Abstract
The origins of novel traits are often studied using species trees and modeling phenotypes as different states of the same character, an approach that cannot always distinguish multiple origins from fewer origins followed by reversals. We address this issue by studying the origins of C4 photosynthesis, an adaptation to warm and dry conditions, in the grass Alloteropsis. We dissect the C4 trait into its components, and show two independent origins of the C4 phenotype via different anatomical modifications, and the use of distinct sets of genes. Further, inference of enzyme adaptation suggests that one of the two groups encompasses two transitions to a full C4 state from a common ancestor with an intermediate phenotype that had some C4 anatomical and biochemical components. Molecular dating of C4 genes confirms the introgression of two key C4 components between species, while the inheritance of all others matches the species tree. The number of origins consequently varies among C4 components, a scenario that could not have been inferred from analyses of the species tree alone. Our results highlight the power of studying individual components of complex traits to reconstruct trajectories toward novel adaptations.
Keywords: Ancestral state, complex trait, co‐option, reticulate evolution, species tree
Inferences of transitions among character states along species phylogenies provide powerful tools to test specific hypotheses about the timing and rate of functional diversification, correlations among functional and ecological traits (e.g., Pagel 1999; Edwards et al. 2010; Danforth et al. 2013; Moreau and Bell 2013; McGuire et al. 2014; Halliday et al. 2016), and speciation rates (Rabosky et al. 2013; Cantalapiedra et al. 2017; Cooney et al. 2017). However, distinguishing between a single origin of a trait with subsequent losses versus multiple independent origins can be problematic (Whiting et al. 2002; Pagel 2004; Wiens et al. 2006; Gamble et. al. 2012), particularly when some character states affect the rates of speciation and/or extinction, when rates of transitions are high and asymmetrical, or variable among clades and through time (Maddison 2006; Goldberg and Igic 2008; Beaulieu et al. 2013; Igic and Busch 2013; King and Lee 2015). Indeed, transition rates might be higher in taxonomic groups possessing evolutionary precursors that increase the likelihood of evolving a specific trait (Blount et al. 2008, 2012; Marazzi et al. 2012; Christin et al. 2013a, 2015; Werner et al. 2014). This can lead to an unbalanced distribution of character states across the tree, with clusters forming in certain clades. However, a low rate of origins would lead to similar patterns if the rate of reversals is high (Wiens 1999; Danforth et al. 2003; Trueman et al. 2004; Pyron and Burbink 2014). Difficulties worsen if hybridization and introgression disconnect the history of underlying traits from the species tree (Pardo‐Diaz et al. 2012; Meier et al. 2017).
An alternative approach to analyzing the phenotypes as different character states is to decompose them into their constituent parts, then carefully analyze the evolution of each element independently to understand how the trait has been assembled or lost (Christin et al. 2010; Oliver et al. 2012; Niemiller et al. 2013; Kadereit et al. 2014). The use of distinct components can be interpreted as evidence for multiple origins, while reversals could leave a signature of the lost trait that can be detected when components are compared with those from species that never evolved it (Protas et al. 2006; Christin et al. 2010; Oliver et al. 2012; Niemiller et al. 2013). Identifying the mutations that underlie a trait further helps to distinguish shared origins and reversals (Igic et al. 2006; Shimizu et al. 2008; Niemiller et al. 2013; Meier et al. 2017). Evaluating the number of origins of each component of a complex trait would reconstruct the order of modifications that led to the trait of interest. This approach is applied here to the photosynthetic diversity exhibited within a five‐species taxonomic group.
