Abstract
Background
Current strategies for cardiovascular disease (CVD) risk assessment among adults without known CVD are limited by suboptimal performance and a narrow focus on only atherosclerotic CVD (ASCVD). We hypothesized that a strategy combining promising biomarkers across multiple different testing modalities would improve global and atherosclerotic CVD risk assessment among individuals without known CVD.
Methods
We included participants from the Multi-Ethnic Study of Atherosclerosis (MESA, n=6621) and Dallas Heart Study (DHS, n=2202) who were free from CVD and underwent measurement of left ventricular hypertrophy by electrocardiogram (ECG-LVH), coronary artery calcium (CAC), N-terminal pro B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT) and high-sensitivity C-reactive protein (hs-CRP). Associations of test results with the global composite CVD outcome (CVD death, myocardial infarction [MI], stroke, coronary or peripheral revascularization, incident heart failure or atrial fibrillation) and ASCVD (fatal or nonfatal MI or stroke) were assessed over > 10 years of follow-up. Multivariable analyses for the primary global CVD endpoint adjusted for traditional risk factors plus statin use and creatinine (base model).
Results
Each test result was independently associated with global composite CVD events in MESA after adjustment for the components of the base model and the other test results (p< 0.05 for each). When the five tests were added to the base model, the c-statistic improved from 0.74 to 0.79 (p=0.001), significant integrated discrimination improvement (0.07, 95% CI 0.06–0.08, p<0.001) and net reclassification improvement (0.47, 95% CI 0.38–0.56, p=0.003) were observed, and the model was well calibrated (χ2=12.2, p=0.20). Using a simple integer score counting the number of abnormal tests, compared with those with a score of 0, global CVD risk was increased among participants with a score of 1 (adjusted HR 1.9, 95% CI 1.4–2.6), 2 (HR 3.2, 95% CI 2.3–4.4), 3 (HR 4.7, 95% CI 3.4, 6.5) and ≥4 (HR 7.5, 95% CI 5.2–10.6). Findings replicated in DHS and were similar for the ASCVD outcome.
Conclusions
Among adults without known CVD, a novel multimodality testing strategy using ECG-LVH, CAC, NT-proBNP, hs-cTnT and hs-CRP significantly improved global CVD and ASCVD risk assessment.
Keywords: biomarker, troponin T, NT-proBNP, C-reactive protein, risk prediction
Subject terms: Epidemiology, Primary Prevention, Cardiovascular Disease, Prognosis, Diagnostic Testing
INTRODUCTION
Strategies for cardiovascular disease (CVD) risk assessment among adults without known CVD remain largely based on traditional atherosclerosis risk factors.1 However, these risk prediction equations provide only moderate discrimination of atherosclerotic cardiovascular disease (ASCVD) risk.1–3 Moreover, these algorithms typically do not consider risk for additional cardiovascular events, such as heart failure and atrial fibrillation, which are increasingly important contributors to the overall burden of CVD in the population.4, 5 A growing body of evidence suggests that preventive interventions such as weight loss, exercise, and more aggressive blood pressure control may favorably impact not only ASCVD, but also these other highly relevant CVD outcomes.6–8
Prior studies have evaluated individual novel risk markers in an attempt to improve CVD risk prediction, and have identified several promising blood and imaging based biomarkers.9–20 However, for individual biomarkers, even those independently associated with outcomes, the incremental improvement in discrimination and risk classification is typically modest.21, 22 As a result, investigators have explored combinations of biomarkers as a potential strategy to augment CVD risk prediction, with mixed results.23–28 Importantly, these prior studies have mostly combined biomarkers within the same testing modality, such as panels of genetic variants or circulating protein biomarkers, have frequently studied biomarkers with limited specificity for cardiovascular disease, and have included combinations of highly correlated biomarkers.29 To our knowledge, no large studies have combined the most promising individual biomarkers across multiple different testing modalities in an attempt to create a risk prediction tool that augments traditional risk factor strategies.
We hypothesized that a panel combining non-redundant CVD biomarkers across multiple different testing modalities would overcome these limitations and improve CVD risk prediction. The tests prospectively selected included 12-lead electrocardiography (ECG) for assessment of left ventricular hypertrophy (ECG-LVH),30–32 coronary artery calcium (CAC) measurement by computed tomography (CT),11–13 and measurement of N-terminal pro-brain natriuretic peptide (NT-proBNP)14–16 high sensitivity cardiac troponin T (hs-cTnT)17–20 and high sensitivity C-reactive protein (hs-CRP).9, 10 These tests were selected because they reflect distinct and relevant pathological processes, multiple reports from population-based studies demonstrate independent associations of these measurements with CVD outcomes, and sufficient data exist from which to generate a priori thresholds to define abnormal test results.
METHODS
Study populations
Study participants were included from Exam 1 of the Multi-Ethnic Study of Atherosclerosis (MESA) and Phase 1 of Dallas Heart Study (DHS). Both MESA and DHS are ongoing multi-ethnic population-based cohort studies, with methods previously described.33, 34 Between 2000–2002, MESA enrolled 6814 participants 45–84 years old who were free from known CVD. For the present study, we excluded participants missing results from any of the 5 tests, with incomplete follow-up, or missing any of the covariates required for the multivariable analyses, resulting in a final study population of 6621 with complete data for all covariates (Supplementary Figure 1). A total of 3072 participants from DHS aged 30–65 completed the three DHS phase-1 visits between 2000–2002, including a detailed in-home survey, laboratory testing, and imaging tests and ECG. For the present study, we excluded participants with prevalent cardiovascular disease (CVD) at baseline as well as those missing data on test results or covariates, or with incomplete follow-up, resulting in 2202 participants with complete data for all covariates (Supplementary Figure 1). MESA was approved by the Institutional Review Boards of the University of Washington and the participating sites and DHS was approved by the Institutional Review Board of UT Southwestern Medical Center. All participants provided written informed consent.
Data Collection and Variable Definitions
Race/ethnicity, history of CVD and smoking status were self-reported. Detailed descriptions of variable definitions for hypertension, diabetes, hypercholesterolemia, and low high-density lipoprotein (HDL) cholesterol have been previously described for MESA 33 and DHS 35 and are based on conventional clinical definitions.
