Abstract
This study aimed to determine the prevalence of fecal carriage of extended spectrum β-lactamase (ESBL) and/or plasmidic AmpC β-lactamase (pAmpC) producing Escherichia coli among dogs (n=428) in Turkey. Polymerase chain reaction (PCR) and sequencing were used to characterize genes encoding β-lactamase and plasmid mediated quinolone resistance (PMQR). Antimicrobial susceptibility testing and PCRs for virulence genes and phylogenetic groups were also performed. Cefotaxime resistant E. coli isolates were detected in 95 (22.2%) of the swab samples. Sequencing analysis results showed occurrence of various β-lactamase genes: blaCTX-M-15 (62), blaTEM-1b (42), blaCMY-2 (22), blaCTX-M-3 (16), blaCTX-M-1 (15), blaOXA-1 (9) and blaSHV-12 (3) alone or in combination. The most frequently encountered phylogenetic group was group A1 (35.8%), followed by group D2 (22.1%), B1 (15.8%), D1 (9.5%), A0 (7.4%), B22 (5.3%) and B23 (4.2%), respectively. PMQR genes, aac(6’)-Ib-cr, qnrS1 and qnrB10 were detected in 25.3, 10.5 and 1.1% of the isolates, respectively. While all isolates were susceptible to imipenem and amikacin, resistance rates to non-β-lactam antibiotics ranged from 20.0% for tobramycin to 56.8% for tetracycline. The virulence genes were only detected in 34 (36.2%) of the isolates and this isolates carried single or various combination of virulence genes of iucD, papC, papE, f17a-A and eaeA. Four isolates were identified as human virulent pandemic CTX-M-15 producing E. coli clone O25b:ST131/B2. To the best of our knowledge, this is the first study to show fecal carriage of ESBL/pAmpC type β-lactamase producing E. coli isolates among dogs in Turkey.
Keywords: dog, Escherichia coli, ESBL, molecular characterization, pAmpC
The extended spectrum cephalosporins (ESCs) are critically important antimicrobials for the treatment of bacterial infections in both human and veterinary medicine [50]. Increased use of these drugs led emergence of ESC resistant Gram negative bacteria in humans and animals [47]. Main resistance mechanisms to ESCs are frequently related to plasmid mediated production of enzymes, extended spectrum β-lactamases (ESBLs) and AmpC β-lactamases (pAmpC) [25, 36]. Carriage of ESBL and AmpC genes on plasmids enhances transfer of these genes to other bacteria by conjugation. Furthermore, these plasmids carry genes conferring resistance to various classes of antimicrobials, such as fluoroquinolones, aminoglycosides, sulphonamides, trimethoprim. This makes treatment options for infections caused by ESBL and/or AmpC producing bacteria very limited [39].
CTX-M type ESBLs was first detected in a laboratory dog in Japan in 1986 [33]. This type of ESBLs showed a significant international dissemination since 2000, becoming the most prevalent type of ESBL throughout the world and, outnumbering other ESBL types such as TEM and SHV. Currently, there are different 172 CTX-M β-lactamases types (http://www.lahey.org/studies/), clustered into five phylogroups (CTX-M-1, -2, -8, -9 and -25). CTX-M-1 phylogroup, which includes CTX-M-15, is the most widely distributed all over the world [8].
The genes encoding AmpC type β-lactamases are chromosomal- or plasmid-mediated and most pAmpC genes are derived from the chromosomal ampC genes of several members of the family Enterobacteriaceae [38]. CMY family is the most prevalent pAmpC β-lactamase [25] and 136 different types of this family have been described to date (http://www.lahey.org/studies/). Among the CMY family, CMY-2 type is most frequently encountered around the world. In Turkey, CMY-2 producing E. coli has been reported in humans, cattle and retail meats [3, 37, 52].
Due to their close contact with humans, companion animals may play a role as a reservoir of transmitting antimicrobial resistant bacteria [18]. Recently, Ljungquist et al. [32] showed household transfer of ESBL-/pAmpC producing E. coli between humans and dogs in Sweden. Another worrying development is the recent emergence of highly virulent human pandemic B2-O25b-ST131 clone of CTX-M-15 producing E. coli strains from companion animals of different species in various European countries [17, 40, 46]. This clone, isolated in 2008, was implicated in human urinary tract and bloodstream infections, and proved difficult to treat due to extensive multidrug resistance [42]. The risk associated with the transfer of these multiresistant bacteria from humans to companion animals and vice versa is a great concern for both canine welfare and public health.
Recently, presence of ESBLs/pAmpC β-lactamase producing fecal E. coli isolates from cattle and chicken has been reported in Turkey [3, 4]. However, data on ESBL/pAmpC β-lactamase producing E. coli in dogs is not available. Therefore, the objectives of this study is to determine the prevalence of ESBL and/or pAmpC producing E. coli from dogs in three cities and to characterize positive isolates with molecular methods.
