ABSTRACT
Novel approaches to the prevention of microbial infections after the insertion of orthopedic external fixators are in great demand because of the extremely high incidence rates of such infections, which can reach up to 100% with longer implant residence times. Monolaurin is an antimicrobial agent with a known safety record that is broadly used in the food and cosmetic industries; however, its use in antimicrobial coatings of medical devices has not been studied in much detail. Here, we report the use of monolaurin as an antibacterial coating on external fixators for the first time. Monolaurin-coated Kirschner wires (K-wires) showed excellent antibacterial properties against three different bacterial strains, i.e., methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus epidermidis. Approximately 6.0-log reductions of both planktonic and adherent bacteria were achieved using monolaurin-coated K-wires, but monolaurin-coated K-wires did not show any observable cytotoxicity with mouse osteoblast cell cultures. Overall, monolaurin-coated K-wires could be promising as potent antimicrobial materials for orthopedic surgery.
KEYWORDS: antibacterial coating, external fixators, monolaurin, orthopedic implants, biofilms
INTRODUCTION
Kirschner wires (K-wires) are used extensively in orthopedic applications ranging from fracture fixation to deformity correction. Although K-wires are very versatile and fairly effective, K-wiring procedures are often associated with pin tract infections, which can lead to even more serious issues such as osteomyelitis (1, 2). These infections arise from the use of percutaneous pinning techniques, as seen in skeletal traction, percutaneous fracture pinning, and external fixation for fracture stabilization or complex deformity reconstruction. Pin sites are niduses for infections since the skin barrier is disrupted, providing a potential passage for opportunistic bacteria to enter a previously privileged area. Multiple findings suggest that the infection rates following K-wiring procedures range from 11 to 100%, depending on the lifetime of the implant (3–5). If appropriate pin care is neglected, then these infections can cause sepsis, osteomyelitis, and sometimes death (3, 6). The economic burden of treatment for this type of infection is expected to reach as high as $1.6 billion annually by 2020 (7). Therefore, surgeons, microbiologists, and material scientists are all working to find the best possible solutions to this challenging issue.
In attempts to prevent pin track sepsis, multiple strategies have been developed. Most of the strategies involve coating the implants with antibiotics, antimicrobial or antiadhesive polymers or peptides, silver or nitric ions, nanoparticles, or other antiseptics such as chlorhexidine or silver-sulfadiazine (8–12). However, a major concern associated with the implementation of antibacterial coatings is their adverse effect on adjacent tissues and bones, which inhibits fracture or fusion healing (13). Another important issue is the in vivo loss of antibacterial activity resulting from the exposure of bacteria to sublethal concentrations of the drug, eventually leading to the development of drug resistance in situ (14). Therefore, the development of novel coatings to reduce pin site infections is of particular importance.
The antibacterial properties of fatty acids and their esters have been studied extensively over the past few decades. Monolaurin, also known as glycerol monolaurate, was found to be the most effective antibacterial agent among these compounds (15). Monolaurin is currently used as a broad-range antimicrobial agent in the food and cosmetic industries. Multiple studies have shown that, besides antibacterial properties, monolaurin possesses antiviral and antifungal activities (16–18). In in vitro studies, monolaurin prevented biofilm formation of various Staphylococcus species, including Staphylococcus aureus and Staphylococcus epidermidis, pathogens that cause approximately 80% of orthopedic implant-associated infections (18–20). It was also shown that, at sublethal concentrations, bacteria did not develop resistance to monolaurin (21). In vivo experiments demonstrated that oral administration of monolaurin appeared to be an effective approach to manage and to treat S. aureus infections (19). In a surgical site infection model (22), monolaurin exhibited antibacterial and antibiofilm activities against both Gram-positive and Gram-negative bacteria. Therefore, a large body of evidence suggests that monolaurin possesses profound antimicrobial properties, which make it an excellent candidate for antibacterial coating on orthopedic devices, particularly K-wires and external fixation devices, because of high incidence rates of infections for these devices. The advantages of monolaurin as a coating include its low cost, its ease of application, and its designation by the FDA as generally recognized as safe (GRAS); in addition, the low water solubility of monolaurin may provide for prolonged drug release from the implant and improved antimicrobial performance.
In the study reported here, monolaurin was employed as a passive coating on K-wires, with the long-term goals of achieving reduced incidence rates of orthopedic implant-associated infections and decreasing the burden of pin tract infections, thus improving patient outcomes. A dip-coating technique was implemented to modify stainless steel K-wires. Following physicochemical characterization of the coating, the antibacterial properties of modified wires were evaluated in vitro in model experiments with S. epidermidis, methicillin-sensitive S. aureus (MSSA), and methicillin-resistant S. aureus (MRSA). Furthermore, the biological effect of monolaurin coating on osteoblasts was studied. The objectives of this study were to demonstrate the efficacy of monolaurin-coated K-wires in vitro and to provide a foundation for further animal and clinical studies.
RESULTS
Binding yield of monolaurin.
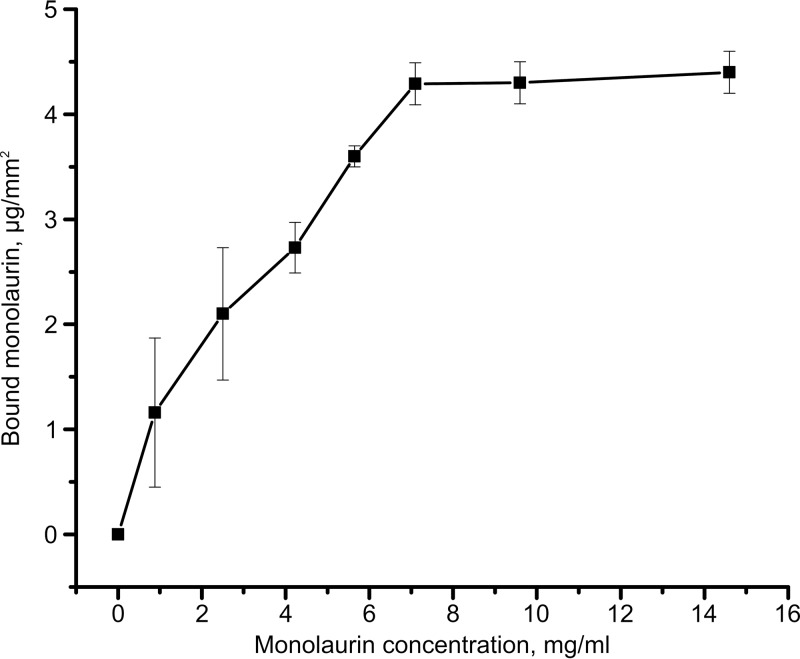
The binding yield of monolaurin was determined from the adsorption isotherm of the drug on stainless steel K-wires (Fig. 1). Due to the low molar extinction coefficient of monolaurin, large errors were observed at low loading concentrations. The adsorption isotherm reached saturation at a monolaurin concentration of approximately 7.5 mg/ml. The saturation loading was determined to be 84 ± 4 μg of monolaurin per cm of wire, corresponding to a density of 4.3 ± 0.2 μg/mm2. Based on these results, 10 mg/ml monolaurin solutions were used to coat K-wires in all subsequent experiments, to ensure saturation coating.
