Abstract
Reactivity patterns to acute stress are important indicators of physical and mental health. However, the relationships between emotion socialization and stress responses are not well understood. This study aimed to examine whether parental responses to negative emotions predicted physiological and psychological responses to acute stress in late adolescence and emerging adulthood, and whether these relationships varied by gender and ethnicity. Participants were 973 individuals (mean age = 19.20 years; 50% male; 63% African American, 34% European American) who reported on parental emotion socialization. Participants completed a standardized social stress test (the Trier Social Stress Test; TSST). Heart rate, blood pressure and salivary samples were assessed from baseline throughout the task and during recovery period. Psychological responses to stress were measured immediately after the TSST. Unsupportive parental responses to children’s negative emotions were associated with blunted cortisol reactivity and greater negative emotions to a psychosocial stress task in females and African American youth, whereas supportive parental responses predicted greater cortisol reactivity and lower negative emotions to stress in European American youth, as well as less negative emotions in males. However, parental responses to negative emotions did not predict heart rate or SBP reactivity to the TSST. Findings suggest that parental emotion socialization may be an important factor influencing HPA axis reactivity and psychological responses to stress, with important differences across gender and ethnic youth subgroups.
Keywords: emotion socialization, stress reactivity, late adolescence, emerging adulthood
1. Introduction
Understanding how children and adolescents respond to stress is important for understanding of normal development, as well as the development of physical illness and psychopathology (Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000). Physiological and psychological responses to stress are closely related to emotion regulation abilities (Gross & Levenson, 1997; Quirin et al., 2011), which are partly determined by parental emotion socialization strategies (Katz et al., 2012; Shortt et al., 2016). For example, when mothers were more supportive of children’s emotions, children showed better emotion regulation abilities (Shipman et al., 2007). Thus, emotion socialization may also play a central role in children’s learning to regulate their physiological and psychological responses to stress. This relationship is also supported by the heuristic model of the socialization of emotion, which posits that emotion socialization is associated with children’s arousal in a given context such as stressful situations (Eisenberg, Cumberland, & Spinrad, 1998), and by the evolutionary perspective that the stress response system is developing adaptively to match the environment (Boyce & Ellis, 2005). Thus, parental support for the expression of emotions may help children to appropriately regulate both their psychological and physiological responses to acute stress. However, empirical studies investigating the relationships between emotion socialization and stress reactivity are lacking.
It has been noted that parents can respond in supportive or unsupportive ways to children’s negative emotions, which is largely determined by parents’ philosophy or attitudes towards negative emotions (Eisenberg et al., 1998; Gottman, Katz, & Hooven, 1996; Magai & O’Neal, 1997). Parents who adopt the “emotion-coaching” philosophy value their children’s negative emotions and consider them as opportunities to improve children’s emotion competence and strengthen parent-child intimacy. Those parents are more likely to encourage appropriate expression of negative emotions. Consequently, their children are more open to communicating their feelings and better at regulating emotions. In contrast, “emotion-dismissing” parents view negative emotions as inappropriate and harmful. Those parents are more likely to ask their children to ignore or suppress the expression of negative emotions. As a result, their children may not develop good emotion regulation skills and may try to avoid expressing negative emotions openly (Sanders, Zeman, Poon, & Miller, 2015).
Available evidence suggests that supportive emotion socialization strategies (e.g., acceptance and assistance with children’s emotions) are associated with children’s greater parasympathetic regulatory capacities (e.g., higher baseline vagal tone and greater vagal tone suppression) during parent-child interactions (Gottman et al., 1996), whereas unsupportive emotion socialization strategies are linked to poorer emotion regulation and coping (Sanders et al., 2015). Additionally, interventions targeting parents’ emotion socialization practices have resulted in fewer somatic complaints in youth (e.g., recurrent headaches without any specific organic cause; Kehoe, Havighurst, & Harley, 2015). Although supportive of the relationships between emotion socialization and physiological functioning, these studies have focused primarily on regulatory physiology in specific social contexts, such as parent-child interactions (Gottman et al., 1996), and not on physiological responses to stress. Thus, little is known about the relationship between emotion socialization and reactivity to acute social stress. To address this question, we used the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), which is one of the most commonly used paradigms to reliably induce acute psychosocial stress in a laboratory setting (Dickerson, Gruenewald, & Kemeny, 2004; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007). The main purpose of this study was to examine whether emotion socialization predicted physiological and psychological responses to stress. This study focused on late adolescence and emerging adulthood, a developmental period characterized by many stresses and challenges that accompany the transition from adolescence into young adulthood (Arnett, 2000). Finally, because little is known about possible gender and ethnic differences in the effects of emotion socialization on stress reactivity, moderating effects of gender and ethnicity were also explored.
1.1 Physiological Responses to Stress
Psychobiological responses to stress encompass two major systems: the autonomic nervous system (ANS) and the hypothalamic pituitary adrenocortical (HPA) axis (Chrousos, 2009). The ANS involves two coordinated, but also opposing systems: the excitatory sympathetic nervous system (SNS) and the inhibitory parasympathetic nervous system (PNS) (Porges, Doussard-Roosevelt, & Maiti, 1994). The SNS mobilizes bodily energy, whereas the PNS conserves and restores energy (Freeman, Dewey, Hadley, Myers, & Froelicher, 2006). In a stressful situation, activity of the SNS becomes dominant and produces a higher level of physiological arousal (e.g., increased heart rate and blood pressure) to cope with the stressor. By contrast, during rest the PNS is dominant and maintains a lower level of physiological arousal (e.g., decreased heart rate and blood pressure) (Appelhans & Luecken, 2006). The complex interactions between the two systems contribute to changes in cardiovascular activities (Stroud et al., 2009). ANS activation is quick (in seconds) and is typically considered as a “defense reaction” (Henry, 1993) – an active response to challenging, but controllable environmental demands (Schommer, Hellhammer, & Kirschbaum, 2003).
Stress also activates the HPA axis, triggering a sequence of events that involves the secretion of the corticotropic-releasing hormone (CRH) from the hypothalamus, stimulating the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland, and the release of glucocorticoid hormones by the adrenal cortex (Tsigos & Chrousos, 2002). The main glucocorticoid hormone in humans is cortisol, which helps mobilize resources to meet the increased metabolic demands required to deal with the stressors (Kudielka & Kirschbaum, 2005). The secretion of cortisol occurs relatively slowly (in minutes) (Chen et al., 2015). HPA activation is typically considered as a “defeat reaction,” occurring when the situation is perceived to be uncontrollable and with no hope of success (Björntorp, 2001; Henry, 1993).
Two hypotheses have been proposed regarding the association between psychosocial stressors and physiological responses to stress. The hyper-reactivity hypothesis posits that psychosocial stressors may heighten physiological stress responses, whereas the hypo-reactivity hypothesis poses that psychosocial stressors are linked to blunted physiological stress responses (Gunnar & Fisher, 2006; Lupien, McEwen, Gunnar, & Heim, 2009). Some studies support the hyper-reactivity hypothesis (Danese & McEwen, 2012; Jezova, Makatsori, Duncko, Moncek, & Jakubek, 2004; Linares, Shrout, Nucci-Sack, & Diaz, 2012; Ulrich-Lai & Herman, 2009), whereas other studies support the hypo-reactivity hypothesis (Evans et al., 2013; Lucas-Thompson, 2012; Saxbe, Margolin, Shapiro, & Baucom, 2012). Many factors may contribute to these discrepancies, including type of stressor exposure, time since stressor onset, stressor duration, and individual characteristics. A recent study also indicates that the relationship may vary based on child’s developmental history of exposure to stressful relationships and experiences (Jaffee et al., 2015). Although activation of the two stress systems is crucial for survival, chronic or repeated physiological responses to stressors may lead to dysregulation of stress systems, contributing to various physical and psychological disorders (Carney, Freedland, & Veith, 2005; Charmandari, Tsigos, & Chrousos, 2005; Stroud et al., 2009).
In the current study, we investigated whether retrospective reports of emotion socialization in childhood predict physiological responses to stress. Specifically, we focused on parental responses to negative emotions and both ANS (heart rate, SBP and DBP) and HPA axis reactivity (salivary cortisol) to acute psychosocial stress (the TSST).
1.2 Psychological Responses to Stress
It has been well documented that acute stress elicits heightened psychological responses, especially negative emotions (Allen, Kennedy, Cryan, Dinan, & Clarke, 2014). Besides emotional reactivity, individuals also react to stress by conscious cognitive effort to deal with the stressor, or mental effort mobilization (Brehm & Self, 1989; Gendolla & Richter, 2010). The intensity of mobilized efforts is determined by subjective task difficulty and performance contingent incentive (Gendolla & Krüsken, 2002; Wright, 2008). Effort typically increases with task difficulty, as long as the demands do not exceed the person’s abilities (e.g., the task is viewed as doable) and the outcomes are justified (e.g., success is worth the efforts) (Gendolla & Richter, 2010).