Multimodality testing
LVH was determined from standard 12-lead ECGs using the Sokolow-Lyon voltage criteria and defined as present or absent.36 In MESA, CAC scans were performed in duplicate using either electron beam or multidetector CT.37 CAC scores were expressed in Agatston units and the mean of the two scans was used. In DHS, CAC measurements were obtained from electron beam CT scans performed in duplicate 1–2 minutes apart as previously described.38 To minimize false-positive CAC classifications due to tissue-associated artifact, a mean EBCT score > 10 U was defined as CAC positive status.38 NT-proBNP and hs-cTnT were measured using the Cobas e601 in MESA and the Elecysys-2010 in DHS18, 39 (both Roche Diagnostics, Indianapolis, IN). hs-CRP was measured using the BNII nephelometer (Dade Behring, Inc., Deerfield IL) in MESA40 and the Roche/Hitachi 912 System, Tina-quant assay (Roche Diagnostics) in DHS.41 The following thresholds were prospectively selected to define elevated biomarker levels: NT-proBNP ≥ 100 pg/mL42 hs-cTnT ≥ 5 ng/L (the limit of detection),43 and hs-CRP ≥ 3 mg/L.44 Values below the limit of blank of the hs-cTnT assay were arbitrarily assigned a level of 1.5 ng/L.
Cohort follow-up and endpoint collection
In MESA, participants were contacted by a telephone interviewer at 9–12 month intervals to inquire about hospital admissions, CVD diagnoses and deaths. Medical records and death certificates were requested for all suspected cases, with records obtained in 98% of reported hospitalized CVD events. In DHS, fatal events were ascertained for all subjects using the National Death Index. Deaths were classified as cardiovascular if they included International Statistical Classification of Diseases, 10th Revision (ICD-10) codes I00–I99. In the DHS, two overlapping approaches were used to capture nonfatal events. 1) A detailed health survey regarding interval cardiovascular events was administered by the Data Coordinating Center during annual calls to study subjects 2) for subjects providing informed consent (>90%), quarterly tracking was performed for hospital admissions using the Dallas-Fort Worth Hospital Council Data Initiative Database that includes all hospital admission data for 70 out of 72 hospitals in the Dallas-Fort Worth area. Primary clinical source documents were collected and reviewed for all suspected non-fatal cardiovascular events in both MESA and DHS and were independently adjudicated by blinded endpoint committees. Follow-up data for both fatal and nonfatal events was complete through 12/31/2012 in MESA and 12/31/2011 in DHS.
Study endpoints
The primary outcome was prospectively defined as time to the first event of a global CVD composite of CV death, MI, stroke, coronary or peripheral revascularization > 3 months after enrollment, incident heart failure, or atrial fibrillation. The major secondary endpoint was hard ASCVD events, including fatal or nonfatal MI, and fatal or nonfatal stroke. Tertiary endpoints included CHD (fatal or nonfatal MI), incident heart failure, all-cause mortality, and CVD mortality. The tertiary endpoints were evaluated with univariable analyses only in DHS due to small numbers of these events, which precluded multivariable adjustment. A blanking period of 3 months for revascularization events was used to account for any influence of the study visit or CAC measurement on revascularization decisions.
Statistical Methods
All analyses were performed separately in MESA and DHS. The analysis strategy considered the test results as both continuous and categorical variables. In the continuous variable analyses, CAC, NT-proBNP, hs-cTnT, hs-CRP were modeled as natural log transformed (Ln) continuous variables, with a value of 1 added to CAC due to large numbers of zero values, and ECG-LVH was modeled as a dichotomous variable. In the categorical analyses, all variables were modeled as dichotomous variables using the pre-specified cutpoints described above. Associations of test results with study outcomes were assessed using unadjusted and adjusted Cox proportional hazards models, with all covariates determined apriori. The base model included traditional risk factors: age, sex, race/ethnicity, smoking status, diabetes, total cholesterol, HDL-cholesterol, systolic blood pressure, blood pressure medications, and statin use. For the primary global CVD outcome, serum creatinine was included in the base model, and for the heart failure outcome, both creatinine and body mass index (BMI) were included in the base model.45, 46 The first multivariable model added individual test results to the base model and the second model added all 5 of the test results to the base model. Assumptions for the Cox proportional hazards models were verified by Schoenfeld residuals.
Improvement in discrimination and reclassification for the primary global CVD outcome and the secondary ASCVD outcome was assessed by comparing the base model with the model that included the base model plus the 5 screening tests. Discrimination was assessed using Harrell’s c-statistic, with confidence intervals determined by a jackknife resampling method. Improvement in the c-statistic was determined using bootstrap resampling. Integrated discrimination improvement, reflecting the difference in discrimination slopes between models with and without the markers, was determined using the failure probabilities from the Cox-proportional hazards models.47 Category free net reclassification improvement (NRI) was performed for all endpoints according to methods described by Pencina et al.48 Calibration of the global CVD and ASCVD models was assessed by the modified Hosmer-Lemeshow test for time-to-event data. For the primary analyses, coefficients were determined separately for each model in MESA and DHS. Sensitivity analyses were performed in which the full multivariable models with continuous biomarker coefficients from MESA were applied directly to the DHS cohort.
To facilitate clinical application of the multimodality strategy, a simple integer score counting the number of abnormal screening test results was created, with values ranging from 0–5. In MESA scores of 4–5 were collapsed and in DHS scores of 3–5 were collapsed, due to small numbers of participants in the highest risk categories. Cumulative rates of the primary composite outcome were determined and displayed using the Nelson-Aalen failure estimator, with groups compared with the log-rank test. Multivariable adjusted Cox proportional hazards analyses were performed, adjusting for the variables contained in the base model. A similar approach using the same integer score was used for secondary and tertiary endpoints. Unadjusted stratified analyses were performed in subgroups defined by sex, younger age (men <55, women < 65), race/ethnicity (Black, White, Hispanic), and estimated 10-year ASCVD risk < 7.5% using the pooled cohort equations.1
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina), and all p-values are two-sided with an alpha of 0.05. Published SAS macros were used to assess measures of fit.49
Results
Participant characteristics are detailed in Table 1. Median age at enrollment was 62 years in MESA and 44 years in DHS. In MESA, over a median 11 years of follow-up, 1026 global CVD events occurred, including 486 ASCVD events. In the DHS, over a median follow-up period of 10.3 years, 179 global CVD events occurred, including 96 ASCVD events. The prevalence of abnormal results on the 5 tests in MESA and DHS is shown in Table 1, and ranged from 9% for ECG-LVH (in both cohorts) to 45% for hs-CRP (in DHS). The individual test results were not highly correlated in either MESA or DHS (Supplementary Tables 1 and 2).
Table 1.