FIG 1.
Isotherm of monolaurin adsorption on stainless steel K-wires.
Characterization of monolaurin coating.
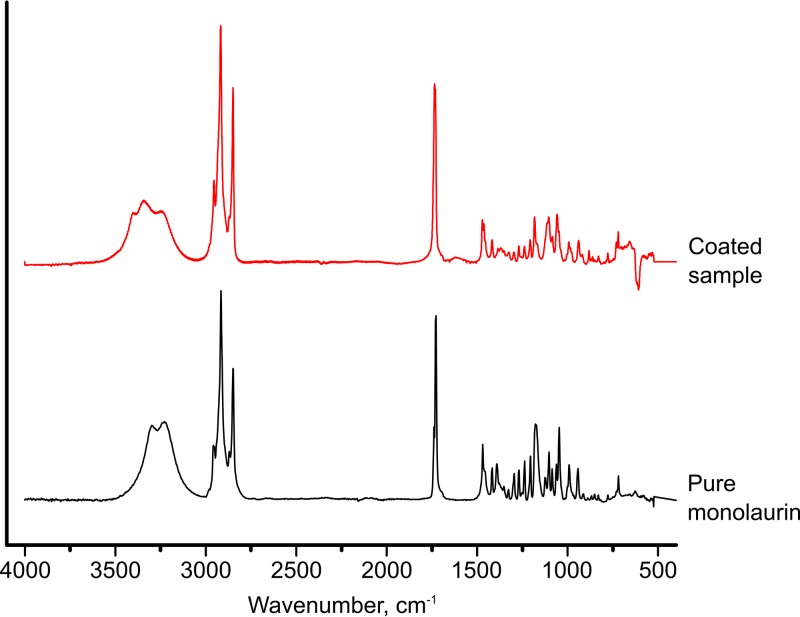
Fourier transform infrared spectroscopy (FTIR) was used to prove monolaurin adsorption on the K-wires. Figure 2 shows the spectra collected from coated and pure monolaurin. As can be seen, there was virtually no difference between the spectrum of the coated sample and that of pure monolaurin; the spectrum of a plain wire (not shown) did not display any absorbance bands. Thus, these data confirm that the coating consists of monolaurin.
FIG 2.
FTIR spectra of a monolaurin-coated wire and pure monolaurin.
The morphological characteristics of the coating were analyzed using scanning electron microscopy (SEM), atomic force microscopy (AFM), and spectroscopic reflectometry. As shown in Fig. 3b, monolaurin-coated wires exhibited smooth surfaces with minor extrusion lines. According to the micrographs, the coating was evenly distributed across the surface of the wires. Little to no difference could be seen in comparisons of the surface morphology of the plain and monolaurin-coated K wires.
FIG 3.
SEM micrographs of plain (a) and monolaurin-coated (b) K-wires.
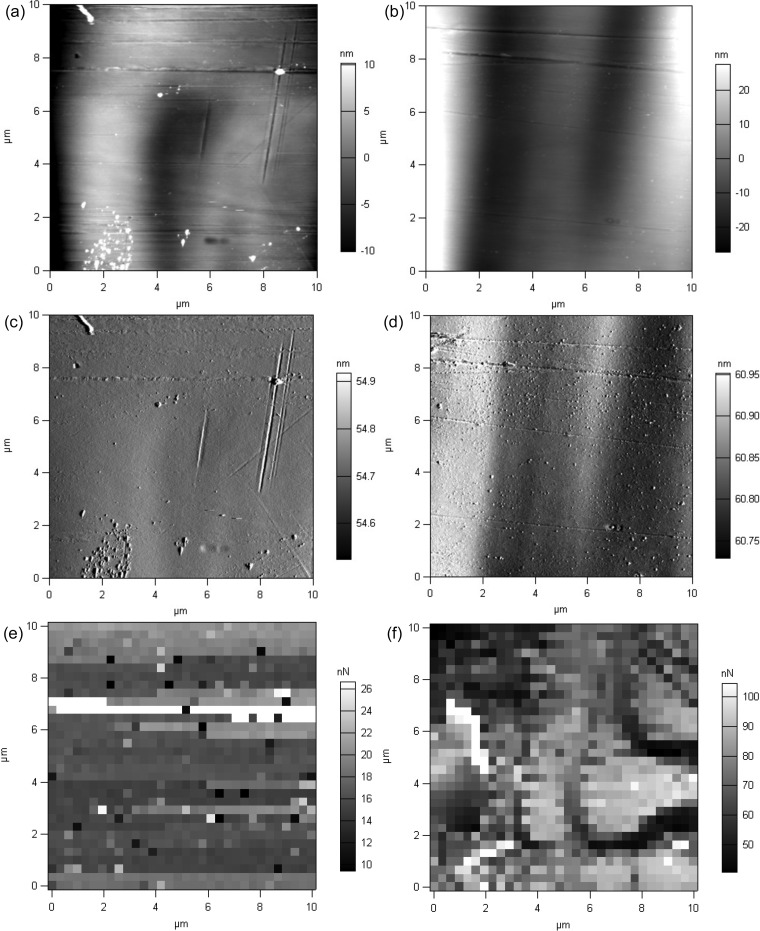
Further analysis of micromorphological characteristics of the monolaurin coating was conducted using the AFM technique. Topographic images acquired by AFM (Fig. 4a to d) confirmed the smoothness of the monolaurin coating. Using a scratching technique, the thickness of the coating was determined to be 144 ± 35 nm. These results are in good correlation with those from spectroscopic reflectometry, which showed the thickness of the coating to be 161 ± 57 nm. Coated samples exhibited considerably greater adhesion force between the surface and the cantilever tip, compared to the uncoated samples (85.2 ± 18.6 and 15.9 ± 2.1 nN for coated and uncoated wires, respectively; P < 0.001), indicating the presence of a uniform coating (Fig. 4e and f).
FIG 4.
AFM images of uncoated and coated samples. The topography (a and b), cantilever deflections (c and d), and adhesion force maps (e and f) of plain (a, c, and e) and monolaurin-coated (b, d, and f) samples were assessed.
Evaluation of antibacterial activity. (i) Antibacterial activity against planktonic bacteria.