C4 photosynthesis is a complex phenotype that improves the efficiency of carbon fixation in warm and dry conditions when compared to the ancestral C3 photosynthetic pathway (Sage et al. 2012; Atkinson et al. 2016). The C4 advantages are achieved by increasing the concentration of CO2 around Rubisco, the enzyme responsible for inorganic carbon fixation in the Calvin cycle of all photosynthetic organisms (von Caemmerer and Furbank 2003; Sage et al. 2012). To function, C4 photosynthesis requires the coordinated action of numerous anatomical and biochemical components that lead to the emergence of a novel biochemical pathway, usually across two types of cells; the mesophyll and bundle sheath cells (Hatch 1987; Prendergast et al. 1987; Gowik et al. 2011; GPWGII 2012; Bräutigam et al. 2014). Besides the increased expression of genes coopted for a C4 function, several other changes are known to occur during the evolution of C4 photosynthesis, including an expansion of bundle sheath tissue, a concentration of chloroplasts within it, and the adaptation of the enzymes to the new catalytic context (Fig. 1; Bläsing et al. 2000; von Caemmerer and Furbank 2003; McKown and Dengler 2007; Sage et al. 2012).
Figure 1.
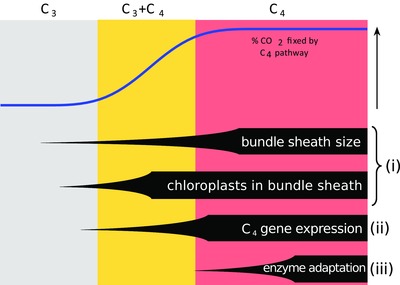
Schematic of expected changes during the transition from C3 to C4.
The continuous variation in anatomical and biochemical components can be simplified using three phenotypic categories; C3 plants, C3+C4 intermediates, and C4 plants. A schematic indicates the proportion of atmospheric CO2 fixed by the C4 cycle, and the expected order of modifications is shown at the bottom for four categories of changes, with the number on the right indicating the section of our analyses where they are investigated.
Despite its apparent complexity, C4 photosynthesis evolved multiple times independently, and is present in distantly related groups of plants (Sinha and Kellogg 1996; Kellogg 1999; Sage et al. 2011). As with any complex trait, C4 photosynthesis likely evolved in incremental steps, via stages that are functionally intermediate and gradually increase carbon assimilation in warm and dry conditions (Fig. 1; Sage et al. 2012; Heckmann et al. 2013; Williams et al. 2013; Mallmann et al. 2014; Christin and Osborne 2014). An increase in bundle sheath size and the relocation of the chloroplasts/Rubisco to these cells can sustain a photorespiratory bypass (Hylton et al. 1988; Bräutigam and Gowik 2016). Subsequent increases in C4 enzyme abundances can generate a weak C4 cycle, which assimilates some of the atmospheric CO2, complementing the C3 cycle in C3+C4 plants (referred to as "type II C3‐C4 intermediates" in the specialized literature; Fig. 1; Heckmann et al. 2013; Mallmann et al. 2014). The transition to a full C4 state involves further increases of the bundle sheath tissue and gene expression, while selective pressures adapt the C4 enzymes for the new biochemical context (Fig. 1; Bläsing et al. 2000; McKown and Dengler 2007).
In the angiosperm phylogeny, C4 taxa form clusters, many of which have multiple C4 clades that are separated by non‐C4 branches (Sage et al. 2011; GPWGII 2012). Thus, establishing past photosynthetic transitions is difficult when photosynthetic type is modeled as a simple binary character (Ibrahim et al. 2009; Christin et al. 2010; Hancock and Edwards 2014; Bohley et al. 2015; Fisher et al. 2015; Washburn et al. 2015). Overall, nonhomology of key C4 components among some closely related C4 groups, including the cells, enzymes, and genes modified to generate the C4 pathway (Prendergast et al. 1987; Soros and Dengler 2001; Bräutigam et al. 2014; Lundgren et al. 2014; Wang et al. 2014), points to a predominance of C4 origins (Sinha and Kellogg 1996; Christin and Besnard 2009; Christin et al. 2010). However, the possibility of evolutionary reversals to a non‐C4 state is still debated (e.g., Kadereit et al. 2014; Bohley et al. 2015; Washburn et al. 2015). Furthermore, some components of the C4 phenotype (e.g., expansion of bundle sheaths and migration of chloroplasts; Fig. 1) may have evolved relatively few times, and have then been recurrently used for independent transitions to C3+C4 or C4 photosynthesis (Christin et al. 2011, 2013a).