Baseline characteristics
| Variable | MESA | DHS |
|---|---|---|
| N | 6621 | 2202 |
| Age | 62 [53, 70] | 44 [37, 52] |
| Male | 47% | 44% |
| Race/ethnicity | ||
| White | 38% | 34% |
| Black | 27% | 47% |
| Hispanic | 22% | 16% |
| Asian/Other | 12% | 2% |
| BMI | 27.6 [24.6, 31.2] | 28.2 [24.6, 32.4] |
| Hypertension | 45% | 31% |
| Medication for hypertension | 37% | 19% |
| SBP | 124 [112, 140] | 121 [112, 133] |
| DBP | 72 [65, 79] | 77 [71, 84] |
| Diabetes | 13% | 9% |
| Current smoker | 13% | 27% |
| Hypercholesterolemia | 37% | 13% |
| Statin medication | 15% | 6% |
| Lipids | ||
| Total Cholesterol | 192 [170, 215] | 180 [157, 205] |
| LDL Cholesterol | 116 [96, 136] | 106 [84, 129] |
| HDL Cholesterol | 48 [40, 59] | 48 [40, 58] |
| Triglycerides | 111 [78, 161] | 97 [69, 146] |
| Test Results | ||
| ECG-LVH | 9% | 9% |
| CAC, Agatston U | 0 [0, 86.5] | 0.5 [0, 4.3] |
| CAC > 10 U | 42% | 19% |
| NT-proBNP, pg/mL | 53.0 [24.0, 107.7] | 27.4 [12.7, 56.0] |
| NT-proBNP ≥100 pg/mL | 27% | 11% |
| Hs-cTnT, ng/L | 4.4 [3.0, 7.5] | 1.5 [1.5, 1.5] |
| hs-cTnT ≥ 5 ng/L | 44% | 14% |
| Hs-CRP, mg/L | 1.9 [0.8, 4.2] | 2.7 [1.1, 6.2] |
| Hs-CRP ≥ 3 mg/L | 36% | 45% |
| Endpoints | ||
| Global CVD | 1026 (15.5%) | 179 (8.1%) |
| ASCVD | 486 (7.3%) | 96 (4.4%) |
| CHD | 314 (4.7%) | 61 (2.8%) |
| CHF | 252 (3.8%) | 28 (1.3%) |
| All cause death | 806 (12.2%) | 94 (4.3%) |
| CV death | 183 (2.8%) | 46 (2.1%) |
Continuous variables are presented as median [interquartile range]. BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure; LDL=low density lipoprotein, HDL=high density lipoprotein; ECG=electrocardiogram; LVH=left ventricular hypertrophy; CAC=coronary artery calcium score; NT-proBNP=N-terminal prohormone of B-type natriuretic peptide; hs-cTnT=high sensitivity cardiac troponin T. CVD=cardiovascular disease; ASCVD=atherosclerotic CVD, CHD=coronary heart disease; CHF=congestive heart failure; CV=cardiovascular
Associations of Test Results with Outcomes
In MESA, each of the 5 tests was associated with the primary global CVD outcome after adjustment for traditional risk factors and the other test results, with results consistent whether the tests were considered as continuous variables or using the prospective dichotomous cutpoints (Table 2). Findings replicated in DHS with the exception that hs-CRP was not independently associated with global CVD after multivariable adjustment (Table 2).
Table 2.
Association of test results the with the primary composite global cardiovascular disease outcome
| Variable | Unadjusted HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|||
|---|---|---|---|---|---|---|
| MESA | DHS | MESA | DHS | MESA | DHS | |
| Continuous Test Results | ||||||
| ECG-LVH* | 1.84 (1.55, 2.19) | 2.60 (1.78, 3.79) | 1.39 (1.17, 1.67) | 1.73 (1.16, 2.57) | 1.24 (1.04, 1.49) | 1.72 (1.15, 2.57) |
| Ln (CAC+1) | 2.14 (2.02, 2.28) | 1.96 (1.76, 2.12) | 1.62 (1.51, 1.74) | 1.31 (1.14, 1.50) | 1.55 (1.44, 1.66) | 1.31 (1.14, 1.50) |
| Ln (NT-proBNP) | 1.88 (1.76, 2.01) | 1.67 (1.44, 1.94) | 1.64 (1.52, 1.77) | 1.27 (1.07, 1.50) | 1.48 (1.37, 1.60) | 1.19 (1.01, 1.41) |
| Ln (hs-cTnT) | 1.74 (1.67, 1.83) | 1.74 (1.58, 1.91) | 1.36 (1.28, 1.45) | 1.21 (1.05, 1.39) | 1.22 (1.14, 1.31) | 1.17 (1.01, 1.35) |
| Ln (hs-CRP) | 1.20 (1.13, 1.28) | 1.22 (1.05, 1.43) | 1.18 (1.10, 1.26) | 0.98 (0.83, 1.16) | 1.16 (1.08, 1.24) | 0.97 (0.82, 1.15) |
| Categorical Test Results | ||||||
| ECG-LVH* | 1.84 (1.55, 2.19) | 2.60 (1.78, 3.79) | 1.39 (1.17, 1.67) | 1.73 (1.16, 2.57) | 1.34 (1.12, 1.61) | 1.71 (1.15, 2.55) |
| CAC > 10 U | 3.86 (3.37, 4.41) | 4.95 (3.69, 6.64) | 2.13 (1.84, 2.47) | 1.78 (1.26, 2.52) | 2.05 (1.76, 2.38) | 1.81 (1.28, 2.55) |
| NT-proBNP ≥100 pg/mL | 2.62 (2.31, 2.96) | 3.81 (2.76, 5.25) | 1.84 (1.60, 2.12) | 2.04 (1.40, 2.98) | 1.70 (1.48, 1.96) | 1.88 (1.29, 2.75) |
| hs-cTnT ≥ 5 ng/L | 3.06 (2.69, 3.49) | 4.05 (2.99, 5.49) | 1.49 (1.28, 1.73) | 1.53 (1.06, 2.19) | 1.38 (1.19, 1.60) | 1.46 (1.01, 2.11) |
| hs-CRP ≥ 3 mg/L | 1.46 (1.29, 1.65) | 1.52 (1.13, 2.04) | 1.45 (1.27, 1.65) | 1.10 (0.80, 1.51) | 1.39 (1.22, 1.59) | 1.06 (0.78, 1.46) |
Model 1 is adjusted for age, sex, race, smoking status, diabetes, total cholesterol, HDL-cholesterol, systolic blood pressure and blood pressure medications, statin medications and creatinine. Model 2 includes the components of model 1 and all 5 test results. The Hazard Ratio (HR) for continuous test results reflects a one standard deviation change in Ln of the test result. MESA=Multiethnic Study of Atherosclerosis; DHS=Dallas Heart Study HR=Hazard ratio: CI=confidence interval; ECG=electrocardiogram; LVH=left ventricular hypertrophy; CAC=coronary artery calcium score; NT-proBNP=N-terminal prohormone of B-type natriuretic peptide; hs-cTnT=high sensitivity cardiac troponin T. N=1026 endpoints in MESA and 179 in DHS.
ECG-LVH was treated as a categorical variable in both analyses. Coefficients were determined separately for each model.