Thus, even with the same stressor, people might mobilize different levels of mental efforts (e.g., trying hard vs. giving up), due to differences in subjective evaluation of the task difficulty and importance of dealing with the stressor. Thus, psychological responses to stress involve negative emotions and effort mobilization. However, these two domains have been typically studied in separate lines of research, so little is known about the relationship of these two types of responses. This study examined if emotion socialization predicts both types of psychological responses to stress. Specifically, we focused on parental responses to negative emotions and psychological responses (negative emotions and mental effort mobilization) to acute psychosocial stress (the TSST).
1.3 The Moderating Role of Gender and Ethnicity
Findings regarding gender differences in stress-related physiological responses are inconsistent. Some studies found no gender differences in either cardiovascular (e.g., SBP and DBP) or HPA axis reactivity to acute stressors (Dickerson & Kemeny, 2004; Girdler, Turner, Sherwood, & Light, 1990; Kelly, Tyrka, Anderson, Price, & Carpenter, 2008). In contrast, other studies revealed that females show greater heart rate increase and males demonstrate greater salivary cortisol responses to acute stress (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Kudielka et al., 2000; Lovallo, 2006; Spangler, 1997). In addition, dysregulation of the HPA axis has been proposed to be a major contributor to higher rates of depression in females (Oldehinkel & Bouma, 2011). With regard to psychological responses to stress, females tend to report higher level of negative emotions compared to males (Kelly et al., 2008; Kudielka et al., 2004; Troisi, 2001). Although gender differences in effort mobilization have received little attention in research, men tend to have higher SBP baseline values than women and no gender differences in effort-related cardiovascular reactivity have been observed (Gendolla, Richter, & Silvia, 2008). Therefore, the current study aimed to explore possible gender differences in both physiological and psychological responses to acute stressor, which may contribute to gender disparities in mental and physical health outcomes (Kudielka & Kirschbaum, 2005).
It has been well documented that racial/ethnic minorities, especially African Americans individuals, have higher rates of stress-related diseases (e.g., cardiovascular disease, hypertension, and stroke), higher mortality rates, and lower life expectancies than European American individuals (Kahn & Fazio, 2005; Williams, Neighbors, & Jackson, 2003). Psychosocial stress has been proposed to be a potential contributor to the observed health disparities, because ethnic minorities tend to be exposed to more psychosocial stressors (Turner & Avison, 2003; Utsey et al., 2013) that can cause adverse physiological effects (Stroud et al., 2009). Mixed findings have been reported for ethnic differences in cardiovascular responses to acute stress, with some studies finding higher levels of cardiovascular baseline activity and reactivity to laboratory stressors in African American adults and others finding lower levels (Gillin et al., 1996).
Surprisingly, few studies have examined ethnic differences in HPA axis reactivity (DeSantis et al., 2007; Finney, Stoney, & Engebretson, 2002). One study found that African American adolescents have flatter cortisol slopes across the waking day than European American youth (DeSantis et al., 2007), a pattern which has been associated with poorer health outcomes (Adam & Gunnar, 2001). Thus, one aim of the current study was to examine possible ethnic differences in physiological and psychological responses to a standardized social-evaluative stressor (the TSST).
1.4 Goals and Hypotheses
Based on the literature reviewed, the purpose of this study was to investigate the associations between parental emotion socialization and physiological and psychological responses to stress. We hypothesized that more unsupportive and less supportive parental response to negative emotions would be linked with stronger physiological reactivity, lower levels of effort mobilization, and heightened negative emotional responses to acute psychosocial stress. Possible gender and ethnic differences in these effects were also explored.
2. Methods
2.1 Participants
Participants were 973 individuals (M age = 19.20 years, SD = 1.13; range = 16–23) participating in a larger community-based study of adolescent health. Youth were recruited from fifth grade classrooms in public schools in a large city in the Southeast U.S. and followed throughout adolescence. Because perceived emotion socialization was assessed only at the last wave (Wave 4), data from previous assessments are not included in this report. Of the current participants, 50% (n = 487) were male and 50% (n = 486) were female. Approximately 63% (n = 617) of participants were African American, 34% (n = 331) were European American, and 3% (n = 25) were other ethnicities. About 7% of current participants dropped out of high school, 9% were still in high school, 30% completed high school but were not in college, and 54% were in college. Regarding parental education, 7% did not complete high school, 21% completed high school but did not attend college, 33% had some college education or a 2 year degree, and 39% graduated from a 4-year college or had a graduate degree.
2.2 Procedures
All study procedures were approved by the University of Alabama at Birmingham Institutional Review Board. After providing informed consent, each participant was interviewed individually by a trained interviewer. Most participants were interviewed in person at a university research lab, but individuals who have moved away from the local area (9%) were interviewed over the phone, and those participants did not attend the TSST task. The interview included self-report questionnaires that were administered through computer-assisted technology, anthropometric measurement of height and weight, as well as Trier Social Stress Task (TSST, Kirschbaum et al., 1993).
After getting acclimated to the lab environment and being interviewed for approximately 60 minutes, participants were asked to rest for five minutes (baseline) and then were introduced to the Trier Social Stress Test (TSST; Kirschbaum et al., 1993). They were asked to do their best on the task, and informed that those performing at the top 20% would receive a special prize. Participants were then given five minutes to prepare a speech for a job interview and then five minutes to present the speech in front of two judges. Next, participants were asked to do a mental arithmetic task (serial subtraction; e.g., 996 minus 13) for another five minutes, with the difficulty level adjusted based on the participants’ performance to achieve similar difficulty level for each participant (e.g., if the participant made 3 or more mistakes in a short period of time, the judges would decrease difficulty by one sequence down). The judges wore white coats and provided no positive feedback throughout the test. The participants were also videotaped during the tasks and informed that experts will be viewing these videotapes and evaluating their behavior.
All interviews were conducted in the afternoon to minimize the effects of diurnal variation in cortisol production (Granger, Johnson, Szanton, Out, & Schumann, 2012). Participants were instructed to avoid the following on the day of visit: strenuous exercise, smoking, alcoholic beverages, caffeinated drinks or pills. Compliance with these requirements was confirmed during the interview. None of the participants were diagnosed with chronic medical conditions or taking medications that would affect cortisol levels.
2.3 Measures
2.3.1 Emotion socialization
Emotion socialization was measured using the Emotions as a Child Scale (EAC; Magai & O’Neal, 1997), a measure assessing youth-reported parental emotion socialization practices for anger, fear and sadness. The original EAC was based on Malatesta-Magai’s model (1991) that parents typically use five independent strategies (Reward, Punish, Override, Neglect, and Magnify) to socialize children’s emotions. The five subscales are retained as separate factors. Alternatively, 2-factor models have been proposed based on the notion that specific emotion socialization strategies can be categorized into those that facilitate vs. inhibit children’s emotional expressions (Magai & O’Neal, 1997). Based on this model, an abbreviated version of EAC has been developed and validated in the population of late adolescence and emerging adulthood, with measurement invariance established across gender and ethnicity (Guo, Mrug, & Knight, 2016). Therefore, the abbreviated version of the EAC was used in the current study. The abbreviated EAC is composed of two subscales across the three emotions: supportive and unsupportive parental responses (7 and 5 items, respectively). The supportive parental responses subscale includes the reward (e.g., “comforted me”), neglect (e.g., “focused on me”; reverse-coded), and override (e.g., “told me to cheer up”) dimensions from the original EAC. The unsupportive parental responses subscale combines the punish (e.g., “let me know s/he did not approve”) and magnify (e.g., “got very sad”) items from the original EAC. Participants were asked to rate how often their parent responded to each emotion in the given way when they were children on a scale ranging from 1 (Never) to 5 (Very often). In the current study, item scores for the supportive and unsupportive subscales were averaged across the three emotions (21 supportive and 15 unsupportive items). Cronbach’s alphas were .96 and .87, respectively. The correlation between the supportive and unsupportive subscales was .12 (p<.01), indicating that the two subscales were relatively independent.
2.3.2 Heart rate and blood pressure
Before the baseline period started, a wrist cuff from Vasotrac blood pressure monitor was attached on each participant’s non-dominant hand. Heart rate and blood pressure were recorded every 30 seconds from baseline throughout the task and a 5-minute recovery period. Baseline levels of heart rate and blood pressure were computed as the average of the last 2 baseline measurements (to allow participants to achieve a true baseline). Heart rate and blood pressure during the remaining periods (preparation, speech, math, and recovery) were averaged within each period (i.e., 10 measurements per each 5-minute period).