MATERIALS AND METHODS
Sample collection, isolation and identification of E. coli
Between January 2014 and June 2014, a total of 428 non-duplicate rectal swabs from companion dogs in Hatay (n=148), Mersin (n=142) and Adana (n=138) cities which are located in southern Turkey. Age of dogs was ranging from one month to 16 year (median age 3 years), including 47 breeds. Among the dogs sampled, 45.1% (n=193) were females and 54.9% (n=235) were males. Dogs had no clinical symptoms and admitted to veterinary clinics for routine physical examination, parasite screening or vaccination procedures.
Selective isolation of ESBL producing E. coli
The swabs were inoculated onto 2 µg/ml cefotaxime containing Brilliance E. coli/Coliform Agar Selective Agar (Oxoid, U.K.) for selective isolation and incubated at 35°C for 24 hr. Screening concentration of cefotaxime indicating ESBL/pAmpC production was determined in accordance with Clinical Laboratories Standards Institute (CLSI) criteria [12]. One typical colony was selected and passaged onto Blood Agar to obtain pure cultures. Identification was done using classical methods (Gram stain, catalase, oxidase, indole, MR-VP, citrate and urease), and using species-specific PCR [49].
Antimicrobial susceptibility testing
Antimicrobial susceptibility of ESBL/pAmpC producing E. coli isolates tested by disk diffusion method and interpreted according to Clinical Laboratories Standards Institute (CLSI) criteria [12]. The antimicrobials tested were ampicillin (10 µg), amoxicillin-clavulanic acid (10/20 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), cefotaxime (10 µg), cefpodoxime (10 µg), cefepime (30 µg), cefoxitin (30 µg), cephalothin (30 µg), aztreonam (30 µg), imipenem (10 µg), chloramphenicol (30 µg), gentamicin (10 µg), tobramycin (10 µg), amikacin (10 µg), kanamycin (30 µg), tetracycline (30 µg) and sulfametoxazole-trimethoprim (1.25/23.75 µg).
Production of ESBL phenotype was evaluated by disk combination method using ceftazidime, cefotaxime, cefpodoxime disks alone or in combination with clavulanic acid and double disk synergy test (DDST) using amoxicillin/clavulanic acid, cefotaxime and ceftazidime disks. Those cefotaxime-resistant E. coli isolates not only showing a negative ESBL-phenotype but also showing resistance to amoxicillin/clavulanic acid and cefoxitin were accepted as pAmpC phenotype. Klebsiella pneumoniae ATCC 700603 and E. coli ATCC 25922 were used as positive and negative controls, respectively. The isolates that were resistant to three or more antimicrobial from different classes were defined as multidrug resistant (MDR).
DNA extraction
DNA extraction was done using boiling method, as previously reported by Ahmed et al. [1].
Determination of ESBL and PMQR genes
The ESBL genes blaSHV, blaTEM, blaCTX-M, blaOXA were investigated by PCR as described by Ahmed et al. [1]. The pAmpC genes were detected with the same method as described by Pérez-Pérez and Hanson [38]. Screening of PMQR genes qnrA, qnrB, qnrC, qnrD, qnrS, aac(6’)-Ib and qepA genes were searched as suggested by Cavaco et al. [9], Kim et al. [28] and Park et al. [35]. All ESBL/pAmpC β-lactamase and PMQR positive amplicons were sequenced from both ends and sequencing results were compared to reported sequences available in GenBank.
Phylogenetic grouping
The phylogenetic analysis of the ESBL/pAmpC β-lactamase producing E. coli isolates were determined by the triplex PCR as previously reported by Clermont et al. [10]. Phylogenetic groups and subgroups (A0, A1, B1, B22, B23, D1 and D2) were determined according to Escobar-Páramo et al. [16].
Determination of pandemic B2 O25b-ST131 clone
Pandemic B2 O25b-ST131 E. coli CTX-M-15 clone was investigated using allele specific PCR developed by Clermont et al. [11].
Determination of virulence genes
The presence of 18 virulence genes [iucD, hlyA, cnf1, papC, papE-F, sfa/focDE, f17A, f17a-A, f17b-A, f17c-A, 17d-A, afa D-8, afa E-8, clpG, cnf2, stx1, stx2, eaeA] were examined by PCR [6, 7, 27, 29, 49, 53].
Pulsed field gel electrophoresis (PFGE) analysis
Clonality of E. coli B2-O25-ST131 CTX-M-15 isolates was determined by pulsed-field gel electrophoresis (PFGE) technique with XbaI restriction of DNA in Public Health Institution of Turkey (Ankara) as described previously [15].