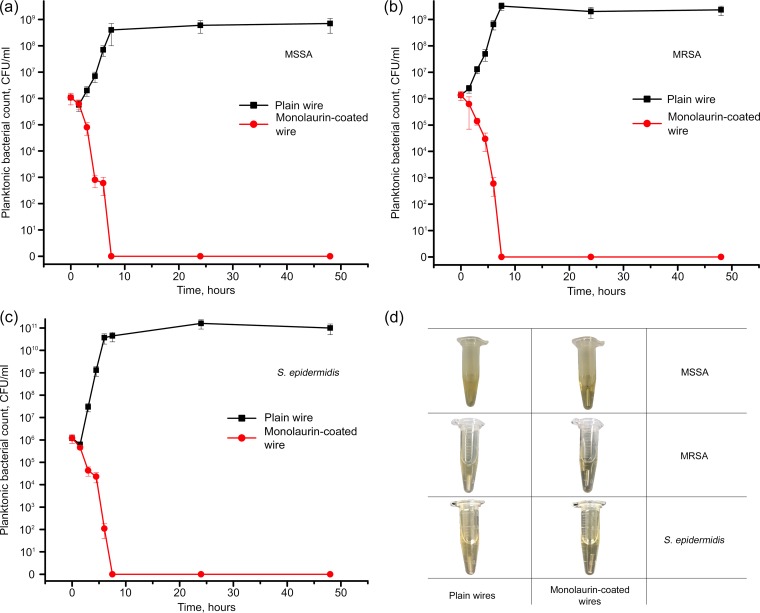
The antimicrobial activity of monolaurin-coated wires against planktonic bacteria was assessed based on the microbial viability curves. As shown in Fig. 5 and in Fig. S3 in the supplemental material, coated wires were highly efficient against MSSA, MRSA, and S. epidermidis, completely eliminating planktonic bacteria in less than 7.5 h. In the presence of uncoated wires, the numbers of bacteria increased by more than 2 orders of magnitude over the first 7.5 h. These data suggested that monolaurin-coated wires achieved 6.0-log reductions of planktonic MSSA, MRSA, and S. epidermidis.
FIG 5.
(a to c) Planktonic bacteria viability curves obtained in the presence of plain and monolaurin-coated K-wires for MSSA (a), MRSA (b), and S. epidermidis (c). (d) Representative photographs of tubes containing plain and monolaurin-coated wires that had been incubated with the bacteria for 48 h.
(ii) Antibacterial activity against adherent bacteria.
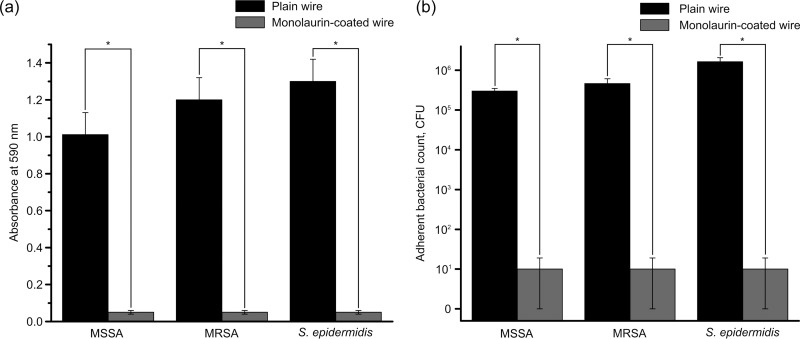
The antibiofilm activity of monolaurin coating was evaluated with the crystal violet (CV) assay and quantitative analysis of adherent bacteria. The results of the CV assay are shown in Fig. 6a. For all three bacterial strains, the absorbance values for coated wires were significantly lower than those for plain wires, indicative of the antibiofilm activity of monolaurin-coated wires against MSSA, MRSA, and S. epidermidis (n = 6; P < 0.001).
FIG 6.
(a) Results of CV assays performed with plain and monolaurin-coated wires that had been incubated in the presence of MSSA, MRSA, or S. epidermidis. (b) Adherent bacterial counts for plain and monolaurin-coated wires that had been incubated for 48 h in the presence of MSSA, MRSA, or S. epidermidis. Asterisks represent statistically significant differences between plain and monolaurin-coated wires (P < 0.05).
Figure 6b and Fig. S3 show the counts of adherent bacteria. Again, monolaurin-coated wires demonstrated pronounced antibiofilm properties, resulting in significant decreases in the numbers of adherent bacteria, compared to plain wires (n = 4; P < 0.05). Monolaurin-coated wires produced 5.7-, 5.8-, and 6.0-log reductions of adherent MSSA, MRSA, and S. epidermidis, respectively. These results are consistent with those obtained for planktonic bacteria.
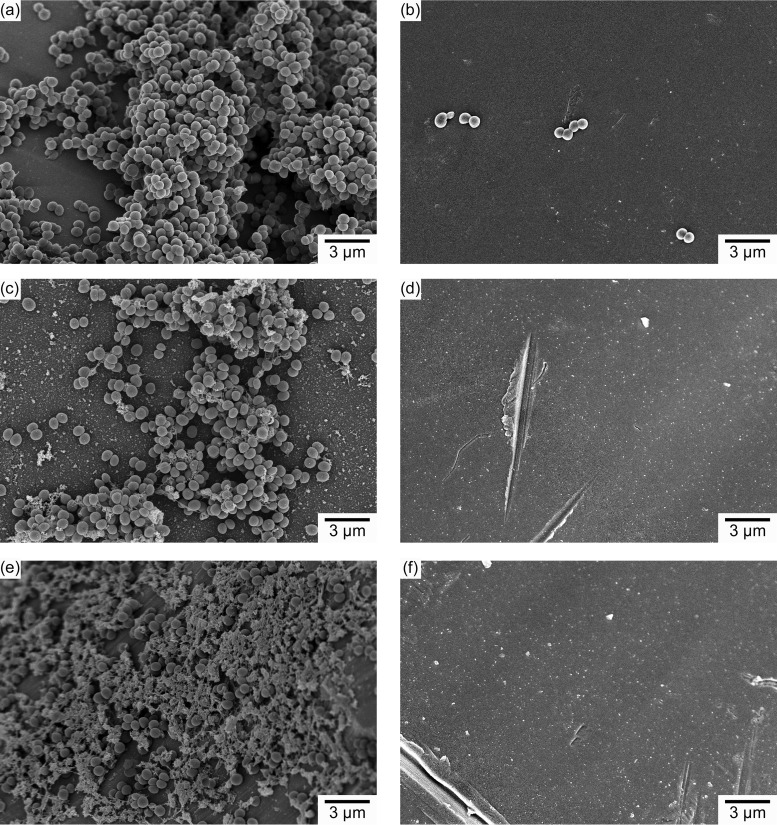
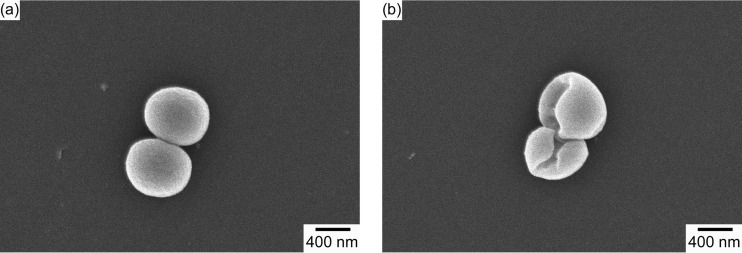
The observed antibiofilm effect was additionally confirmed by SEM imaging. As can be seen in Fig. 7, there was little or no evidence of adherent bacteria on the surface of the coated wires for all three bacterial strains studied. In contrast, large numbers of adherent bacteria surrounded by extracellular matrix were evident in the SEM images of the uncoated wires for all three strains studied. Analysis of high-magnification images (magnification of ×30,000) revealed that the morphology of bacteria adherent to the plain wires was different from that of bacteria found on monolaurin-coated wires (Fig. 8; also see Fig. S4 and S5). It was evident that, in the latter case, the integrity of the bacterial cell wall was disrupted, resulting in deformation of the bacterial cell surface. In contrast, the bacteria attached to the plain wire exhibited the conventional spherical shape.