2.3.3 Saliva collection
Salivary cortisol is the most commonly used non-invasive biomarker of HPA axis responses (Skoluda et al., 2015). Saliva samples were collected using passive drool immediately before the baseline period (pre-task), 30 minutes after the 15-minute task began (15 minutes post-task; peak stress), and 55 minutes after the task began (40 minutes post-task; recovery). The rationale for defining peak stress at 15 minutes post-task is that peaks in salivary cortisol commonly occur between 10–20 minutes post-stress (Allen et al., 2014; Dickerson & Kemeny, 2004; Rohleder, Schommer, Hellhammer, Engel, & Kirschbaum, 2001). Samples were immediately frozen at −20º C and later shipped overnight on dry ice for cortisol analyses at the Institute for Interdisciplinary Salivary Bioscience Research at Arizona State University.
After thawing, samples were centrifuged at 3,000 rpm for 15 minutes to remove mucins. Samples were assayed for cortisol using commercially available competitive immunoassays without modification to the manufacturers recommended protocol (Salimetrics, State College, PA). The cortisol assay used 25 μl of saliva for singlet determinations and had a range of sensitivity from 0.007 to 3 μg/dl. Samples were all assayed in duplicate and the average of the duplicate assays were used in the statistical analysis. On average, intra- and inter-assay coefficients of variation were less than 10% and 15%.
2.3.4 Psychological responses to stress
A self-report questionnaire was administered immediately after the TSST to assess participants’ psychological responses to psychosocial stress. Participants were asked to rate their experiences with the speech and math tasks using 16 items on a scale ranging from 1 (Not at all) to 5 (Very much). Two subscales were derived with exploratory factor analysis – effort mobilization and negative emotions subscales (4 and 9 items, respectively; see Data Analyses and Results). Sample items include: “I tried hard to make a good impression on the audience” (effort mobilization); “During the tasks, I felt ashamed” (negative emotions). Cronbach’s alphas were .83 and .89 for the effort mobilization and negative emotion subscales, respectively.
2.3.6 Covariates
Covariates include participants’ age, sex, ethnicity, academic status, parental education, body mass index (BMI), and time of day when saliva samples were taken. Sex was coded 0 for male and 1 for female; ethnicity was coded 0 for European American and 1 for African American or other. Academic status was coded into three dummy coded variables: dropped out of high school, still in high school, and completed high school but not in college; being in college served as the reference group. Parental education was reported on an ordinal scale ranging from ‘did not complete high school’ (1) to ‘graduated from a 4-year college or had a graduate degree’ (4).
2.3.7 Moderators
Sex and ethnicity served as moderators in multigroup modeling to explore possible gender and ethnic differences in physiological and psychological responses to stress. Because 97% of the participants were European American or African American, ethnic differences were tested across these two subgroups only (ethnicity was coded 0 for European American and 1 for African American); those from other ethnic groups were excluded from these analyses.
2.4 Data Analyses
Descriptive statistics were performed and outliers were truncated to 3 SD from the mean (Gravetter & Wallnau, 2016). Then, bivariate correlations among main variables were examined. Gender and ethnic differences among all main variables were examined using independent-samples t-test. Missing data were handled using Full Information Maximum Likelihood (FIML) in all analyses (Wothke, 2000). Log-transformation was performed on skewed variables to approach a normal distribution. Because some of the variables were still not normally distributed after log-transformation, the maximum likelihood estimation with robust standard errors (MLR) was used. The effects of emotion socialization on cortisol reactivity were tested with multiple regressions predicting overall cortisol secretion and its increase throughout the test, computed as areas under the curve with respect to ground and increase (AUCG and AUCI), respectively (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Latent growth curve modeling was not used for cortisol reactivity since saliva samples were collected only at three time points; thus, only a linear trajectory could be estimated, which would not allow accurate modeling of the expected curvilinear cortisol response to acute stress (increase followed by decrease) (Curran, Obeidat, & Losardo, 2010). The effects of emotion socialization on ANS reactivity to stress were tested using latent growth curve models in Mplus version 7.11. First, the Intraclass Correlation Coefficients (ICC) were estimated from unconditional means models (no predictors). Then, the growth curves of the ANS responses across the five periods (baseline, preparation, speech task, math task, and recovery) were characterized using unconditional growth models with an intercept, linear slope, and quadratic slope. Next, emotion socialization dimensions (supportive and unsupportive parental responses) were included as predictors of the growth curve parameters (intercept and slopes). Good fit was indicated by comparative fit index (CFI; Bentler, 1990) > .95, root-mean-square error of approximation (RMSEA; Steiger & Lind, 1980) ≤ .06, and standardized root-mean-square residual (SRMR; Bentler, 1995) ≤ .08. Covariates in all analyses included participants’ age, sex, ethnicity, academic status, parental education, and body mass index (BMI). The cortisol analyses were also adjusted for time of day when saliva samples were taken.
For the psychological response model, exploratory factor analysis (EFA) was first conducted to explore the factorial structure of the TSST questionnaire. Factorability of the items was first examined with item correlations, the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO), and the Bartlett’s test of sphericity. Then, EFA was conducted using principal axis factor extraction and oblique rotation. If factor correlations were less than .32, the EFA was rerun with orthogonal rotation (Tabachnick & Fiddell, 2007). Multiple criteria were utilized to inform factor retention, including (a) eigenvalues > 1 (Kaiser, 1960), (b) the scree test (Cattell, 1966), (c) Horn’s parallel analysis (HPA; Horn, 1965) and (d) Velicer’s minimum average partial correlation (MAP; Velicer, 1976). Among these approaches, HPA and MAP tend to be the best criteria for determining the number of factors (Velicer, Eaton, & Fava, 2000). For the final factor solution, several items were eliminated based on low factor loadings (<.40), low communalities (≤.40), or cross-loadings across factors (>.40). Cronbach’s alpha was used to examine internal consistency of the final factors.
Next, the effects of emotion socialization on psychological responses to stress were tested using path analysis in Mplus Version 7.11. Independent variables included supportive and unsupportive parental responses to negative emotions. Dependent variables included effort mobilization and negative emotions to stress. The independent and dependent variables were allowed to covary. Covariates included participants’ age, gender, ethnicity, academic status, and parental education.
Finally, multigroup modeling was conducted to explore possible gender and ethnic (African American vs. European American) differences for each physiological and psychological response model. The multigroup analyses compared the fit of a constrained model (all path estimates were constrained to be equal across gender or ethnic groups) with the fit of an unconstrained model (all path estimates were allowed to vary across groups). Moderation was indicated by a significantly better fit of the unconstrained model compared to the constrained model. If a model showed group differences, follow-up analyses investigated specific differences by freeing a single path at a time and comparing it to the fully constrained model. Again, a significantly better fit of the less constrained model indicated that the freed path varied across the two groups. To prevent inflation of Type I error due to multiple testing, these follow up analyses used Bonferroni correction with p level set at .025 for the cortisol reactivity model, .008 for the ANS reactivity models and .0125 for the psychological responses model.
3. Results
3.1 Preliminary Analyses
Preliminary analyses identified 122 observations as outliers. The outliers were distributed among heart rate and blood pressure reactivity across the five periods (baseline, preparation, speech task, math task, and recovery) and cortisol reactivity across the three periods (baseline, 15 min post-task and 40 min post-task). These values were truncated to 3SD above the mean. Correlations among main variables showed that supportive parental responses to negative emotions were associated with higher heart rate during the speech part of TSST (r = .08, p < .05) and higher reported effort mobilization (r = .07, p < .05), whereas unsupportive parental responses were linked to lower heart rate during the math part (r = −.07, p < .05) and more negative self-reported emotions during the task (r = .11, p < .01). In addition, SBP and DBP were highly correlated across the five time periods (r = .92 to .94, p < .001). Thus, only SBP was used in further analyses. Finally, effort mobilization was related to higher heart rate and SBP during the speech part (r = .06, p < .05 for heart rate, r = .07, p < .05 for SBP) and negative self-reported emotion was associated with higher heart rate at baseline and during the recovery period (r = .08, p < .05 for baseline, r = .07, p < .05 for recovery).