Statistical analysis
Differences in frequencies of ESBL/pAmpC producing E. coli isolates according to provinces, different age groups and genders were evaluated using Pearson’s or likelihood ratio chi-square tests. IBM SPSS Statistics package Version 23 (IBM Corp., Armonk, NY, U.S.A.) was used for the statistical analysis. P value <0.05 was considered as statistically significant.
Ethics
The study was approved by the Animal Ethical Committe of Mustafa Kemal University (2012/08/15).
RESULTS
Isolation results
Cefotaxime resistant isolates were recovered in 95 (22%) of 428 dog. Out of 95 cefotaxime resistant E. coli isolates, 72 (75.8%) isolates exhibited ESBL phenotype, while the remaining 23 (24.2%) isolates showed an pAmpC phenotype, characterized by resistance to amoxicillin-clavulanic acid and cefoxitin. Prevalence of ESBL/pAmpC positive E. coli was 18.3% (26/142) in Mersin, 31.8% (47/148) in Hatay and 22% (22/138) in Adana. Significant differences between fecal carriage and provinces were observed (P=0.004). However, no significant differences between genders were observed (P=0.595). Furthermore, there were also no significant diferences between different age groups (P=0.089).
Antimicrobial susceptibility
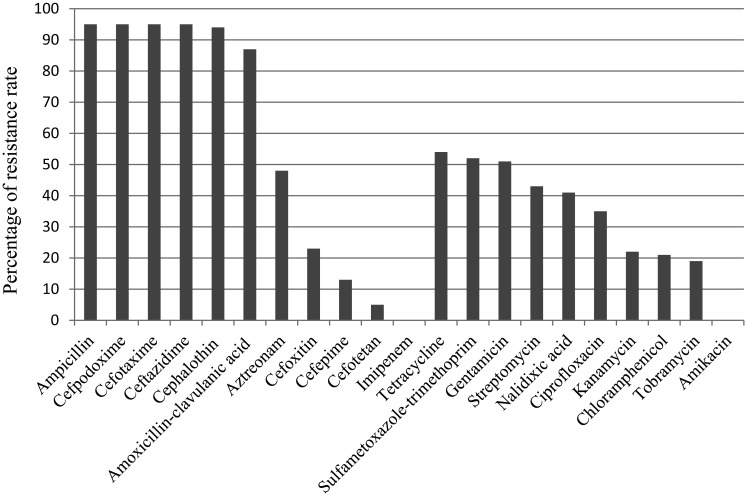
The results of antimicrobial susceptibility of ESBL/pAmpC producing E. coli isolates are given in Fig. 1. All isolates were resistant to ampicillin, cefotaxime, ceftazidime, cefpodoxime, but susceptible to imipenem. Resistance rates for the rest of other β-lactams were: 98.9, 91.6, 50.5, 24.2, 13.7 and 5.3% for cephalothin, amoxicillin-clavulanic acid, aztreonam, cefoxitin, cefepime and cefotetan, respectively. As for the non-β lactam antimicrobials, while all isolates were susceptible to amikacin, various rates of resistance were observed for tetracycline (56.8%), gentamicin (53.7%), sulfametoxazole-trimethroprim (45.3%), streptomycin (45.3%), nalidixic acid (43.2%), ciprofloxacin (36.8%), kanamycin (22.2%), chloramphenicol (22.1%) and tobramycin (20%). The most of the isolates (67.4%) showed MDR phenotype. MDR to six, five, four and three antimicrobial classes were observed in 14 (%), 23 (%), 10 (%), 17 (%) isolates, respectively. Resistance to one and two classes of antimicrobials were detected in 17 and 14 isolates, respectively.
Fig. 1.
Antimicrobial susceptibility of ESBL/pAmpC producing E. coli isolates.
Detection of ESBL/pAmpC β-lactamase genes
ESBL/pAmpC β-lactamase genes identified in E. coli isolates were: CTX-M-15 (n=46), CTX-M-1 (n=13), CTX-M-3 (n=11), CTX-M-15+CMY-2 (n=14), CTX-M-3+CMY-2 (n=5), CTX-M-1+CMY-2 (n=2), CTX-M-3+SHV-12 (n=1), CTX-M-15+SHV-12 (n=2) and CMY-2 (n=1).
Determination of PMQR genes
Among 35 ciprofloxacin resistant isolates, PMQR genes were found in 22 (62.9%) isolates including 17 aac(6’)-Ib-cr, four qnrS1, one aac(6’)-Ib-cr + qnrS1. In addition, PMQR genes were also detected in nine ciprofloxacin susceptible isolates, of which four had aac(6’)-Ib-cr, four carried qnrS1 and one harbored qnrB10.