Associations of the test results with the secondary composite ASCVD outcomes are shown in Table 3. In MESA, each of the test results except hs-CRP was independently associated with ASCVD. In DHS, associations of hs-CRP and hs-cTnT with ASCVD were attenuated after adjustment for risk factors (Table 3). Associations of the screening tests with tertiary endpoints were largely concordant in MESA and DHS, with variation seen depending on which endpoint was evaluated (Supplementary Tables 3–6). CAC was most strongly associated with coronary heart disease events, followed by NT-proBNP and hs-cTnT, with no association seen for ECG-LVH or hs-CRP (Supplementary Table 3). All 5 test results were independently associated with incident heart failure, with largest hazards seen for NT-proBNP, ECG-LVH, and hs-cTnT. NT-proBNP demonstrated the largest hazard ratio for fatal outcomes, followed by hs-cTnT. ECG-LVH associated with CVD mortality, but not all-cause mortality (Supplementary Tables 3–6).
Table 3.
Association of test results the with the composite atherosclerotic cardiovascular disease (ASCVD) outcome
| Variable | Unadjusted HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
|||
|---|---|---|---|---|---|---|
| MESA | DHS | MESA | DHS | MESA | DHS | |
| Continuous Test Results | ||||||
| ECG-LVH * | 1.71 (1.33, 2.21) | 2.76(1.67, 4.56) | 1.24 (0.95, 1.61) | 1.83 (1.07, 3.12) | 1.14 (0.87, 1.48) | 1.84 (1.08, 3.14) |
| Ln (CAC+1) | 2.04 (1.87, 2.22) | 2.17 (1.88, 2.50) | 1.56 (1.41, 1.73) | 1.43 (1.20, 1.72) | 1.49 (1.34, 1.65) | 1.43 (1.19, 1.72) |
| Ln (NT-proBNP) | 1.73 (1.62, 1.85) | 1.56 (1.27, 1.92) | 1.45 (1.30, 1.61) | 1.25 (1.01, 1.55) | 1.29 (1.15, 1.43) | 1.18 (0.95, 1.47) |
| Ln (hs-cTnT) | 1.73 (1.62, 1.85) | 1.74 (1.53, 1.97) | 1.37 (1.25, 1.49) | 1.16 (0.98, 1.38) | 1.23 (1.12, 1.35) | 1.11 (0.93, 1.34) |
| Ln (hs-CRP) | 1.17 (1.08, 1.28) | 1.19 (0.97, 1.47) | 1.09 (0.99, 1.21) | 0.97 (0.77, 1.22) | 1.07 (0.97, 1.17) | 0.93 (0.74, 1.18) |
| Categorical test results | ||||||
| ECG-LVH * | 1.71 (1.33, 2.21) | 2.45 (1.49, 4.02) | 1.24 (0.95, 1.61) | 1.69 (0.99, 2.87) | 1.19 (0.92, 1.55) | 1.88 (1.10, 3.20) |
| CAC > 10 U | 3.87 (3.18, 4.72) | 7.27 (4.87, 10.85) | 2.17 (1.74, 2.70) | 2.31 (1.44, 3.70) | 2.09 (1.67, 2.60) | 2.31 (1.44, 3.71) |
| NT-proBNP ≥100 pg/mL | 2.44 (2.04, 2.92) | 3.22 (2.10, 4.92) | 1.69 (1.35, 2.11) | 2.25 (1.38, 3.68) | 1.55 (1.26, 1.91) | 1.99 (1.19, 3.32) |
| hs-cTnT ≥ 5 ng/L | 2.78 (2.31, 3.36) | 4.20 (2.87, 6.17) | 1.40 (1.13, 1.74) | 1.43 (0.91, 2.25) | 1.29 (1.04, 1.59) | 1.23 (0.75, 2.02) |
| hs-CRP ≥ 3 mg/L | 1.38 (1.16, 1.65) | 1.54 (1.05, 2.25) | 1.24 (1.02, 1.50) | 1.12 (0.74, 1.69) | 1.19 (0.98, 1.44) | 1.10 (0.72, 1.70) |
Model 1 is adjusted for age, sex, race, smoking status, diabetes, total cholesterol, HDL-cholesterol, systolic blood pressure and blood pressure medications, and statin use. Model 2 includes the components of model 1 and all 5 test results. The Hazard Ratio (HR) for continuous test results reflects a one standard deviation change in the Ln of the test result. N=486 events in MESA and 96 in DHS.
ECG-LVH was treated as a categorical variable in both analyses. Coefficients were determined separately for each model.
Evaluation of Risk Prediction Metrics
Addition of the 5 tests to the base model (variables from model 2) improved the c-statistic for global and ASCVD in both DHS and MESA and for each of the tertiary endpoints in MESA (P<0.01 for each, Table 4), with the largest increase in c-statistic seen for the global CVD and HF endpoints. Addition of the test results also resulted in significant category-free net reclassification improvement (NRI) and integrated discrimination improvement for the global CVD endpoint in both MESA and DHS (Table 4). Models including the 5 tests were well calibrated in both MESA and DHS for both global and ASCVD endpoints (Supplementary Figures 2 and 3). In exploratory analyses focusing only on the ASCVD endpoint in MESA, the largest improvement in risk prediction metrics was observed when CAC was added to the base model, with modest but significant increments beyond CAC in the c-statistic, NRI, and IDI observed for addition NT-proBNP and hs-cTnT, but not for addition of ECG-LVH or hs-CRP (Supplementary Table 7).
Table 4.
Change in risk prediction metrics with additional of test results to base models
| MESA | DHS | |||||||
|---|---|---|---|---|---|---|---|---|
| C-statistic Base Model* | C-statistic Base Model + Test Results | Category free NRI | IDI | C-statistic Base Model* | C-statistic Base Model + Test Results | Category free NRI | IDI | |
| Global CVD | 0.743 | 0.786† | 0.473 (0.383,0.563) | 0.073 (0.063, 0.083) | 0.832 | 0.850‡ | 0.261 (0.052,0.47) | 0.024 (0.008, 0.04) |
| ASCVD | 0.748 | 0.779† | 0.394 (0.275,0.512) | 0.042 (0.032, 0.052) | 0.859 | 0.873‡ | 0.355 (0.129,0.581) | 0.021 (0.001, 0.041) |
| CHD | 0.749 | 0.794† | 0.498 (0.357,0.638) | 0.043 (0.031, 0.055) | 0.865 | 0.889‡ | 0.585 (0.294,0.877) | 0.058 (0.023, 0.093) |
| Heart Failure | 0.786 | 0.847† | 0.549 (0.407,0.692) | 0.086 (0.064, 0.108) | 0.840 | 0.871§ | 0.719 (0.381,0.989) | 0.13 (0.042, 0.218) |
| All-Cause Mortality | 0.746 | 0.789† | 0.315 (0.199,0.431) | 0.038 (0.03, 0.046) | 0.817 | 0.829 | 0.061 (−0.516,0.638) | 0.03 (0.005, 0.055) |
| Cardiovascular Mortality | 0.822 | 0.840‡ | 0.438 (0.185,0.691) | 0.05 (0.032, 0.068) | 0.840 | 0.858 | 0.308 (−0.045,0.662) | 0.012 (−0.01, 0.034) |
Base model includes variables in the pooled cohort equations plus statin therapy. Global CVD models additionally include creatinine and heart failure models additionally include body mass index and creatinine. Coefficients were determined separately for each model.
p<0.001;
p<0.01;
p<0.05 vs. base model
In sensitivity analyses, the MESA base models and models including all 5 five test results were applied directly to DHS using coefficients for all variables derived from MESA. In these analyses, both base models and fully adjusted models had lower c-statistics than the models in which the coefficients were derived in the DHS dataset. However, improvements in the c-statistic, NRI, and IDI were similar using the two modeling strategies (Supplementary Table 8). Calibration remained adequate in DHS when the MESA models were directly applied (Supplementary Figure 4), but as expected was worse compared with the models in which coefficients were derived in DHS.