Independent-samples t tests (Table 1) indicated that females reported more supportive parental responses and fewer unsupportive parental responses to negative emotions compared to males. In addition, females had higher heart rate and lower SBP and cortisol levels (AUCG and AUCI) than males. Finally, females reported more negative emotions but similar level of effort mobilization during the TSST compared to males. In terms of ethnic differences, African American participants reported more unsupportive parental responses to negative emotions than European American participants, but similar levels of supportive responses. In addition, African American participants had lower heart rate and cortisol levels (AUCG and AUCI), as well as higher baseline SBP. Finally, African American participants reported more negative emotions but similar level of effort mobilization during the TSST compared to European American participants.
Table 1.
Gender and Ethnic Differences in Main Variables
| Female M (SD) | Male M (SD) | t-value | African American M (SD) | European American M (SD) | t-value | |
|---|---|---|---|---|---|---|
| Supportive | 3.87 (0.78) | 3.72 (0.71) | 3.08** | 3.78 (0.80) | 3.84 (0.64) | −1.24 |
| Unsupportive | 2.21 (0.65) | 2.38 (0.64) | −4.13*** | 2.35 (0.71) | 2.21 (0.54) | 3.46** |
| Heart rate, baseline | 75.81 (11.75) | 69.35 (12.54) | 8.28*** | 71.77 (12.64) | 73.64 (12.20) | −2.20* |
| Heart rate, preparation | 88.15 (15.17) | 79.00 (15.07) | 9.44*** | 81.56 (15.22) | 87.14 (16.21) | −5.26*** |
| Heart rate, speech | 93.69 (18.85) | 83.88 (17.86) | 8.31*** | 85.61 (17.78) | 94.66 (19.79) | −6.92*** |
| Heart rate, math | 89.42 (17.80) | 80.86 (16.22) | 7.82*** | 83.16 (17.09) | 88.71 (17.73) | −4.68*** |
| Heart rate, recovery | 78.94 (13.35) | 72.11 (13.38) | 7.93*** | 74.23 (13.78) | 77.75 (13.45) | −3.76*** |
| SBP, baseline | 121.69 (15.99) | 128.88 (20.13) | −6.16*** | 126.43 (18.19) | 123.56 (18.73) | 2.29* |
| SBP, preparation | 133.06 (20.30) | 143.35 (23.75) | −7.26*** | 138.48 (22.02) | 138.22 (23.55) | 0.17 |
| SBP, speech | 139.85 (24.66) | 150.60 (28.37) | −6.31*** | 145.03 (25.99) | 146.12 (28.74) | −0.59 |
| SBP, math | 138.47 (23.77) | 150.43 (27.93) | −7.18*** | 144.07 (26.31) | 146.08 (27.08) | −1.11 |
| SBP, recovery | 131.02 (19.90) | 139.68 (21.59) | −6.49*** | 136.11 (21.16) | 134.44 (21.16) | 1.15 |
| AUCG | 11.30 (9.15) | 13.83 (8.10) | −4.43*** | 11.10 (7.31) | 14.90 (9.99) | −5.95*** |
| AUCI | 1.40 (5.38) | 3.43 (6.35) | −5.19*** | 1.61 (4.96) | 3.68 (6.90) | −4.71*** |
| Effort mobilization | 3.55 (1.09) | 3.59 (1.03) | −0.48 | 3.53 (1.12) | 3.65 (0.94) | −1.77 |
| Negative emotions | 2.80 (1.06) | 2.38 (0.97) | 6.41*** | 2.79 (1.09) | 2.24 (0.85) | 8.50*** |
Note: Supportive - supportive parental responses to child emotions; Unsupportive - unsupportive parental responses to child emotions; SBP - systolic blood pressure; AUCG – area under the curve, ground; AUCI – area under the curve, increase.
p<.05,
p<.01,
p<.001
3.2 Main Analyses
3.2.1 Emotion Socialization and Physiological Responses to Stress
3.2.1.1 Cortisol reactivity model
Multiple regression analyses tested whether supportive and unsupportive parental responses predicted cortisol secretion. We hypothesized that individuals who received less supportive and more unsupportive parental response to negative emotions would show higher cortisol reactivity to the TSST. However, after adjusting for covariates, neither type of parental responses predicted cortisol reactivity (see Table 2).
Table 2.
Overall Path Coefficients of the Cortisol Reactivity Model
| Variable | AUCG
|
AUCI
|
||
|---|---|---|---|---|
| β | p | β | p | |
|
|
|
|||
| Supportive | −0.00 | 0.92 | 0.01 | 0.66 |
| Unsupportive | −0.04 | 0.21 | −0.05 | 0.11 |
Note: Supportive - supportive parental responses to child emotions; Unsupportive - unsupportive parental responses to child emotions; AUCG – area under the curve, ground; AUCI – area under the curve, increase.
Covariates included participants’ age, gender, race, academic status, parental education, body mass index, and time of day when saliva samples were taken.
Next, multigroup modeling tested gender and ethnic differences in the effects of parental responses on cortisol secretion. Significant gender differences emerged for both AUCG and AUCI models (AUCG: χ2 (11) = 22.15, p < .05; AUCI: χ2 (11) = 44.25, p < .001). Using Bonferroni correction for multiple testing, unsupportive parental responses uniquely predicted lower levels of AUCG and AUCI in females, whereas these effects were not significant for males (see Table 3). Although the effects of supportive parental responses also varied by gender, the path coefficients were not significant for either gender.
Table 3.
Gender and Ethnic Differences in Unstandardized Path Coefficients of the Cortisol Reactivity Models
| Male | Female | Δχ2(1) | p | European American | African American | Δχ2(1) | p | |
|---|---|---|---|---|---|---|---|---|
| AUCG | ||||||||
| Supportive | 0.28 | −0.37 | 13.25 | <.001 | 0.69* | −0.16 | 25.27 | <.001 |
| Unsupportive | −0.07 | −1.09* | 14.17 | <.001 | 0.78 | −0.54 | 19.82 | <.001 |
|
| ||||||||
| AUCI | ||||||||
| Supportive | 0.44 | −0.14 | 25.55 | <.001 | 0.60* | 0.20 | 9.59 | <.01 |
| Unsupportive | 0.10 | −0.80** | 25.24 | <.001 | 0.23 | −0.54* | 12.62 | <.001 |
Note: Supportive - supportive parental responses to child emotions; Unsupportive - unsupportive parental responses to child emotions; AUCG – area under the curve, ground; AUCI – area under the curve, increase.
Bolded items indicate significantly stronger path coefficients
p<.05,
p<.01.
Similarly, multigroup modeling indicated significant ethnic differences in parental responses predicting cortisol levels (AUCG: χ2 (11) = 48.62, p < .001; AUCI: χ2 (11) = 40.66, p < .001). In this case, supportive parental responses to negative emotions uniquely predicted greater cortisol levels only in European American youth for both AUCG and AUCI, whereas unsupportive parental responses uniquely predicted lower cortisol levels only in African American youth for AUCI (see Table 3).
3.2.1.2 ANS reactivity models
Unconditional means models yielded Intraclass Correlation Coefficients (ICC) of .20 for heart rate and .23 for SBP, indicating that 20% and 23% of the total variation in heart rate and SBP was due to differences among individuals (vs. changes within individuals over time). Then, unconditional growth models with an intercept, linear slope, and quadratic slope were conducted for heart rate and SBP across the five periods (baseline, preparation, speech task, math task, and recovery). As shown in Table 4, both unconditional growth models fit the data well. In both models, the linear slopes were significant and positive, indicating initial increase in heart rate and SBP after baseline. In addition, the quadratic slopes were significant and negative, reflecting a deceleration and eventual decline. The variance of intercept, linear slope and quadratic slope were significantly different from zero, indicating individual variability in these growth parameters.
Table 4.