FIG 7.
SEM micrographs of bacteria lodging on the surfaces of plain and monolaurin-coated wires. (a) MSSA adherent to a plain wire. (b) MSSA adherent to a monolaurin-coated wire. (c) MRSA adherent to a plain wire. (d) MRSA adherent to a monolaurin-coated wire. (e) S. epidermidis adherent to a plain wire. (f) S. epidermidis adherent to a monolaurin-coated wire.
FIG 8.
High-magnification SEM images of S. aureus adherent to the surfaces of plain (a) and monolaurin-coated (b) wires. Magnification, ×30,000.
(iii) Inhibition zone test.
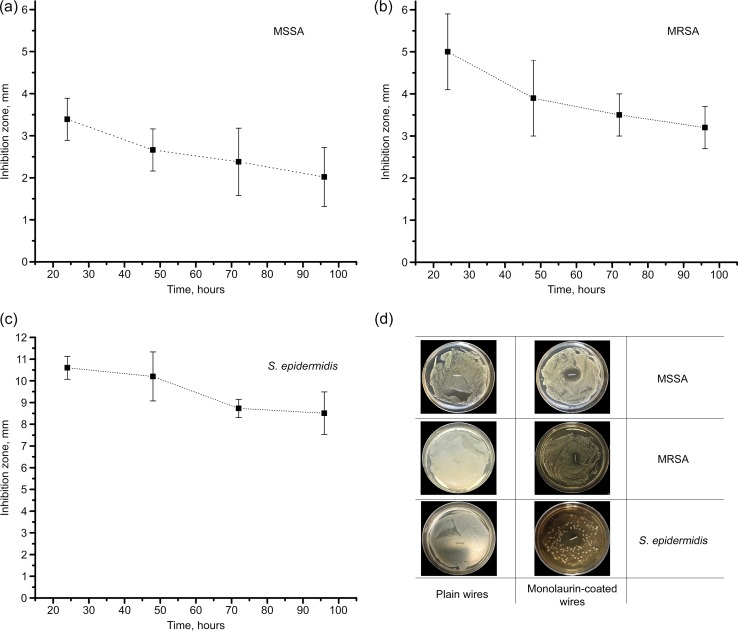
Additional evaluation of the antimicrobial activity of monolaurin-coated wires was performed with the inhibition zone assay. As shown in Fig. 9d, coated wires were efficient against all three bacterial strains tested. The results of time-dependent antimicrobial efficacy testing suggested that the inhibition diameter decreased significantly with time (n = 6; P < 0.05) (Fig. 9a to c). Even after 96 h of incubation, however, monolaurin-coated K-wires were capable of inhibiting bacterial growth for MSSA, MRSA, and S. epidermidis. Overall, the results of the inhibition zone test are indicative of good prolonged antibacterial efficacy of monolaurin-coated wires, demonstrating their ability to inhibit the growth of all three strains for at least 4 days.
FIG 9.
(a to c) Inhibition zones for MSSA (a), MRSA (b), and S. epidermidis (c) around monolaurin-coated K-wires. (d) Representative photographs of inoculated agar plates containing plain and monolaurin-coated wires.
Evaluation of cytotoxicity.
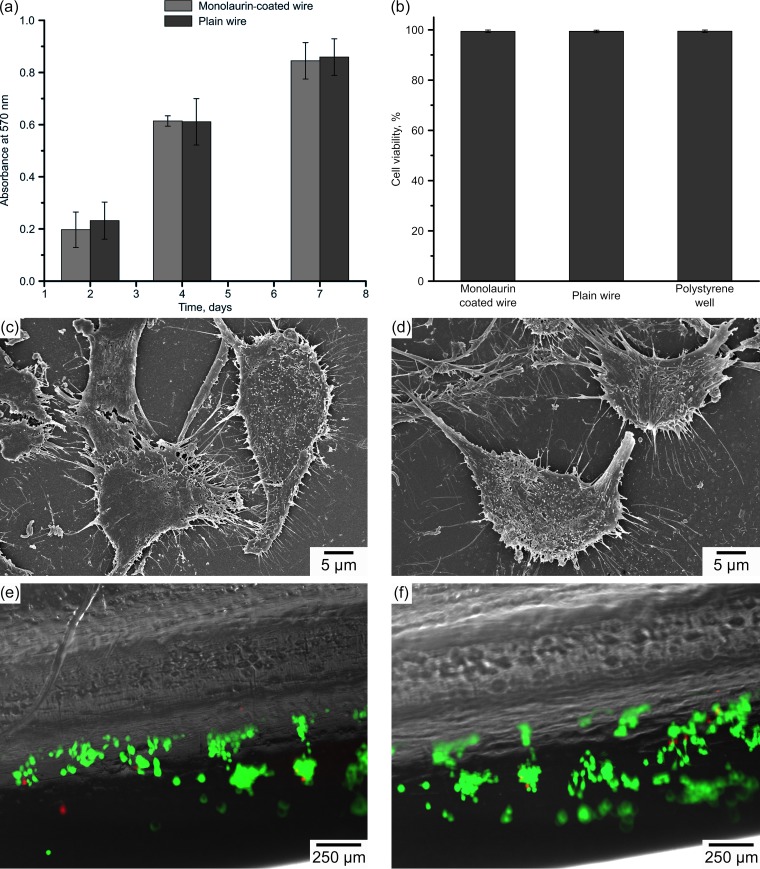
The influence of the monolaurin coating on cell proliferation was assessed using the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (Fig. 10a). The image demonstrates that culturing osteoblasts in the presence of monolaurin-coated wires did not affect the cell proliferation rate; the optical density at 570 nm (OD570), which indirectly corresponds to the number of cells, was not significantly different for coated versus uncoated wires at different time points (n = 4; day 2, P = 0.687; day 4, P = 0.781; day 7, P = 0.757).
FIG 10.