Multimodality risk score
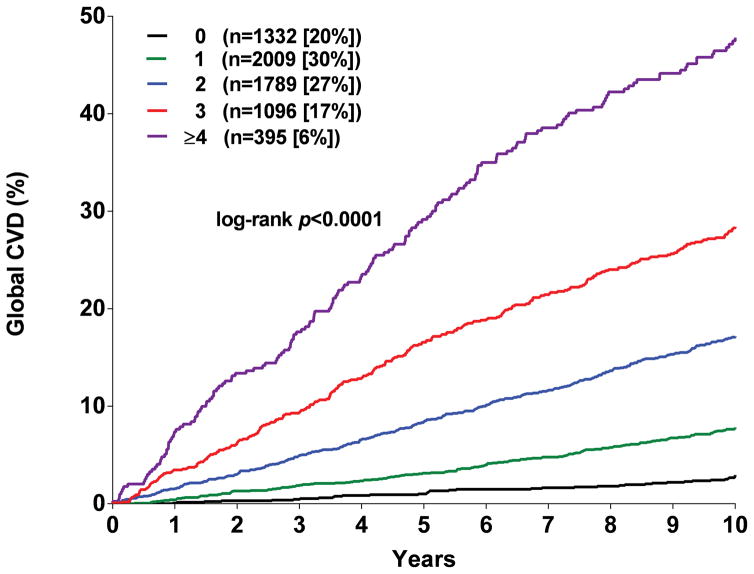
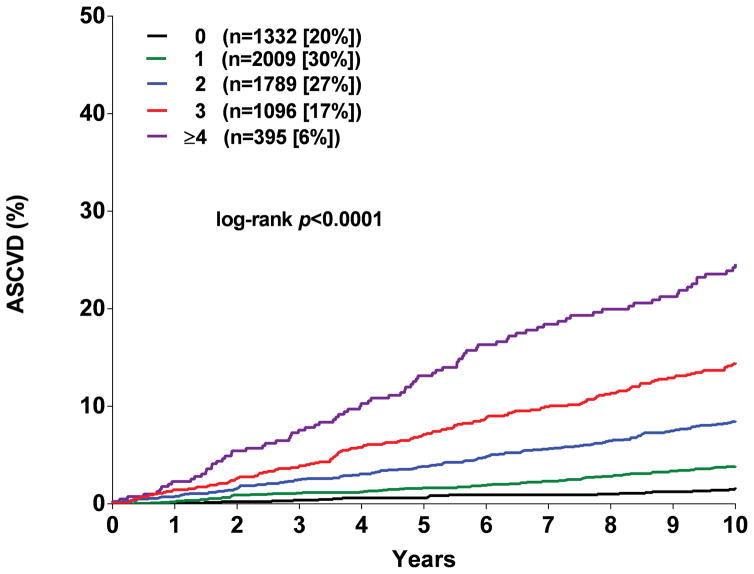
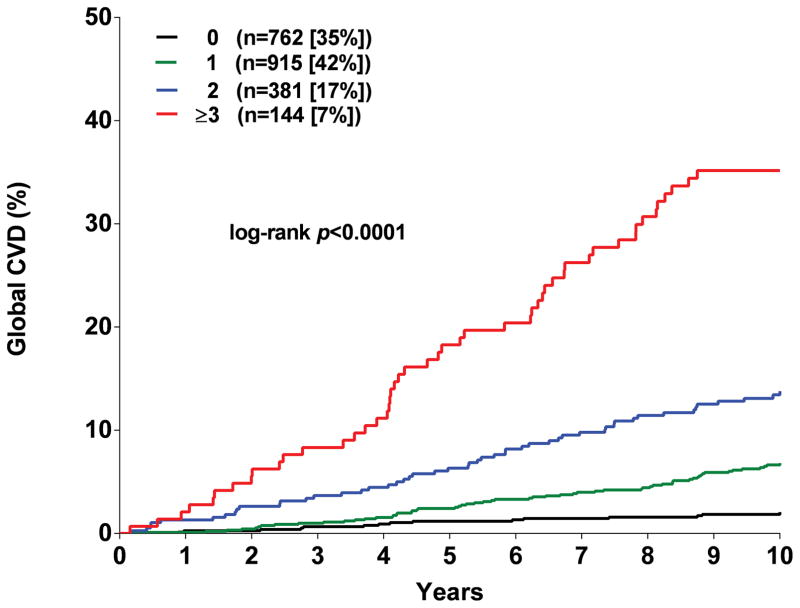
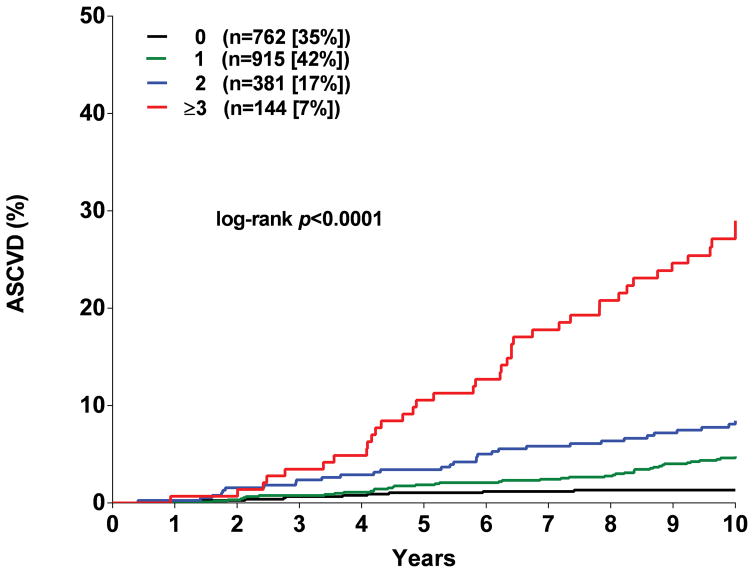
Participants were assigned one point for each abnormal test result, yielding an integer score ranging from 0–5. The proportion of individuals with scores of 0, 1, 2, 3 or ≥ 4 was 20, 30, 27, 17, and 6 % in MESA and the proportion with scores of 0, 1, 2, and ≥ 3 in DHS was 35, 42, 17, and 7 %, respectively (Supplementary Figure 5). A > 20-fold gradient of risk for both global CVD and ASCVD was observed across higher scores in both MESA and DHS (Figure 1). In MESA, participants with scores ≥2 comprised <50% of the cohort but accounted for 79% of the events. In the younger DHS population, participants with a score ≥2 comprised 24% of the cohort but accounted for 58% of events (Supplementary Figure 5). In both MESA and DHS, consistent graded associations with global CVD and ASCVD risk were seen with increasing scores across sex and race/ethnic subgroups, and in younger and lower risk individuals (Figure 2, Supplementary Figure 6).
