ABSTRACT
Macroautophagy/autophagy is a catabolic process that is widely found in nature. Over the past few decades, mounting evidence has indicated that noncoding RNAs, ranging from small noncoding RNAs to long noncoding RNAs (lncRNAs) and even circular RNAs (circRNAs), mediate the transcriptional and post-transcriptional regulation of autophagy-related genes by participating in autophagy regulatory networks. The differential expression of noncoding RNAs affects autophagy levels at different physiological and pathological stages, including embryonic proliferation and differentiation, cellular senescence, and even diseases such as cancer. We summarize the current knowledge regarding noncoding RNA dysregulation in autophagy and investigate the molecular regulatory mechanisms underlying noncoding RNA involvement in autophagy regulatory networks. Then, we integrate public resources to predict autophagy-related noncoding RNAs across species and discuss strategies for and the challenges of identifying autophagy-related noncoding RNAs. This article will deepen our understanding of the relationship between noncoding RNAs and autophagy, and provide new insights to specifically target noncoding RNAs in autophagy-associated therapeutic strategies.
KEYWORDS: autophagy, bioinformatics, circRNAs, long noncoding RNAs, microRNAs
Introduction
Noncoding RNAs, which account for nearly 98% of the transcriptome, lack the capacity to be translated into proteins.1 Conventional notions regarding noncoding RNAs were restricted to rRNA and tRNA for a long period of time, and indeed, both of these noncoding RNAs play irreplaceable roles in the translation of protein-coding genes.2 However, with accumulating knowledge, previously identified yet disregarded noncoding RNAs are now receiving new attention (detailed in Box I). Noncoding RNAs participate in variety of biological processes, including modulating gene expression both at the transcription and post-transcription levels, protecting genomes from exogenous nucleic acids to guide genome rearrangement or DNA synthesis, and others.3 Additionally, noncoding RNA dysfunction is related to imbalances in cellular homeostasis and leads to pathologies such as tumorigenesis.4,5
Box I—. Disregarded noncoding RNAs receive new attention
As the old saying goes, the seeds of revolution are invariably sown decades before it erupts. This is an accurate portrayal of changing attitudes regarding noncoding RNAs.3 Throughout the development of the RNA field, rRNAs and tRNAs, which were discovered in the 1950s, first gained attention for their roles in gene expression and protein synthesis.2 However, it took a long period of time to innovate and obtain knowledge to move from small nuclear RNAs to small nucleolar RNAs.6 In the process, the old rules providing a rational framework for inertial thinking were overthrown. Noncoding RNAs take part in a remarkably broad spectrum of cellular processes. Based on the number of nucleotides, noncoding RNAs are classified as small noncoding RNAs and long noncoding RNAs.7 Small noncoding RNAs are RNAs that contain approximately 20–24 nucleotides, exemplified by microRNAs (miRNAs). In 1993, the Ambros and Ruvkun laboratories first announced the discovery of a short RNA that base-pairs to partially complementary sequences in the 3′ untranslated region of mRNA to control the timing of developmental transitions.8 Through base pairing at specific intervals on target mRNAs, miRNAs do not cause cleavage but initiate translational repression to achieve mRNA decay.9 Compared to small noncoding RNAs, the number of nucleotides in long noncoding RNAs (lncRNAs) is usually greater than 200 base pairs.7 LncRNAs were once considered “transcriptional noise” or abandoned RNA transcribed from junk DNA.7,10 It is daunting to investigate their biological functions and mechanisms. On the one hand, available evidence indicates cytoplasmic lncRNAs scavenge or alter the expression of miRNAs as competing endogenous RNAs (ceRNAs) or interact with translational machinery by targeting mRNAs.11 On the other hand, nuclear lncRNAs recruit histone-modifying complexes such as Xist for transcriptional repression or bind chromatin-modifying complexes such as PRC2 to affect target gene expression and decoy proteins to inhibit their actions.12–14 The recent development of detection technology facilitated the identification of a novel type of circular RNA (circRNA). In contrast to typical linear noncoding RNAs, endogenous circRNAs form a 3-dimensional covalently closed continuous loop structure by ligating the 3′ and 5′ ends.15 The special closed loop protects circRNAs from degradation by exoribonucleases and may endow special biologic functions such as avidity for miRNAs, allowing circRNAs to act as intracellular sponges, resulting in the hierarchical regulation of one noncoding RNA by another.16,17 CircRNAs remain mysterious, and much has yet to be revealed about their nature once certain obstacles are overcome. However, we are moving from ignorance to awareness regarding noncoding RNAs, and we are gradually closing in on their biologic origins. The discovery of multiple types of noncoding RNAs heralds better prospects for their characterization.
Macroautophagy, hereafter referred to as autophagy, is a highly conserved catabolic process that is essential for maintaining homeostasis.18 In 1962, Ashford and Porter first observed an increase in lysosomes and a phenomenon involving lysosomes digesting cytoplasmic components into proteins in hepatocytes during glucagon perfusion into rat livers.19 In the following year, de Duve named this phenomenon “autophagy” to describe cellular self-destruction.20 Autophagosomes, the major units in the autophagy process, are characterized by the formation of double-membrane vesicles. Intracellular phagophores engulf damaged proteins and organelles to generate autophagosomes and then combine with lysosomes to form autolysosomes (detailed in Box II).21 The engulfed cargoes are degraded by lysosomal hydrolases, and the decomposition products are reused or further decomposed.22 The degradation of intracellular material enables cell survival to cope with external stress. At the same time, external stresses also affect cellular autophagic activity.23 Stresses such as starvation or glucagon enhance cellular autophagy levels compared with reductions by exogenous insulin or amino acids.24–27 By studying the ultrastructure of lysosomes and the mechanisms underlying cytoplasmic component sequestration into lysosomes, autophagy itself can be subdivided into specific subgroups.28 Mammalian cells primarily undergo macroautophagy and also experience other types of autophagy, such as microautophagy and chaperone-mediated autophagy.23 Among these subgroups, the major differences are the types of cargo to be degraded and the mode of transportation for cargo into the lysosomes.29 Since the initial identification of Atg5 in 1996, more than 40 Atg genes have been found in yeast, and many of these have mammalian orthologs.30 Autophagy deregulation due to ATG genes is related to various pathological states in humans, such as neurodegeneration, cardiovascular disease, pathogenic infections and cancer.31–34 In some breast cancers, autophagy is restored by exogenous BECN1 to suppress tumorigenesis.35 At the same time, autophagy itself is also beneficial for tumor cells to survive metabolic stresses.36 For example, the accumulation of SQSTM1/p62, which is important for autophagosome maturation, promotes tumorigenesis.37 Thus, the exact role of autophagy is still open for debate.
Box II—. The molecular mechanisms of autophagy: lessons from yeast
Macroautophagy is primarily a degradation pathway to turn over and recycle intracellular materials through autophagosome-dependent vacuolar hydrolysis.18 Autophagy was initially discovered in mammalian cells, but many prominent breakthroughs were made in yeast by the ease and applicability of genetic and molecular techniques.19,40 The autophagy process consists of several steps, including phagophore induction, nucleation and expansion, autophagosome maturation and fusion with the vacuole/lysosome, and breakdown and efflux of the autophagic cargo. The nutrient-sensing kinase MTOR (in mammals)/TOR (in yeast) acts as the main adaptor junction to precisely sense and accumulate stress signals from different sources. Under normal circumstances, MTOR exists in an active state to repress phagophore initiation by blockading assembly of the ULK1 complex. The ULK1 complex consists of ULK1, ATG13, RB1CC1/FIP200 and ATG101 in mammalian cells, which correspond to the Atg1, Atg13, Atg17, Atg29, Atg31 complex in yeast.41,42 Stresses such as starvation or hypoxia inactivate MTOR to disassociate it from the ULK1 complex, and the assembled ULK1 complex phosphorylates ATG13 and RB1CC1 to induce the phagophore.41 Following induction, autophagy cascades sequentially proceed to the phosphatidylinositol 3-kinase (PtdIns3K) complex, of which PIK3C3/Vps34, BECN1/Vps30 and PIK3R4/Vps15 are the core components.43 In this multi-subunit complex BECN1 functions as a scaffold to recruit and activate coenzyme factors, including ATG14/Atg14, UVRAG/Vps38, AMBRA1, SH3GLB1/Bif-1 and RUBCN/Rubicon.44–46 BECN1 also interacts with BCL2 as a mutually antagonistic factor to balance autophagy and apoptosis.35 Subsequently during autophagosome formation, 2 ubiquitin-like protein conjugation systems, specifically the LC3/Atg8–phophatidylethanolamine (PE) conjugation system and the ATG12–ATG5 conjugation system, as well as the ATG9/Atg9 cycling system are essential. The E1-like enzyme ATG7/Atg7 activates the ubiquitin-like modifiers ATG12/Atg12 and LC3/Atg8, which are transferred to the E2-like enzymes ATG10/Atg10 and ATG3/Atg3, respectively.20 LC3 forms an amide bond with PE that is dependent on the isopeptide ATG12–ATG5.47 The ATG12–ATG5-ATG16L1 complex functions as an E3-like enzyme that determines the site of LC3 lipidation. At the same time, the ATG12–ATG5-ATG16L1 complex is required for elongation of the phagophore membrane.48 LC3 conjugates to PE on the membrane subsequent to ATG4/Atg4 proteolysis, which is important for membrane biogenesis.49 Ultimately, the ‘mature’ autophagosome traffics to and fuses with the lysosomal/vacuolar membrane to form an autolysosome wherein the cargo is degraded by hydrolases, and concomitant metabolic byproducts are released through permeases in the autolysosomal membrane (the intuitive flow is shown in Figs. 1 and 2, and orthologous contrast in Table S1).
Increasing evidence suggests noncoding RNAs are associated with autophagy regulation. The first small noncoding RNA identified as an autophagy regulator was MIR30A, which targets the BECN1 gene in a variety of cancer cells.38 Numerous researchers have reported the ability of lncRNAs to regulate miRNAs by binding to and separating them from their binding sites on mRNAs to affect autophagic activity.39 In this review, we focus on summarizing the important roles of noncoding RNAs and their diverse regulatory mechanisms in autophagy. Additionally, we integrate public resources to predict autophagy-related noncoding RNAs and discuss experimental research methods in combination with bioinformatics tools and analysis. A profound understanding of the interactions between noncoding RNAs and autophagy may benefit clinical therapeutics.
miRNAs and the regulation of autophagy
As an important member of noncoding RNAs, miRNAs have been confirmed to take part in each phase of autophagy, including phagophore induction, nucleation and expansion, and autophagosome and autolysosome maturation, and play regulatory roles. The details are as follows:
Phagophore induction
The ULK1 complex integrates upstream nutrient and energy signals to coordinate phagophore induction, and phosphorylation of the ULK1 complex is controlled by MTOR, a major nutrient/energy sensor.50,51 The upstream nutrient signaling pathways include the class I phosphoinositide 3-kinase (PI3K)-AKT-MTOR, Ca2+-AMPK-MTOR, TP53-MTOR and others.52–55 Some miRNAs interfere with upstream nutrient signaling pathways to affect downstream phagophore induction (Table 1). For example, MIR451, MIR155 and MIR21 regulate the expression of certain key enzymes such as TSC1, RHEB and PTEN in the PI3K-AKT-MTOR signaling pathway (Table 1 and Fig. 1). During hypertrophic cardiomyopathy, MIR451 is downregulated to activate autophagy by suppressing TSC1, which forms a heterodimer with the product of TSC2.52,56 In another study of Mycobacterium tuberculosis infection in macrophages, MIR155 induces autophagy to decrease the survival of intracellular Mycobacteria by interfering with RHEB, which is a negative regulatory factor in autophagy.53 However, TSC1 and RHEB negatively regulate each other. The phosphorylation of AKT prevents TSC1 from inhibiting RHEB (Fig. 1). In this way, MIR451 and MIR155 interactively regulate the upstream signaling pathway.52,53 Certain calcium-metabolizing enzymes such as TRPM3 and Drosophila IP3K2 are conditioned by MIR204 and Drosophila mir-14 in the Ca2+-AMPK-MTOR pathway (Table 1 and Fig. 1). In clear renal carcinoma, TRPM3, which is enriched in cancer cells to raise the AMPK-activing Ca2+ influx, promotes tumor growth. MIR204 represses TRPM3 to inhibit autophagy and shorten tumor cell survival.54 In a separate study of Drosophila, mir-14 was vital to salivary gland cell death by inhibiting IP3K2, the product of which phosphorylates inositol trisphosphate (IP3) to prevent the release of calcium, leading to improved autophagy.57 Intriguingly, TP53, which is involved in the crosstalk between autophagy and apoptosis, exerts dual properties in terms of autophagy regulation. Under genotoxic stress, TP53 and HMGB1 form complexes in the cytoplasm and nucleus, respectively, and lead to opposing outcomes (detailed below).58 Confirmed miRNAs such as MIR212, MIR144 and MIR129–5p regulate autophagy through the TP53-MTOR pathway (Table 1 and Fig. 1). In prostate cancer, MIR212 is downregulated both in cancer tissues and blood serum and disrupts the upstream signaling pathway by antagonizing SIRT1 to inhibit cellular autophagy.55 In addition, upstream nutrient and energy signals are also affected by ambient stresses such as hypoxia. Hypoxia caused by oxygen deprivation in the intracellular environment attenuates aerobic oxidation, leading to a lack of energy supply. For example, MIR301A/B targets the 3′ untranslated region of NDRG2 to decrease its expression, causing an increase in autophagy as opposed to the reduced apoptosis observed under hypoxia.59
Table 1.
miRNAs targeting different Autophagy-related pathways in phagophore induction.
| Pathway | Target | Description | Autophagy | miRNA | Refs |
|---|---|---|---|---|---|
| Hypoxia | NDRG2 | NDRG family member 2 | activated | MIR301A/B | 59 |
| PI3K-AKT-MTOR | TGFBR2 | transforming growth factor β receptor 2 | inhibited | MIR19A/B-3p | 138 |
| IGF1R | insulin like growth factor 1 receptor | activated | MIRLET7I | 139,140 | |
| MIR100 | |||||
| PTEN | phosphatase and tensin homolog | activated | MIR21 | 117 | |
| TSC1 | tuberous sclerosis 1 | inhibited | MIR451 | 52 | |
| RHEB | Ras homolog enriched in brain | activated | MIR155 | 53,141 | |
| RICTOR | RPTOR independent companion of MTOR complex 2 | activated | MIR155 | 141,142 | |
| MIR15A | |||||
| MIR16 | |||||
| RPS6KB2 | ribosomal protein S6 kinase B2 | activated | MIR155 | 141 | |
| Ca2+-AMPK-MTOR | IP3K2 | inositol 1,4,5-trisphosphate kinase 2 | activated | Drosophila | 57 |
| mir-14 | |||||
| TRPM3 | transient receptor potential cation subfamily M member 3 | inhibited | MIR204 | 54 | |
| TP53 | TIGAR | TP53 induced glycolysis regulatory phosphatase | activated | MIR144 | 143 |
| CDKN1A | cyclin dependent kinase inhibitor 1A | activated | MIR182 | 144 | |
| DRAM1 | DNA damage regulated autophagy modulator 1 |
inhibited | MIR199A-5p | 145 | |
| SIRT1 | sirtuin 1 | inhibited | MIR140–3p | 55,146,147 | |
| MIR212 | |||||
| MIR34A | |||||
| HMGB1 | high mobility group box 1 | inhibited | MIR129–5p | 148–153 | |
| MIR141 | |||||
| MIR218 | |||||
| MIRLET7F | |||||
| MIR22 | |||||
| HMGB2 | high mobility group box 2 | inhibited | MIR23B-3p | 154 | |
| MAPK1/ERK2-MAPK3/ERK1 | DUSP4/5 | dual specificity phosphatase 4/5 | activated | MIR26A | 155 |
| Translational factors | FOXO3 | forkhead box O3 | inhibited | MIR182 | 118,156 |
| MIR212/132 | |||||
| TFAP2A | transcription factor AP-2 α | inhibited | MIR638 | 157 | |
| STAT3 | signal transducer and activator of transcription 3 | inhibited | MIR17–5p | 70 | |
| Mitophagy | BNIP3L | BCL2 interacting protein 3 like | inhibited | MIR137 | 158 |
| FUNDC1 | FUN14 domain containing 1 | inhibited | MIR137 | 158 | |
| UCP2 | uncoupling proteins 2 | inhibited | MIR214 | 159 | |
| CDKN1B -CDK2-MTOR | CDKN1B | cyclin-dependent kinase inhibitor 1B | inhibited | MIR221 | 119 |
| Hedgehog | Gas1 | growth arrest specific 1 | activated | MIR148A | 160 |
| Others | PSME4 | proteasome activator subunit 4 | inhibited | MIR29B | 161 |
| ARC | activity regulated cytoskeleton associated protein | activated | MIR325 | 162 | |
| UBQLN1 | ubiquilin 1 | inhibited | MIR200C | 163 |
Figure 1.
