed to be [PP]2-IP3 (12), but a [PP]3-IP4 is an equally possible product (see ref. 3). Another uncertainty is that the [PP]2-IP4 isomer in yeast and mammals may be different from the 5,6-diphospho version found in Dictyostelium discoideum (20). The molecular nature of the PP-IP5 kinase(s) is also unknown because no one has succeeded in cloning it. Finally, kcs1Δ yeast can synthesize PP-IP5 by using another enzyme (5). Could this be a novel PP-IP5 isomer produced by off-target phosphorylation of IP6 by the PP-IP5 kinase?
Regulation of Telomere Length
Telomeres are the nucleoprotein complexes that occur at the ends of eukaryotic linear chromosomes. They consist of long, repetitive DNA sequences that attract a number of sequence- and structure-specific binding proteins (reviewed in refs. 6 and 7). These chromosomal caps prevent nucleolytic degradation and provide a mechanism for cells to distinguish natural termini from broken ends; the latter signal DNA damage. Without telomere protection, the activation of DNA damage response pathways by chromosomal ends would provoke cell cycle arrest, senescence, or apoptosis. Another feature of telomeres is that their 3′ single-strand nucleotide overhang cannot be replicated, so most human somatic cells lose terminal DNA with each division. Thus, telomeres also guard the more internally located coding region of the genome, but this protection requires ongoing telomere attrition to be countered by elongation mechanisms. Yet, telomere elongation is a double-edged sword. Although long-term proliferation of eukaryotic cells requires mechanisms to maintain telomere length, a telomere-driven limitation of the proliferative capacity of transformed cells can also be considered a tumor suppressor system (7). Therefore, an understanding of the molecular processes of telomere maintenance has potential therapeutic benefit.
Among important players in telomere maintenance in yeast are Tel1 and Mec1 (8), members of the phosphatidylinositol 3-kinase-related kinase (PIKK) family; despite homology in their catalytic domain to phosphatidylinositol 3-kinases, the PIKKs phosphorylate proteins rather than lipids. Wortmannin and caffeine are known PIKK inhibitors (9). Snyder's group (4) thinks that there might be a hitherto unrecognized relationship between inositol pyrophosphates and PIKK-related processes, after they observed that yeast became immune to the toxic, growth-impeding effects of wortmannin and caffeine, if the IP6 kinase gene, KCS1, was deleted. These results were a little surprising, because earlier reports contrarily indicated that kcs1Δ cells were hypersensitive to both wortmannin (10) and caffeine (11). Nevertheless, Snyder's group was prompted by its observations to study the effects on telomere length when inositol pyrophosphate synthesis was targeted genetically. Working independently, York's laboratory (5), in collaboration with T. D. Petes' group (University of North Carolina, Chapel Hill), were also interested in studying the effects of inositol pyrophosphates on PIKK-related functions such as telomere length.
A New Role for PP-IP4?
To fully appreciate the experiments that were performed by both teams, it should be noted that Kcs1 phosphorylates both IP6 and IP5 (ref. 12 and the green arrows in Fig. 1). IP5 is initially converted by Kcs1 to PP-IP4, which is itself further phosphorylated; the final product could be either [PP]3-IP4 (as shown in Fig. 1) or [PP]2-IP4. Either way, the vacant 2-OH distinguishes this whole group of IP5 derivatives from the other inositol pyrophosphates, PP-IP5 and [PP]2-IP4 (Fig. 1). Selective elimination of PP-IP5 and [PP]2-IP4 by deletion of Ipk1 (the purple arrow in Fig. 1) led to increased synthesis of PP-IP4, which in turn was associated with decreased telomere length (4, 5). Elimination of PP-IP4 (and all of the other inositol pyrophosphates) by deletion of the KCS1 gene was accompanied by telomere lengthening (4, 5). York's group (5) added an additional, critical experiment, in view of the pleiotropic nature of Kcs1 (11): whereas transformation of kcs1Δ yeast with WT Kcs1 rescued telomere length, transformation with a kinase-dead mutant of Kcs1 (D791A; K793A) did not. These results specifically attribute telomere maintenance to the kinase activity of Kcs1 and not some additional domain in this protein. Both groups suggest that a fluctuating PP-IP4 concentration acts as a rheostat that can either extend or shorten telomeres.
How favorably will this newcomer be welcomed by the telomere field? York and colleagues (5) noted that regulation of telomere length by an intracellular signal (PP-IP4 or a downstream metabolite) potentially connects telomere maintenance to environmental inputs. However, these genetic studies caused either complete elimination of PP-IP4 from the cell or its overwhelming accumulation. Both conditions lie far outside the range of normal fluctuations in the cellular levels of this pyrophosphate. It will be necessary to show that physiologically relevant changes in PP-IP4 levels have the capacity to regulate telomere length. In mammals, such events might involve differential expression of the three isoforms of IP6 kinase, because each has different affinities for the competing IP5 and IP6 substrates (12, 13). Unfortunately, perhaps, the one isoform of IP6 kinase that is predominantly nuclear (type 2) has the weakest ability to phosphorylate IP5 to PP-IP4 (12, 13). Furthermore, no one has yet shown that there is an extracellular stimulus that can specifically regulate PP-IP4 levels, thereby providing opportunities for telomere length to be regulated independent of the many effects that other inositol pyrophosphates can elicit (see above).
The regulation of telomere length by an intracellular signal potentially connects telomere maintenance to environmental inputs.