Figure 1. Kaplan Meir estimates of the rates of the composite global cardiovascular disease (CVD) and atherosclerotic cardiovascular disease (ASCVD) outcomes stratified by the number of abnormal test results.
Panel A: Global CVD outcome in MESA. Panel B: ACVD outcome in MESA. Panel C: Global CVD outcome in DHS. Panel D: ASCVD outcome in DHS.
Figure 2. Unadjusted association between the number of abnormal test results and the composite global cardiovascular disease (CVD) and atherosclerotic cardiovascular disease (ASCVD) outcomes in selected subgroups in MESA.
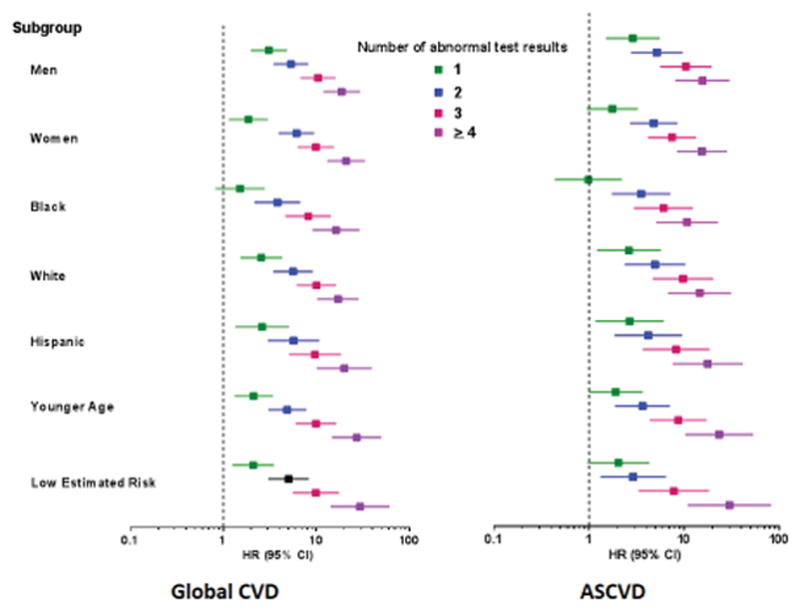
All comparisons are versus participants with a risk score =0. No significant interactions were seen across subgroups (p>0.05 for each). Younger age is defined as age <55 in men and <65 in women. Low estimated risk is defined as 10-year estimated ASCVD risk < 7.5% using the pooled cohort equations.
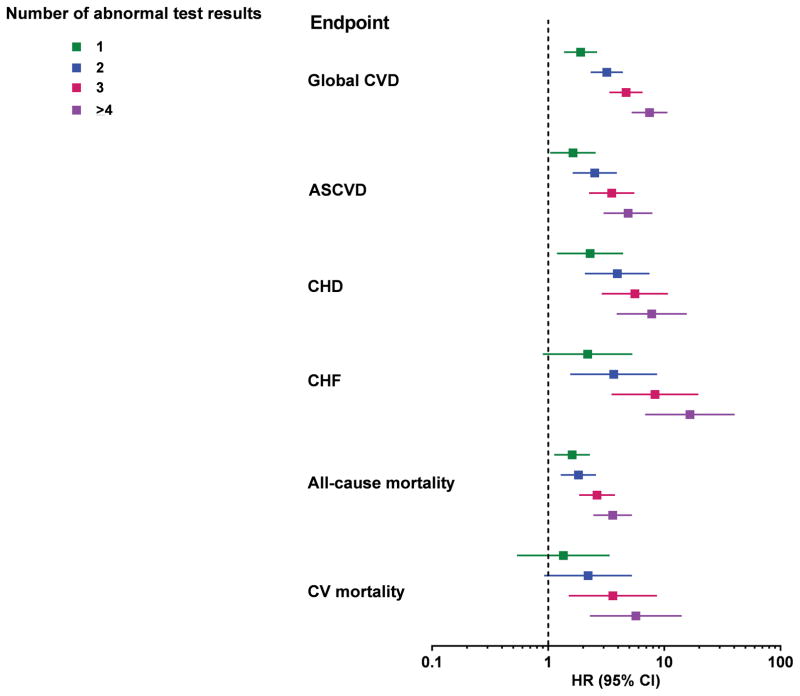
As expected, higher scores were associated with a greater burden of traditional risk factors (Supplementary Tables 9 and 10). However, in multivariable analyses accounting for traditional risk factors, compared with those with a score of 0 in MESA, CVD risk increased among participants with a score of 1 (HR 1.9, 95% CI 1.4–2.6), 2 (HR 3.2, 95% CI 2.3–4.4), 3 (HR 4.7, 95% CI 3.4, 6.5) and ≥ 4 (HR 7.5, 95% CI 5.2–10.6) (Figure 3). Similar graded associations across higher scores were seen for the secondary ASCVD endpoint and with tertiary endpoints, with findings most robust for incident heart failure (Figure 3). In the DHS, higher scores were also associated with Global CVD, ASCVD, and all-cause mortality in the fully adjusted models (Supplementary Figure 7).
Figure 3. Multivariable-adjusted association between the number of abnormal test results and cardiovascular endpoints in MESA.