Phylogenetic grouping
Most of ESBL/AmpC producing E. coli isolates belonged to phylogenetic group A1 (34/95; 35.8%), followed by group D2 (21/95; 22.1%), B1 (15/95; 15.8%), D1 (9/95, 9.5%), A0 (7/95, 7.4%), B22 (5/95; 5.3%) and B23 (4/95; 4.2%), respectively.
Determination of pandemic O25:ST131 clone
Pandemic O25:ST131 clone was detected in four (4.3%) isolates producing CTX-M-15, which belongs to B2 phylogenetic group. This clone was detected in Adana (n=3) and Mersin (n=1) isolates but not in Hatay.
PFGE analysis and detection of virulence genes
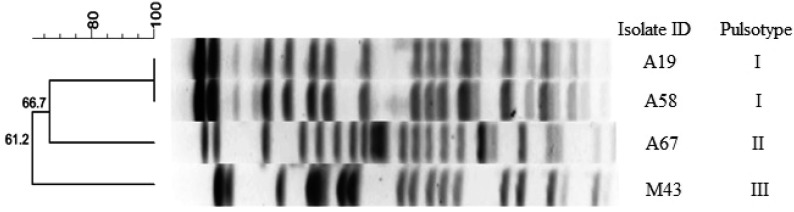
PFGE results revealed presence of three pulsotype among E. coli B2-O25-ST131 CTX-M-15 isolates (Fig. 2). Virulence genes were detected in 36.2% (n=34) of 95 ESBL/pAmpC β-lactamase producing E. coli isolates and these isolates were only found as positive for iucD, f17a-A, papC, papE and eaeA genes. Other virulence genes were not detected. Single or various combination of virulence genes were observed among the isolates. The most common gene encountered was iucD gene, which was found in 31 isolates. Also, f17a-A fimbrial adhesin in two (2.1%) isolates and papE-papC, eaeA and papE genes in one (1.1%) isolate were detected.
Fig. 2.
Dendrogram showing the relationship of four B2-O25b-ST131 CTX-M-15 positive E. coli isolates based on XbaI-generated PFGE profiles.
DISCUSSION
This is the first study on the prevalence of ESBL/pAmpC producing E. coli from dogs conducted in Turkey. Our study showed that prevalence of fecal carriage of ESBL/pAmpC producing E. coli was 22.2% (95/428). Our results are higher than those reported in France (18.5%), in Mexico (17%), in Algeria (14.7%) [20, 41, 54], but comparable to those reported in China (24.5%) [45]. In addition, significant differences between fecal carriage and provinces were observed (P=0.004). Although past treatment histories were not recorded during sampling, it was speculated that the significant differences observed between fecal carriage and provinces could be related to variations in overall antibiotic use among these populations but not to individual risk factors.
According to studies carried out in different countries, it seems that types and subtypes of ESBL/pAmpC enzymes in E. coli strains isolated among dogs vary geographically. CTX-M-15 was the most common enzyme determined in this study, produced by 21 isolates alone and 41 isolates combined with one or more β-lactamase genes. In addition to blaCTX-M-15, blaSHV-12 was observed in three isolates combined with other β-lactamase genes. CTX-M-15 were reported in healthy dogs by several studies [17, 24, 34, 41, 54]. CTX-M-15 is also the most commonly reported enzyme type in E. coli isolates from human clinical samples [2, 13, 19]. Recent studies in Turkey shows presence of this enzyme among ESBL producing E. coli isolates in cattle and retail meat [3, 37]. To a lesser extent, CTX-M-3 and CTX-M-1, were detected in nine and three isolates alone, also eight and 12 isolates in combination with other β-lactamase genes, respectively.
In this study, aac(6’)-Ib-cr was the most common PMQR gene, detected in 24 (25.3%) of ESBL/pAmpC producing E. coli isolates. Moreover, aac(6’)-Ib-cr was more frequently found in CTX-M-15 producing isolates (14/24, 58.3%), and to lesser extent, with other CTX-Ms including CTX-M-1 (4) and CTX-M-3 (6). Recently, Liu et al. [31] reported the frequent combination of blaCTX-M-15 and aac(6’)-Ib-cr in CTX-M producing E. coli from dogs and cats in United States. In this study, PMQR genes were also detected in nine ciprofloxacin susceptible isolates. Similar finding was also reported by Zhao et al. [55], who found that 21 of 39 qnr positive isolates were ciprofloxacin susceptible. Liao et al. [30] recently reported that the PMQR genes were not associated with selective pressure caused by quinolones, the presence of PMQR genes were more linked to cephalosporin use than quinolone use, suggesting that other resistance genes might have more effect on PMQR prevalence.