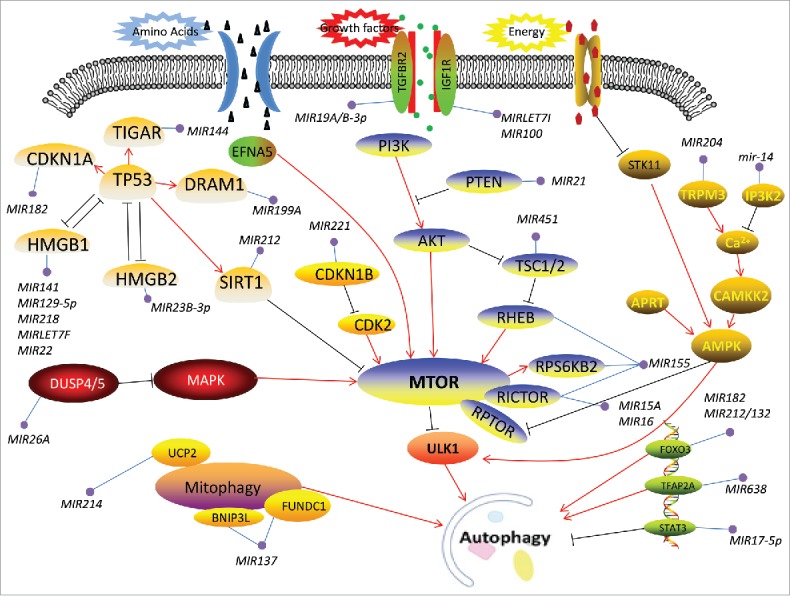
Overview of the miRNAs involved in the regulation of autophagy-related signaling pathways. The interplay of autophagy with multiple upstream signaling pathways occurs through MTOR, which is a master regulator of autophagy that is involved in several regulatory pathways including PI3K-AKT-MTOR, Ca2+-AMPK-MTOR, TP53-MTOR and others. Except for the classic nutrient-sensing MTOR pathways, autophagy is implicated in various other signaling events, such as the mitochondrial pathway and transcription factor pathways.
Notably, the engine consisting of the ULK1 complex and MTOR is not only affected by upstream signals but also directly controlled by miRNAs. For example, MTOR inactivation is modulated by miRNAs such as MIR99A, MIR15A and MIR100; correspondingly, activation of the ULK1 complex is inhibited by MIR20A, Mir106a and others (Table 2 and Fig. 2). Without exception, these miRNAs dephosphorylate MTOR, resulting in the recovery of ULK1 complex assembly to accelerate autophagy (e.g., MIR99A and MIR144 in cardiomyocytes), or boycott ULK1 complex phosphorylation to reduce autophagy (e.g., MIR20A in myoblasts).60–62 Additionally, some mitochondrial membrane proteins such as BNIP3L/NIX and FUNDC1 and translational factors such as FOXO3/FoxO3a and STAT3 also contribute to phagophore induction and are regulated by miRNAs such as MIR137, MIR182 and MIR17–5p (Table 1 and Fig. 1).
Table 2.
miRNAs modulating autophagy signaling networks.
| Autophagy phase | Target | Characteristics | miRNA | Autophagy | Refs |
|---|---|---|---|---|---|
| Induction | MTOR | Intracellular protein complex of an atypical Ser/Thr with kinase activity | MIR99A | Pro-autophagy | 60,61,112,142,164–166 |
| MIR144 | |||||
| MIR15A | |||||
| MIR16 | |||||
| MIR7 | |||||
| MIR100 | |||||
| MIR222 | |||||
| ULK1/2 | Serine/threonine protein kinase | MIR25 | Anti-autophagy | 62,123,167–171 | |
| MIR595 | |||||
| MIR20A | |||||
| MIR26B | |||||
| MIR4487 | |||||
| Mir17–5p | |||||
| Mir106b | |||||
| MIR885–3p | |||||
| MIR290/295 | |||||
| RB1CC1 | RB1 inducible coiled-coil 1 | MIR224–3p | Anti-autophagy | 172,173 | |
| MIR20A | |||||
| MIR20B | |||||
| ATG13 | Component of the ULK1 complex | MIR4459 | Anti-autophagy | 174 | |
| Phagophore nucleation | BCL2 | Integral outer mitochondrial membrane protein that blocks the apoptotic death of cells | MIR21 | Pro-autophagy | 67,68,147,175–181 |
| MIR497 | |||||
| MIR182 | |||||
| MIR34A | |||||
| MIR210 | |||||
| MIR205 | |||||
| Mir195 | |||||
| MIR24–2 | |||||
| MIR365–2 | |||||
| MIR146A | |||||
| MCL1 | Anti-apoptotic BCL2 family member | MIR106A | Pro-autophagy | 70,182–184 | |
| MIR17–5p | |||||
| MIR204 | |||||
| MIR101 | |||||
| BECN1 | Component of class III PtdIns3K complex | MIR30A/B/C/D | Anti-autophagy | 38,66,181,185–191 | |
| MIR409–3p | |||||
| MIR376A/B | |||||
| MIR17–5p | |||||
| MIR216A/B | |||||
| MIR519A | |||||
| MIR129 | |||||
| ATG14 | Component of class III PtdIns3K complex | MIR195 | Anti-autophagy | 74,79,192 | |
| Bos Taurus MIR29B | |||||
| MIR152 | |||||
| UVRAG | Component of class III PtdIns3K complex | MIR630 | Anti-autophagy | 73,193–195 | |
| MIR374A | |||||
| MIR125A MIR183 | |||||
|
Mir351 | |||||
| ATG2 | Peripheral membrane protein | MIR30D | Anti-autophagy | 196–199 | |
| MIR143 | |||||
| MIR130A | |||||
| MIR1303 | |||||
| ATG9 | Transmembrane protein | Caenorhabditis elegans | Anti-autophagy | 78,79 | |
| mir-34 | |||||
| Bos Taurus | |||||
| MIR29B | |||||
| Elongation and completion | ATG3 | E2 like enzyme for LC3/ATG8 conjugation | MIR495 | Anti-autophagy | 200 |
| ATG4 | Cysteine proteinase | MIR376B | Anti-autophagy | 66,75,201–204 | |
| MIR101 | |||||
| MIR34A | |||||
| Mir144 | |||||
| MIR24–3p | |||||
| ATG5 | Conjugated with ATG12 | MIR181A | Anti-autophagy | 83,124,172,193,205–207 | |
| MIR374A | |||||
| MIR30A/B/C | |||||
| MIR224–3p | |||||
| MIR299–5p | |||||
| ATG7 | E1 like enzyme | MIR188–3p | Anti-autophagy | 80–82,104,171,208 | |
| MIR375 | |||||
| MIR17 | |||||
| MIR290–295 | |||||
| MIR210 | |||||
| MIR199 | |||||
| ATG12 | Ubiquitin like protein | MIR630 | Anti-autophagy | 154,193,209,210 | |
| MIR23B-3p | |||||
| MIR200B | |||||
| ATG10 | E2 like enzyme for ATG12 conjugation | MIR519A | Anti-autophagy | 193 | |
| ATG16L1 | Component of ATG12–ATG5-ATG16L1 protein complex | MIR885–3p | Anti-autophagy | 83,84,170,211,212 | |
| MIR30A/C | |||||
| MIR20A | |||||
| MIR106B | |||||
| MIR93 | |||||
| MAP1LC3A | Microtubule associated protein 1 light chain 3 α | MIR214 | Anti-autophagy | 54 | |
| MAP1LC3B | Microtubule associated protein 1 light chain 3 β | MIR497 | Anti-autophagy | 54,68,85 | |
| MIR204 | |||||
| MIR214 | |||||
| SQSTM1 | Autophagy receptor and ubiquitin-binding protein | Mir106 | Anti-autophagy | 88,213 | |
| Mir17 | |||||
| Mir20 | |||||
| Mir93 | |||||
| MIR372 | |||||
| Fusion and Degradation |
LAMP2 | Lysosomal associated membrane protein 2 | MIR207 | Anti-autophagy | 89 |
| MIR352 | |||||
| LARP1 | La ribonucleoprotein domain family member 1 | MIR4459 | Anti-autophagy | 39,214 | |
| MIR26A/B | |||||
| RAB1A | RAB family of the small GTPase superfamily | MIR184 | Anti-autophagy | 215 | |
| MIR150 | |||||
| RAB5A | MIR101 | 75,216 | |||
| MIR130A | |||||
| RAB11A | MIR21 | 217–219 | |||
| MIR320A | |||||
| MIR520E | |||||
| RAB7 | MIR17–5p | 220 | |||
| RAB1B | MIR502 | 221 | |||
| RAB31 | MIR184 | 215 | |||
| MIR150 |
Figure 2.
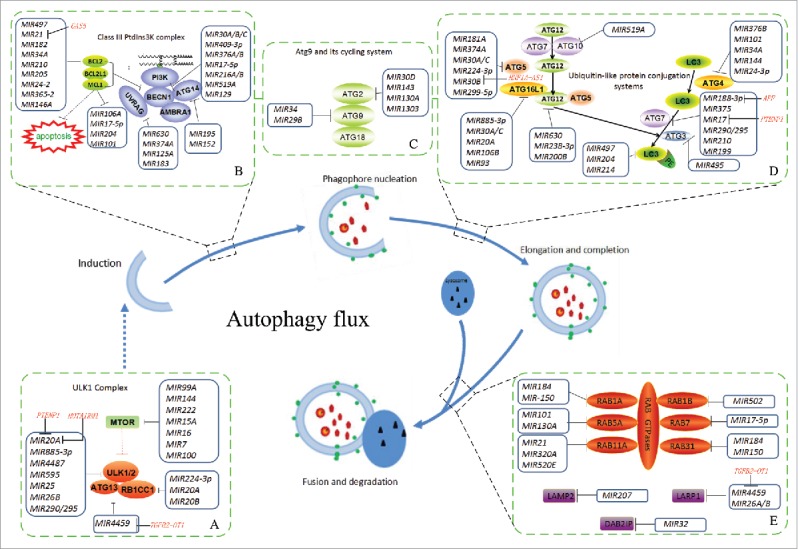
Detailed schematic of the roles of related miRNAs and lncRNAs during the core phase of autophagy. The core proteins or genes regulated by miRNAs and lncRNAs are marked during the dynamic steps. Autophagy induction is directly controlled by MTOR or other translational factors and signaling pathways (Fig. 1). Under an unfavorable stimulus, such as hypoxia or starvation, the inactivated MTOR assembles and activates ULK1/2 complexes to trigger the autophagy cascade (steps A-B). Then, initiation of the phagophore and phagophore nucleation is driven by the BECN1-associated PtdIns3K complex. In this critical stage, crosstalk exists between autophagy and apoptosis (step B). During autophagosome formation, phagophore elongation and completion involve 2 ubiquitin-like protein conjugation systems (ATG12–ATG5 conjugation and LC3–phophatidylethanolamine [PE] conjugation) and the ATG9 cycling system (steps C-D). The RAB family of small GTPases is essential for endocytosed proteins to function throughout the autophagy flux (step E). Finally, the mature autophagosome fuses with a lysosome to form the autolysosome, which degrades its cargo via hydrolases.
Phagophore nucleation
In one model of autophagosome biogenesis, isolated membranes gather and assemble into phagophores. The PtdIns3K complex, which is recruited by the activated ULK1 complex, plays an essential role in phagophore nucleation.43 Among the components of this complex, BECN1 has an irreplaceable role and functions as a scaffolding protein to recruit and assemble cofactors such as ATG14, UVRAG and others.63 The importance of BECN1 is also reflected in the crosstalk between autophagy and apoptosis.64 BECN1 and BCL2 are mutually antagonistic such that BCL2 suppresses autophagy by sequestering BECN1, and BECN1 potentiates apoptosis by binding to BCL2.64,65 Many miRNAs, such as MIR30, MIR376A/B and others, target the BECN1 gene to affect autophagy (Table 2 and Fig. 2). For example, MIR376B attenuates starvation-induced autophagy by blocking BECN1 in breast cancer.66 Furthermore, miRNAs enhance autophagy by interfering with the BCL2 gene (Table 2 and Fig. 2). Notably, the downregulation of MIR21 and MIR497 promotes autophagy while reducing apoptotic injury by inhibiting the BCL2 gene.67,68 MCL1, an antiapoptotic BCL2 homolog, also accelerates autophagy.69 In macrophages infected by Mycobacterium tuberculosis, the upregulation of MIR17–5p accelerates protective autophagy to eliminate infection by downregulating MCL1.70 In both autophagy and apoptosis, the role of the tumor suppressor TP53 cannot be ignored. The dual regulatory roles of this protein facilitate its interaction with HMGB1 in the cytoplasm and nucleus.58 TP53 knockout enhances the expression of cytosolic HMGB1, which induces autophagy by directly binding with BECN1 to displace BCL2, compared with autophagy inhibition by HMGB1 in the nucleus.71 Several miRNAs target HMGB1 and TP53 to regulate autophagy, including MIR22, MIR218, MIR23B-3p and others (Table 1 and Fig. 1).
As cofactors of BECN1, ATG14 and UVRAG also play important roles in phagophore nucleation, and miRNAs are involved in this process (Table 2 and Fig. 2). For example, Mir125a- and Mir351-mediated Uvrag reduction is associated with autophagy inhibition; additionally, autophagy attenuation caused by MIR125A is also involved in immune escape by Mycobacterium tuberculosis.72,73 During ovarian cancer treatment, MIR152 attenuates cisplatin-induced autophagy by downregulating ATG14 while enhancing cisplatin-induced apoptosis and inhibiting tumor cell proliferation.74
Additionally, the RAB family, which includes small GTPases that regulate early endocytosis, acts at the early phagophore stage in mammalian cells to activate the PtdIns3K complex to localize into the ATG5-positive phagophore. Several miRNAs, including MIR101, MIR130A, MIR150 and others, affect the PtdIns3K complex activity by regulating the RAB family (Table 2 and Fig. 2). MIR101 expression is lacking in some cancers, such as breast cancer, liver cancer and prostate cancer. MIR101-mediated autophagy inhibition through RAB5a accelerates the drug sensitivity of tumor cells.75 After phagophore nucleation, the compartment gradually expands to assemble the autophagosome in a stepwise manner.