(a) Results of the MTT assay performed on osteoblasts grown in the presence of plain and monolaurin-coated K-wires. (b) Viability of osteoblasts adherent to plain and monolaurin-coated samples, determined by the Live/Dead assay. (c and d) SEM micrographs of osteoblasts adherent to plain (c) and monolaurin-coated (d) samples. (e and f) Fluorescence microscopic images of osteoblasts lodging on plain (e) and monolaurin-coated (f) wires. Cells stained green are viable, and red staining corresponds to dead cells.
The analysis of adherent cells was performed using SEM imaging and the Live/Dead assay. Figure 10c to f display micrographs of osteoblasts adherent to the surface of plain and monolaurin-coated wires. Good cell attachment and spreading across the surface were evident in the images; the osteoblasts exhibited normal polygonal morphology with extended filopodia. Little to no difference could be noted in comparisons of plain and modified samples.
Figure 10b shows fluorescence images of osteoblasts stained with Live/Dead reagents. Similar to plain wires, monolaurin-coated wires exhibited a large number of adherent osteoblasts, the vast majority of which were found to be viable (Table S1). Quantification of the results obtained revealed that the percentages of viable cells were not significantly different for plain (99.4 ± 0.1%) and monolaurin-coated (99.4 ± 0.1%) wires and tissue-grade polystyrene (99.5 ± 0.1%), which was used as a positive control (n = 4; P = 0.311). Thus, the results of SEM imaging and the Live/Dead assay are in good agreement with the results of the MTT assay and suggest good biocompatibility and no cytotoxicity of monolaurin-coated K-wires.
DISCUSSION
The present work focuses on the potential benefits of using monolaurin as an antibacterial coating on orthopedic K-wires, in an attempt to reduce the burden of pin tract infections, particularly those caused by S. aureus (including MRSA) and S. epidermidis. In this study, stainless steel K-wires were coated with monolaurin using a simple but effective dip-coating method. The proposed methodology appears to be fairly inexpensive and simple and does not require the use of toxic chemical compounds or thermal or mechanical treatments, which may cause structural, chemical, or physical alterations in the implants. The GRAS status of ethanol and monolaurin, the only compounds used for the manufacturing of the coated K-wires, can facilitate the translation of modified wires into clinical practice.
Given that the binding yield of monolaurin at saturation was found to be ∼4.3 μg/mm2, the total dose of monolaurin absorbed on a 1-cm K-wire is 84 μg. Although the antibacterial activity of monolaurin has been studied previously, there are still debates regarding the therapeutic dose and the MIC of this compound. Having analyzed the antibacterial activity of monolaurin against 29 strains of S. aureus, including MRSA, Holland et al. (23) determined that its MIC was in the range of 10 to 20 μg/ml. However, Preuss et al. (24) calculated the MIC of monolaurin against S. aureus to be 62.5 μg/ml. Another study suggested that the MIC of monolaurin against S. aureus was in the range of 25 to 50 μg/ml (25). We were unable to find the MIC of monolaurin against S. epidermidis in the literature. However, the antimicrobial activity of lauric acid against S. epidermidis is well studied, and the MIC was determined to be 3.9 μg/ml (26). Multiple studies suggest that monolaurin has better antimicrobial properties than lauric acid (21, 25, 27). Therefore, the MIC of monolaurin against S. epidermidis can be expected to be lower than 3.9 μg/ml. Overall, although it is difficult to estimate the volume of liquid to which a K-wire is exposed in vivo after implantation, the dose of monolaurin introduced with K-wires in this study (84 μg per 1 cm of wire) is expected to impart antibacterial properties to the implants at least during the first days after the implantation. The results of our inhibition zone tests support this conclusion. Nonetheless, animal studies are required to determine whether the current dose is sufficient to provide a therapeutic effect in vivo.
There is a general consensus that increased surface roughness leads to enhanced osteointegration, primarily due to the more developed bone-to-implant contact (28, 29). In certain cases, however, improved osteointegration can be undesirable (30). The use of external fixators and K-wires implies the subsequent removal of the implants, and a more developed bone-to-implant contact would increase frictional forces between the implant and the bone upon removal, causing severe discomfort for patients. Therefore, alterations of surface characteristics, particularly roughness, of external fixators and K-wires should be avoided. The results of SEM and AFM imaging conducted herein showed that the topography of the monolaurin-coated wires was similar to that of the plain wires (Fig. 3 and 4). The only parameter that was affected by the presence of the monolaurin coating was the surface stickiness, as measured by AFM. However, the observed increase in stickiness is not expected to affect cell adhesion and osteointegration in any way, since those responses are guided biochemically (through adhesion proteins) and not physically. Hence, the modification of K-wires with monolaurin coating is not anticipated to influence the osteointegrative properties of the implants. One possible issue that might be caused by the altered stickiness of the wires is their handling by the surgeon during implantation and removal. We qualitatively tested the handling of the coated wires in a pilot experiment, by using a wire to pierce a silicon rubber Septa flask stopper, and we did not see any differences in handling between coated and uncoated wires. However, handling could still be an issue during actual surgery and will be assessed in detail in future animal studies.
The present study was primarily focused on in vitro analysis of the antibacterial activity of monolaurin-coated K-wires. S. aureus (MSSA and MRSA) and S. epidermidis were tested here mainly because these pathogens cause the majority of implant-associated infections. It was estimated that all existing pathogens except for staphylococci account for only 22% of orthopedic infections (31). Hence, the importance of fighting staphylococcal infections cannot be overestimated. The high level of activity against MRSA noted here for monolaurin-coated wires is of special interest, because these antibiotic-resistant strains have the highest morbidity and mortality rates.
Since most pin site infections are caused by the formation of bacterial biofilms on the implant surface, the modified wires are required to possess pronounced antibiofilm activity. However, the efficacy against planktonic pathogens should not be underestimated. In 1987, Anthony Gristina introduced the concept called “race to the surface” (32). During the initial postoperative period, the host defense system is impaired and is vulnerable to the bacteria introduced during surgery. Gristina suggested that there is a race to the surface of the implant between host cells and bacteria from adjacent tissues; if the race is won by bacteria, then the risk of implant infection increases considerably. Therefore, treatment needs to be directed also against nearby planktonic bacteria. Given the low water solubility of monolaurin, it is predicted that coated K-wires can potentially provide prolonged drug release upon implantation. Thus, monolaurin coating is anticipated to be effective against planktonic bacteria.
As predicted, monolaurin-coated K-wires exhibited excellent antimicrobial properties against planktonic MSSA, MRSA, and S. epidermidis, completely eliminating up to 106 CFU for each of these strains (Fig. 5; also see Fig. S3 in the supplemental material). Monolaurin-coated wires also prevented biofilm formation, as indicated by the results of the CV staining and SEM imaging (Fig. 6 to 8; also see Fig. S3). It is important to emphasize that the few bacteria found on the surfaces of the modified wires had disrupted cell walls, which suggests that these microorganisms were not viable. Although a consensus regarding monolaurin's mechanism of antibacterial action has not been reached, alteration of the plasma membrane and disruption of the bacterial cell wall is considered to be one of the two most likely mechanisms of action (21, 25). An alternative mechanism assumes that monolaurin is able to interfere with bacterial two-component regulatory systems. Monolaurin was found to inhibit WalK/R, one of the 16 two-component regulatory systems in S. aureus, leading to the death of the bacteria (33). In this study, we observed direct confirmation of the disruption of bacterial cell walls; however, the second mechanism could also play a role, as it was not studied here.