All comparisons are versus participants with a risk score=0. All models adjusted for age, sex, race, smoking status, diabetes, total cholesterol, HDL-cholesterol, systolic blood pressure and blood pressure medications, and statin medications. The global CVD and mortality models were additionally adjusted for serum creatinine, and the heart failure models were additional adjusted for body mass index and creatinine. CVD=cardiovascular disease; ASCVD=atherosclerotic CVD, CHD=coronary heart disease; CHF=congestive heart failure; CV=cardiovascular
Discussion
In the present study, we combined 5 promising tests for cardiovascular risk stratification among adults without known CVD: the 12-lead ECG to assess LVH, CAC scanning, and measurement of NT-proBNP, hs-cTnT, and hs-CRP. Although this combination of tests captures multiple well-defined cardiac pathological processes, including cardiac hypertrophy, coronary atherosclerosis, neurohormonal activation, cardiomyoctye injury and inflammation, to our knowledge they have not been considered together previously. Each test provided non-redundant incremental information to traditional risk factors, and when the test results were combined in a simple integer score, a > 20-fold gradient in risk for the primary global CVD outcome was seen across the range of scores after > 10 years of follow-up. The findings were consistent among women, ethnic minorities, younger individuals, and those at low predicted risk for ASCVD. Results were robust to multivariable adjustment, and across different CVD endpoints, and performed similarly in two distinct cohorts with different age ranges and race/ethnic distributions. Discrimination and risk classification were improved for both global and ASCVD outcomes, and models incorporating the screening test results were generally well calibrated. These findings provide strong evidence that a simple strategy including the most promising biomarkers from several different testing modalities substantially improves CVD risk prediction among individuals without known CVD.
CVD risk stratification has traditionally been focused on predicting only CHD events. More recently, in concert with changes in prevention guidelines,1, 50 the focus of CVD risk prediction has expanded to include stroke. It is notable that while rates of MI and stroke have been steadily declining,51 the prevalence of HF is projected to increase by 25% over the next 20 years.4 Among middle-aged adults, the 10-year risk of incident heart failure is approximately 10%,52 with lifetime risks of 30–40%.53 Tools to predict incident HF may allow targeted therapies to prevent its development, which could have important public health implications given its associated morbidity and mortality. To date only a single global CVD risk model has been developed that considers ASCVD and HF together,54 and differs from our approach as it only contained traditional CHD risk factors as covariates and did not include AF as part of the global CVD outcome.54 Like HF, AF is rapidly increasing in prevalence, is difficult to treat once present, and carries substantial costs and morbidity.5, 55 A focus on global CVD risk prediction, incorporating endpoints of HF and AF as was done in the present study, is likely to become increasingly important. Importantly, global CVD risk assessment should be considered as a complement and not a replacement for cause-specific risk estimation (i,e for ASCVD).
Although the individual biomarkers were each associated with the primary composite global CVD endpoint, they differed in their relative associations with secondary and tertiary CVD endpoints, as would be expected based on the pathological processes captured by each biomarker.11, 15, 18, 31, 39 For example, CAC demonstrated the largest hazard ratio for ASCVD and CHD events, while NT-proBNP and hs-cTnT were associated with the highest hazards for all-cause and CVD mortality and heart failure. Importantly, although NT-proBNP and hs-cTnT were included to enhance global CVD risk prediction, they also provided independent prognostic value for ASCVD and CHD in MESA. While hs-CRP provided modest incremental information for the global CVD endpoint and HF endpoints, this biomarker generally demonstrated the weakest and least consistent associations across the portfolio of endpoints. Importantly, the multimodality strategy provided robust discrimination and reclassification for ASCVD as well as global CVD events. Thus, this strategy may contribute to more accurate identification of appropriate candidates for ASCVD preventive therapies, while at the same time capturing risk for broader CVD events.
Several limitations of the present study merit consideration. First, this study was not designed to determine the optimal number or combination of the screening tests for risk stratification purposes. The number of potential combinations of the 5 tests is 120, and each potential combination could be considered for multiple endpoints. Second, the number of endpoints in the DHS was too low to perform multivariable adjustment for the tertiary endpoints. However, the adjusted results for the primary and secondary outcomes demonstrated consistent results compared with MESA, as did unadjusted analyses for the tertiary endpoints. Finally, we acknowledge that comparing strength of association between the different tests presents challenges, and can be influenced by the incidence of the different endpoints and the distributions of the test results in the study cohorts.
The goal of our study was to evaluate prospectively a multimodality risk prediction strategy, and replicate the findings in a second population-based dataset. We did not design our primary analyses to validate the MESA multivariable models in DHS, but rather to determine if the scientific approach replicated in a second data set. We did perform sensitivity analyses in which the MESA models were applied directly to the DHS, and while overall performance of the models was modestly impacted (as would be expected), the improvement in model performance with addition of the 5 tests was generally similar to the primary analysis approach in which the coefficients were derived in the DHS. The models from MESA were also less well calibrated when applied to DHS, particularly for the ASCVD endpoint, although calibration remained adequate. Additional prospective validation is required before the multivariable models can be considered for clinical application.
Clinical Implications
Current consensus recommendations support only selective additional testing beyond traditional cardiovascular risk factors.1, 22 However, combinations of tests were not assessed in these guidelines, and the gradients of risk seen with the individual tests considered in these documents were not as large in magnitude as those seen with the multimodality risk score in the current study. Our robust findings support the potential value of a multimodality testing strategy using these markers in selected individuals in whom additional risk stratification is desired.
The multimodality testing strategy may help to individualize and more efficiently target cardiovascular prevention efforts in primary care. Although current prevention guidelines recommend a risk-based approach only when implementing statin and aspirin therapy, the role for targeting therapy based on risk in primary prevention is likely to expand in the future. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that lowering blood pressure below currently recommended targets was associated with reduced rates of heart failure and all-cause mortality,8 endpoints that were predicted well by the tests studied here. The favorable effects of more aggressive blood pressure lowering in SPRINT were balanced by side effects and some safety concerns, and the resource implications of broad implementation of lower blood pressure targets would be substantial. In addition, a novel agent for the management of diabetes, empagliflozin, recently demonstrated a 38% reduction in death from cardiovascular causes and 35% reduction in heart failure, with lesser impact on ASCVD endpoints.56 Targeting empagiflozin to patients at highest risk for death and heart failure events may be a prudent strategy given the high cost of the drug. Thus, an individualized, risk-based approach utilizing traditional risk factors plus biomarkers may be appropriate when determining blood pressure targets or implementing newer therapies that favorably impact CVD endpoints beyond ASCVD.
The multimodality strategy could also facilitate targeting of global and disease-specific CVD prevention efforts as population health care becomes an increasing focus of health care delivery. For example, among individuals with risk score of 0, global CVD risk was extremely low in both MESA and DHS (<3% over 10 years in each study), and this large group of individuals could be managed with a low intensity/low cost approach. On the other hand, higher scores clearly captured risk not recognized with traditional risk factor algorithms, as consistent results were seen even among individuals estimated to be at low risk with the pooled cohort equations. Individuals with scores ≥2, for example, represented fewer than half of MESA participants and 1/4 of the younger DHS cohort, yet accounted for 79% and 58% of global CVD events, respectively. A tailored and incrementally more intensive approach to global CVD risk reduction would be appropriate for individuals with a greater number of abnormal test results. For example, higher risk individuals could be referred to lifestyle intervention programs, focusing on improving low fitness and obesity, which are important contributors to multiple components of the global CVD endpoint. Triage for cardiovascular specialist evaluation may be considered for the highest risk individuals, a strategy recently evaluated for a biomarker screening program in primary care with promising preliminary results. 57 Although each of the 5 tests is available clinically and thus measurement is currently feasible, larger studies will be needed both to validate the present findings and also to elucidate the optimal strategy for clinical implementation. Moreover, additional consideration of costs, both those directly related to the tests and those engendered by abnormal test results, would be necessary prior to implementation.