Phagophore expansion and maturation into the autophagosome
There exist 2 key mechanisms that underlie the expansion of phagophore membranes to form the autophagosome: the ATG9 cycling system and 2 ubiquitin-like protein conjugation systems, the LC3–phophatidylethanolamine (PE) conjugation system and the ATG12–ATG5 conjugation system. ATG9 is widespread in eukaryotes, and its trafficking is proposed to be critical in providing membrane to the expanding phagophore.76 In yeast, Atg11, Atg23 and Atg27 are involved in the anterograde transport of Atg9, whereas the peripheral membrane proteins Atg2 and Atg18 form a complex with Atg9 that is required for Atg9 retrieval.77 Among these proteins, miRNA regulation has been observed (Table 2 and Fig. 2). For example, in Caenorhabditis elegans, mir-34 inhibits autophagy by disrupting ATG-9 cycling, shortening the life span.78 Conversely, through the same mechanism, Bos taurus MIR29B attenuates autophagy to repress the replication of bovine viral diarrhea virus in host cells.79 In the 2 ubiquitin-like protein conjugation systems, multiple miRNAs have been reported to be involved, such as MIR106B, MIR200, MIR210 and others (Table 2 and Fig. 2). During the course of chronic obstructive pulmonary disease, MIR210 attenuates protective autophagy by targeting ATG7, which accelerates bronchial myofibroblast differentiation.80 ATG7 is also targeted by MIR17 and MIR199 to activate autophagy to inhibit the cytotoxicity of chemotherapeutic and low-dose ionizing radiation in glioblastomas and hepatocellular cancers.81,82 Furthermore, MIR30A/C and MIR106B are upregulated in the intestinal tissues of patients with Crohn disease and interfere with both ATG5 and ATG16L1 expression, leading to the inhibition of cellular autophagy. As a result of autophagy weakening in intestinal epithelial cells, local inflammation of the intestinal tract becomes exacerbated.83,84 In other ubiquitin-like conjugation systems, MIR204, for example, attenuates autophagy in cardiomyocytes when switching from hypoxia to reoxygenation by targeting LC3 and simultaneously repressing BCL2 expression.85
Furthermore, SQSTM1, a multifunctional receptor protein, binds LC3 and is incorporated by the phagophore, ultimately becoming degraded along with ubiquitinated cargo proteins in autolysosomes.86 SQSTM1 is not only a specific substrate of autophagy but also a strong inducer of autophagy, similar to the oxidative stress response.87 Mir17, Mir20, Mir93, Mir106 and MIR372 are involved in the degradation of SQSTM1 to regulate autophagy (Table 2 and Fig. 2). For example, Mir17, Mir20, Mir93 and Mir106 promote haematopoietic cell expansion through autophagy attenuation by targeting Sqstm1 in mice.88 The above miRNAs regulate key proteins during phagophore expansion into the autophagosome and influence autophagosome maturation. Ultimately, the ‘mature’ autophagosome fuses with the lysosomal membrane to enter the autolysosome maturation phase.
Autolysosome maturation
Completion of the autophagic process relies on the fusion of autophagosomes with lysosomes to form autolysosomes. The docking and fusion processes are promoted by RAB7, LAMP2 and other proteins (Table 2 and Fig. 2). MIR207 and MIR352 modulate LAMP2 gene expression to block the lysosomal-autophagy pathway. Furthermore, MIR207 mimics also reduce the number of cellular lysosomes and autophagosomes.89 Conversely, MIR4459 inhibits LARP1 expression, which is involved in SQSTM1 protein synthesis to attenuate autophagy in vascular endothelial cells.39 The identification of these miRNAs as regulators of autophagy-lysosomal genes will allow us to identify regulatory mechanisms and may have implications for further clinical applications.
Long noncoding RNAs and autophagy regulation
In terms of traditional concepts regarding the sequential transfer of biological information, individual thinking can be constrained by central dogma, which in this case entails the detailed residue-by-residue transfer of sequential information that cannot be transferred back from protein to either protein or nucleic acid, as noted by Francis Crick in 1958.90 However, accumulating evidence indicates that this simplification ignores the existence of reverse information flow from RNA to DNA. Therefore, the central dogma was restated by Francis Crick in 1970.91 Similar to the complements in central dogma, studies on the other forms of noncoding RNAs will supplement the cognition of noncoding RNAs in regulating autophagy. Multiple miRNAs underlie the regulation of autophagy. As another important type of noncoding RNA, long noncoding RNAs (lncRNAs) are estimated to exceed 15,000.92,93 Are lncRNAs merely functionless transcription byproducts of coding genes, or are they special envoys? The latter hypothesis is not a figment of the imagination. Emerging evidence indicates lncRNAs act as competitive platforms for both miRNAs and mRNAs.11 The lncRNA category is diverse and includes not only antisense, intronic, and intergenic molecules but also pseudogenes and retrotransposons.94,95 Meanwhile, lncRNAs demonstrate specificity among diverse tissues and cells in physiological or pathological conditions.96 In addition to expanding the transcriptome, some lncRNAs unite to carry out autophagy regulation, including PTENP1, MEG3, APF and others (Table 3 and Fig. 2).
Table 3.
lncRNAs targeting special targets in autophagy.
| Name | Accession noa. | Disease phenotype | Regulation | Mechanism | Refs |
|---|---|---|---|---|---|
| APF | GEO profile: AK079427 | myocardial infarction | upregulate | Binds MIR188–3p to affect ATG7 expression | 104 |
| TGFB2-OT1 (FLJ11812) | HGNC_ID: 50629 | inflammation | — | Binds MIR3960, MIR4488 and MIR4459 to target ATG13, CERS1, NAT8L and LARP1 | 39,222 |
| PTENP1 | HGNC_ID: 9589 | hepatocellular carcinoma | downregulate | Represses oncogenic PI3K-AKT signaling pathway and elicit autophagy via sequestering MIR17, MIR19B and MIR20Ain vitro | 97 |
| NBR2 | HGNC_ID: 20691 | human cancers | downregulate | Induced by the STK11-AMPK pathway under energy stress and interacts with AMPK to promotes kinase activity in turn | 223,224 |
| PVT1 | HGNC_ID: 9709 | diabetes | upregulate | unknown | 225 |
| MEG3 | HGNC_ID: 14575 | mycobacterial infection bladder cancer | downregulate | Linked to MTOR activity and PI3K-AKT signaling pathway to regulate autophagy | 99,226 |
| PCGEM1 | HGNC_ID: 30145 | osteoarthritis | upregulate | Increases the expression of ATG12, ATG5, ATG3 and BECN1 | 227 |
| BANCR | HGNC_ID: 43877 | papillary thyroid carcinoma | upregulate | unknown | 228 |
| GAS5 | HGNC_ID: 16355 | osteoarthritis non-small cell lung cancer | upregulate | Acts as a negative regulator of MIR21 in autophagy | 229,230 |
| Chast | Ensembl_ID: ENSMUST00000130556 | cardiovascular | downregulate | Impedes Plekhm1 to autophagy inhibition and cardiomyocyte hypertrophy | 101 |
| H19 | HGNC_ID:4713 | diabetic cardiomyopathy | downregulate | Regulates DIRAS3 expression and promote MTOR phosphorylation to inhibit autophagy as cardiomyocytes exposed to high glucose | 231 |
| loc146880 | HGNC_ID:28630 | lung cancer | upregulate | PM2.5 exposure induces ROS, which activates loc146880 expression, and the lncRNA upregulates autophagy in return | 232 |
| HOTAIRM1 | HGNC_ID: 37117 | myeloid differentiation | upregulate | Acts as a miRNA sponge in a pathway that included MIR20A, MIR106B, MIR125B and their targets ULK1, E2F1 and DRAM2. | 233 |
| AlncRNA | — | hepatocellular carcinoma | upregulate | Targets multiple miRNAs including MIR21, MIR153, MIR216A, MIR217, MIR494 and MIR10A-5p | 234 |
| MALAT1 | HGNC_ID:29665 | hepatocellular carcinoma aggressive pancreatic cancer | upregulate | EPAS1/HIF-2α-MALAT1-MIR216B axis regulating MDR of HCC cells via modulating autophagy in hepatocellular carcinoma and via HuR-TIA-1-mediated autophagy activation in aggressive pancreatic cancer | 235,236 |
| AK156230 | GEO profile: AK156230 | mouse embryonic fibroblasts | upregulate | unknown | 237 |
| HOTAIR | HGNC_ID: 33510 | hepatocellular carcinoma | upregulate | Activates autophagy by increasing ATG3 and ATG7 expression | 238 |
| HNF1A-AS1 | HGNC_ID: 26785 | hepatocellular carcinoma | upregulate | Acts as an oncogene in tumor growth and apoptosis through sponging tumor-suppressive MIR30B-5p (MIR30B) and derepress BCL2 | 207 |
Ensembl Genome Browser, Gene Expression Omnibus (GEO), HUGO Gene Nomenclature Committee (HGNC)
Specifically, noncoding PTENP1, a pseudogene of the tumor suppressor gene PTEN, contains miRNA-binding sites that act as natural miRNA sponges, which bind shared miRNAs to regulate the cognate PTEN gene.97 In hepatocellular carcinoma, lncRNA suppresses the oncogenic PI3K-AKT-MTOR pathway to induce cellular autophagy and apoptosis through decoy MIR17, MIR19B, and MIR20A, which interact with PTEN and PHLPP, resulting in reduced autophagy levels (Fig. 3).97 At the same time, the PTENP1 pseudogene encodes 2 antisense RNA isoforms, α and β. The α isoform locates to the promoter region of PTEN to modulate its transcription via DNA methylation. In contrast, the β isoform combines with the PTENP1 lncRNA through RNA-RNA pairing to destabilize PTEN protein output.98 Similarly, the APF lncRNA regulates autophagic cell death by adsorbing MIR188–3p, and MIR188–3p inhibits ATG7 to suppress autophagy (Fig. 3).
Figure 3.
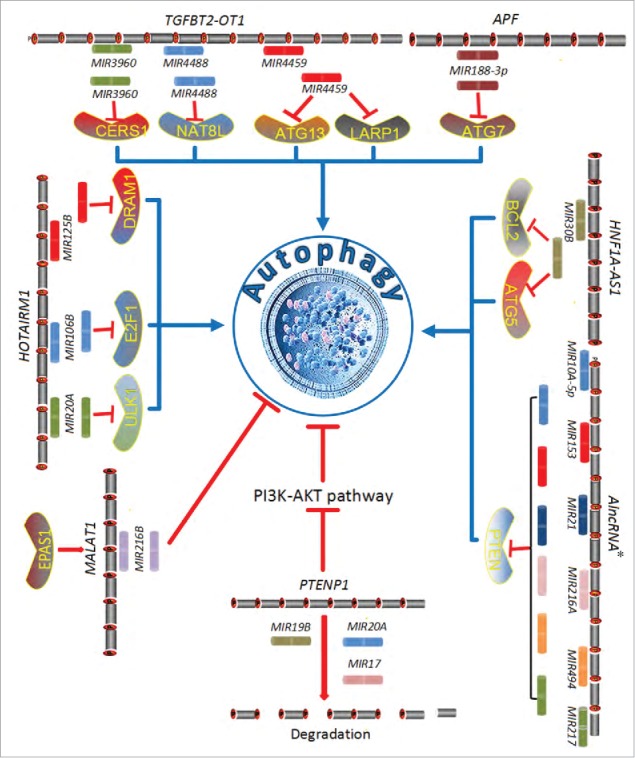
Conceptual schematic of regulation mechanism between miRNAs and lncRNAs in autophagy. AlncRNA* is an abbreviation of “an artificial long noncoding RNA.”
Crosstalk between autophagy and apoptosis is not converted by only miRNAs but also lncRNAs. For example, the downregulation of the lncRNA encoded by MEG3 increases autophagy but inhibits apoptosis to extend cell survival in bladder cancer.99 MEG3 lncRNA increases apoptosis to suppress cancer cell proliferation through TP53 regulation and, as mentioned in Section miRNAs and the regulation of autophagy, the downregulation of TP53 increases cytosolic HMGB1 to form the HMGB1-BECN1 complex, which promotes autophagy.71,99 Additionally, lncRNAs function as guide strands to influence cis or trans autophagy-related gene expression.100 The Chast lncRNA inhibits cardiac autophagy and exacerbates cardiomyocyte hypertrophy by impeding Plekhm1 gene expression during cardiac remodeling.101 PLEKHM1 is an autophagy regulator that plays an important role in vesicular transport and impedes autophagy in various cell lines.102,103 Suppression of Chast attenuates or reverses cardiomyocyte hypertrophy.101 Above all, lncRNAs act as competitive platforms for trans- or cis-regulation, and co-repression on target genes are crucial regulators of autophagy regulatory networks.99,101,104 Deepening knowledge will allow us to further understand mechanisms involving lncRNAs and autophagy. In terms of technology platforms, particularly sequencing technology such as high-throughput sequencing and “next-generation” sequencing, the depth and breadth of sequencing instrumentation are continuously improving to achieve higher accuracy in less time. Scientific research methods should also keep pace with this technological revolution. Methodology will be discussed in detail in the section below on integration of public resources and prediction of noncoding RNAs associated with autophagy.