Emergence of virulent human pandemic O25b-ST131/B2 CTX-M-15 producing E. coli clone draws significant international attention as a major cause of human infections [42]. Recently, emergence of B2-O25b-ST131 clone producing CTX-M-15 and CTX-M-27 enzymes have been reported in canine clinical samples in Japan [21], in Portugal [40] and in U.K. [46], Similarly, Ewers et al. [17] reported presence of CXT-M-15 producing E. coli from dogs with urinary tract infections from different European countries (Netherland, Germany, Spain, Denmark and France) belonged to this clone. This is the first report of the pandemic O25b-ST131/B2 E. coli CTX-M-15 clone in commensal dog E. coli isolates in Turkey. PFGE analysis showed that the isolates had different profiles (Fig. 2), suggesting genomic diversity within this clone. This result is not adequate to assertain the source and direction of possible transmission. For that reason, to elucidate possible transmission route of this clone from human to companion animals or vice versa, genetic relatedness of human and animal isolates should be evaluated using more isolate in Turkey. However, considering the current findings, it is possible to say that dogs might play a role in spreading of this pandemic clone to community.
Trott [47] reported that ceftiofur use in veterinary clinical settings increased CMY-2 type AmpC β-lactamase selection both in Salmonella spp. and E. coli isolates. In this study, 23 (24.5%) isolates were found as pAmpC β-lactamase producer and all carried the blaCMY-2 gene, but the blaCMY-2 gene was almost found in combination with other β-lactamase genes, except an isolate. A similar result has been reported in the Republic of Korea by So et al. [44], who found the blaCMY-2 gene in 12 isolates in combination with the blaCTX-M genes and alone in three isolates. Although presence of CMY-2 has been previously reported in E. coli isolates in Turkey from cattle [3] and broilers (unpublished data), this is the first study that report on occurrence of blaCMY-2 carrying E. coli among dogs in Turkey.
ESBL and/or pAmpC producing bacteria have also been reported resistant to other classes of antibiotics rather than beta-lactams and treatment options for infections caused by these bacteria are very limited [22, 23, 39]. In this study, the isolates were also resistant to non-β-lactams in various rates. This could be explained by co-existence of ESBL and/or pAmpC genes with other resistance determinants on same plasmids [14, 51].
It is considered that virulent extra-intestinal E. coli (ExPEC) strains usually belonged to phylogenetic groups B2 and D in comparison with other phylogenetic groups. In contrast, commensal strains frequently belonged to phylogenetic groups A and B1. Therefore, phylogenetic grouping is used to determine potential pathogenic strains for screening purposes and to establish a link between virulence and phylogenetic groups [11]. In this study, the isolates carrying virulence genes belonged mainly to phylogenetic group A (17 isolates) and D (10 isolates), but to a lesser extent, B2 (six isolates) and B1 (one isolate). Similar results were also reported by Ben Sallem et al. [5] and Wagner et al. [48]. Johnson et al. [26] reported that ExPEC strains may take part in intestinal microbiota of healthy dogs without showing any clinical symptoms and may pose a risk for the transfer of these bacteria to humans. Wagner et al. [48] indicated that multidrug resistant (MDR) E. coli isolates from canine urinary tract infections have a reduced virulence genotype compared to susceptible ones.
Regarding with the distribution of ESBL/pAmpC strains according to phylogenetic groups, all phylogenetic groups were determined among isolates with various rates. However, most of the isolates belonged mainly to phylogenetic group A (41/95) and D (30/95), but to a lesser extent, B1 (15/95) and B2 (9/95). Our results are consistent with studies indicating that majority of ESBL/pAmpC producing isolates belonged to commensal phylogenetic groups A and B1 [43, 54].
In conclusion, this is the first study on the presence of ESBL and/or pAmpC producing E. coli strains among dogs in Turkey and distribution of genes encoding β-lactamase genes. High prevalence of blaCTX-M and blaCMY-2 genes emphasize the possibility of transfer of these resistant bacteria to humans. Companion animals due to their close contact with their owners should be monitored regularly to prevent the dissemination of resistant bacteria. Detailed molecular analyses are needed to reveal the genetic relationship of ESBL/pAmpC producing strains isolated from different sources including dogs and humans.
Acknowledgments
The study was funded by the Scientific and Technical Research Council of Turkey (TUBITAK, Project Number: TOVAG 113 O 843).