Since the cytotoxicity of antibacterial agents is an important issue (34), the cytocompatibility of monolaurin-coated K-wires was extensively studied herein (Fig. 10). According to International Standard ISO 10993, a material is considered to be cytotoxic if cell viability drops below 70% in its presence (35). In this study, we did not observe evidence of any adverse effects of monolaurin on osteoblast viability; cell viability was found to exceed 99%. The size, shape, morphology, and number of osteoblasts adherent to the surface of modified wires suggest good biocompatibility and a lack of cytotoxicity for the monolaurin-coated wires. These results were not unexpected, considering the known safety record of monolaurin (21, 25, 36).
In conclusion, monolaurin-coated Kirschner wires were manufactured, and their performance was evaluated in vitro. Prepared wires showed pronounced antibacterial and antibiofilm activities against methicillin-sensitive and methicillin-resistant S. aureus and S. epidermidis. Excellent biocompatibility of monolaurin-coated implants was demonstrated in a mice osteoblast model. Data obtained here can serve as the foundation for further animal and clinical studies of monolaurin-coated K-wires. Taking into account the advantages of monolaurin as an antibacterial coating, the approach proposed in this work can have huge potential for orthopedic applications.
MATERIALS AND METHODS
Materials.
Stainless steel K-wires were obtained from Smith & Nephew (Andover, MA). Monolaurin, high-performance liquid chromatography (HPLC)-grade acetonitrile, 200 proof ethanol, and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO). Difco tryptic soy agar and BBL tryptic soy broth (TSB) were obtained from BD (Franklin Lakes, NJ). Alpha minimum essential medium (αMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Corning Inc. (Manassas, VA). MTT and the Live/Dead viability/cytotoxicity kit were purchased from Thermo Fisher Scientific (Waltham, MA).
K-wire modification.
Stainless steel K-wire pieces with a length of 10 mm and a diameter of 1.575 mm were used in this study. The surface area of the samples was calculated to be approximately 19.5 mm2, assuming cylindrical geometry. Prior to the modification, the wires were cleaned in air plasma (input power, 30 W) for 15 min. For coating, 1 ml of monolaurin solution in ethanol (10 mg/ml) was transferred into a sterile centrifuge tube with a cleaned K-wire. After 10 min of incubation, the wire was removed from the solution and air dried for 10 min at room temperature under sterile conditions. Uncoated controls were prepared by incubating the wires in pure ethanol for 10 min, followed by air drying for 10 min, similar to the coated wires.
Characterization of the coating. (i) FTIR analysis of the samples.
FTIR spectra were collected by averaging 16 scans obtained with a FTIR spectrometer equipped with a Thermo Scientific Spectra-Tech Foundation Series Endurance diamond attenuated total reflectance (ATR) accessory (Magna 550; Thermo-Nicolet). Scans were performed at a resolution of 4 cm−1 and recorded in absorbance units from 500 to 4,000 cm−1. Baseline correction was applied to determine net peak heights.
(ii) Surface morphology of the samples.
Monolaurin-coated K-wires were characterized using SEM (model S-4800; Hitachi, Schaumburg, IL). Prior to imaging, samples were sputter coated with a gold film (thickness, ∼5 to 7 Å). Micrographs with magnifications of up to ×50,000 were obtained using an accelerated voltage of 5 kV and a working distance of 6 mm.
Additionally, surface morphology was characterized by AFM using a MFP-3D instrument (Asylum, Santa Barbara, CA) equipped with metal-coated Olympus AC240TS microcantilevers, with a nominal resonance frequency of 70 kHz and a spring constant of approximately 2 nN/nm. Imaging was performed in both tapping and force-mapping modes. Samples of uncoated and coated wires were fixed on a glass slide using double-sided duct tape. The topography of all samples was measured in contact mode; additionally, coated samples were scratched to measure the thickness of the coating. The stickiness of the studied samples was analyzed by force mapping. The precise spring constant for each of the cantilevers was determined before each measurement.
The thickness of the monolaurin coating was also measured using spectroscopic reflectometry (Edmund Optics, Barrington, NJ), according to a protocol adapted from reference 37. For this experiment, silicon wafers coated with monolaurin according to the procedure described above were used. Measurements were conducted with an angle of incidence of 0°, at wavelengths ranging from 400 to 900 nm. The Fresnel equation was used to fit the acquired data. A total of 3 replicates were analyzed.
(iii) Determination of monolaurin binding yield.
Coated wires were prepared using a series of monolaurin solutions in ethanol at different concentrations (1, 2.5, 5, 6, 7.5, 10, and 15 mg/ml). After 10 min of incubation, each wire was carefully removed from the solution, air dried for 10 min, and transferred to a vial containing 1 ml of anhydrous acetonitrile, where it was sonicated for 30 min. The absorption spectra of the resulting monolaurin solution in acetonitrile were recorded using a UV-visible spectrophotometer (UV-2 UV-visible spectrophotometer; ATi UNICAM, Cambridge, UK), in the range of 190 to 300 nm. The amount of deposited monolaurin was calculated from the value for the absorption at 220 nm by using a calibration curve (see Fig. S2 in the supplemental material). The latter was obtained by using monolaurin solutions in acetonitrile with standard concentrations ranging from 0.1 to 10 mg/ml.
In vitro evaluation of antibacterial activity. (i) Species studied.
The antimicrobial activity of monolaurin-coated wires was evaluated against methicillin-sensitive S. aureus (ATCC 14775), methicillin-resistant Staphylococcus aureus (ATCC 33591), and S. epidermidis (ATCC 12228).
(ii) Studies of planktonic bacteria.
Prior to the experiments, bacteria were cultured in soy broth until they reached the stationary phase. Subsequently, the concentrations of the inocula were adjusted to 106 CFU/ml by using fresh broth. Coated and plain wires were placed in sterile test tubes containing 1 ml of a bacterial suspension of 106 CFU/ml. Samples were incubated at 37°C, with mild shaking. Aliquots of the bacterial suspension were taken at the following time points: 0, 1.5, 3, 4.5, 6, 7.5, 24, and 48 h. The aliquots were analyzed by the spread-plate method according to International Standard ISO 4833-2 (38). Based on the data, the antibacterial efficacy was calculated as log reduction of bacteria. A total of 4 replicates were performed to obtain planktonic bacteria viability curves.
(iii) Studies of adherent bacteria.