Circular RNAs and autophagy regulation
As important and complementary members of the noncoding RNA family, the high-profile discovery of natural circRNAs was met with a great deal of interest. CircRNAs are novel endogenous noncoding RNAs that differ from traditional linear RNAs. The biogenesis of circRNAs is confusing and remains unclear, although circularization signals, exon-skipping events and splicing machinery are thought to participate in the circularization process.105,106 The exact mechanism by which the splicing machinery selects particular regions to circularize has not been fully elucidated.106 Among numerous convincing hypotheses, several theoretical models have been proposed to explain the possible formation of circRNAs, including lariat-driven circularization, intron-pairing-driven circularization and resplicing-driven circularization.16,107 In theory, any exon-skipping event holds the potential to cause cyclization, and a spliced lariat containing skipped exons will rapidly undergo internal splicing.16 Originally, circularized transcripts were thought to be byproducts of imperfect splicing, like lncRNAs, a notion supported by their low yield, lack of specific protective modifications and sequence conservation.108 However, this concept has been recently challenged.16,106 CircRNAs were not discovered earlier and received less attention because classic RNA detection methods specifically identify only RNA molecules with polyadenylated tails, and the generation of circRNAs involves polyadenylated tail loss.17
Potentially, circRNAs are cleaved by autophagic degradation and regulate autophagy in turn. Their higher stability endows circRNAs with more biological functions as intermediates in RNA processing reactions. For example, the circRNA CDR1-AS/ciRS-7/circRNA sponge for MIR7 (CDR1 antisense RNA) functions as a sponge for MIR7.109 CDR1-AS itself contains more than 70 conserved seed matches for MIR7. The seed matches are limited in their complementarity, which prevents bound MIR7 from being sliced from CDR1-AS by RISC.109,110 Interestingly, MIR7 suppresses cell viability and induces autophagy by inhibiting EGFR expression and efficiently regulates the PI3K-AKT-MTOR pathway to reduce AKT, MTOR and RPS6KB1 to inhibit tumor growth.111,112 Thus, as a natural MIR7 sponge, CDR1-AS may perturb its concentration and function. According to 2 different groups, the conserved, stable structure of CDR1-AS may be related to the activity and function of MIR7.109 Furthermore, CDR1-AS is sensitive to MIR671, and MIR671 directs the miRNA-mediated endonucleolytic cleavage of CDR1-AS.113 Therefore, CDR1-AS may be responsible for bringing MIR7 to a subcellular location where MIR671 promotes MIR7 slicing from CDR1-AS.17,114 Another circRNA, circular Foxo3, which is encoded along with the linear Foxo3 mRNA by the Foxo3 gene, appears to possess a high affinity for CDK2 and CDKN1A/p21.115 Deregulation of the Foxo3 gene is associated with AKT activity and PTEN silencing, both of which reduce autophagy.116,117 On the one hand, additional tests are required to determine whether circular Foxo3 affects Foxo3 gene transcription and translation to regulate Foxo3 mRNA and proteins during autophagy.118 On the other hand, there is a high affinity between circular Foxo3 and CDK2 that allows them to form a ternary complex with CDKN1A or interact with CDKN1B/p27.115 CDKN1A and CDKN1B are both inhibitors of CDK2. CDK2 phosphorylates CDKN1B to promote its degradation, and CDKN1B negatively regulates CDK2 to induce autophagy.119 Circular Foxo3 may construct a special molecular space structure with CDK2 to absorb or capture downstream proteins such as CDKN1B to regulate autophagy. Thus, autophagy is closely associated with RNA or protein dysfunction. The emergence of circRNAs represents a new perspective from which we will review the hierarchical regulation of one noncoding RNA by another in the context of autophagy-related noncoding RNAs. Depending on their unique 3-dimensional covalent structure, circRNAs effectively capture or sequester RNAs or proteins and release them in subcellular locations to mediate autophagy regulation. New types of noncoding RNAs hold great prospects for research and applications. Given the peculiarities of controlled inhibition and subsequent derepression, circRNAs also have the potential to be autophagy-related research tools.
Integration of public resources and the prediction of noncoding RNAs associated with autophagy
Based on our discussion and analysis in the preceding 3 modules, noncoding RNAs are crucial regulators of autophagy, evidenced by their intensive interactions with this process.120 However, this role is only the tip of the iceberg, and there remains a great deal for us to explore. The 21st century is the century of biologic information. In the post-genomic era, given massive workload requirements, requests for higher technology, scattered research sites, and vast amounts of experimental data, the need to develop public resources is urgent.121
Our team compiled relevant information on noncoding RNAs from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/pubmed/), including 4 noncoding autophagy-associated RNA databases (Table 4). Based on these databases and resources, we consolidated cross-species data, including data from Homo sapiens, Pan troglodytes, Macaca mulatta and others (Fig. 4). The consolidated data comprise 375 predicted miRNAs related to autophagy, including 46 miRNAs in Bos Taurus, 47 in Canis lupus, 3 in Danio rerio, 15 in Gallus gallus, 51 in Homo sapiens, 52 in Macaca mulatta, 62 in Mus musculus, 52 in Pan troglodytes and 47 in Rattus norvegicus (Table S2). The 4 core steps of autophagy, specifically induction, phagophore nucleation and expansion, and autophagosome and autolysosome maturation, as well as the intersection of the 9 species with predicted miRNAs is shown in a Venn diagram (Fig. 4). These graphs provide valuable and instructive predictive information regarding the regulatory relationships between noncoding RNAs and autophagy for scientists in this field. However, due to the lack of extensive overlap among the databases and the literature, these predicted noncoding RNAs may undergo biological regulation at certain key steps (Fig. 4). An integrative analysis of these databases and resources may provide new insights for solutions to the tough issues being investigated by small- to moderately-sized autophagy research groups. The new unconfirmed regulatory mechanisms between noncoding RNAs and the autophagy regulation network may be clarified by analyzing these predicted noncoding RNAs in different species. Reality, however, may prove different. On the one hand, we may amass a large number of predicted autophagy-related noncoding RNAs. On the other hand, these noncoding RNAs may be related to autophagy regulation. Confusion may lie in revealing specific regulatory mechanisms to connect the 2.
Table 4.
Noncoding RNA-associated autophagy databases.
| Database Name | website | Ref |
|---|---|---|
| Autophagy Regulatory Network | http://autophagy-regulation.org/search | 239 |
| The Autophagy Database | http://www.tanpaku.org/autophagy/index.html | 240 |
| ncRDeathDB | http://www.rna-society.org/ncrdeathdb/index.php | 241 |
| GAMDB | http://gamdb.liu-laboratory.com/index.php | 121 |
Figure 4.
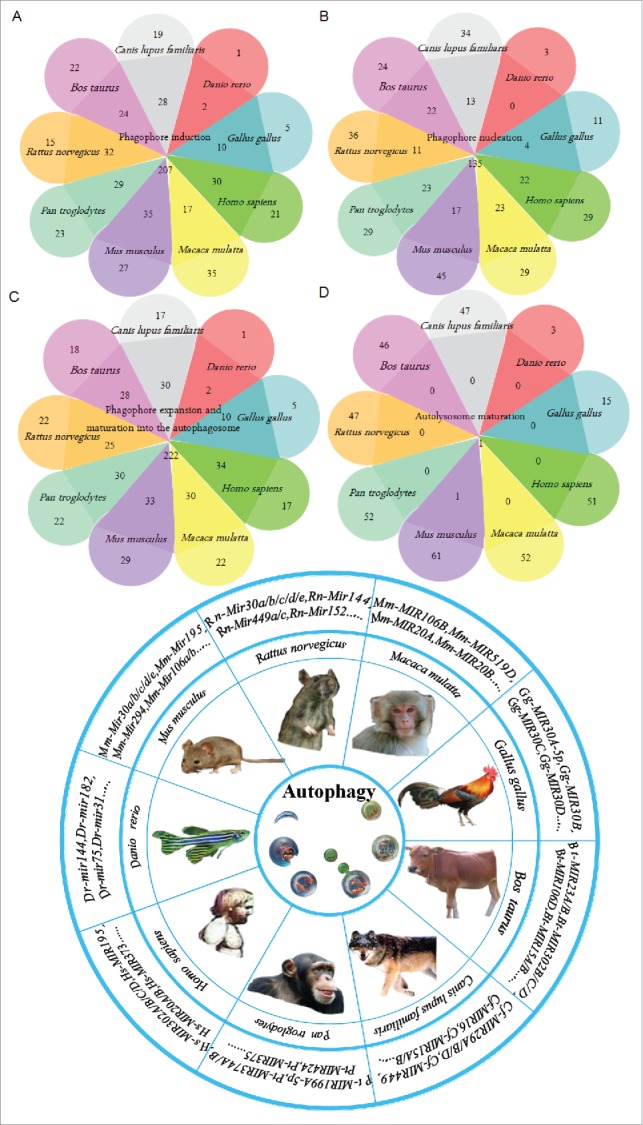
An overview of the functional and physical interactions between multiple predicted miRNAs from different species and autophagy. The Venn diagram includes 375 predicted miRNAs in the analysis. According to species, we assigned the miRNAs to 9 groups during the different steps of autophagy, and the data intersection is shown in the Venn diagram. The pie chart presents the different species involved in this biological miRNA prediction and the representative predicted miRNAs that are involved in autophagy.
Predicted noncoding RNAs have been compiled from the 4 noncoding autophagy-associated RNA databases (Table 4 and Table S2), and the development of sequencing instruments with greater depth and breadth will allow us to identify many more unknown RNAs associated with autophagy. These noncoding RNAs are merely nodes in the autophagy regulation network. The interactions between them require analysis on multiple levels. Thus, we urge the development of affordable bioinformatics tools to solve these problems as well as the construction of computational databases or the analysis of noncoding RNA transcriptome sequences, as detailed in Table 5. Such resources will allow us to predict putative, related upstream or downstream noncoding RNAs and proteins in a relatively objective manner. In particular, computational analysis will act as a beacon to guide us. However, these methods alone are not sufficient for us to carry out the research. In practice, there are many uncertainties; therefore, we will likely need to carry out bioinformatics analyses to calculate and analyze potential correlations in the autophagy regulation network to narrow our research scope as much as possible. For example, MIR188–3p is predicted to take part in autophagy regulation, but we lack knowledge of its upstream and downstream relationships. Given these circumstances, bioinformatics tools such as RNAhybrid and bioinformatics analyses were used to predict hidden relationships, followed by experimental verification, and researchers ultimately identified an autophagy regulatory axis: APF-MIR188–3p-ATG7.104 In this way, research methodology matches technological progress: we not only rely on upgraded technology to discover novel autophagy-related noncoding RNAs but also use this methodology in combination with experimental technology to explore specific regulatory mechanisms.
Table 5.
Noncoding RNA-associated databases and resources.
| Category | Name | Description | Type | Link |
|---|---|---|---|---|
| miRNA databases | StarBase | StarBase is designed for decoding miRNA-lncRNA, miRNA-mRNA, miRNA-circRNA, lncRNA-protein and other interactions and ceRNA networks from 108 CLIP-Seq data sets. It also provides Pan-Cancer Analysis for miRNAs, lncRNAs, circRNAs and protein-coding genes | database | http://starbase.sysu.edu.cn/ |
| StarScan | StarScan is developed for scanning small RNA (e.g., miRNA)-mediated RNA cleavage events in lncRNA, circRNA and mRNA from degradome sequencing data | software | http://mirlab.sysu.edu.cn/starscan/ | |
| Cupid | Cupid is a method for simultaneous prediction of miRNA-target interactions and their mediated ceRNA interactions. It is an integrative approach that significantly improves on miRNA-target prediction accuracy | software | http://cupidtool.sourceforge.net/ | |
| TargetScan | Predicts biological targets of miRNAs by searching for the presence of sites that match the seed region of each miRNA | database | http://www.targetscan.org/vert_71/ | |
| TarBase | A comprehensive database of experimentally supported animal miRNA targets | database | http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index | |
| miRecords | An integrated resource for miRNA-target interactions | database | http://c1.accurascience.com/miRecords/ | |
| PicTar | PicTar is combinatorial miRNA target predictions | database | http://pictar.bio.nyu.edu/ | |
| PITA | PITA is an integrated resource to predict by base-pairing interactions within the mRNA and in miRNA target recognition | software | https://genie.weizmann.ac.il/pubs/mir07/mir07_data.html | |
| miRTarBase | The experimentally validated miRNA-target interactions database | database | http://mirtarbase.mbc.nctu.edu.tw/ | |
| deepBase | deepBase is a database for annotating and discovering small and long ncRNAs from high-throughput deep sequencing data | database | http://deepbase.sysu.edu.cn/ | |
| microRNA.org | miRNA.org is designed for experimentally observed miRNA expression patterns and predicted miRNA targets | database | http://www.microrna.org/microrna/getExprForm.do | |
| lncRNA databases | lncRNABase | Designed for decoding miRNA-lncRNA and miRNA-ceRNA interaction networks from 108 CLIP-Seq data sets and provides Pan-Cancer interaction networks of lncRNAs, miRNAs, ceRNAs, mRNAs and RNA-Binding Proteins | database | http://starbase.sysu.edu.cn/mirLncRNA.php |
| ChIPBase | ChIPBase is a database for annotating and exploring the expression profiles and the transcriptional regulation of lncRNAs and other ncRNAs | database | http://deepbase.sysu.edu.cn/chipbase/ | |
| lncRNAdb | lncRNAdb is a database providing comprehensive annotations of functional long noncoding RNAs | database | http://lncrnadb.com/ | |
| NONCODE | NONCODE provides an integrative annotation of long noncoding RNAs | database | http://www.noncode.org/ | |
| lncRNome | lncRNome is a comprehensive searchable biologically oriented knowledgebase for long noncoding RNAs in humans | database | http://genome.igib.res.in/lncRNome/ | |
| NRED | A database of long noncoding RNA expression | database | http://nred.matticklab.com/cgi-bin/ncrnadb.pl | |
| LNCipedia | A database for annotated human lncRNA transcript sequences and structures | database | http://www.lncipedia.org/ | |
| CircRNA databases | circRNABase | The circRNABase is designed for decoding miRNA-circRNA interaction networks from thousands of circRNAs and 108 CLIP-Seq data sets | database | http://starbase.sysu.edu.cn/mirCircRNA.php |
| circBase | The circBase collects public circRNA data sets and support to download the custom python scripts | database | http://www.circbase.org/ | |
| CircNet | CircNet records public and novel circRNAs and putative circRNA-miRNA interactions | database | http://circnet.mbc.nctu.edu.tw/ | |
| Circ2Traits | Circ2Traits is a comprehensive database for circular RNA potentially associated with disease and traits | database | http://gyanxet-β.com/circdb/ |
Discussion
As described in the sections above, autophagy in response to stress is an evolutionary mechanism for survival that involves protein and organelle recycling.122 Noncoding RNAs, considered “transcriptional trash,” participate in many biologic processes and play important roles in autophagy.38 The field investigating autophagy regulation by noncoding RNAs continues to grow both in terms of volume and impact. However, autophagy and noncoding RNA research is still in its infancy, and a great deal of information remains to be elucidated, such as the paradox of autophagy effects versus noncoding RNA control, deficiencies in research methods, imperfect practical applications and others.
The effects of autophagy directed by noncoding RNAs have remained controversial for many years. Whether autophagy regulated by noncoding RNAs is a cell death mechanism or a cell survival mechanism, both sides of the argument are independent.104,123,124 Meanwhile noncoding RNAs also appear to exert bilateral regulation.125 The uncertainty of autophagy and the dual roles of noncoding RNAs complicate our understanding of associated regulatory mechanisms, making explanations difficult. Quality control plays a critical role in cellular autophagy and is involved in protein dynamics.126 Unfortunately, the concrete mechanism of quality control and the full dynamic process by which misfolded or damaged proteins are incorporated into phagophores still remains unclear.
Further improvements should allow us to visualize the dynamic machinery of autophagy with higher spatiotemporal resolution. The emergence of circRNAs exhibiting stronger stability and cytoplasm localization through molecular engineering will potentially result in the development of capture and imaging devices that are superior to LC3 and SQSTM1 for monitoring dynamics.127 However, the construction of genetic animal models remains a research predicament. A major deficit of traditional genetic animal models is the inability to reproduce major age-dependent characteristics starting from birth.34 Thus, it is impossible to compare the effects of impairing noncoding RNAs on autophagy over time. The introduction of conditional knockouts such as through CRISPR/Cas9 may partially help us overcome this problem.128 Additionally, previous studies exploring a single autophagy gene have given different results for partial and nonsystematic interference. We should turn to multidisciplinary and integrated public databases to examine interference by single or multiple factors with noncoding RNAs and to elucidate the multiple genes and steps involved in the complex autophagy network regulated by noncoding RNAs. In parallel with mechanistic research, the application of dysregulated noncoding RNAs in autophagy has received a great deal of attention.129,130
In terms of clinical applications to elicit selective cell death, the induction of apoptosis via therapeutic targeting of the apoptosis pathway demonstrates significant benefits.131 However, given the resistance to traditional chemotherapeutic drugs that induce apoptosis, it is not appropriate to simply abandon survival in favor of cell death.131 Autophagy features prominent crosstalk between cell survival and death. Abnormal autophagy regulated by noncoding RNAs is associated with the occurrence of certain diseases, and these dysregulated noncoding RNAs are latent therapeutic targets.132 The introduction of RNA interference may shed light upon diseases involving deficient or sufficient autophagy directed by noncoding RNAs.133 The development of RNAi demonstrating high efficiency and specificity has proven valuable.134,135 However, there are concerns regarding the biosafety and reliability of RNAi delivery systems.136 Technology optimization may help solve such problems. For example, a dual-purpose probe consisting of magnetic nanoparticles and Cy5.5 dye conjugated to an RNAi duplex may function as an imaging tracer.137 Such a design represents a new way of using dysregulated noncoding RNAs as specific targets in autophagy-associated therapeutic strategies.