REFERENCES
- 1.Ahmed A. M., Motoi Y., Sato M., Maruyama A., Watanabe H., Fukumoto Y., Shimamoto T.2007. Zoo animals as reservoirs of gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl. Environ. Microbiol. 73: 6686–6690. doi: 10.1128/AEM.01054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altinkum S. M., Ergin S., Bahar H., Mamal Torun M.2013. CTX-M-15 type extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: A developing problem in infected outpatients and hospitalised patients in Istanbul, Turkey. Afr. J. Microbiol. Res. 7: 692–697. [Google Scholar]
- 3.Aslantaş Ö., Elmacıoğlu S., Yılmaz E. Ş.2016. Prevalence and characterization of ESBL- and AmpC-producing Escherichia coli from cattle. Kafkas Univ. Vet. Fak. Derg.. [Google Scholar]
- 4.Başaran Kahraman B., Diren Sığırcı B., Çelik B., Gümüş B., Metiner K., Adıgüzel M. C., Bağcıgil A. F., İkiz S., Özgür N. Y., Ak S.2016. Detection of extended-spectrum β-lactamase and AmpC β-lactamase producing Escherichia coli isolates from chickens. Kafkas Univ. Vet. Fak. Derg. 22: 591–596. [Google Scholar]
- 5.Ben Sallem R., Ben Slama K., Sáenz Y., Rojo-Bezares B., Estepa V., Jouini A., Gharsa H., Klibi N., Boudabous A., Torres C.2012. Prevalence and characterization of extended-spectrum beta-lactamase (ESBL)- and CMY-2-producing Escherichia coli isolates from healthy food-producing animals in Tunisia. Foodborne Pathog. Dis. 9: 1137–1142. doi: 10.1089/fpd.2012.1267 [DOI] [PubMed] [Google Scholar]
- 6.Bertin Y., Martin C., Oswald E., Girardeau J. P.1996. Rapid and specific detection of F17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex PCR. J. Clin. Microbiol. 34: 2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertin Y., Martin C., Girardeau J. P., Pohl P., Contrepois M.1998. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol. Lett. 162: 235–239. doi: 10.1111/j.1574-6968.1998.tb13004.x [DOI] [PubMed] [Google Scholar]
- 8.Brolund A., Sandegren L.2016. Characterization of ESBL disseminating plasmids. Infect. Dis. (Lond) 48: 18–25. doi: 10.3109/23744235.2015.1062536 [DOI] [PubMed] [Google Scholar]
- 9.Cavaco L. M., Hasman H., Xia S., Aarestrup F. M.2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 53: 603–608. doi: 10.1128/AAC.00997-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clermont O., Bonacorsi S., Bingen E.2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66: 4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clermont O., Dhanji H., Upton M., Gibreel T., Fox A., Boyd D., Mulvey M. R., Nordmann P., Ruppé E., Sarthou J. L., Frank T., Vimont S., Arlet G., Branger C., Woodford N., Denamur E.2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 64: 274–277. doi: 10.1093/jac/dkp194 [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standart Institute 2012. Performance standarts for antimicrobial susceptibility testing; twenty-second informational supplement; M100-S20. Wayne. [Google Scholar]
- 13.Copur Cicek A., Saral A., Ozad Duzgun A., Yasar E., Cizmeci Z., Ozlem Balci P., Sari F., Firat M., Altintop Y. A., Ak S., Caliskan A., Yildiz N., Sancaktar M., Esra Budak E., Erturk A., Birol Ozgumus O., Sandalli C.2013. Nationwide study of Escherichia coli producing extended-spectrum β-lactamases TEM, SHV and CTX-M in Turkey. J. Antibiot. 66: 647–650. doi: 10.1038/ja.2013.72 [DOI] [PubMed] [Google Scholar]
- 14.Coque T. M., Novais A., Carattoli A., Poirel L., Pitout J., Peixe L., Baquero F., Cantón R., Nordmann P.2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg. Infect. Dis. 14: 195–200. doi: 10.3201/eid1402.070350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durmaz R., Otlu B., Koksal F., Hosoglu S., Ozturk R., Ersoy Y., Aktas E., Gursoy N. C., Caliskan A.2009. The optimization of a rapid pulsed-field gel electrophoresis protocol for the typing of Acinetobacter baumannii, Escherichia coli and Klebsiella spp. Jpn. J. Infect. Dis. 62: 372–377. [PubMed] [Google Scholar]
- 16.Escobar-Páramo P., Grenet K., Le Menac’h A., Rode L., Salgado E., Amorin C., Gouriou S., Picard B., Rahimy M. C., Andremont A., Denamur E., Ruimy R.2004. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 70: 5698–5700. doi: 10.1128/AEM.70.9.5698-5700.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewers C., Grobbel M., Stamm I., Kopp P. A., Diehl I., Semmler T., Fruth A., Beutlich J., Guerra B., Wieler L. H., Guenther S.2010. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-beta-lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 65: 651–660. doi: 10.1093/jac/dkq004 [DOI] [PubMed] [Google Scholar]
- 18.Guardabassi L., Schwarz S., Lloyd D. H.2004. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 54: 321–332. doi: 10.1093/jac/dkh332 [DOI] [PubMed] [Google Scholar]