One of the proposed candidates for an evolutionary reversal from C4 to C3 is in the grass genus Alloteropsis (Ibrahim et al. 2009). Within this genus, the species Alloteropsis semialata contains C3, C3+C4, and C4 genotypes (Ellis 1974; Brown 1975; Lundgren et al. 2016). In molecular phylogenies based on either plastid or nuclear markers, this species is sister to the C4 Alloteropsis angusta, and the two species form a monophyletic clade sister to the three remaining closely‐related C4 species: Alloteropsis cimicina, A. paniculata, and A. papillosa (Ibrahim et al. 2009; Christin et al. 2012; Olofsson et al. 2016). The C4 A. semialata and A. cimicina use different cell types for the segregation of C4 reactions (Renvoize 1987), which suggests independent realizations of C4 photosynthesis (Christin et al. 2010). However, the evolutionary origins of C4 biochemistry and the situation within the A. angusta/A. semialata group remain largely unexplored.
In this study, we focus on the genus Alloteropsis and its C3 outgroup, to test the competing hypotheses of multiple origins versus fewer origins followed by reversals, independently for each C4 component. A C4 phenotype generated via distinct cells, genes, or amino acid mutations would indicate independent origins. In contrast, a reversal may lead to a derived state that retains traces of its past C4 state when compared to the ancestral one (i.e., approximated by the C3 outgroup here). We combined different approaches to investigate different components of the complex C4 trait. (i) Focusing on anatomical characters, we evaluate the most likely number of episodes of movement of chloroplasts to the bundle sheath, and expansion of this tissue. (ii) Using transcriptome analyses to estimate gene expression, we then determine the most likely number of origins of a C4 cycle via the upregulation of known C4 photosynthetic genes. (iii) The number of episodes of enzyme adaptation for the C4 cycle is estimated using positive selection analyses, with scenarios corresponding to episodes of adaptation along different sets of branches. (iv) Finally, we compare divergence times across genes, to detect potential introgression of C4 components, as suggested within this genus for two C4 genes (Olofsson et al. 2016). Our multifaceted effort highlights the power of comparative analyses that directly consider genes and other components involved in the trait of interest, rather than modeling complex phenotypes as states of a single character. Using this approach, we show that recurrent origins of C4 photosynthesis in Alloteropsis arose via a complex mixture of co‐option of traits increasing C4 accessibility, hybridization, and independent adaptation of the phenotype.
Methods
TAXON SAMPLING
The different datasets were obtained from plants grown under controlled conditions (See Supporting Information Methods 1.1 for detailed description of growth conditions), including one Alloteropsis cimicina (C4) accession, one A. paniculata (C4) accession, two A. angusta (C4) accessions, and up to 10 different A. semialata accessions collected from separate populations that encompass the global genetic and photosynthetic diversity of this species (one C3, two C3+C4 intermediates with a weak C4 cycle, and seven C4 accessions; Table S1; Lundgren et al. 2016). The over representation of C4 A. semialata accessions mirrors their natural abundance, with C4 accessions spread throughout Africa, Asia, and Australia, C3 accessions only reported in Southern Africa, and C3+C4 individuals restricted to central East Africa (Lundgren et al. 2015). We also make use of species representing the C3 sister group to Alloteropsis (Panicum pygmaeum and Entolasia marginata), previously identified using plastid markers (GPWGII 2012). Using the above taxa, we conduct four complementary sets of analyses, each providing insight into the origins or spread of distinct components of C4 in Alloteropsis.