More information regarding conformation errors and the improper localization of lipid molecules during phagophore nucleation and autophagosome formation caused by dysregulated noncoding RNAs will be obtained, and structure, functional polymer and genetic analyses of isolated membranes and regulatory noncoding RNAs will be undertaken. Ultimately, a complete understanding of autophagy and noncoding RNAs as well as relevant applications should be an objective for all scientists working in this field.
Supplementary Material
Abbreviations
- AKT
AKT serine/threonine kinase
- AMPK
AMP-activated protein kinase
- APF
autophagy-promoting factor
- ATG
autophagy-related
- BANCR
BRAF-activated non-protein coding RNA
- BCL2BCL2
BCL2apoptosis regulator
- BECN1
beclin 1
- circRNA
circular RNA
- ceRNA
competing endogenous RNA
- Chast
cardiac hypertrophy-associated transcript
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9
- GAS5
growth arrest specific 5 (non-protein coding)
- LARP1
La ribonucleoprotein domain family member 1
- lncRNA
long noncoding RNA
- MAP1LC3
microtubule associated protein 1 light chain 3
- MCL1
myeloid cell leukemia sequence 1
- MEG3
maternally expressed 3 (non-protein coding)
- miRNA
microRNA
- MTOR
mechanistic target of rapamycin
- NBR2
neighbor of BRCA1 gene 2 (non-protein coding)
- PLEKHM1
pleckstrin homology and RUN domain containing M1
- PtdIns3K
class III phosphatidylinositol 3-kinase
- PTENP1
phosphatase and tensin homolog pseudogene 1
- PVT1
Pvt1 oncogene (non-protein coding)
- RISCs
RNA-induced silencing complexes
- RNAi
RNA interference
- rRNA
rRNA
- TGFB2-OT1
TGFB2 overlapping transcript 1
- tRNA
tRNA
- ULK1
unc-51 like kinase 1
- UVRAG
UV irradiation resistance associated gene
- Vps
vacuolar protein sorting
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professors Hongbo Liu for assistance with figure preparation, critical reading of the manuscript and document submission. The data used in this review were partially supported by 4 public databases from the Autophagy Regulatory Network, the Autophagy Database, ncRDeathDB and GAMDB.
Funding
This work was supported by funding from the Project of Heilongjiang Province Applied Technology Research and Development (grant number GA13C201), the National Health and Family Planning Commission of the People's Republic of China (grant number 201402003), the National Key Technology Support Program (grant number 2014BAI09B08) and the National Natural Science Foundation of China (grant number 81602323).
References
- [1].Gupta SK, Thum T. Non-coding RNAs as orchestrators of autophagic processes. J Mol Cell Cardiol 2016; 95:26-30; PMID:26654780; https://doi.org/ 10.1016/j.yjmcc.2015.11.012 [DOI] [PubMed] [Google Scholar]
- [2].Sharp PA. The centrality of RNA. Cell 2009; 136:577-80; PMID:19239877; https://doi.org/ 10.1016/j.cell.2009.02.007 [DOI] [PubMed] [Google Scholar]
- [3].Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014; 157:77-94; PMID:24679528; https://doi.org/ 10.1016/j.cell.2014.03.008 [DOI] [PubMed] [Google Scholar]
- [4].Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al.. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464:1071-6; PMID:20393566; https://doi.org/ 10.1038/nature08975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao G, Su Z, Song D, Mao Y, Mao X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-kappaB. FEBS Lett 2016; 590:2884–95; PMID:27434861 [DOI] [PubMed] [Google Scholar]
- [6].Samarsky DA, Fournier MJ, Singer RH, Bertrand E. The snoRNA box C/D motif directs nucleolar targeting and also couples snoRNA synthesis and localization. EMBO J 1998; 17:3747-57; PMID:9649444; https://doi.org/ 10.1093/emboj/17.13.3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Knowling S, Morris KV. Non-coding RNA and antisense RNA. Nature's trash or treasure? Biochimie 2011; 93:1922-7; PMID:21843589; https://doi.org/ 10.1016/j.biochi.2011.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843-54; PMID:8252621; https://doi.org/ 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- [9].Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 2012; 336:233-7; PMID:22422859; https://doi.org/ 10.1126/science.1215704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Costa FF. Non-coding RNAs: meet thy masters. BioEssays 2010; 32:599-608; PMID:20544733; https://doi.org/ 10.1002/bies.200900112 [DOI] [PubMed] [Google Scholar]
- [11].Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52:1527-39; PMID:19007588; https://doi.org/ 10.1016/j.jacc.2008.07.051 [DOI] [PubMed] [Google Scholar]
- [12].Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 2008; 322:750-6; PMID:18974356; https://doi.org/ 10.1126/science.1163045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al.. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 2009; 106:11667-72; PMID:19571010; https://doi.org/ 10.1073/pnas.0904715106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu MY, Gui G, Wei B, Preston JF 3rd, Oakford L, Yuksel U, Giedroc DP, Romeo T. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem 1997; 272:17502-10; PMID:9211896; https://doi.org/ 10.1074/jbc.272.28.17502 [DOI] [PubMed] [Google Scholar]
- [15].Shao Y, Chen Y. Roles of circular RNAs in neurologic disease. Front Mol Neurosci 2016; 9:25; PMID:27147959; https://doi.org/ 10.3389/fnmol.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna 2013; 19:141-57; PMID:23249747; https://doi.org/ 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al.. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495:333-8; PMID:23446348; https://doi.org/ 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- [18].Xie Z, Klionsky DJ. Autophagosome formation: Core machinery and adaptations. Nat Cell Biol 2007; 9:1102-9; PMID:17909521; https://doi.org/ 10.1038/ncb1007-1102 [DOI] [PubMed] [Google Scholar]
- [19].Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol 1962; 12:198-202; PMID:13862833; https://doi.org/ 10.1083/jcb.12.1.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 2010; 12:814-22; PMID:20811353; https://doi.org/ 10.1038/ncb0910-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al.. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016; 12:1-222; PMID:26799652; https://doi.org/ 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005; 12(Suppl 2):1542-52; PMID:16247502; https://doi.org/ 10.1038/sj.cdd.4401765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011; 147:728-41; PMID:22078875; https://doi.org/ 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- [24].Novikoff AB, Essner E, Quintana N. Golgi apparatus and lysosomes. Fed Proc 1964; 23:1010–22; PMID:14209792 [PubMed] [Google Scholar]
- [25].Deter RL, Baudhuin P, De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol 1967; 35:C11-6; PMID:6055998; https://doi.org/ 10.1083/jcb.35.2.C11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pfeifer U. Inhibition by insulin of the physiological autophagic breakdown of cell organelles. Acta Biol Med Ger 1977; 36:1691–4; PMID:616715 [PubMed] [Google Scholar]
- [27].Mortimore GE, Schworer CM. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 1977; 270:174-6; PMID:927529; https://doi.org/ 10.1038/270174a0 [DOI] [PubMed] [Google Scholar]
- [28].Mortimore GE, Lardeux BR, Adams CE. Regulation of microautophagy and basal protein turnover in rat liver. Effects of short-term starvation. J Biol Chem 1988; 263:2506–12; PMID:3257493 [PubMed] [Google Scholar]
- [29].Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci 2011; 124:161-70; PMID:21187343; https://doi.org/ 10.1242/jcs.064576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klionsky DJ. Cell biology: Regulated self-cannibalism. Nature 2004; 431:31-2; PMID:15343317; https://doi.org/ 10.1038/431031a [DOI] [PubMed] [Google Scholar]
- [31].Xilouri M, Stefanis L. Chaperone mediated autophagy in aging: Starve to prosper. Ageing Res Rev 2016; 32:13–21; PMID:27484893 [DOI] [PubMed] [Google Scholar]
- [32].Yang Y, Zhao C, Yang P, Wang X, Wang L, Chen A. Autophagy in cardiac metabolic control: Novel mechanisms for cardiovascular disorders. Cell Biol Int 2016; 40:944-54; PMID:27191043; https://doi.org/ 10.1002/cbin.10626 [DOI] [PubMed] [Google Scholar]
- [33].Chargui A, El May MV. Autophagy mediates neutrophil responses to bacterial infection. APMIS 2014; 122:1047–58; PMID:24735202 [DOI] [PubMed] [Google Scholar]
- [34].Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069-75; PMID:18305538; https://doi.org/ 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402:672-6; PMID:10604474; https://doi.org/ 10.1038/45257 [DOI] [PubMed] [Google Scholar]
- [36].Cheong H. Integrating autophagy and metabolism in cancer. Arch Pharm Res 2015; 38:358-71; PMID:25614051; https://doi.org/ 10.1007/s12272-015-0562-2 [DOI] [PubMed] [Google Scholar]
- [37].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al.. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009; 137:1062-75; PMID:19524509; https://doi.org/ 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, Liu CG, Yang JM. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 2009; 5:816-23; PMID:19535919; https://doi.org/ 10.4161/auto.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang S, Lu W, Ge D, Meng N, Li Y, Su L, Zhang S, Zhang Y, Zhao B, Miao J. A new microRNA signal pathway regulated by long noncoding RNA TGFB2-OT1 in autophagy and inflammation of vascular endothelial cells. Autophagy 2015; 11:2172-83; PMID:26565952; https://doi.org/ 10.1080/15548627.2015.1106663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 1992; 119:301-11; PMID:1400575; https://doi.org/ 10.1083/jcb.119.2.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 2009; 284:12297-305; PMID:19258318; https://doi.org/ 10.1074/jbc.M900573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22:132-9; PMID:20056399; https://doi.org/ 10.1016/j.ceb.2009.12.004 [DOI] [PubMed] [Google Scholar]
- [43].Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 2007; 12:209–18; PMID:17295840; https://doi.org/ 10.1111/j.1365-2443.2007.01050.x [DOI] [PubMed] [Google Scholar]
- [44].Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, et al.. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 2007; 9:1142-51; PMID:17891140; https://doi.org/ 10.1038/ncb1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 2006; 8:688-99; PMID:16799551; https://doi.org/ 10.1038/ncb1426 [DOI] [PubMed] [Google Scholar]
- [46].Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 2009; 11:468-76; PMID:19270693; https://doi.org/ 10.1038/ncb1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al.. A ubiquitin-like system mediates protein lipidation. Nature 2000; 408:488-92; PMID:11100732; https://doi.org/ 10.1038/35044114 [DOI] [PubMed] [Google Scholar]
- [48].Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 2001; 152:657-68; PMID:11266458; https://doi.org/ 10.1083/jcb.152.4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720-8; PMID:11060023; https://doi.org/ 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal 2009; 2:pe51; PMID:19690328; https://doi.org/ 10.1126/scisignal.284pe51 [DOI] [PubMed] [Google Scholar]
- [51].Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, et al.. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 2012; 31:1095-108; PMID:22343943; https://doi.org/ 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Song L, Su M, Wang S, Zou Y, Wang X, Wang Y, Cui H, Zhao P, Hui R, Wang J. MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med 2014; 18:2266-74; PMID:25209900; https://doi.org/ 10.1111/jcmm.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang J, Yang K, Zhou L, Minhaowu Wu Y, Zhu M, Lai X, Chen T, Feng L, Li M, et al.. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS pathog 2013; 9:e1003697; PMID:24130493; https://doi.org/ 10.1371/journal.ppat.1003697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hall DP, Cost NG, Hegde S, Kellner E, Mikhaylova O, Stratton Y, Ehmer B, Abplanalp WA, Pandey R, Biesiada J, et al.. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014; 26:738-53; PMID:25517751; https://doi.org/ 10.1016/j.ccell.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S, Collins S, Kumar D. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget 2015; 6:34446–57; PMID:26439987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, et al.. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem 2002; 277:35364-70; PMID:12167664; https://doi.org/ 10.1074/jbc.M205838200 [DOI] [PubMed] [Google Scholar]
- [57].Nelson C, Ambros V, Baehrecke EH. miR-14 regulates autophagy during developmental cell death by targeting ip3-kinase 2. Mol Cell 2014; 56:376-88; PMID:25306920; https://doi.org/ 10.1016/j.molcel.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ 3rd, Li L, et al.. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res 2012; 72:1996-2005; PMID:22345153; https://doi.org/ 10.1158/0008-5472.CAN-11-2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo YJ, Liu JX, Guan YW. Hypoxia induced upregulation of miR-301a/b contributes to increased cell autophagy and viability of prostate cancer cells by targeting NDRG2. Eur Rev Med Pharmacol Sci 2016; 20:101–8; PMID:26813459 [PubMed] [Google Scholar]
- [60].Yang Z, Han Y, Cheng K, Zhang G, Wang X. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif 2014; 47:587-95; PMID:25348507; https://doi.org/ 10.1111/cpr.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 2014; 109:423; PMID:25060662; https://doi.org/ 10.1007/s00395-014-0423-z [DOI] [PubMed] [Google Scholar]
- [62].Wu H, Wang F, Hu S, Yin C, Li X, Zhao S, Wang J, Yan X. MiR-20a and miR-106b negatively regulate autophagy induced by leucine deprivation via suppression of ULK1 expression in C2C12 myoblasts. Cell Signal 2012; 24:2179-86; PMID:22781751; https://doi.org/ 10.1016/j.cellsig.2012.07.001 [DOI] [PubMed] [Google Scholar]
- [63].He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol 2010; 22:140-9; PMID:20097051; https://doi.org/ 10.1016/j.ceb.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005; 122:927-39; PMID:16179260; https://doi.org/ 10.1016/j.cell.2005.07.002 [DOI] [PubMed] [Google Scholar]
- [65].Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 2008; 4:600-6; PMID:28186856; https://doi.org/ 10.4161/auto.6260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Korkmaz G, le Sage C, Tekirdag KA, Agami R, Gozuacik D. miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 2012; 8:165-76; PMID:22248718; https://doi.org/ 10.4161/auto.8.2.18351 [DOI] [PubMed] [Google Scholar]