Conclusion
A novel multi-modality CVD risk assessment strategy using the non-redundant markers of ECG LVH, CAC, NT-proBNP, hs-cTnT, and hs-CRP substantially improved global and atherosclerotic CVD risk stratification among individuals from the general population free from CVD at study entry. Additional study of preventive strategies incorporating these complementary tests is indicated.
Supplementary Material
Clinical Perspective.
What is New?
We evaluated a novel strategy for assessment of CVD risk among adults without known CVD that combined promising biomarkers across multiple different testing modalities, including 12-lead electrocardiography for assessment of left ventricular hypertrophy, coronary artery calcium, N-terminal pro-brain natriuretic peptide, high sensitivity cardiac troponin T and high sensitivity C-reactive protein.
Each test result provided incremental information with regard to global CVD risk in the Multi-Ethnic Study of Atherosclerosis (MESA), and a score containing the 5 results provided robust stratification of global and atherosclerotic CVD risk, with findings replicated in the Dallas Heart Study.
What are the Clinical Implications?
Our findings support the potential value of a multimodality testing strategy in selected individuals in whom additional risk stratification is desired beyond measurement of traditional atherosclerosis risk factors.
Additional studies are needed to validate the present findings, determine the optimal approach to implementation, and address direct and indirect cost implications of the additional testing.
Acknowledgments
The authors thank the other investigators, the staff and participants of the MESA and DHS studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This study was funded by research grant CA03801 awarded to Dr. de Lemos from the National Space Biomedical Research Institute (NSBRI). MESA was supported by research was supported by R01 HL071739 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. The DHS was funded by a grant from the Donald W. Reynolds Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center. Biomarker measurements were supported by investigator initiated grants to Dr. de Lemos and DeFilippi from Roche Diagnostics.
Footnotes
Role of the sponsor: Roche diagnostics had no role in the design and conduct of the study and did not participate in analysis or interpretation of the data. They were not provided a summary report of the data and have not reviewed this submission.
Disclosures
Dr. de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics, and consulting income from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Prevencio, Diadexus, Siemen’s Healthcare, Radiometer, and Amgen. Dr. DeFilippi has received research support from Roche Diagnostics, Abbott Diagnostics, Critical Diagnostics and consulting fees or honoraria from Roche Diagnostics, Siemens Healthcare Diagnostics, Thermo-Fisher, and Radiometer. Dr. Wang has received consulting fees from Ultragenyx. Dr. Seliger has received grant support from Roche Diagnostics. Dr. Budoff has received grant support from General Electric. Dr. Ballantyne has received grant support from Roche Diagnostics and Abbott Diagnostics and has a provision patent (filed by Baylor College of Medicine and Roche) for the use of biomarkers to improve prediction of heart failure.
References
- 1.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Cook NR. Statins: New american guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the UnitedAtates: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JD, Aronis KN, Chrispin J, Patil KD, Marine JE, Martin SS, Blaha MJ, Blumenthal RS, Calkins H. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol. 2015;66:2899–2906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 7.defilippi CR, de Lemos JA, Tkaczuk AT, Christenson RH, Carnethon MR, Siscovick DS, Gottdiener JS, Seliger SL. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol. 2012;60:2539–2547. doi: 10.1016/j.jacc.2012.08.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of c-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. Jama. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 11.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Gronemeyer D, Seibel R, Kalsch H, Brocker-Preuss M, Mann K, Siegrist J, Jockel KH. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett BM, Berger JS, Manson JE, Ridker PM, Cook NR. B-type natriuretic peptides improve cardiovascular disease risk prediction in a cohort of women. J Am Coll Cardiol. 2014;64:1789–1797. doi: 10.1016/j.jacc.2014.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 16.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B-type natriuretic peptides and cardiovascular risk: Systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 17.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, Ridker PM, Pradhan AD. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: The women’s health study. Circulation. 2011;123:2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 24.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 25.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathiresan S, Melander O, Anevski D, Guiducci C, Burtt NP, Roos C, Hirschhorn JN, Berglund G, Hedblad B, Groop L, Altshuler DM, Newton-Cheh C, Orho-Melander M. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358:1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 27.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Munzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The monica, risk, genetics, archiving, and monograph (morgam) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: The Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB, Abbott RD. A prognostic comparison of asymptomatic left ventricular hypertrophy and unrecognized myocardial infarction: The Framingham Study. Am Heart J. 1986;111:391–397. doi: 10.1016/0002-8703(86)90156-0. [DOI] [PubMed] [Google Scholar]
- 31.Desai CS, Ning H, Lloyd-Jones DM. Competing cardiovascular outcomes associated with electrocardiographic left ventricular hypertrophy: The Atherosclerosis Risk in Communities study. Heart. 2012;98:330–334. doi: 10.1136/heartjnl-2011-300819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Nazarian S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2014;63:2007–2013. doi: 10.1016/j.jacc.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic Study of Atherosclerosis: Objectives and design. American journal of epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 34.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 35.Deo R, Khera A, McGuire DK, Murphy SA, de PMNJ, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 36.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 37.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of Multi-ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 38.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African Americans and caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 39.de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: Results from the Dallas Heart Study. Am Heart J. 2009;157:746–753. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB, Jr, Herrington DM. Gender and c-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr, Grundy SM, McGuire DK. Relationship between c-reactive protein and subclinical atherosclerosis: The Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 42.Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133:631–638. doi: 10.1161/CIRCULATIONAHA.115.017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 44.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 45.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: The Health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: Extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 48.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kremers WK. Technical report series no. 80, concordance for survival time data:Fixed and time-dependent covariates and possible ties in predictor and time. Rochester, Minnesota: Mayo Clinic College of Medicine; 2007. [Google Scholar]
- 50.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 51.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 52.Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, Boerwinkle E, Hoogeveen RC, Aguilar D, Astor BC, Srinivas PR, Deswal A, Mosley TH, Coresh J, Folsom AR, Heiss G, Ballantyne CM. Troponin T and n-terminal pro-b-type natriuretic peptide: A biomarker approach to predict heart failure risk--the Atherosclerosis Risk in Communities study. Clin Chem. 2013;59:1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML, Lloyd-Jones DM. Lifetime risk for heart failure among white and black americans: Cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 55.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 56.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.