- 19.Gür D., Gülay Z., Akan O. A., Aktaş Z., Kayacan C. B., Cakici O., Eraç B., Gültekin M., Oğünç D., Söyletir G., Unal N., Uysal S.2008. [Resistance to newer beta-lactams and related ESBL types in gram-negative nosocomial isolates in Turkish hospitals: results of the multicentre HITIT study]. Mikrobiyol. Bul. 42: 537–544 (in Turkish). [PubMed] [Google Scholar]
- 20.Haenni M., Saras E., Métayer V., Médaille C., Madec J. Y.2014. High prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in France. Antimicrob. Agents Chemother. 58: 5358–5362. doi: 10.1128/AAC.02545-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada K., Nakai Y., Kataoka Y.2012. Mechanisms of resistance to cephalosporin and emergence of O25b-ST131 clone harboring CTX-M-27 β-lactamase in extraintestinal pathogenic Escherichia coli from dogs and cats in Japan. Microbiol. Immunol. 56: 480–485. doi: 10.1111/j.1348-0421.2012.00463.x [DOI] [PubMed] [Google Scholar]
- 22.Hawkey P. M., Jones A. M.2009. The changing epidemiology of resistance. J. Antimicrob. Chemother. 64Suppl 1: i3–i10. doi: 10.1093/jac/dkp256 [DOI] [PubMed] [Google Scholar]
- 23.Ho P. L., Chow K. H., Lai E. L., Lo W. U., Yeung M. K., Chan J., Chan P. Y., Yuen K. Y.2011. Extensive dissemination of CTX-M-producing Escherichia coli with multidrug resistance to ‘critically important’ antibiotics among food animals in Hong Kong, 2008-10. J. Antimicrob. Chemother. 66: 765–768. doi: 10.1093/jac/dkq539 [DOI] [PubMed] [Google Scholar]
- 24.Hordijk J., Schoormans A., Kwakernaak M., Duim B., Broens E., Dierikx C., Mevius D., Wagenaar J. A.2013. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front. Microbiol. 4: 242. doi: 10.3389/fmicb.2013.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby G. A.2009. AmpC beta-lactamases. Clin. Microbiol. Rev. 22: 161–182. doi: 10.1128/CMR.00036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J. R., Stell A. L., Delavari P.2001. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect. Immun. 69: 1306–1314. doi: 10.1128/IAI.69.3.1306-1314.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaipainen T., Pohjanvirta T., Shpigel N. Y., Shwimmer A., Pyörälä S., Pelkonen S.2002. Virulence factors of Escherichia coli isolated from bovine clinical mastitis. Vet. Microbiol. 85: 37–46. doi: 10.1016/S0378-1135(01)00483-7 [DOI] [PubMed] [Google Scholar]
- 28.Kim H. B., Park C. H., Kim C. J., Kim E. C., Jacoby G. A., Hooper D. C.2009. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 53: 639–645. doi: 10.1128/AAC.01051-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalioui L., Jouve M., Gounon P., Le Bouguenec C.1999. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 67: 5048–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao C. H., Hsueh P. R., Jacoby G. A., Hooper D. C.2013. Risk factors and clinical characteristics of patients with qnr-positive Klebsiella pneumoniae bacteraemia. J. Antimicrob. Chemother. 68: 2907–2914. doi: 10.1093/jac/dkt295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Thungrat K., Boothe D. M.2016. Occurrence of OXA-48 carbapenemase and other β-Lactamase genes in ESBL-producing multidrug resistant Escherichia coli from dogs and cats in the United States, 2009–2013. Front. Microbiol. 7: 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljungquist O., Ljungquist D., Myrenås M., Rydén C., Finn M., Bengtsson B.2016. Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs - a pilot study. Infect. Ecol. Epidemiol. 6: 31514–31520. doi: 10.3402/iee.v6.31514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto Y., Ikeda F., Kamimura T., Yokota Y., Mine Y.1988. Novel plasmid-mediated beta-lactamase from Escherichia coli that inactivates oxyimino-cephalosporins. Antimicrob. Agents Chemother. 32: 1243–1246. doi: 10.1128/AAC.32.8.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Keefe A., Hutton T. A., Schifferli D. M., Rankin S. C.2010. First detection of CTX-M and SHV extended-spectrum β-lactamases in Escherichia coli urinary tract isolates from dogs and cats in the United States. Antimicrob. Agents Chemother. 54: 3489–3492. doi: 10.1128/AAC.01701-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park C. H., Robicsek A., Jacoby G. A., Sahm D., Hooper D. C.2006. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50: 3953–3955. doi: 10.1128/AAC.00915-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson D. L., Bonomo R. A.2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18: 657–686. doi: 10.1128/CMR.18.4.657-686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pehlivanlar Önen S., Aslantaş Ö., Şebnem Yılmaz E., Kürekci C.2015. Prevalence of β-lactamase producing Escherichia coli from retail meat in Turkey. J. Food Sci. 80: M2023–M2029. doi: 10.1111/1750-3841.12984 [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Pérez F. J., Hanson N. D.2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40: 2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitout J. D., Laupland K. B.2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern Review. Lancet Infect. Dis. 8: 159–166. doi: 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 40.Pomba C., da Fonseca J. D., Baptista B. C., Correia J. D., Martínez-Martínez L.2009. Detection of the pandemic O25-ST131 human virulent Escherichia coli CTX-M-15-producing clone harboring the qnrB2 and aac(6′)-Ib-cr genes in a dog. Antimicrob. Agents Chemother. 53: 327–328. doi: 10.1128/AAC.00896-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha-Gracia R. C., Cortés-Cortés G., Lozano-Zarain P., Bello F., Martínez-Laguna Y., Torres C.2015. Faecal Escherichia coli isolates from healthy dogs harbour CTX-M-15 and CMY-2 β-lactamases. Vet. J. 203: 315–319. doi: 10.1016/j.tvjl.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 42.Rogers B. A., Sidjabat H. E., Paterson D. L.2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66: 1–14. doi: 10.1093/jac/dkq415 [DOI] [PubMed] [Google Scholar]
- 43.Schaufler K., Bethe A., Lübke-Becker A., Ewers C., Kohn B., Wieler L. H., Guenther S.2015. Putative connection between zoonotic multiresistant extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli in dog feces from a veterinary campus and clinical isolates from dogs. Infect. Ecol. Epidemiol. 5: 25334. doi: 10.3402/iee.v5.25334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.So J. H., Kim J., Bae I. K., Jeong S. H., Kim S. H., Lim S. K., Park Y. H., Lee K.2012. Dissemination of multidrug-resistant Escherichia coli in Korean veterinary hospitals. Diagn. Microbiol. Infect. Dis. 73: 195–199. doi: 10.1016/j.diagmicrobio.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 45.Sun Y., Zeng Z., Chen S., Ma J., He L., Liu Y., Deng Y., Lei T., Zhao J., Liu J. H.2010. High prevalence of bla(CTX-M) extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 16: 1475–1481. doi: 10.1111/j.1469-0691.2010.03127.x [DOI] [PubMed] [Google Scholar]
- 46.Timofte D., Maciuca I. E., Kemmett K., Wattret A., Williams N. J.2014. Detection of the human-pandemic Escherichia coli B2-O25b-ST131 in UK dogs. Vet. Rec. 174: 352–353. doi: 10.1136/vr.101893 [DOI] [PubMed] [Google Scholar]
- 47.Trott D.2013. β-lactam resistance in gram-negative pathogens isolated from animals Review. Curr. Pharm. Des. 19: 239–249. doi: 10.2174/138161213804070339 [DOI] [PubMed] [Google Scholar]
- 48.Wagner S., Gally D. L., Argyle S. A.2014. Multidrug-resistant Escherichia coli from canine urinary tract infections tend to have commensal phylotypes, lower prevalence of virulence determinants and ampC-replicons. Vet. Microbiol. 169: 171–178. doi: 10.1016/j.vetmic.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G., Clark C. G., Rodgers F. G.2002. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J. Clin. Microbiol. 40: 3613–3619. doi: 10.1128/JCM.40.10.3613-3619.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO 2012. World Health Organisation: critically important antimicrobials for human medicine. 3rd revision 2011, 31. Available: http://apps.who.int/iris/bitstream/10665/77376/1/ 9789241504485 _eng.pdf.
- 51.Wieler L. H., Ewers C., Guenther S., Walther B., Lübke-Becker A.2011. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinical samples. Int. J. Med. Microbiol. 301: 635–641. doi: 10.1016/j.ijmm.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz N. O., Agus N., Bozcal E., Oner O., Uzel A.2013. Detection of plasmid-mediated AmpC β-lactamase in Escherichia coli and Klebsiella pneumoniae. Indian J. Med. Microbiol. 31: 53–59. doi: 10.4103/0255-0857.108723 [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto S., Terai A., Yuri K., Kurazono H., Takeda Y., Yoshida O.1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12: 85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x [DOI] [PubMed] [Google Scholar]
- 54.Yousfi M., Mairi A., Touati A., Hassissene L., Brasme L., Guillard T., De Champs C.2016. Extended spectrum β-lactamase and plasmid mediated quinolone resistance in Escherichia coli fecal isolates from healthy companion animals in Algeria. J. Infect. Chemother. 22: 431–435. doi: 10.1016/j.jiac.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 55.Zhao X., Xu X., Zhu D., Ye X., Wang M.2010. Decreased quinolone susceptibility in high percentage of Enterobacter cloacae clinical isolates caused only by Qnr determinants. Diagn. Microbiol. Infect. Dis. 67: 110–113. doi: 10.1016/j.diagmicrobio.2009.12.018 [DOI] [PubMed] [Google Scholar]