- [67].Seca H, Lima RT, Lopes-Rodrigues V, Guimaraes JE, Almeida GM, Vasconcelos MH. Targeting miR-21 induces autophagy and chemosensitivity of leukemia cells. Curr Drug Targets 2013; 14:1135-43; PMID:23834154; https://doi.org/ 10.2174/13894501113149990185 [DOI] [PubMed] [Google Scholar]
- [68].Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H, Luo G, Liao W, Bin J, Huang X, et al.. Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget 2015; 6:18829-44; PMID:26299920; https://doi.org/ 10.18632/oncotarget.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Germain M, Nguyen AP, Le Grand JN, Arbour N, Vanderluit JL, Park DS, Opferman JT, Slack RS. MCL-1 is a stress sensor that regulates autophagy in a developmentally regulated manner. EMBO J 2011; 30:395-407; PMID:21139567; https://doi.org/ 10.1038/emboj.2010.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kumar R, Sahu SK, Kumar M, Jana K, Gupta P, Gupta UD, Kundu M, Basu J. MicroRNA 17-5p regulates autophagy in Mycobacterium tuberculosis-infected macrophages by targeting Mcl-1 and STAT3. Cell Microbiol 2016; 18:679-91; PMID:26513648; https://doi.org/ 10.1111/cmi.12540 [DOI] [PubMed] [Google Scholar]
- [71].Kang R, Livesey KM, Zeh HJ, Loze MT, Tang D. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy 2010; 6:1209-11; PMID:20935509; https://doi.org/ 10.4161/auto.6.8.13651 [DOI] [PubMed] [Google Scholar]
- [72].Kim Y, Kang YS, Lee NY, Kim KY, Hwang YJ, Kim HW, Rhyu IJ, Her S, Jung MK, Kim S, et al.. Uvrag targeting by Mir125a and Mir351 modulates autophagy associated with Ewsr1 deficiency. Autophagy 2015; 11:796-811; PMID:25946189; https://doi.org/ 10.1080/15548627.2015.1035503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim JK, Yuk JM, Kim SY, Kim TS, Jin HS, Yang CS, Jo EK. MicroRNA-125a inhibits autophagy activation and antimicrobial responses during mycobacterial infection. J Immunol 2015; 194:5355-65; PMID:25917095; https://doi.org/ 10.4049/jimmunol.1402557 [DOI] [PubMed] [Google Scholar]
- [74].He J, Yu JJ, Xu Q, Wang L, Zheng JZ, Liu LZ, Jiang BH. Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy. Autophagy 2015; 11:373-84; PMID:25650716; https://doi.org/ 10.1080/15548627.2015.1009781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, Jaattela M, Lund AH. microRNA-101 is a potent inhibitor of autophagy. EMBO J 2011; 30:4628-41; PMID:21915098; https://doi.org/ 10.1038/emboj.2011.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol 2010; 12:831-5; PMID:20811355; https://doi.org/ 10.1038/ncb0910-831 [DOI] [PubMed] [Google Scholar]
- [77].Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell 2004; 6:79-90; PMID:14723849; https://doi.org/ 10.1016/S1534-5807(03)00402-7 [DOI] [PubMed] [Google Scholar]
- [78].Yang J, Chen D, He Y, Melendez A, Feng Z, Hong Q, Bai X, Li Q, Cai G, Wang J, et al.. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age (Dordr) 2013; 35:11-22; PMID:22081425; https://doi.org/ 10.1007/s11357-011-9324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fu Q, Shi H, Ni W, Shi M, Meng L, Zhang H, Ren Y, Guo F, Wang P, Qiao J, et al.. Lentivirus-mediated Bos taurus bta-miR-29b overexpression interferes with bovine viral diarrhoea virus replication and viral infection-related autophagy by directly targeting ATG14 and ATG9A in Madin-Darby bovine kidney cells. J Gen Virol 2015; 96:85-94; PMID:25234643; https://doi.org/ 10.1099/vir.0.067140-0 [DOI] [PubMed] [Google Scholar]
- [80].Fujita Y, Araya J, Ito S, Kobayashi K, Kosaka N, Yoshioka Y, Kadota T, Hara H, Kuwano K, Ochiya T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles 2015; 4:28388; PMID:26563733; https://doi.org/ 10.3402/jev.v4.28388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Comincini S, Allavena G, Palumbo S, Morini M, Durando F, Angeletti F, Pirtoli L, Miracco C. microRNA-17 regulates the expression of ATG7 and modulates the autophagy process, improving the sensitivity to temozolomide and low-dose ionizing radiation treatments in human glioblastoma cells. Cancer Biol Ther 2013; 14:574-86; PMID:23792642; https://doi.org/ 10.4161/cbt.24597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F, Xia Q. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun 2012; 423:826-31; PMID:22713463; https://doi.org/ 10.1016/j.bbrc.2012.06.048 [DOI] [PubMed] [Google Scholar]
- [83].Nguyen HT, Dalmasso G, Muller S, Carriere J, Seibold F, Darfeuille-Michaud A. Crohn's disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 2014; 146:508-19; PMID:24148619; https://doi.org/ 10.1053/j.gastro.2013.10.021 [DOI] [PubMed] [Google Scholar]
- [84].Zhai Z, Wu F, Chuang AY, Kwon JH. miR-106b fine tunes ATG16L1 expression and autophagic activity in intestinal epithelial HCT116 cells. Inflamm Bowel Dis 2013; 19:2295-301; PMID:23899543; https://doi.org/ 10.1097/MIB.0b013e31829e71cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jian X, Xiao-yan Z, Bin H, Yu-feng Z, Bo K, Zhi-nong W, Xin N. MiR-204 regulate cardiomyocyte autophagy induced by hypoxia-reoxygenation through LC3-II. Int J Cardiol 2011; 148:110-2; PMID:21316776; https://doi.org/ 10.1016/j.ijcard.2011.01.029 [DOI] [PubMed] [Google Scholar]
- [86].Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 2009; 452:181–97; PMID:19200883 [DOI] [PubMed] [Google Scholar]
- [87].Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 2010; 285:22576-91; PMID:20452972; https://doi.org/ 10.1074/jbc.M110.118976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Meenhuis A, van Veelen PA, de Looper H, van Boxtel N, van den Berge IJ, Sun SM, Taskesen E, Stern P, de Ru AH, van Adrichem AJ, et al.. MiR-17/20/93/106 promote hematopoietic cell expansion by targeting sequestosome 1-regulated pathways in mice. Blood 2011; 118:916-25; PMID:21628417; https://doi.org/ 10.1182/blood-2011-02-336487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tao J, Liu W, Shang G, Zheng Y, Huang J, Lin R, Chen L. MiR-207/352 regulate lysosomal-associated membrane proteins and enzymes following ischemic stroke. Neuroscience 2015; 305:1-14; PMID:26232047; https://doi.org/ 10.1016/j.neuroscience.2015.07.064 [DOI] [PubMed] [Google Scholar]
- [90].Crick FH. On protein synthesis. Symp Soc Exp Biol 1958; 12:138–63; PMID:13580867 [PubMed] [Google Scholar]
- [91].Crick F. Central dogma of molecular biology. Nature 1970; 227:561-3; PMID:4913914; https://doi.org/ 10.1038/227561a0 [DOI] [PubMed] [Google Scholar]
- [92].Fritah S, Niclou SP, Azuaje F. Databases for lncRNAs: a comparative evaluation of emerging tools. Rna 2014; 20:1655-65; PMID:25323317; https://doi.org/ 10.1261/rna.044040.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, et al.. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012; 22:1775-89; PMID:22955988; https://doi.org/ 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lee JT. Epigenetic regulation by long noncoding RNAs. Science 2012; 338:1435-9; PMID:23239728; https://doi.org/ 10.1126/science.1231776 [DOI] [PubMed] [Google Scholar]
- [95].Papait R, Kunderfranco P, Stirparo GG, Latronico MV, Condorelli G. Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl Res 2013; 6:876-83; PMID:23835777; https://doi.org/ 10.1007/s12265-013-9488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012; 482:339-46; PMID:22337053; https://doi.org/ 10.1038/nature10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, Hu YC. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 2015; 44:71-81; PMID:25617127; https://doi.org/ 10.1016/j.biomaterials.2014.12.023 [DOI] [PubMed] [Google Scholar]
- [98].Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grander D, Morris KV. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol 2013; 20:440-6; PMID:23435381; https://doi.org/ 10.1038/nsmb.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ying L, Huang Y, Chen H, Wang Y, Xia L, Chen Y, Liu Y, Qiu F. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol BioSystems 2013; 9:407-11; PMID:23295831; https://doi.org/ 10.1039/c2mb25386k [DOI] [PubMed] [Google Scholar]
- [100].Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Ann Rev Biochem 2012; 81:145-66; PMID:22663078; https://doi.org/ 10.1146/annurev-biochem-051410-092902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, et al.. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med 2016; 8:326ra22; PMID:26888430; https://doi.org/ 10.1126/scitranslmed.aaf1475 [DOI] [PubMed] [Google Scholar]
- [102].Del Fattore A, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B, Cappariello A, Rucci N, Spera G, Helfrich MH, et al.. A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts. J Bone Miner Res 2008; 23:380-91; PMID:17997709; https://doi.org/ 10.1359/jbmr.071107 [DOI] [PubMed] [Google Scholar]
- [103].McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, Coxon FP, Miranda de Stegmann D, Bhogaraju S, Maddi K, et al.. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell 2015; 57:39-54; PMID:25498145; https://doi.org/ 10.1016/j.molcel.2014.11.006 [DOI] [PubMed] [Google Scholar]
- [104].Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B, Zhao WK, Xu SJ, Fan LH, Zhang XJ, et al.. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun 2015; 6:6779; PMID:25858075; https://doi.org/ 10.1038/ncomms7779 [DOI] [PubMed] [Google Scholar]
- [105].Zaphiropoulos PG. Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol Cell Biol 1997; 17:2985-93; PMID:9154796; https://doi.org/ 10.1128/MCB.17.6.2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science 2013; 340:440–1; PMID: 23620042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chen I, Chen CY, Chuang TJ. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip Rev RNA 2015; 6:563-79; PMID:26230526; https://doi.org/ 10.1002/wrna.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 2014; 15:409; PMID:25070500; https://doi.org/ 10.1186/s13059-014-0409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495:384-8; PMID:23446346; https://doi.org/ 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- [110].Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, et al.. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 2015; 58:870-85; PMID:25921068; https://doi.org/ 10.1016/j.molcel.2015.03.027 [DOI] [PubMed] [Google Scholar]
- [111].Tazawa H, Yano S, Yoshida R, Yamasaki Y, Sasaki T, Hashimoto Y, Kuroda S, Ouchi M, Onishi T, Uno F, et al.. Genetically engineered oncolytic adenovirus induces autophagic cell death through an E2F1-microRNA-7-epidermal growth factor receptor axis. Int J Cancer 2012; 131:2939-50; PMID:22492316; https://doi.org/ 10.1002/ijc.27589 [DOI] [PubMed] [Google Scholar]
- [112].Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 2012; 55:1852-62; PMID:22234835; https://doi.org/ 10.1002/hep.25576 [DOI] [PubMed] [Google Scholar]
- [113].Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J 2011; 30:4414-22; PMID:21964070; https://doi.org/ 10.1038/emboj.2011.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kosik KS. Molecular biology: circles reshape the RNA world. Nature 2013; 495:322–4; PMID:23446351 [DOI] [PubMed] [Google Scholar]
- [115].Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 2016; 44:2846-58; PMID:26861625; https://doi.org/ 10.1093/nar/gkw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7:847-59; PMID:17943136; https://doi.org/ 10.1038/nrc2223 [DOI] [PubMed] [Google Scholar]
- [117].Liu X, Luo F, Ling M, Lu L, Shi L, Lu X, Xu H, Chen C, Yang Q, Xue J, et al.. MicroRNA-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cells. Toxicol Lett 2016; 252:1-10; PMID:27107786; https://doi.org/ 10.1016/j.toxlet.2016.04.015 [DOI] [PubMed] [Google Scholar]
- [118].Hudson MB, Rahnert JA, Zheng B, Woodworth-Hobbs ME, Franch HA, Price SR. miR-182 attenuates atrophy-related gene expression by targeting FoxO3 in skeletal muscle. Am J Physiol Cell Physiol 2014; 307:C314-9; PMID:24871856; https://doi.org/ 10.1152/ajpcell.00395.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Su M, Wang J, Wang C, Wang X, Dong W, Qiu W, Wang Y, Zhao X, Zou Y, Song L, et al.. MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ 2015; 22:986-99; PMID:25394488; https://doi.org/ 10.1038/cdd.2014.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yang Y, Liang C. MicroRNAs: an emerging player in autophagy. ScienceOpen Res 2015; 2015; PMID:26744638; https://doi.org/ 10.14293/S2199-1006.1.SOR-LIFE.A181CU.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang L, Xie T, Tian M, Li J, Song S, Ouyang L, Liu B, Cai H. GAMDB: a web resource to connect microRNAs with autophagy in gerontology. Cell Prolif 2016; 49:246-51; PMID:27037912; https://doi.org/ 10.1111/cpr.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Orhon I, Dupont N, Codogno P. Primary cilium and autophagy: the Avengers of cell-size regulation. Autophagy 2016; 12(11):2258–9; PMID:27485792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chen Y, Wang S, Zhang L, Xie T, Song S, Huang J, Zhang Y, Ouyang L, Liu B. Identification of ULK1 as a novel biomarker involved in miR-4487 and miR-595 regulation in neuroblastoma SH-SY5Y cell autophagy. Sci Rep 2015; 5:11035; PMID:26183158; https://doi.org/ 10.1038/srep11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, Wang H, Gong X, He X, Lu G, et al.. MiR-124 regulates apoptosis and autophagy process in MPTP model of parkinson's disease by targeting to bim. Brain Pathol 2016; 26:167-76; PMID:25976060; https://doi.org/ 10.1111/bpa.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y, Kong XM, Huang YR. Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget 2014; 5:9169-82; PMID:25333253; https://doi.org/ 10.18632/oncotarget.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ohsumi Y. Historical landmarks of autophagy research. Cell research 2014; 24:9-23; PMID:24366340; https://doi.org/ 10.1038/cr.2013.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lasda E, Parker R. Circular RNAs: diversity of form and function. Rna 2014; 20:1829-42; PMID:25404635; https://doi.org/ 10.1261/rna.047126.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012; 337:816-21; PMID:22745249; https://doi.org/ 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jia HZ, Zhang W, Zhu JY, Yang B, Chen S, Chen G, Zhao YF, Feng J, Zhang XZ. Hyperbranched-hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J Control Release 2015; 216:9-17; PMID:26272764; https://doi.org/ 10.1016/j.jconrel.2015.08.006 [DOI] [PubMed] [Google Scholar]