Bacterial cultures with concentrations of 106 CFU/ml were prepared as described above. Coated and plain wires were placed in a sterile 24-well plate; 1 ml of bacterial inoculum was added to the wells containing the wires. In order for bacteria to adhere and to form a biofilm, the wires were statically incubated for 48 h at 35°C (39). Biofilm formation was quantified using the CV assay described by Kobayashi et al. (40). Briefly, after the 48-h biofilm growth period, coated and plain wires were transferred to a sterile 24-well plate, rinsed three times with PBS, and incubated for 10 min with a 0.1% aqueous solution of CV. After the wires were washed three times with PBS, they were placed in 33% acetic acid and incubated for 15 min, with mild shaking. The optical density of the resulting solution was measured at 590 nm using a microplate reader (Synergy HT; Bio-Tek, Winooski, VT). All experiments were replicated six times.
In addition, the numbers of CFU in the biofilms were measured according to the protocol described in the literature (41). Briefly, after plain and monolaurin-coated wires were exposed to the bacterial inocula for 48 h to create a biofilm, each wire was gently rinsed three times with sterile PBS and placed in a test tube with 1 ml of sterile PBS. To remove the adherent bacteria, wires were vortex-mixed for 30 s, followed by sonication in an ultrasonic bath for 60 s. Colony counting was performed for the resulting bacterial suspensions using the protocol described above (Fig. S1). The log reduction of adherent bacteria was then calculated. Four replicates were performed for both coated and uncoated samples.
Biofilm formation was also imaged using SEM. Sample preparation for SEM was performed using the protocol described by El Abed et al. (42). Briefly, samples were fixed with 2.5% glutaraldehyde, followed by postfixation with 1% osmium tetroxide. The wires were then dehydrated by successive exposure to an ethanol gradient (50, 75, 90, and 100%), followed by treatment with hexamethyldisilazane (HMDS). The samples were then sputter coated with a layer of gold, and SEM images were obtained using accelerating voltages of up to 5 kV and a working distance of 5 mm.
(iv) Inhibition zone diameter test.
The antibacterial activity of the wires was also evaluated using the inhibition zone test. The protocol for these measurements was adapted from the literature (35). Briefly, 106 CFU of bacteria were seeded on agar plates. Coated and plain wires were transferred to the center of the inoculated plates and incubated at 37°C; the zone of inhibition was measured at different time points (1, 2, 3, and 4 days), as described in the literature (35). These experiments were replicated six times.
In vitro evaluation of cytotoxicity. (i) Culture conditions for studies.
The analysis of the cytotoxicity of monolaurin-coated K-wires was performed in an experimental model with mouse 7F2 osteoblasts (ATCC CRL-12557). Cells were cultured at 37°C, in 5% CO2, in αMEM supplemented with 10% FBS and 1% penicillin and streptomycin.
(ii) MTT assay.
The effect of the monolaurin coating on osteoblast proliferation was evaluated using the MTT assay. The protocol for this experiment was adapted from the literature (43). Osteoblasts were passaged after reaching confluence, and aliquots containing ∼40,000 cells were transferred into a sterile 24-well plate containing monolaurin-coated and plain wires. Samples were incubated in the presence of cells at 37°C, in 5% CO2, for 2, 4, and 7 days. Following the incubation, the wires were removed from the wells, and osteoblasts were exposed to the MTT reagent at 5 mg/ml for 4 h. Then, dimethyl sulfoxide (DMSO) was added to completely dissolve formazan crystals, and the optical density of the resulting solution was measured at 570 nm using a microplate reader (Synergy HT; Bio-Tek). Four replicates were performed for each of the time points.
(iii) Live/Dead assay.
The viability of osteoblasts attached to the wires was also assessed using the Live/Dead assay. To achieve cell attachment, the wires were incubated with 200 μl of osteoblast suspension (200,000 cells/ml) for 4 h at 37°C. Then, the wires were transferred to wells containing fresh αMEM and were incubated for 4 days at 37°C, in 5% CO2. After the incubation, the plain and coated wires were removed from the medium, gently rinsed three times with sterile PBS, and treated with calcein AM (20 μM) and ethidium homodimer-1 (4 μM). Following an incubation period of 30 min, wires were imaged using a fluorescence microscope (EVOS FL Auto; Thermo-Fisher). The viability of cells attached to the surface of the wires was measured according to the Live/Dead assay kit manual. Cells attached to tissue-grade polystyrene were used as the control. Four replicates were performed for this assay.
(iv) Cell visualization.
Attached osteoblasts were also imaged using SEM, and cell morphology was analyzed based on the images obtained. After cells were allowed to attach to the wires, samples were fixed and dehydrated as described above. SEM images were obtained using accelerating voltages of up to 5 kV and a working distance of 5 mm.
Statistical analysis.
All numerical data are presented as the mean value ± standard deviation. One-way analysis of variance (ANOVA) was used for variance comparisons. Statistical differences with P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was partially supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health (grant P20GM103444) and a Clemson University URGC grant.
We thank Nikolay Borodinov and Kimberly Ivey for conducting FTIR and reflectometry measurements, Jeannette Rodriguez, Mikhail Bredikhin, and Raisa Kiseleva for helping with the microbial colony counting experiments, George Wetzel for assisting with SEM imaging, and Olga Reukova for helping with illustrations.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00442-17.