Results of Fitting Unconditional Latent Growth Curve Models for Quadratic Individual Change in Heart Rate and Systolic Blood Pressure
| Variable | Parameter | Outcome
|
|
|---|---|---|---|
| Heart Rate | Systolic Blood Pressure | ||
| Fixed effects | |||
| Initial status | μπ0 | 72.42*** | 125.24*** |
| Linear rate of change | μπ1 | 14.66*** | 16.86*** |
| Quadratic rate of change | μπ2 | −3.48*** | −3.59*** |
| Variance components | |||
| Level 1 | |||
| Baseline | σ2ε1 | 16.64 | −3.07 |
| Preparation | σ2ε2 | 56.78*** | 119.80*** |
| Speech | σ2ε3 | 91.84*** | 185.12*** |
| Math | σ2ε4 | 71.11*** | 155.64*** |
| Recovery | σ2ε5 | 41.65*** | 97.45*** |
| Level 2 | |||
| Initial status | σ2π0 | 144.43*** | 356.52*** |
| Linear rate of change | σ2π1 | 135.63*** | 239.51*** |
| Quadratic rate of change | σ2π2 | 6.82*** | 10.28*** |
| Initial status × Linear rate of change | σ2π01 | −15.44 | −40.55 |
| Initial status × Quadratic rate of change | σ2π02 | 2.33 | 3.25 |
| Linear rate of change × Quadratic rate of change | σ2π12 | −30.14*** | −48.66*** |
| Goodness-of-fit | |||
| χ2 (df = 6) | 32.92*** | 22.46** | |
| CFI | 0.98 | 0.98 | |
| RMSEA | 0.07 | 0.05 | |
| SRMR | 0.02 | 0.05 | |
Note:
p <.01
P<.001
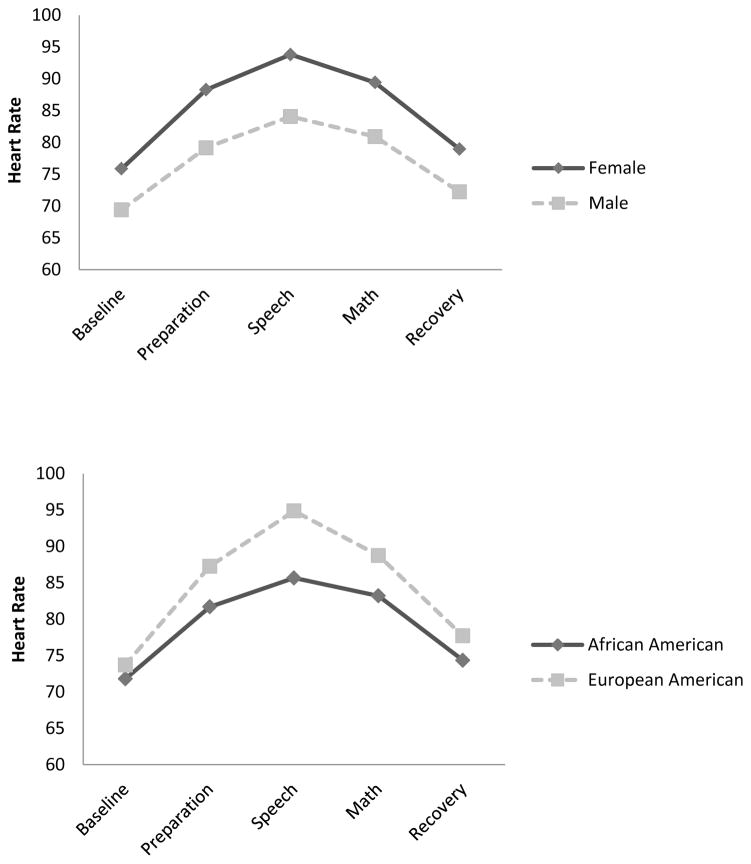
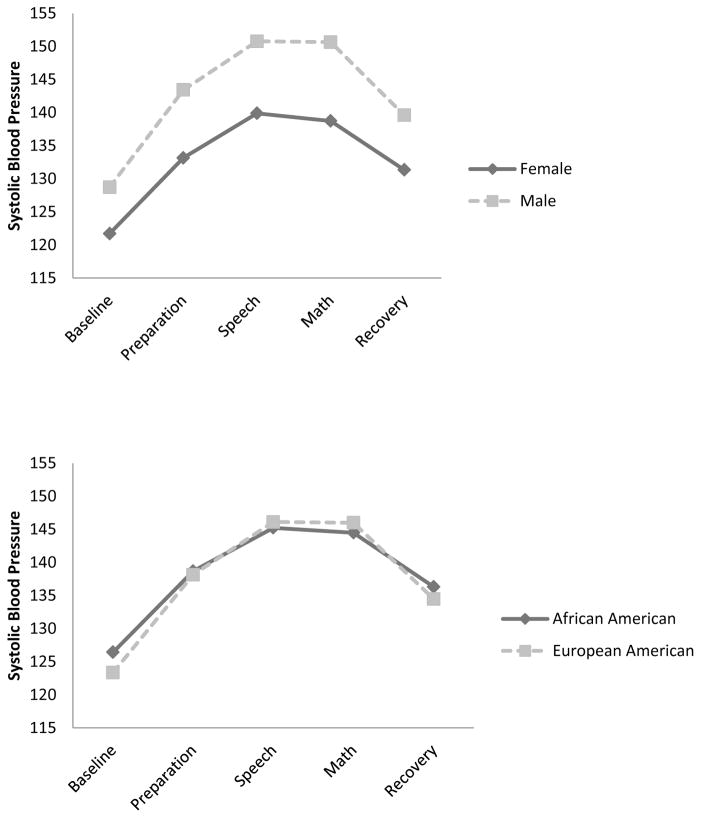
Next, supportive and unsupportive parental responses to negative emotions were included as predictors of the three growth parameters, together with all covariates. As shown in Table 5, these conditional models had excellent model fit. Although supportive and unsupportive parental responses did not predict growth curve parameters of the two models, gender and ethnicity did. Specifically, females and European American participants had higher initial heart rate, followed by faster increase and greater quadratic deceleration than males and African American participants, respectively (see Figure 1). Additionally, females had lower initial SBP, followed by slower increase and slower quadratic deceleration than males, and African American participants had slower increase in SBP than European American participants, although these groups did not differ in initial level and quadratic deceleration (see Figure 2).
Table 5.
Selected Parameter Estimates and Standard Errors for Heart Rate and Systolic Blood Pressure Reactivity Using Conditional Latent Growth Curve Models
| Variable | Intercept
|
Linear growth
|
Quadratic growth
|
|||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| Heart Rate | ||||||
| Growth factor mean | 64.79*** | 8.07 | 31.43*** | 8.33 | −6.91*** | 1.93 |
| Supportive | 0.00 | 0.03 | 0.01 | 0.04 | −0.00 | 0.04 |
| Unsupportive | 0.05 | 0.03 | −0.02 | 0.04 | 0.01 | 0.04 |
| Female | 0.28*** | 0.04 | 0.14*** | 0.04 | −0.15*** | 0.04 |
| African American | −0.11** | 0.04 | −0.14** | 0.04 | 0.15** | 0.05 |
|
| ||||||
| Systolic Blood Pressure | ||||||
| Growth factor mean | 139.38*** | 12.73 | 50.88*** | 12.90 | −11.41*** | 2.84 |
| Supportive | −0.03 | 0.03 | 0.05 | 0.04 | −0.03 | 0.04 |
| Unsupportive | −0.03 | 0.03 | −0.05 | 0.04 | 0.06 | 0.05 |
| Female | −0.22*** | 0.03 | −0.18*** | 0.04 | 0.20*** | 0.04 |
| African American | 0.06 | 0.04 | −0.09* | 0.05 | 0.10 | 0.05 |
|
| ||||||
| Goodness-of-fit | ||||||
| Heart Rate | Systolic Blood Pressure | |||||
|
|
|
|||||
| χ2 (df = 26) | 88.43*** | 52.08** | ||||
| CFI | 0.98 | 0.99 | ||||
| RMSEA | 0.05 | 0.03 | ||||
| SRMR | 0.01 | 0.02 | ||||
Note: Supportive - supportive parental responses to negative emotions; Unsupportive - unsupportive parental responses to negative emotions
p < .05,
p <.01,
P<.001
Figure 1. Heart Rate Responses to the TSST across Gender and Ethnicity.
Note: Figures are based on model-based estimates.
Figure 2. Systolic Blood Pressure Responses to the TSST across Gender and Ethnicity.
Note: Figures are based on model-based estimates.
Then, multigroup modeling was used to examine gender and ethnic differences in the effects of parental responses on heart rate and SBP responses. However, no significant differences were detected (for gender: heart rate χ2 (27) = 34.00, p = .17; SBP χ2 (27) = 27.28, p = .45; for ethnicity: heart rate χ2 (27) = 43.17, p = .03, but no ethnic differences were found for supportive and unsupportive parental responses paths; SBP χ2 (27) = 23.00, p = .68).
3.2.2 Emotion Socialization and Psychological Responses to Stress
3.2.2.1 Factor Analysis of the TSST Questionnaire
Factorability of the 16 items was supported by a number of correlations greater than .30, KMO values of .89 (above the recommended value of .60), and significant Bartlett’s tests of sphericity (χ2(120) = 6953.86, p < .001). The criterion of eigenvalue greater than 1 suggested the extraction of 3 factors accounting for 33.60%, 19.41%, and 6.62% of the total variance, respectively. However, the eigenvalue of the third factor (1.06) was only slightly greater than 1, making the retention of the third factor an arbitrary decision (Zwick & Velicer, 1986). The scree plot showed a significant slope change after 2 factors, indicating a two-factor solution. For the Velicer’s MAP Test, it took 2 steps to get to the lowest average squared partial correlation, also suggesting 2 factors. Finally, parallel analysis showed that only the first two eigenvalues exceeded the 95th percentile eigenvalues from random data, indicating the presence of two factors. Given these results, two factors were retained.