- [130].Basak I, Patil KS, Alves G, Larsen JP, Moller SG. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell Mol Life Sci 2016; 73:811-27; PMID:26608596; https://doi.org/ 10.1007/s00018-015-2093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer 2008; 8:121-32; PMID:18202696; https://doi.org/ 10.1038/nrc2297 [DOI] [PubMed] [Google Scholar]
- [132].Sullenger BA, Nair S. From the RNA world to the clinic. Science 2016; 352:1417-20; PMID:27313039; https://doi.org/ 10.1126/science.aad8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009; 457:426-33; PMID:19158789; https://doi.org/ 10.1038/nature07758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002; 2:243-7; PMID:12242156; https://doi.org/ 10.1016/S1535-6108(02)00122-8 [DOI] [PubMed] [Google Scholar]
- [135].Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004; 10:816-20; PMID:15235598; https://doi.org/ 10.1038/nm1076 [DOI] [PubMed] [Google Scholar]
- [136].Chen Y, Gu H, Zhang DS, Li F, Liu T, Xia W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials 2014; 35:10058-69; PMID:25277774; https://doi.org/ 10.1016/j.biomaterials.2014.09.003 [DOI] [PubMed] [Google Scholar]
- [137].Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med 2007; 13:372-7; PMID:17322898; https://doi.org/ 10.1038/nm1486 [DOI] [PubMed] [Google Scholar]
- [138].Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, Wang T, Zhou X, Zhu W, Li P, et al.. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-beta R II during TGF-beta1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep 2016; 6:24747; PMID:27098600; https://doi.org/ 10.1038/srep24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hou C, Zhu M, Sun M, Lin Y. MicroRNA let-7i induced autophagy to protect T cell from apoptosis by targeting IGF1R. Biochem Biophys Res Commun 2014; 453:728-34; PMID:25305490; https://doi.org/ 10.1016/j.bbrc.2014.10.002 [DOI] [PubMed] [Google Scholar]
- [140].Ge YY, Shi Q, Zheng ZY, Gong J, Zeng C, Yang J, Zhuang SM. MicroRNA-100 promotes the autophagy of hepatocellular carcinoma cells by inhibiting the expression of mTOR and IGF-1R. Oncotarget 2014; 5:6218-28; PMID:25026290; https://doi.org/ 10.18632/oncotarget.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N, Cui K, Liao M, He J, Jiang Y, et al.. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy 2014; 10:70-9; PMID:24262949; https://doi.org/ 10.4161/auto.26534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Huang N, Wu J, Qiu W, Lyu Q, He J, Xie W, Xu N, Zhang Y. MiR-15a and miR-16 induce autophagy and enhance chemosensitivity of Camptothecin. Cancer Biol Ther 2015; 16:941-8; PMID:25945419; https://doi.org/ 10.1080/15384047.2015.1040963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chen S, Li P, Li J, Wang Y, Du Y, Chen X, Zang W, Wang H, Chu H, Zhao G, et al.. MiR-144 inhibits proliferation and induces apoptosis and autophagy in lung cancer cells by targeting TIGAR. Cell Physiol Biochem 2015; 35:997-1007; PMID:25660220; https://doi.org/ 10.1159/000369755 [DOI] [PubMed] [Google Scholar]
- [144].Peng X, Li W, Yuan L, Mehta RG, Kopelovich L, McCormick DL. Inhibition of proliferation and induction of autophagy by atorvastatin in PC3 prostate cancer cells correlate with downregulation of Bcl2 and upregulation of miR-182 and p21. PloS One 2013; 8:e70442; PMID:23936432; https://doi.org/ 10.1371/journal.pone.0070442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yi H, Liang B, Jia J, Liang N, Xu H, Ju G, Ma S, Liu X. Differential roles of miR-199a-5p in radiation-induced autophagy in breast cancer cells. FEBS Lett 2013; 587:436-43; PMID:23337876; https://doi.org/ 10.1016/j.febslet.2012.12.027 [DOI] [PubMed] [Google Scholar]
- [146].Pando R, Even-Zohar N, Shtaif B, Edry L, Shomron N, Phillip M, Gat-Yablonski G. MicroRNAs in the growth plate are responsive to nutritional cues: association between miR-140 and SIRT1. J Nutr Biochem 2012; 23:1474-81; PMID:22402365; https://doi.org/ 10.1016/j.jnutbio.2011.09.010 [DOI] [PubMed] [Google Scholar]
- [147].Yang Y, Cheng HW, Qiu Y, Dupee D, Noonan M, Lin YD, Fisch S, Unno K, Sereti KI, Liao R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Cir Res 2015; 117:450-9; PMID:26082557; https://doi.org/ 10.1161/CIRCRESAHA.117.305962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang Y, Wang Y, Zhao W, Wang W. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol 2014; 35:7025-34; PMID:24752578; https://doi.org/ 10.1007/s13277-014-1965-2 [DOI] [PubMed] [Google Scholar]
- [149].Li X, Wang S, Chen Y, Liu G, Yang X. miR-22 targets the 3′ UTR of HMGB1 and inhibits the HMGB1-associated autophagy in osteosarcoma cells during chemotherapy. Tumour Biol 2014; 35:6021-8; PMID:24609901; https://doi.org/ 10.1007/s13277-014-1797-0 [DOI] [PubMed] [Google Scholar]
- [150].Luo J, Chen J, He L. mir-129-5p attenuates irradiation-induced autophagy and decreases radioresistance of breast cancer cells by targeting HMGB1. Med Sci Monit 2015; 21:4122-9; PMID:26720492; https://doi.org/ 10.12659/MSM.896661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pannuru P, Dontula R, Khan AA, Herbert E, Ozer H, Chetty C, Lakka SS. miR-let-7f-1 regulates SPARC mediated cisplatin resistance in medulloblastoma cells. Cell Signal 2014; 26:2193-201; PMID:25014664; https://doi.org/ 10.1016/j.cellsig.2014.06.014 [DOI] [PubMed] [Google Scholar]
- [152].Ran X, Yang J, Liu C, Zhou P, Xiao L, Zhang K. MiR-218 inhibits HMGB1-mediated autophagy in endometrial carcinoma cells during chemotherapy. Int J Clin Exp Pathol 2015; 8:6617–26; PMID:26261543 [PMC free article] [PubMed] [Google Scholar]
- [153].Zhu H, Huang L, Zhu S, Li X, Li Z, Yu C, Yu X. Regulation of autophagy by systemic admission of microRNA-141 to target HMGB1 in l-arginine-induced acute pancreatitis in vivo. Pancreatology 2016; 16(3):337–46; PMID:27017485 [DOI] [PubMed] [Google Scholar]
- [154].An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis 2015; 6:e1766; PMID:25996293; https://doi.org/ 10.1038/cddis.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Han W, Fu X, Xie J, Meng Z, Gu Y, Wang X, Li L, Pan H, Huang W. MiR-26a enhances autophagy to protect against ethanol-induced acute liver injury. J Mol Med 2015; 93:1045-55; PMID:25877859; https://doi.org/ 10.1007/s00109-015-1282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, et al.. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012; 3:1078; PMID:23011132; https://doi.org/ 10.1038/ncomms2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Bhattacharya A, Schmitz U, Raatz Y, Schonherr M, Kottek T, Schauer M, Franz S, Saalbach A, Anderegg U, Wolkenhauer O, et al.. miR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy. Oncotarget 2015; 6:2966-80; PMID:25650662; https://doi.org/ 10.18632/oncotarget.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Li W, Zhang X, Zhuang H, Chen HG, Chen Y, Tian W, Wu W, Li Y, Wang S, Zhang L, et al.. MicroRNA-137 is a novel hypoxia-responsive microRNA that inhibits mitophagy via regulation of two mitophagy receptors FUNDC1 and NIX. J Biol Chem 2014; 289:10691-701; PMID:24573672; https://doi.org/ 10.1074/jbc.M113.537050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Yu X, Luo A, Liu Y, Wang S, Li Y, Shi W, Liu Z, Qu X. MiR-214 increases the sensitivity of breast cancer cells to tamoxifen and fulvestrant through inhibition of autophagy. Mol Cancer 2015; 14:208; PMID:26666173; https://doi.org/ 10.1186/s12943-015-0480-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Liu XY, He YJ, Yang QH, Huang W, Liu ZH, Ye GR, Tang SH, Shu JC. Induction of autophagy and apoptosis by miR-148a through the sonic hedgehog signaling pathway in hepatic stellate cells. Am J Cancer Res 2015; 5:2569–89; PMID:26609469 [PMC free article] [PubMed] [Google Scholar]
- [161].Jagannathan S, Vad N, Vallabhapurapu S, Vallabhapurapu S, Anderson KC, Driscoll JJ. MiR-29b replacement inhibits proteasomes and disrupts aggresome+autophagosome formation to enhance the antimyeloma benefit of bortezomib. Leukemia 2015; 29:727-38; PMID:25234165; https://doi.org/ 10.1038/leu.2014.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bo L, Su-Ling D, Fang L, Lu-Yu Z, Tao A, Stefan D, Kun W, Pei-Feng L. Autophagic program is regulated by miR-325. Cell Death Differ 2014; 21:967-77; PMID:24531537; https://doi.org/ 10.1038/cdd.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sun Q, Liu T, Yuan Y, Guo Z, Xie G, Du S, Lin X, Xu Z, Liu M, Wang W, et al.. MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int J Cancer 2015; 136:1003-12; PMID:25044403; https://doi.org/ 10.1002/ijc.29065 [DOI] [PubMed] [Google Scholar]
- [164].Xiang C, Cui SP, Ke Y. MiR-144 inhibits cell proliferation of renal cell carcinoma by targeting MTOR. J Huazhong Univ Sci Technol Med Sci 2016; 36:186-92; PMID:27072960; https://doi.org/ 10.1007/s11596-016-1564-0 [DOI] [PubMed] [Google Scholar]
- [165].Zhu Z, Wang CP, Zhang YF, Nie L. MicroRNA-100 resensitizes resistant chondrosarcoma cells to cisplatin through direct targeting of mTOR. Asian Pac J Cancer Prev 2014; 15:917-23; PMID:24568519; https://doi.org/ 10.7314/APJCP.2014.15.2.917 [DOI] [PubMed] [Google Scholar]
- [166].Su M, Chen Z, Wang C, Song L, Zou Y, Zhang L, Hui R, Wang J. Cardiac-specific overexpression of miR-222 induces heart failure and inhibits autophagy in mice. Cell Physiol Biochem 2016; 39:1503-11; PMID:27614440; https://doi.org/ 10.1159/000447853 [DOI] [PubMed] [Google Scholar]
- [167].Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie T, Zhang J, Peng C, Lin Y, Chen J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014; 5:7013-26; PMID:25026296; https://doi.org/ 10.18632/oncotarget.2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].John Clotaire DZ, Zhang B, Wei N, Gao R, Zhao F, Wang Y, Lei M, Huang W. miR-26b inhibits autophagy by targeting ULK2 in prostate cancer cells. Biochem Biophys Res Commun 2016; 472:194-200; PMID:26920049; https://doi.org/ 10.1016/j.bbrc.2016.02.093 [DOI] [PubMed] [Google Scholar]
- [169].Duan X, Zhang T, Ding S, Wei J, Su C, Liu H, Xu G. microRNA-17-5p modulates bacille calmette-guerin growth in RAW264.7 Cells by targeting ULK1. PloS One 2015; 10:e0138011; PMID:26384021; https://doi.org/ 10.1371/journal.pone.0138011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Huang Y, Chuang AY, Ratovitski EA. Phospho-DeltaNp63alpha/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle 2011; 10:3938-47; PMID:22071691; https://doi.org/ 10.4161/cc.10.22.18107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Chen Y, Liersch R, Detmar M. The miR-290-295 cluster suppresses autophagic cell death of melanoma cells. Sci Rep 2012; 2:808; PMID:23150779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Guo X, Xue H, Guo X, Gao X, Xu S, Yan S, Han X, Li T, Shen J, Li G. MiR224-3p inhibits hypoxia-induced autophagy by targeting autophagy-related genes in human glioblastoma cells. Oncotarget 2015; 6:41620–37; PMID:26536662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Li S, Qiang Q, Shan H, Shi M, Gan G, Ma F, Chen B. MiR-20a and miR-20b negatively regulate autophagy by targeting RB1CC1/FIP200 in breast cancer cells. Life Sci 2016; 147:143-52; PMID:26829385; https://doi.org/ 10.1016/j.lfs.2016.01.044 [DOI] [PubMed] [Google Scholar]
- [174].Lu W, Han L, Su L, Zhao J, Zhang Y, Zhang S, Zhao B, Miao J. A 3′UTR-associated RNA, FLJ11812 maintains stemness of human embryonic stem cells by targeting miR-4459. Stem Cells Dev 2015; 24:1133-40; PMID:25437332; https://doi.org/ 10.1089/scd.2014.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, Wang NS. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol 2011; 34:549-59; PMID:22123611; https://doi.org/ 10.1159/000333809 [DOI] [PubMed] [Google Scholar]
- [176].Kouri FM, Hurley LA, Daniel WL, Day ES, Hua Y, Hao L, Peng CY, Merkel TJ, Queisser MA, Ritner C, et al.. miR-182 integrates apoptosis, growth, and differentiation programs in glioblastoma. Genes Dev 2015; 29:732-45; PMID:25838542; https://doi.org/ 10.1101/gad.257394.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Singh R, Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis–a combined computational and experimental approach. J Cell Sci 2012; 125:1568-78; PMID:22328513; https://doi.org/ 10.1242/jcs.095976 [DOI] [PubMed] [Google Scholar]
- [178].Verdoodt B, Neid M, Vogt M, Kuhn V, Liffers ST, Palisaar RJ, Noldus J, Tannapfel A, Mirmohammadsadegh A. MicroRNA-205, a novel regulator of the anti-apoptotic protein Bcl2, is downregulated in prostate cancer. Int J Oncol 2013; 43:307–14; PMID:23612742 [DOI] [PubMed] [Google Scholar]
- [179].Xu TX, Zhao SZ, Dong M, Yu XR. Hypoxia responsive miR-210 promotes cell survival and autophagy of endometriotic cells in hypoxia. Eur Rev Med Pharmacol Sci 2016; 20:399–406; PMID:26914112 [PubMed] [Google Scholar]
- [180].Zhang F, Wang J, Chu J, Yang C, Xiao H, Zhao C, Sun Z, Gao X, Chen G, Han Z, et al.. MicroRNA-146a Induced by Hypoxia Promotes Chondrocyte Autophagy through Bcl-2. Cell Physiol Biochem 2015; 37:1442-53; PMID:26492575; https://doi.org/ 10.1159/000438513 [DOI] [PubMed] [Google Scholar]
- [181].Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis 2012; 33:2018-25; PMID:22902544; https://doi.org/ 10.1093/carcin/bgs266 [DOI] [PubMed] [Google Scholar]
- [182].Chen Z, Sangwan V, Banerjee S, Mackenzie T, Dudeja V, Li X, Wang H, Vickers SM, Saluja AK. miR-204 mediated loss of Myeloid cell leukemia-1 results in pancreatic cancer cell death. Mol Cancer 2013; 12:105; PMID:24025188; https://doi.org/ 10.1186/1476-4598-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Rao YM, Shi HR, Ji M, Chen CH. MiR-106a targets Mcl-1 to suppress cisplatin resistance of ovarian cancer A2780 cells. J Huazhong Univ Sci Technol Med Sci 2013; 33:567-72; PMID:23904379; https://doi.org/ 10.1007/s11596-013-1160-5 [DOI] [PubMed] [Google Scholar]
- [184].Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y, Zhuang SM. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res 2009; 69:1135-42; PMID:19155302; https://doi.org/ 10.1158/0008-5472.CAN-08-2886 [DOI] [PubMed] [Google Scholar]
- [185].Tan S, Shi H, Ba M, Lin S, Tang H, Zeng X, Zhang X. miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Mol Med 2016; 37:1030–8; PMID:26935807 [DOI] [PubMed] [Google Scholar]