REFERENCES
- 1.Zhu J, Zhao Y, Yang L, Hou S, Su Y, Yang R. 2015. Antibacterial modification of Kirschner wires with polyluteolin toward methicillin-resistant Staphylococcus aureus (MRSA). Materials (Basel) 8:4876–4883. doi: 10.3390/ma8084876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. 2006. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 37(Suppl 2):S105–S112. doi: 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Sharma H, Taylor GR, Clarke NMP. 2007. A review of K-wire related complications in the emergency management of paediatric upper extremity trauma. Ann R Coll Surg Engl 89:252–258. doi: 10.1308/003588407X155482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira N, Marais LC. 2012. Prevention and management of external fixator pin track sepsis. Strategies Trauma Limb Reconstr 7:67–72. doi: 10.1007/s11751-012-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hargreaves DG, Drew SJ, Eckersley R. 2004. Kirschner wire pin tract infection rates: a randomized controlled trial between percutaneous and buried wires. J Hand Surg Br 29:374–376. doi: 10.1016/j.jhsb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. 2011. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am 36:610–616. doi: 10.1016/j.jhsa.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61–65. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, He X, Gao Y, Zhang X, Yao X, Tang B. 2017. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater Sci Eng C Mater Biol Appl 75:7–15. doi: 10.1016/j.msec.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 9.Muzykantov VR. 2010. NO gets a test ride on high-tech transporting nanodevices: a commentary on “Sustained-release nitric oxide from long-lived circulating nanoparticles.” Free Radic Biol Med 49:528–529. doi: 10.1016/j.freeradbiomed.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Liu G, Zhai Z, Liu L, Li H, Yang K, Tan L, Wan P, Liu X, Ouyang Z, Yu Z, Tang T, Zhu Z, Qu X, Dai K. 2014. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother 58:7586–7591. doi: 10.1128/AAC.03936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehl R, Brunetto PS, Woischnig AK, Varisco M, Rajacic Z, Vosbeck J, Terracciano L, Fromm KM, Khanna N. 2016. Preventing implant-associated infections by silver coating. Antimicrob Agents Chemother 60:2467–2475. doi: 10.1128/AAC.02934-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park K-H, Greenwood-Quaintance KE, Schuetz AN, Mandrekar JN, Patela R. 2017. Activity of tedizolid in methicillin-resistant Staphylococcus epidermidis experimental foreign body-associated osteomyelitis. Antimicrob Agents Chemother 61:e01644–. doi: 10.1128/AAC.01644-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetrick EM, Schoenfisch MH. 2006. Reducing implant-related infections: active release strategies. Chem Soc Rev 35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 14.Romanò CL, Scarponi S, Gallazzi E, Romanò D, Drago L. 2015. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res 10:157. doi: 10.1186/s13018-015-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruzin A, Novick RP. 2000. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J Bacteriol 182:2668–2671. doi: 10.1128/JB.182.9.2668-2671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verallo-Rowell VM, Dillague KM, Syah-Tjundawan BS. 2008. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis 19:308–315. [PubMed] [Google Scholar]
- 17.Peterson ML, Schlievert PM. 2006. Glycerol monolaurate inhibits the effects of Gram positive select agents on eukaryotic cells. Biochemistry 45:2387–2397. doi: 10.1021/bi051992u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preuss HG, Echard B, Dadgar A, Talpur N, Manohar V, Enig M, Bagchi D, Ingram C. 2005. Effects of essential oils and monolaurin on Staphylococcus aureus: in vitro and in vivo studies. Toxicol Mech Methods 15:279–285. doi: 10.1080/15376520590968833. [DOI] [PubMed] [Google Scholar]
- 20.Murray M, Pizzorno J, Pizzorno L. 2005. The encyclopedia of healing foods. Atria Books, New York, NY. [Google Scholar]
- 21.Schlievert PM, Peterson ML. 2012. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7:e40350. doi: 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller EA, Schlievert PM. 2015. Non-aqueous glycerol monolaurate gel exhibits antibacterial and anti-biofilm activity against Gram-positive and Gram-negative pathogens. PLoS One 10:e0120280. doi: 10.1371/journal.pone.0120280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland KT, Taylor D, Farrell AM. 1994. The effect of glycerol monolaurate on growth of, and production of toxic shock syndrome toxin-1 and lipase by, Staphylococcus aureus. J Antimicrob Chemother 33:41–55. doi: 10.1093/jac/33.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Preuss HG, Echard B, Enig M, Brook I, Elliott TB. 2005. Minimum inhibitory concentrations of herbal essential oils and monolaurin for Gram-positive and Gram-negative bacteria. Mol Cell Biochem 272:29–34. doi: 10.1007/s11010-005-6604-1. [DOI] [PubMed] [Google Scholar]
- 25.Lin YC, Schlievert PM, Anderson MJ, Fair CL, Schaefers MM, Muthyala R, Peterson ML. 2009. Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PLoS One 4:e7499. doi: 10.1371/journal.pone.0007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsuji T, Kao MC, Fang J-Y, Zouboulis CC, Zhang L, Gallo RL, Huang C-M. 2009. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Investig Dermatol 129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman S, Enig MG, Preuss HG. 2006. A review of monolaurin and lauric acid. Alternat Complement Ther 12:310–314. doi: 10.1089/act.2006.12.310. [DOI] [Google Scholar]
- 28.Abron A, Hopfensperger M, Thompson J, Cooper LF. 2001. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J Prosthet Dent 85:40–46. doi: 10.1067/mpr.2001.112415. [DOI] [PubMed] [Google Scholar]
- 29.Rungsiyakull C, Li Q, Sun G, Li W, Swain MV. 2010. Surface morphology optimization for osseointegration of coated implants. Biomaterials 31:7196–7204. doi: 10.1016/j.biomaterials.2010.05.077. [DOI] [PubMed] [Google Scholar]
- 30.Yu JH, Wu LC, Hsu JT, Chang YY, Huang HH, Huang HL. 2011. Surface roughness and topography of four commonly used types of orthodontic archwire. J Med Biol Eng 31:367–370. doi: 10.5405/jmbe.700. [DOI] [Google Scholar]
- 31.Campoccia D, Montanaro L, Arciola CR. 2006. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 32.Gristina A. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 33.Delaune A, Poupel O, Mallet A, Coic YM, Msadek T, Dubrac S. 2011. Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PLoS One 6:e17054. doi: 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jennison T, McNally M, Pandit H. 2014. Prevention of infection in external fixator pin sites. Acta Biomater 10:595–603. doi: 10.1016/j.actbio.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Hou D, Wang L, Zhang Q, Zou J, Sun G. 2015. Antibacterial surgical silk sutures using a high-performance slow-release carrier coating system. ACS Appl Mater Interfaces 7:22394–22403. doi: 10.1021/acsami.5b06239. [DOI] [PubMed] [Google Scholar]
- 36.Pereira CCB, da Silva MAP, Langone MAP. 2004. Enzymatic synthesis of monolaurin. Appl Biochem Biotechnol 114:433. doi: 10.1385/ABAB:114:1-3:433. [DOI] [PubMed] [Google Scholar]
- 37.Borodinov N, Soliani AP, Galabura Y, Zdyrko B, Tysinger C, Novak S, Du Q, Huang Y, Singh V, Han Z, Hu J, Kimerling L, Agarwal AM, Richardson K, Luzinov I. 2016. Gradient polymer nanofoams for encrypted recording of chemical events. ACS Nano 10:10716–10725. doi: 10.1021/acsnano.6b06044. [DOI] [PubMed] [Google Scholar]
- 38.International Organization for Standardization. 2013. Microbiology of the food chain: horizontal method for the enumeration of microorganisms, part 2. Colony count at 30 °C by the surface plating technique. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 39.Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1:Unit 1B.1. doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW. 2009. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin Orthop Relat Res 467:1360–1364. doi: 10.1007/s11999-008-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillaume O, Garric X, Lavigne J-P, Van Den Berghe H, Coudane J. 2012. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: preparation and antimicrobial efficacy in vitro. J Control Release 162:492–501. doi: 10.1016/j.jconrel.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 42.El Abed S, Ibnsouda SK, Latrache H, Hamadi F. 2012. Scanning electron microscopy (SEM) and environmental SEM: suitable tools for study of adhesion stage and biofilm formation, p 717–730. INTECH Open Access Publisher, Rijeka, Croatia. [Google Scholar]
- 43.Wu F, Meng G, He J, Wu Y, Wu F, Gu Z. 2014. Antibiotic-loaded chitosan hydrogel with superior dual functions: antibacterial efficacy and osteoblastic cell responses. ACS Appl Mater Interfaces 6:10005–10013. doi: 10.1021/am502537k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.