Based on the EFA results, three items were eliminated because of low communalities (<.40; items 15 and 16) and cross-loadings (>.40; item 3) (see Table 6). The EFA was repeated with the remaining 13 items, yielding two factors with 4 and 9 items, respectively. The first factor was named ‘effort mobilization’, including items 5, 6, 9, and 11 (Cronbach’s alpha = .83). The second factor was named ‘negative emotions’, including items 1, 2, 4, 7, 8, 10, 12, 13, and 14 (Cronbach’s alpha = .89). Overall, the two factors accounted for 58.19% of the total variance. All items had factor loadings above .60 and were free from cross-loadings. The factor loadings for the original 16-item solution and the final 13-item solution are presented in Table 6, together with eigenvalues and percentages of variance explained by each factor.
Table 6.
Factor Loadings, Eigenvalues and Variance Explained by Each Factor for the Original TSST Questionnaire (16 items) and the Final Solution (13 items)
| Item | 16 Items | 13 items | ||
|---|---|---|---|---|
|
|
||||
| During the tasks, | I | II | I | II |
| 4. I felt angry | .77 | .79 | ||
| 1. I felt upset | .74 | .76 | ||
| 14. I felt hostile | .73 | .75 | ||
| 10. I felt irritated | .74 | .75 | ||
| 12. I gave up trying to do well | .73 | .72 | ||
| 2. I gave up because the tasks were too difficult | .71 | .71 | ||
| 7. I felt ashamed | .70 | .69 | ||
| 8. Trying hard would increase my humiliation | .68 | .68 | ||
| 13. The tasks were very stressful | .65 | .66 | ||
| 16. I performed very well | −.48 | |||
| 9. I tried hard to make a good impression | .82 | .84 | ||
| 6. I did not want to be underestimated so tried my best | .82 | .84 | ||
| 5. I tried hard to prove that I can do better than others | .77 | .79 | ||
| 11. It was important for me to do well | .76 | .77 | ||
| 3. I tried hard not to embarrass myself | .46 | .52 | ||
| 15. The tasks took a lot of effort | .44 | |||
|
| ||||
| Eigenvalue | 5.38 | 3.11 | 4.38 | 2.61 |
| Explained Variance (%) | 33.60 | 19.41 | 36.48 | 21.76 |
Note: Loadings < .40 omitted.
3.2.2.2 Psychological reactivity model
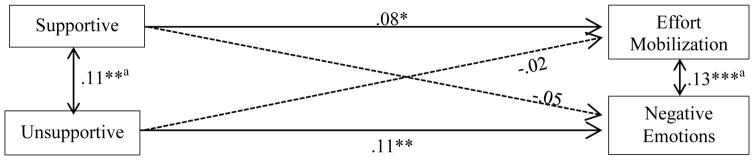
The results from the psychological responses model (see Figure 3) showed that, after adjusting for all covariates, supportive parental responses to negative emotions predicted higher effort mobilization (β = .08, p = .02), whereas unsupportive parental responses predicted higher negative emotions (β = .11, p = .001).
Figure 3. Global Emotion Socialization on Psychological Responses to Stress.
Note: a correlations between residual
Dashed lines indicate nonsignificant paths
*p<.05, **p<.01, ***p<.001
Multigroup modeling showed significant gender differences in the psychological reactivity model (χ2 (16) = 60.46, p < .001). Follow up tests using Bonferroni correction indicated that supportive parental responses to negative emotions predicted less negative emotions in males only, whereas unsupportive parental responses predicted more negative emotions in females only (see Table 7). There were no significant gender differences in the links between parental responses and effort mobilization.
Table 7.
Gender and Ethnic Differences in Unstandardized Path Coefficients of the Psychological Reactivity Model
| Male | Female | Δ χ2(1) | p | European American | African American | Δ χ2(1) | p | |
|---|---|---|---|---|---|---|---|---|
| Supportive-Effort | .12* | .10* | 1.20 | .27 | .16** | .12* | 3.34 | .07 |
| Supportive-Emotion | −.13** | −.01 | 52.09 | <.001 | −.14** | −.03 | 26.52 | <.001 |
| Unsupportive-Effort | −.02 | −.06 | 2.35 | .12 | −.01 | −.05 | 1.33 | .25 |
| Unsupportive-Emotion | .09 | .28*** | 50.95 | <.001 | .06 | .23*** | 26.60 | <.001 |
Note: Supportive - supportive parental responses to child emotions; Unsupportive - unsupportive parental responses to child emotions Bolded items indicate significantly stronger path coefficients
p<.05,
p<.01,
p<.001.
Multigroup modeling also showed significant ethnic differences in the psychological reactivity model (χ2 (16) = 41.43, p < .001). Follow up tests using Bonferroni correction indicated that supportive parental responses to negative emotions predicted less negative emotions in European American participants only, whereas unsupportive parental responses predicted more negative emotions in African American participants only (see Table 7). There were no significant ethnic differences in the links between parental responses and effort mobilization.
4. Discussion
This study examined the relationships between parental emotion socialization and physiological and psychological responses to acute stress in late adolescence and emerging adulthood. Although parental emotion socialization strategies were not related to overall physiological reactivity, supportive parental responses predicted higher self-reports of effort mobilization and unsupportive parental responses predicted greater negative emotions during acute social stress. Additional unique effects emerged when examining gender and ethnic differences, indicating the importance of examining the effects of parental emotion socialization from a gender- and culture-specific perspective. Specifically, unsupportive parental responses to negative emotions uniquely predicted lower cortisol reactivity and more negative emotions in females and African American youth, whereas supportive parental responses predicted higher cortisol reactivity and less negative emotions in European American youth, as well as less negative emotions in males. The differential effects of parental unsupportive and supportive responses to emotions are consistent with prior research documenting the unique roles of supportive and harsh parenting in child developmental outcomes (Krenichyn, Saegert, & Evans, 2001).
4.1 Parental Emotion Socialization and Physiological Reactivity to Stress
This study found that unsupportive parental responses to children’s negative emotions were related to lower cortisol reactivity to the TSST in females. The association may reflect physiological hypo-reactivity to stress, wherein repeated exposure to stressful events reduces physiological reactivity to other stressors (Badanes, Watamura, & Hankin, 2011; Lucas-Thompson, 2012). There is evidence that stressful early life experience, such as neglect or hostile and harsh parenting, may adversely affect the HPA axis functioning, with hyper-reactive cortisol reactivity to stress in early life (Chorpita & Barlow, 1998). For example, preschool-aged children whose mothers demonstrated hostile parenting behaviors showed greater cortisol reactivity to a laboratory task (Dougherty, Klein, Rose, & Laptook, 2011). However, the repeated or prolonged activation of the HPA axis may compromise its resilience over time, leading to blunted reactivity to acute stressors (Taylor, 2010). For instance, childhood physical abuse was linked to reduced cortisol reactivity to the TSST in adult females (Carpenter, Shattuck, Tyrka, Geracioti, & Price, 2011).
In this case, unsupportive parental responses to children’s negative emotions may serve as a chronic stressor that may intensify children’s HPA axis reactivity in early life, but reduce these stress responses over time. Because females are more sensitive to social stress (Hampel & Petermann, 2006), they may perceive unsupportive parental responses to negative emotions as more stressful than males. As a result, unsupportive parental responses may lead to lower cortisol reactivity only in females. This general effect may be strengthened by the nature of the TSST, which involves unsupportive responses from the judges in the TSST that may be similar to the unsupportive parental responses experienced by some participants in their childhood. Importantly, females reported higher negative emotions and similar level of effort mobilization to the TSST than males in the current study, suggesting that the blunted cortisol reactivity in females reporting more unsupportive parental responses is not due to evaluation of the stressor as less stressful or exerting less effort.