(i) COMPARING LEAF ANATOMIES AMONG PHOTOSYNTHETIC TYPES
Leaf cross‐sections were analyzed to identify the leaf compartment being used for the segregation of Rubisco and the modifications that increased the proportion of bundle sheath tissue in C3+C4 and C4 accessions. Co‐option of different tissues and distinct modifications among accessions would support independent origins, while a reversal should result in the leaves of C3 individuals having reverted to a state that retain traces of their past C4 state when compared to the ancestral condition (e.g., enlarged bundle sheath cells and/or chloroplasts in the bundle sheath).
We generated new anatomical data for nine A. semialata accessions and A. angusta (Table S2), which supplemented previously published anatomical data for E. marginata, P. pygmaeum, A. cimicina, and A. paniculata (Christin et al. 2013a). Images of A. semialata and A. angusta leaves in cross‐section were obtained by fixing the center portion of a mature leaf blade in 4:1 ethanol:acetic acid, embedding them in methacrylate embedding resin (Technovit 7100, Heraeus Kulzer GmbH, Wehrhein, Germany), sectioning on a manual rotary microtome (Leica Biosystems, Newcastle, U.K.), staining with Toluidine Blue O (Sigma‐Aldrich, St. Louis, MO), then photographing them with a camera mounted atop a microscope (Olympus DP71 and BX51, respectively, Olympus, Hamburg, Germany), as described in Lundgren et al. (2016).
All species used in this study have two bundle sheath layers, differentiated as inner and outer bundle sheaths, which create concentric circles around each vein (Fig. S1). The sheath co‐opted for the segregation of Rubisco was identified by a concentration of chloroplasts producing starch. We also recorded the presence of minor veins, and measured the following traits on one cross‐sectional image per accession, as described in Christin et al. (2013a), using ImageJ software (Schneider et al. 2012): the interveinal distance (IVD; the average distance between centers of consecutive veins), the number of mediolateral mesophyll cells between veins, the average width of all outer and inner bundle sheath cells within a leaf segment, and the ratio of outer to inner bundle sheath cell widths (OS:IS). One leaf cross‐section was used per accession, with previous work showing the traits we are measuring exhibit little variation within populations (Lundgren et al. 2016).
(ii) COMPARING GENE EXPRESSION PROFILES AMONG PHOTOSYNTHETIC TYPES
We use RNA‐Seq to identify the genes co‐opted by the different accessions performing a C4 cycle, as those encoding C4‐related enzymes that reach high abundance in C4 leaves. Variation in the co‐opted loci would support multiple origins of a weak C4 cycle, while a reversal might lead to high expression of C4‐related genes in individuals without a C4 cycle or loss of functions of genes previously used for the C4 cycle.
For RNA‐Seq, we sampled the highly photosynthetically active distal halves of fully expanded new leaves and fresh roots midway into the photoperiod, which were subsequently flash frozen. Two different photoperiods (i.e., 10 and 14 h) were used to ensure that the identification of the most highly expressed genes did not differ among light regimes. Data from root libraries were only used in this study for transcriptome assembly, while all leaf samples were used for both assembly and quantification of transcript abundances. For a full list of individuals, conditions, and tissues sampled see Table S3.