Mec1 and Tel1 are potential targets of PP-IP4 (see above), but the lethal consequences of MEC1 deletion prohibited its role from being studied. In contrast, the tel1Δ strain is viable. PP-IP4 has no effect in these cells, which already have short telomeres (8), implying that PP-IP4 and Tel1 act in a common signaling pathway (4, 5). Snyder's group (4) seems confident that PP-IP4 will be found to inhibit Tel1, but this possibility still needs to be directly tested. Such future experiments should test the apparent specificity of action of PP-IP4 revealed by the in vivo work.
The mammalian homologues of Tel1 and Mec1 are, respectively, ATM (ataxia-telangiectasia mutated) and ATR (ATM-related). Can PP-IP4 regulate these PIKKs? Are other PIKKs modulated by inositol pyrophosphates? Snyder and colleagues (4) speculate that a previously described, IP6-mediated stimulation of a PIKK that is a DNA repair protein, DNA-PK, might in fact be physiologically caused by inositol pyrophosphates rather than IP6. This hypothesis seems unlikely to be correct, because it is the Ku subunit, and not DNA-PK, that is now known to bind IP6 (14, 15). At least in yeast, Ku seems to act independent of PP-IP4 (5). Nevertheless, the possibility of more widespread interactions of inositol pyrophosphates with PIKKs is, potentially, the most dramatic and far-reaching development that could come from these studies. Organismal survival depends on appropriate adaptive responses to cellular stress, and we already know that this involves signaling pathways driven by PIKKs (9) and inositol pyrophosphates (11, 16). Now we should consider that these two signal transduction systems may be interconnected.
See companion article on page 1911.
References
- 1.Stephens, L. R., Radenberg, T., Thiel, U., Vogel, G., Khoo, K.-H., Dell, A., Jackson, T. R., Hawkins, P. T. & Mayr, G. W. (1993) J. Biol. Chem. 268, 4009–4015. [PubMed] [Google Scholar]
- 2.Menniti, F. S., Miller, R. N., Putney, J. W., Jr. & Shears, S. B. (1993) J. Biol. Chem. 268, 3850–3856. [PubMed] [Google Scholar]
- 3.Shears, S. B. (2004) Biochem. J. 377, 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saiardi, A., Resnick, A. C., Snowman, A. M., Wendland, B. & Snyder, S. H. (2005) Proc. Natl. Acad. Sci. USA 102, 1911–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.York, S. J., Armbruster, B. N., Greenwell, P., Petes, T. D. & York, J. D. (2004) J. Biol. Chem. 10.1074/jbc.M412070200. [DOI] [PubMed]
- 6.d'Adda, D. F., Teo, S. H. & Jackson, S. P. (2004) Genes Dev. 18, 1781–1799. [DOI] [PubMed] [Google Scholar]
- 7.Smogorzewska, A. & de Lange, T. (2004) Annu. Rev. Biochem. 73, 177–208. [DOI] [PubMed] [Google Scholar]
- 8.Mallory, J. C. & Petes, T. D. (2000) Proc. Natl. Acad. Sci. USA 97, 13749–13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham, R. T. (2004) DNA Repair 3, 883–887. [DOI] [PubMed] [Google Scholar]
- 10.Zewail, A., Xie, M. W., Xing, Y., Lin, L., Zhang, P. F., Zou, W., Saxe, J. P. & Huang, J. (2003) Proc. Natl. Acad. Sci. USA 100, 3345–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois, E., Scherens, B., Vierendeels, F., Ho, M. W. Y., Messenguy, F. & Shears, S. B. (2002) J. Biol. Chem. 277, 23755–23763. [DOI] [PubMed] [Google Scholar]
- 12.Saiardi, A., Caffrey, J. J., Snyder, S. H. & Shears, S. B. (2000) J. Biol. Chem. 275, 24686–24692. [DOI] [PubMed] [Google Scholar]
- 13.Saiardi, A., Nagata, E., Luo, H. R., Snowman, A. M. & Snyder, S. H. (2001) J. Biol. Chem. 276, 39179–39185. [DOI] [PubMed] [Google Scholar]
- 14.Ma, Y. & Lieber, M. R. (2002) J. Biol. Chem. 277, 10756–10759. [DOI] [PubMed] [Google Scholar]
- 15.Hanakahi, L. A. & West, S. C. (2002) EMBO J. 21, 2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pesesse, X., Choi, K., Zhang, T. & Shears, S. B. (2004) J. Biol. Chem. 279, 43378–43381. [DOI] [PubMed] [Google Scholar]
- 17.Albert, C., Safrany, S. T., Bembenek, M. E., Reddy, K. M., Reddy, K. K., Falck, J. R., Bröker, M., Shears, S. B. & Mayr, G. W. (1997) Biochem. J. 327, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saiardi, A., Erdjument-Bromage, H., Snowman, A. M., Tempst, P. & Snyder, S. H. (1999) Curr. Biol. 9, 1323–1326. [DOI] [PubMed] [Google Scholar]
- 19.Caffrey, J. J., Safrany, S. T., Yang, X. & Shears, S. B. (2000) J. Biol. Chem. 275, 12730–12736. [DOI] [PubMed] [Google Scholar]
- 20.Laussmann, T., Reddy, K. M., Reddy, K. K., Falck, J. R. & Vogel, G. (1997) Biochem. J. 322, 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]