Additionally, unsupportive parental responses were associated with blunted cortisol reactivity in African American participants, also indicating physiological hypo-reactivity to stress. African American individuals are more sensitive to social stress than European American individuals (Sellers, Caldwell, Schmeelk-Cone, & Zimmerman, 2003). Thus, it is possible that they perceive unsupportive parental responses to negative emotions as more stressful, which may over time lead to blunted HPA axis reactivity to social stressors. In general, African American participants reported higher levels of negative emotions and similar levels of effort mobilization to the TSST as European American participants, indicating that the attenuated cortisol response in African American participants endorsing more unsupportive parental responses is not due to evaluating the stressor as less stressful or exerting less effort. Although there has been scarce research on HPA axis reactivity in African American individuals (DeSantis et al., 2007), our study indicates that psychosocial experiences may have different effects on HPA axis functioning in this population. Considering the cross-sectional design of the study, it should be noted that individuals’ stress reactivity may also influence perceptions of parental responses to negative emotions. For example, previous research has suggested that toddlers’ stress reactivity exerted significant influence on mothers’ responsiveness to their negative emotions (Gudmundson & Leerkes, 2012).
Finally, parental responses to negative emotions did not predict heart rate or SBP reactivity to the TSST in the current study. Early parenting environment, such as family conflict, abuse, cohesion, and expressiveness, has been found to impact short-term cardiovascular reactivity to stress, which likely contribute to physical health in adulthood (Luecken & Lemery, 2004; Streeck-Fisher & van der Kolk, 2000; Taylor, Lerner, Sage, Lehman, & Seeman, 2004). However, little research has specifically focused on emotion socialization and cardiovascular reactivity to stress. Our study indicates that other aspects of parenting may play a more important role in cardiovascular reactivity than emotion socialization in late adolescence and emerging adulthood. Future research should also explore those associations in other age groups to better understand the possible developmental trajectories.
4.2 Parental Emotion Socialization and Psychological Reactivity to Stress
Supportive parental responses to negative emotions were associated with higher effort mobilization across gender and ethnic groups, providing evidence for the possibility that emotion socialization influences children’s cognitive effort to deal with social stressors. It is possible that supportive parental responses increase problem-solving skills in youth. It is also possible that supportive parental responses foster adaptive attitudes toward social judgments and appraisals. For instance, these youth may be more focused on completing the task rather than the negative evaluations. Indeed, warm and supportive parental behaviors have been linked to better social problem-solving skills and social competence in youth (Domitrovich & Bierman, 2001).
In addition, unsupportive parental responses to negative emotions were associated with higher negative emotions to acute social stressor in females and African American youth, whereas supportive parental responses were linked to lower negative emotions in males and European American youth. These gender and ethnic differences may be related to female and African American youths’ greater sensitivity to social stress discussed above (Hampel & Petermann, 2006; Sellers et al., 2003). This greater sensitivity to social stress, coupled with a history of unsupportive parental responses to negative emotions, may prime these youth to experience more negative emotions when confronted with negative or stressful social situations, perhaps due to poorer emotion regulation skills. On the other hand, supportive parental responses may be more effective in supporting emotion regulation and reducing lower negative emotions in response to stress among individuals less sensitive to social stress, such as males and European American youth.
Considering the physiological and psychological reactivity results together, unsupportive parental response to negative emotions were associated with blunted cortisol reactivity but greater emotional reactivity to a psychosocial stressor in females and African American youth. This pattern of mismatch between physiological and psychological stress responses has been observed in individuals with stressful life experiences. For instance, adolescents who were exposed to marital conflict experienced dampened physiological reactivity and heightened emotional reactivity to the TSST, indicating a disruption between physiological and emotional stress responses (Lucas-Thompson, 2012). Another study showed that females who have been abused during childhood or adolescence showed reduced cortisol reactivity and higher level of perceived stress on the TSST (Pierrehumbert et al., 2009). Similar results were found for adults with fearful attachment (Kidd, Hamer, & Steptoe, 2011). These last two empirical studies, as well as our study, failed to find a correlation between subjective and cortisol stress responses, suggesting that these two processes are generally independent.
4.3 Limitations and Future Directions
There are several limitations of this study. First, this study used cross-sectional design, thus no causal inferences about the studied relationships could be made. It is possible that individuals’ reactivity to stress may have influenced perceptions of parental responses to negative emotions. Alternatively, reports of parental responses and stress reactivity may be influenced by a third variable, such as emotional functioning. Longitudinal and intervention research would provide stronger support for the hypothesized directionality of the effects. Second, we relied on retrospective recall of parental emotion socialization from childhood, whose accuracy might be influenced by the linguistic and cognitive skills of the participants (Klimes-Dougan & Zeman, 2007). Third, although multiple indicators of physiological stress response were included, the number of cortisol samples was relatively small. Obtaining more cortisol samples during and after the TSST would provide a more nuanced picture of HPA axis response to stress. Fourth, the average parental education level was high in the current sample, indicating that the sample may not be representative of various socioeconomic backgrounds. It is possible that emotion socialization processes and their associations with stress reactivity may vary by socioeconomic status. Finally, the present study focused on reactivity to a social evaluative stressor. It is possible that physiological and psychological responses vary based on the nature of the stressor. For example, females are more sensitive to interpersonal tasks, whereas males are more motivated by achievement tasks (Kelly et al., 2008). Future research should examine the role of parental emotion socialization in stress reactivity across different types of acute stressors.
5. Conclusions
This study investigated the associations between emotion socialization and physiological and psychological responses to acute psychosocial stress in late adolescence and emerging adulthood. It contributed to the literature by examining both objective and subjective components of stress reactivity in an experimental laboratory study. Additionally, this study employed multiple indexes of stress reactivity, including salivary cortisol, heart rate, and blood pressure for physiological responses, and negative emotions and effort mobilization for psychological responses. Finally, this study demonstrated the unique roles of emotion socialization practices on stress responses in different gender and ethnic groups. Overall, the findings indicate that unsupportive parental responses were associated with blunted cortisol reactivity and greater negative emotions to a psychosocial stress task in females and African American youth, whereas supportive parental responses predicted greater cortisol reactivity and lower negative emotions to stress in European American youth, as well as less negative emotions in males. A better understanding of the underlying mechanisms is needed to inform the development of interventions for specific subgroups of youth.
Highlights.
Parental emotion socialization may be an important factor influencing HPA axis reactivity and psychological responses to stress.
Unsupportive parental emotion socialization was associated with blunted cortisol reactivity and greater negative emotions to a psychosocial stress task in females and African American youth.
Supportive parental emotion socialization predicted greater cortisol reactivity and lower negative emotions to stress in European American youth, as well as less negative emotions in males.
Parental emotion socialization did not predict heart rate or SBP reactivity to a psychosocial stress task.