Total RNA was extracted, Illumina TruSeq libraries generated, and sequencing performed using standard laboratory procedures, and transcriptomes were assembled using available pipelines (see Supporting Information Methods 1.2 for a detailed description of RNA‐Seq protocol and assembly statistics). For each assembled contig, the transcript abundance was calculated as reads per million of mapped reads (rpm). Using a previously developed phylogenetic annotation pipeline (Christin et al. 2013b, 2015), the transcript abundance was then calculated for each gene lineage encoding C4‐related enzymes. For each gene family, all sequences descending from a single gene in the common ancestor of grasses via speciation and/or duplication were considered as the same gene lineage (i.e., these are grass co‐orthologs). These groups include potential lineage specific paralogs (i.e., also known as inparalogs). When different Alloteropsis genes were identified within the same group of co‐orthologs through detailed phylogenetic analyses, the abundance of each group was estimated independently. In Alloteropsis, this is the case only for genes previously shown to have been acquired laterally from distantly related C4 lineages (Christin et al. 2012; see Results). In short, the reference datasets, composed of Arabidopsis thaliana coding sequences annotated as encoding C4‐related enzymes, and homolog sequences from other completely sequenced plants including five grasses, were retrieved from Christin et al. (2013b; 2015), or generated following the same approach for additional C4‐related enzymes identified in more recent studies (Mallmann et al. 2014; Li et al. 2015; Fig. S2). Contigs with similar sequences from the transcriptomes generated here were identified using BLASTn, with a minimal e‐value of 0.01, and a minimal matching length of 50 bp. Only the portion of the contig matching the references was considered to remove UTRs, potential introns, and other very variable segments. Each sequence retrieved this way was then aligned independently to the reference dataset using Muscle (Edgar 2004), and a phylogenetic tree was inferred using Phyml (Guindon and Gascuel 2003) with a GTR+G+I model, a model that fits the vast majority of genes (e.g., Fisher et al. 2016) and is appropriate to infer a large number of trees. Phylogenetic trees were automatically screened, and each contig was assigned to the previously identified gene lineages in which it was nested. The sum of rpm values of all transcriptome contigs assigned to the same gene lineage produced transcript abundance per group of grass co‐orthologs or distinct genes within these groups, which were subsequently transformed into rpm per kilobase (rpkm) values. Rpkm values were then compared among accessions to identify similarities and differences in the expression of C4 photosynthetic genes.
(iii) GENE TREES AND DETECTION OF ENZYME ADAPTATION FOR C4 PHOTOSYNTHESIS
Phylogenetic trees were inferred for C4‐related genes that were highly abundant in the leaf transcriptomes of at least two C4 Alloteropsis samples (identified from transcriptome data; see Results) and their co‐orthologs in other C3 and C4 grasses (see Supporting Information Methods 1.3 for a detailed description of phylogeny construction). The inferred gene trees were used to verify that C4‐related genes were placed as expected based on the species tree, as opposed to a position suggesting an acquisition from distant C4 relatives. In addition, the gene tree topologies were used for positive selection analyses to detect traces of past episodes of enzyme adaptation for the new catalytic context after the initial emergence of a C4 cycle (Fig. 1; Blasing et al. 2000; Christin et al. 2007; Besnard et al. 2009; Wang et al. 2009; Heckmann et al. 2013; Mallmann et al. 2014; Huang et al. 2017). Positive selection on branches leading to each C4 group would support independent transitions to a full C4 cycle via enzyme adaptation, while an early origin followed by a reversal should result in positive selection in the common ancestor of all C4 accessions and possibly in the lineages that reversed back to the previous state.
For each set of genes encoding core C4 enzymes in at least two Alloteropsis accessions, identified via transcriptome analyses, we optimized several codon models (site and branch‐site models) to test for adaptive evolution using codeml as implemented in PAML (Yang 2007). The best‐fit model was identified among those that assume (0) no positive selection (M1a null model), and the branch‐site models that assume shifts in selection pressure, either to relaxed selection (model BSA) or to positive section (model BSA1), at the base of: (1) Alloteropsis (one round of enzyme adaptation), (2) both A. cimicina and A. angusta + A. semialata (two rounds of enzyme adaptation), and (3) A. cimicina, A. angusta, and A. semialata (three independent episodes of enzyme adaptation). Foreground branches for all models were specified as the branch leading to the identified node plus all descending branches (i.e., using a "$" sign as opposed to a "#"). Models involving positive selection in only one of the C4 lineages were also considered (see Supporting Information Methods 1.3 for additional details of positive selection analysis). For each gene lineage, the best‐fit model was identified based on the corrected Akaike information criterion (AICc), selecting the model with the lowest AICc after checking that its ΔAICc score was at least 5.22 units below that of the M1a null model. An ΔAICc score = 5.22 corresponds to a P‐value threshold of 0.01 for a likelihood ratio test comparing these two models using 2 degrees of freedom (df). C4 species other than Alloteropsis were removed prior to analysis to avoid an influence of positive selection in these taxa affecting our conclusion. Analyses were repeated using only codons with fixed nucleotides within each lineage (i.e., A. angusta, C3 A. semialata, C3+C4 A. semialata, and C4 A. semialata), to verify that short terminal branches with unfixed mutations did not significantly inflate the dN/dS ratio, and therefore alter our conclusion. Finally, to assess the effect of gene tree topology on our conclusions, we repeated the positive selection analyses using 100 bootstrap pseudoreplicate topologies.