- [186].Korkmaz G, Tekirdag KA, Ozturk DG, Kosar A, Sezerman OU, Gozuacik D. MIR376A is a regulator of starvation-induced autophagy. PloS One 2013; 8:e82556; PMID:24358205; https://doi.org/ 10.1371/journal.pone.0082556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chatterjee A, Chattopadhyay D, Chakrabarti G. miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin1 expression. PloS One 2014; 9:e95716; PMID:24755562; https://doi.org/ 10.1371/journal.pone.0095716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A, et al.. MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 2014; 5:e1029; PMID:24481443; https://doi.org/ 10.1038/cddis.2013.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Xu X, Fu Y, Tong J, Fan S, Xu K, Sun H, Liang Y, Yan C, Yuan Z, Ge Y. MicroRNA-216b/Beclin 1 axis regulates autophagy and apoptosis in human Tenon's capsule fibroblasts upon hydroxycamptothecin exposure. Exp Eye Res 2014; 123:43-55; PMID:24681041; https://doi.org/ 10.1016/j.exer.2014.03.008 [DOI] [PubMed] [Google Scholar]
- [190].Deng Y, Xu J, Zhang X, Yang J, Zhang D, Huang J, Lv P, Shen W, Yang Y. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy 2014; 10:1776-86; PMID:25126729; https://doi.org/ 10.4161/auto.29746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Chen X, Zhang Y, Shi Y, Lian H, Tu H, Han S, Yin J, Peng B, Zhou B, He X, et al.. MiR-129 triggers autophagic flux by regulating a novel Notch-1/E2F7/Beclin-1 axis to impair the viability of human malignant glioma cells. Oncotarget 2016; 7:9222–35; PMID:26824182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z, Ding J, Jia L, Yuan W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013; 61:504-12; PMID:23361941; https://doi.org/ 10.1002/glia.22451 [DOI] [PubMed] [Google Scholar]
- [193].Huang Y, Guerrero-Preston R, Ratovitski EA. Phospho-DeltaNp63alpha-dependent regulation of autophagic signaling through transcription and micro-RNA modulation. Cell Cycle 2012; 11:1247-59; PMID:22356768; https://doi.org/ 10.4161/cc.11.6.19670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Huangfu L, Liang H, Wang G, Su X, Li L, Du Z, Hu M, Dong Y, Bai X, Liu T, et al.. miR-183 regulates autophagy and apoptosis in colorectal cancer through targeting of UVRAG. Oncotarget 2016; 7:4735–45; PMID:26717041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Huan LC, Wu JC, Chiou BH, Chen CH, Ma N, Chang CY, Tsen YK, Chen SC. MicroRNA regulation of DNA repair gene expression in 4-aminobiphenyl-treated HepG2 cells. Toxicology 2014; 322:69-77; PMID:24857880; https://doi.org/ 10.1016/j.tox.2014.05.003 [DOI] [PubMed] [Google Scholar]
- [196].Au KY, Pong JC, Ling WL, Li JC. MiR-1303 regulates mycobacteria induced autophagy by targeting Atg2B. PloS One 2016; 11:e0146770; PMID:26771516; https://doi.org/ 10.1371/journal.pone.0146770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kovaleva V, Mora R, Park YJ, Plass C, Chiramel AI, Bartenschlager R, Dohner H, Stilgenbauer S, Pscherer A, Lichter P, et al.. miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and trigger killing of chronic lymphocytic leukemia cells. Cancer Res 2012; 72:1763-72; PMID:22350415; https://doi.org/ 10.1158/0008-5472.CAN-11-3671 [DOI] [PubMed] [Google Scholar]
- [198].Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun 2013; 431:617-22; PMID:23274497; https://doi.org/ 10.1016/j.bbrc.2012.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Borralho PM, Kren BT, Castro RE, da Silva IB, Steer CJ, Rodrigues CM. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J 2009; 276:6689-700; PMID:19843160; https://doi.org/ 10.1111/j.1742-4658.2009.07383.x [DOI] [PubMed] [Google Scholar]
- [200].Li W, Yang Y, Hou X, Zhuang H, Wu Z, Li Z, Guo R, Chen H, Lin C, Zhong W, et al.. MicroRNA-495 regulates starvation-induced autophagy by targeting ATG3. FEBS Lett 2016; 590:726-38; PMID:26910393; https://doi.org/ 10.1002/1873-3468.12108 [DOI] [PubMed] [Google Scholar]
- [201].Liao H, Xiao Y, Hu Y, Xiao Y, Yin Z, Liu L, Kang X, Chen Y. Methylation-induced silencing of miR-34a enhances chemoresistance by directly upregulating ATG4B-induced autophagy through AMPK/mTOR pathway in prostate cancer. Oncol Rep 2016; 35:64–72; PMID:26499184 [DOI] [PubMed] [Google Scholar]
- [202].Pan B, Chen Y, Song H, Xu Y, Wang R, Chen L. Mir-24-3p downregulation contributes to VP16-DDP resistance in small-cell lung cancer by targeting ATG4A. Oncotarget 2015; 6:317–31; PMID:25426560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zhou L, Guo L, Tang J, Zhang A, Liu X, Xu G. [miR-144 regulates BCG- and rapamycin-induced autophagy by targeting Atg4a in RAW264.7 cells]. Xi bao yu fen zi mian yi xue za zhi 2015; 31:163–7; PMID:25652854 [PubMed] [Google Scholar]
- [204].Wu Y, Ni Z, Yan X, Dai X, Hu C, Zheng Y, He F, Lian J. Targeting the MIR34C-5p-ATG4B-autophagy axis enhances the sensitivity of cervical cancer cells to pirarubicin. Autophagy 2016; 12(7):1105–17; PMID:27097054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Tekirdag KA, Korkmaz G, Ozturk DG, Agami R, Gozuacik D. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy 2013; 9:374-85; PMID:23322078; https://doi.org/ 10.4161/auto.23117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, Tang D, Cao L. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia 2012; 26:1752-60; PMID:22395361; https://doi.org/ 10.1038/leu.2012.65 [DOI] [PubMed] [Google Scholar]
- [207].Liu Z, Wei X, Zhang A, Li C, Bai J, Dong J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem Biophys Res Commun 2016; 473:1268-75; PMID:27084450; https://doi.org/ 10.1016/j.bbrc.2016.04.054 [DOI] [PubMed] [Google Scholar]
- [208].Chang Y, Yan W, He X, Zhang L, Li C, Huang H, Nace G, Geller DA, Lin J, Tsung A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 2012; 143:177-87 e8; PMID:22504094; https://doi.org/ 10.1053/j.gastro.2012.04.009 [DOI] [PubMed] [Google Scholar]
- [209].Pan B, Feng B, Chen Y, Huang G, Wang R, Chen L, Song H. MiR-200b regulates autophagy associated with chemoresistance in human lung adenocarcinoma. Oncotarget 2015; 6:32805–20; PMID:26416454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Wang P, Zhang J, Zhang L, Zhu Z, Fan J, Chen L, Zhuang L, Luo J, Chen H, Liu L, et al.. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology 2013; 145:1133-43 e12; PMID:23916944 [DOI] [PubMed] [Google Scholar]
- [211].Lu C, Chen J, Xu HG, Zhou X, He Q, Li YL, Jiang G, Shan Y, Xue B, Zhao RX, et al.. MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-mediated autophagy. Gastroenterology 2014; 146:188-99; PMID:24036151; https://doi.org/ 10.1053/j.gastro.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sun KT, Chen MY, Tu MG, Wang IK, Chang SS, Li CY. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 2015; 73:145-53; PMID:25485521; https://doi.org/ 10.1016/j.bone.2014.11.026 [DOI] [PubMed] [Google Scholar]
- [213].Feng L, Ma Y, Sun J, Shen Q, Liu L, Lu H, Wang F, Yue Y, Li J, Zhang S, et al.. YY1-MIR372-SQSTM1 regulatory axis in autophagy. Autophagy 2014; 10:1442-53; PMID:24991827; https://doi.org/ 10.4161/auto.29486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Kato M, Goto Y, Matsushita R, Kurozumi A, Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T, et al.. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int J Oncol 2015; 47:710–8; PMID:26063484 [DOI] [PubMed] [Google Scholar]
- [215].Liu X, Fu B, Chen D, Hong Q, Cui J, Li J, Bai X, Chen X. miR-184 and miR-150 promote renal glomerular mesangial cell aging by targeting Rab1a and Rab31. Exp Cell Res 2015; 336:192-203; PMID:26165933; https://doi.org/ 10.1016/j.yexcr.2015.07.006 [DOI] [PubMed] [Google Scholar]
- [216].Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long G, Yang K. MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A. Int J Clin Exp Pathol 2015; 8:384–93; PMID:25755726 [PMC free article] [PubMed] [Google Scholar]
- [217].Liu X, Hong Q, Wang Z, Yu Y, Zou X, Xu L. MiR-21 inhibits autophagy by targeting Rab11a in renal ischemia/reperfusion. Exp Cell Res 2015; 338:64-9; PMID:26302266; https://doi.org/ 10.1016/j.yexcr.2015.08.010 [DOI] [PubMed] [Google Scholar]
- [218].Wang B, Yang Z, Wang H, Cao Z, Zhao Y, Gong C, Ma L, Wang X, Hu X, Chen S. MicroRNA-320a inhibits proliferation and invasion of breast cancer cells by targeting RAB11A. Am J Cancer Res 2015; 5:2719-29; PMID:26609479; https://doi.org/ 10.1158/1538-7445.AM2015-2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Capobianco V, Nardelli C, Ferrigno M, Iaffaldano L, Pilone V, Forestieri P, Zambrano N, Sacchetti L. miRNA and protein expression profiles of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J Proteome Res 2012; 11:3358-69; PMID:22537031; https://doi.org/ 10.1021/pr300152z [DOI] [PubMed] [Google Scholar]
- [220].Serva A, Knapp B, Tsai YT, Claas C, Lisauskas T, Matula P, Harder N, Kaderali L, Rohr K, Erfle H, et al.. miR-17-5p regulates endocytic trafficking through targeting TBC1D2/Armus. PloS One 2012; 7:e52555; PMID:23285084; https://doi.org/ 10.1371/journal.pone.0052555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Zhai H, Song B, Xu X, Zhu W, Ju J. Inhibition of autophagy and tumor growth in colon cancer by miR-502. Oncogene 2013; 32:1570-9; PMID:22580605; https://doi.org/ 10.1038/onc.2012.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Ge D, Han L, Huang S, Peng N, Wang P, Jiang Z, Zhao J, Su L, Zhang S, Zhang Y, et al.. Identification of a novel MTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells. Autophagy 2014; 10:957-71; PMID:24879147; https://doi.org/ 10.4161/auto.28363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Liu X, Xiao ZD, Han L, Zhang J, Lee SW, Wang W, Lee H, Zhuang L, Chen J, Lin HK, et al.. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol 2016; 18(4):431–42; PMID:26999735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Liu X, Xiao ZD, Gan B. An lncRNA switch for AMPK activation. Cell Cycle 2016; 15:1948-9; PMID:27152502; https://doi.org/ 10.1080/15384101.2016.1184515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Li Z, Hao S, Yin H, Gao J, Yang Z. Autophagy ameliorates cognitive impairment through activation of PVT1 and apoptosis in diabetes mice. Behav Brain Res 2016; 305:265-77; PMID:26971628; https://doi.org/ 10.1016/j.bbr.2016.03.023 [DOI] [PubMed] [Google Scholar]
- [226].Pawar K, Hanisch C, Palma Vera SE, Einspanier R, Sharbati S. Down regulated lncRNA MEG3 eliminates mycobacteria in macrophages via autophagy. Sci Rep 2016; 6:19416; PMID:26757825; https://doi.org/ 10.1038/srep19416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Kang Y, Song J, Kim D, Ahn C, Park S, Chun CH, Jin EJ. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J Orthop Res 2016; 34:412-8; PMID:26340084; https://doi.org/ 10.1002/jor.23046 [DOI] [PubMed] [Google Scholar]
- [228].Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu J, Sun Y. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett 2014; 8:1947–52; PMID:25289082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Song J, Ahn C, Chun CH, Jin EJ. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res 2014; 32:1628-35; PMID:25196583; https://doi.org/ 10.1002/jor.22718 [DOI] [PubMed] [Google Scholar]
- [230].Zhang N, Yang GQ, Shao XM, Wei L. GAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cells. Eur Rev Med Pharmacol Sci 2016; 20:2271–7; PMID:27338051 [PubMed] [Google Scholar]
- [231].Zhuo C, Jiang R, Lin X, Shao M. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget 2016; 8(1):1429–1437; PMID:27903964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Deng X, Feng N, Zheng M, Ye X, Lin H, Yu X, Gan Z, Fang Z, Zhang H, Gao M, et al.. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta 2017; 1861:112-25; PMID:27836757; https://doi.org/ 10.1016/j.bbagen.2016.11.009 [DOI] [PubMed] [Google Scholar]
- [233].Chen ZH, Wang WT, Huang W, Fang K, Sun YM, Liu SR, Luo XQ, Chen YQ. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 2017; 24: 212-24; PMID:27740626; https://doi.org/ 10.1038/cdd.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Tang S, Tan G, Jiang X, Han P, Zhai B, Dong X, Qiao H, Jiang H, Sun X. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget 2016; 7(45):73257–69; PMID:27689326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Li L, Chen H, Gao Y, Wang YW, Zhang GQ, Pan SH, Ji L, Kong R, Wang G, Jia YH, et al.. Long Noncoding RNA MALAT1 Promotes Aggressive Pancreatic Cancer Proliferation and Metastasis via the Stimulation of Autophagy. Mol Cancer Ther 2016; 15:2232-43; PMID:27371730; https://doi.org/ 10.1158/1535-7163.MCT-16-0008 [DOI] [PubMed] [Google Scholar]
- [236].Yuan P, Cao W, Zang Q, Li G, Guo X, Fan J. The HIF-2alpha-MALAT1-miR-216b axis regulates multi-drug resistance of hepatocellular carcinoma cells via modulating autophagy. Biochem Biophys Res Commun 2016; 478:1067-73; PMID:27524242; https://doi.org/ 10.1016/j.bbrc.2016.08.065 [DOI] [PubMed] [Google Scholar]
- [237].Chen YN, Cai MY, Xu S, Meng M, Ren X, Yang JW, Dong YQ, Liu X, Yang JM, Xiong XD. Identification of the lncRNA, AK156230, as a novel regulator of cellular senescence in mouse embryonic fibroblasts. Oncotarget 2016; 7:52673–84; PMID:27343551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Yang L, Zhang X, Li H, Liu J. The long noncoding RNA HOTAIR activates autophagy by upregulating ATG3 and ATG7 in hepatocellular carcinoma. Mol BioSystems 2016; 12:2605-12; PMID:27301338; https://doi.org/ 10.1039/C6MB00114A [DOI] [PubMed] [Google Scholar]
- [239].Turei D, Foldvari-Nagy L, Fazekas D, Modos D, Kubisch J, Kadlecsik T, Demeter A, Lenti K, Csermely P, Vellai T, et al.. Autophagy Regulatory Network - a systems-level bioinformatics resource for studying the mechanism and regulation of autophagy. Autophagy 2015; 11:155-65; PMID:25635527; https://doi.org/ 10.4161/15548627.2014.994346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Homma K, Suzuki K, Sugawara H. The Autophagy Database: an all-inclusive information resource on autophagy that provides nourishment for research. Nucleic Acids Res 2011; 39:D986-90; PMID:20972215; https://doi.org/ 10.1093/nar/gkq995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Wu D, Huang Y, Kang J, Li K, Bi X, Zhang T, Jin N, Hu Y, Tan P, Zhang L, et al.. ncRDeathDB: A comprehensive bioinformatics resource for deciphering network organization of the ncRNA-mediated cell death system. Autophagy 2015; 11:1917-26; PMID:26431463; https://doi.org/ 10.1080/15548627.2015.1089375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