Acknowledgments
This work was supported by the National Institute of Mental Health of the National Institutes of Health [grant number R01MH098348].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26(2):189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience & Biobehavioral Reviews. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10(3):229–240. [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23(03):881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS structural equations program manual [Computer software manual] Encino, CA: Multivariate Software; 1995. [Google Scholar]
- Björntorp P. Heart and soul: stress and the metabolic syndrome. Scandinavian Cardiovascular Journal. 2001;35(3):172–177. doi: 10.1080/140174301750305045. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(02):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brehm JW, Self EA. The intensity of motivation. Annual Review of Psychology. 1989;40:109–131. doi: 10.1146/annurev.ps.40.020189.000545. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine. 2005;67:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214(1):367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chen FR, Raine A, Rudo-Hutt AS, Glenn AL, Soyfer L, Granger DA. Harsh discipline and behavior problems: The moderating effects of cortisol and alpha-amylase. Biological Psychology. 2015;104:19–27. doi: 10.1016/j.biopsycho.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychological Bulletin. 1998;124(1):3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology. 2000;68(6):976–992. [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. Journal of Cognition and Development. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: Shame, physiology, and health. Journal of Personality. 2004;72(6):1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domitrovich CE, Bierman KL. Parenting practices and child social adjustment: Multiple pathways of influence. Merrill-Palmer Quarterly. 2001;47(2):235–263. [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-aged offspring of depressed parents: moderation by early parenting. Psychological Science. 2011;22(5):650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL. Parental socialization of emotion. Psychological Inquiry. 1998;9(4):241–273. doi: 10.1207/s15327965pli0904_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Tulen JH, Franken IH, Huizink AC. Determinants of physiological and perceived physiological stress reactivity in children and adolescents. PloS one. 2013;8(4):e61724. doi: 10.1371/journal.pone.0061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney ML, Stoney CM, Engebretson TO. Hostility and anger expression in African American and European American men is associated with cardiovascular and lipid reactivity. Psychophysiology. 2002;39(3):340–349. doi: 10.1017/s0048577201393101. [DOI] [PubMed] [Google Scholar]
- Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Progress in Cardiovascular Diseases. 2006;48(5):342–362. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gendolla GH, Krüsken J. The joint effect of informational mood impact and performance-contingent consequences on effort-related cardiovascular response. Journal of Personality and Social Psychology. 2002;83(2):271–283. doi: 10.1037//0022-3514.83.2.271. [DOI] [PubMed] [Google Scholar]
- Gendolla GH, Richter M. Effort mobilization when the self is involved: Some lessons from the cardiovascular system. Review of General Psychology. 2010;14(3):212–226. [Google Scholar]
- Gendolla GH, Richter M, Silvia PJ. Self-focus and task difficulty effects on effort-related cardiovascular reactivity. Psychophysiology. 2008;45(4):653–662. doi: 10.1111/j.1469-8986.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Gillin JL, Mills PJ, Nelesen RA, Dillon E, Ziegler MG, Dimsdale JE. Race and sex differences in cardiovascular recovery from acute stress. International Journal of Psychophysiology. 1996;23(1):83–90. doi: 10.1016/0167-8760(96)00041-4. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosomatic Medicine. 1990;52(5):571–591. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Parental meta-emotion philosophy and the emotional life of families: Theoretical models and preliminary data. Journal of Family Psychology. 1996;10(3):243–268. [Google Scholar]
- Granger DA, Johnson SB, Szanton SL, Out D, Schumann LL. Incorporating salivary biomarkers into nursing research: an overview and review of best practices. Biological Research for Nursing. 2012;14(4):347–356. doi: 10.1177/1099800412443892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravetter FJ, Wallnau LB. Statistics for the behavioral sciences. Cengage Learning; 2016. [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77(5):419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Gudmundson JA, Leerkes EM. Links between mothers’ coping styles, toddler reactivity, and sensitivity to toddler’s negative emotions. Infant Behavior and Development. 2012;35(1):158–166. doi: 10.1016/j.infbeh.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Mrug S, Knight DC. Factor Structure of the Emotions as a Child Scale in Late Adolescence and Emerging Adulthood. Psychological Assessment. doi: 10.1037/pas0000412. (in press) http://dx.doi.org/10.1037/pas0000412. [DOI] [PMC free article] [PubMed]
- Gunnar MR, Fisher PA. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18(03):651–677. [PubMed] [Google Scholar]
- Hampel P, Petermann F. Perceived stress, coping, and adjustment in adolescents. Journal of Adolescent Health. 2006;38(4):409–415. doi: 10.1016/j.jadohealth.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Henry JP. Biological basis of the stress response. Physiology. 1993;8(2):69–73. [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, McFarquhar T, Stevens S, Ouellet-Morin I, Melhuish E, Belsky J. Interactive effects of early and recent exposure to stressful contexts on cortisol reactivity in middle childhood. Journal of Child Psychology and Psychiatry. 2015;56(2):138–146. doi: 10.1111/jcpp.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(8):1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kahn JR, Fazio EM. Economic status over the life course and racial disparities in health. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(2):76–84. doi: 10.1093/geronb/60.special_issue_2.s76. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20:141–151. [Google Scholar]
- Katz LF, Maliken AC, Stettler NM. Parental meta-emotion philosophy: A review of research and theoretical framework. Child Development Perspectives. 2012;6(4):417–422. [Google Scholar]
- Kehoe CE, Havighurst SS, Harley AE. Somatic Complaints in Early Adolescence The Role of Parents’ Emotion Socialization. The Journal of Early Adolescence. 2015;35(7):966–989. [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39(1):87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Hamer M, Steptoe A. Examining the association between adult attachment style and cortisol responses to acute stress. Psychoneuroendocrinology. 2011;36(6):771–779. doi: 10.1016/j.psyneuen.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Zeman J. Introduction to the special issue of social development: Emotion socialization in childhood and adolescence. Social Development. 2007;16(2):203–209. [Google Scholar]
- Krenichyn K, Saegert S, Evans GW. Parents as moderators of psychological and physiological correlates of inner-city children’s exposure to violence. Journal of Applied Developmental Psychology. 2001;22(6):581–602. [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. International Journal of Behavioral Medicine. 2004;11(2):116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Encyclopedia of stress. New York: Academic Press; 2000. Sex differences in human stress response. [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Linares LO, Shrout PE, Nucci-Sack A, Diaz A. Child maltreatment, dating perpetration of physical assault, and cortisol reactivity among disadvantaged female adolescents. Neuroendocrinology. 2012;97(3):252–259. doi: 10.1159/000342958. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. International Journal of Psychophysiology. 2006;59(3):195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Thompson RG. Associations of marital conflict with emotional and physiological stress: Evidence for different patterns of dysregulation. Journal of Research on Adolescence. 2012;22(4):704–721. [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24(2):171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magai C, O’Neal CR. Emotions as a child (child version) Long Island University; Brooklyn: 1997. Unpublished manuscript. [Google Scholar]
- Oldehinkel AJ, Bouma EM. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neuroscience & Biobehavioral Reviews. 2011;35(8):1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine. 2001;63(6):966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, Halfon O. The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology. 2009;34(6):924–938. doi: 10.1016/j.psyneuen.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2–3):167–186. [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Sanders W, Zeman J, Poon J, Miller R. Child regulation of negative emotions and depressive symptoms: The moderating role of parental emotion socialization. Journal of Child and Family Studies. 2015;24(2):402–415. [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro LA, Baucom BR. Does dampened physiological reactivity protect youth in aggressive family environments? Child Development. 2012;83(3):821–830. doi: 10.1111/j.1467-8624.2012.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the HPA-axis and the sympathetic adrenal medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Sellers RM, Caldwell CH, Schmeelk-Cone KH, Zimmerman MA. Racial identity, racial discrimination, perceived stress, and psychological distress among African American young adults. Journal of Health and Social Behavior. 2003;43:302–317. [PubMed] [Google Scholar]
- Shipman KL, Schneider R, Fitzgerald MM, Sims C, Swisher L, Edwards A. Maternal Emotion Socialization in Maltreating and Non-maltreating Families: Implications for Children’s Emotion Regulation. Social Development. 2007;16(2):268–285. [Google Scholar]
- Shortt JW, Katz LF, Allen NB, Leve C, Davis B, Sheeber LB. Emotion socialization in the context of risk and psychopathology: Mother and father socialization of anger and sadness in adolescents with depressive disorder. Social Development. 2016;25(1):27–46. doi: 10.1111/sode.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoluda N, Strahler J, Schlotz W, Niederberger L, Marques S, Fischer S, Thoma MV, Spoerri C, Ehlert U, Nater UM. Intra-individual psychological and physiological responses to acute laboratory stressors of different intensity. Psychoneuroendocrinology. 2015;51:227–236. doi: 10.1016/j.psyneuen.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Spangler G. Psychological and physiological responses during an exam and their relation to personality characteristics. Psychoneuroendocrinology. 1997;22(6):423–441. doi: 10.1016/s0306-4530(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Steiger JH, Lind JC. Statistically based tests for the number of common factors. Paper presented at the annual meeting of the Psychometric Society; Iowa City, IA. 1980. [Google Scholar]
- Streeck-Fisher A, van der Kolk BA. Down will come baby, cradle and all: Diagnostic and therapeutic implications of chronic trauma on child development. Australian and New Zealand Journal of Psychiatry. 2000;34:903–918. doi: 10.1080/000486700265. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72(6):1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Troisi A. Gender differences in vulnerability to social stress: a Darwinian perspective. Physiology & Behavior. 2001;73(3):443–449. doi: 10.1016/s0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Avison WR. Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. Journal of Health and Social Behavior. 2003;44(4):488–505. [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsey SO, Belvet B, Hubbard RR, Fischer NL, Opare-Henaku A, Gladney LL. Development and Validation of the Prolonged Activation and Anticipatory Race-Related Stress Scale. Journal of Black Psychology. 2013;39(6):532–559. [Google Scholar]
- Velicer WF. Determining the number of components from the matrix of partial correlations. Psychometrika. 1976;41(3):321–327. [Google Scholar]
- Velicer WF, Eaton CA, Fava JL. Problems and solutions in human assessment. Springer US; 2000. Construct explication through factor or component analysis: A review and evaluation of alternative procedures for determining the number of factors or components; pp. 41–71. [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American Journal of Public Health. 2003;93(2):200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wothke W. Longitudinal and multi-group modeling with missing data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple group data: Practical issues, applied approaches, and specific examples. Hillsdale, NJ: Erlbaum; 2000. pp. 219–240. [Google Scholar]
- Wright RA. Refining the prediction of effort: Brehm’s distinction between potential motivation and motivation intensity. Social and Personality Psychology Compass. 2008;2(2):682–701. [Google Scholar]
- Zwick WR, Velicer WF. Comparison of five rules for determining the number of components to retain. Psychological Bulletin. 1986;99(3):432–442. [Google Scholar]