(iv) DATING THE DIVERGENCE OF ADAPTIVE LOCI TO IDENTIFY INTROGRESSION
To determine whether introgression has spread C4 adaptations among species, we performed molecular dating of markers from across the transcriptomes, including those used for C4 by at least two Alloteropsis accession and their paralogs. The divergence times between species estimated from introgressed genes are expected to be younger than those estimated from other genes (e.g., Smith & Kronforst 2013; Li et al. 2014; Marcussen et al. 2014; Li et al. 2016), resulting either in outliers (if few genes are introgressed) or a multimodal distribution of ages (if many genes are introgressed).
Groups of genes descending from a single gene in the common ancestor of Panicoideae (Panicoideae co‐orthologs), the grass subfamily that includes Alloteropsis, were identified through phylogenetic analyses of our transcriptomes and completely sequenced genomes that were publicly available. Our automated pipeline started with gene families previously inferred for eight plant genomes (homologs: i.e., all the paralogs and orthologs; Vilella et al. 2009), including two Panicoideae grasses (Setaria italica and Sorghum bicolor), two non‐Panicoideae grasses (Brachypodium distachyon and Oryza sativa), and four nongrass species (Amborella trichopoda, A. thaliana, Populus trichocarpa, and Selaginella moellendorffii). To ensure accurate annotation, we restricted the analysis to gene families that included at least one A. thaliana sequence. The coding sequences (CDS) from the above genomes were then used to identify similar sequences in our transcriptomes using BLASTn with a minimum alignment length of 500 bp.
Stringent alignment and filtering methods were used to ensure reliable alignments of the above sequences for each gene family for phylogenetic inference (see Supporting Information Methods 1.4 for full details). In total, 2,797 1:1 Panicoideae co‐ortholog datasets were used for subsequent molecular dating, as implemented in Beast version 1.5.4 (Drummond and Rambaut 2007). For each dataset, divergence times were estimated based on third codon positions, to decrease the risk of selective pressures biasing the outputs. A log‐normal relaxed clock was used, with a GTR+G+I substitution model, and a constant coalescent prior. The Sorghum sequence was selected as the outgroup and the root of the tree was fixed to 31 Ma (using a normal distribution with a SD of 0.0001), based on estimates from Christin et al. (2014). There is uncertainty around this date, and the low species sampling used here probably leads to overestimation of both divergence times and confidence intervals, but the use of consistent sampling and calibration points among markers allows for the comparison of relative (rather than absolute) ages, which is the point of these analyses. Each Beast analysis was run for 2,000,000 generations, sampling a tree every 1,000 generations after a burn‐in period of 1,000,000. For nodes of interest, divergence times were extracted from the posterior distribution as medians.
Divergence times were also estimated for key genes used for C4 photosynthesis in Alloteropsis (identified based on transcriptomes; see Results), using the same parameters. To guarantee a consistent species sampling, the taxa included in the transcriptome‐wide analyses were retrieved from manually curated alignments for C4‐specific genes as well as other groups of orthologs from the same gene families, obtained as described above for C4‐specific forms. In addition, plastid genomes for the same species were retrieved from Lundgren et al. (2015), and reanalyzed with the same parameters. For each of these datasets, the median, 95% CI, and 0.25 and 0.75 quantiles were extracted from the posterior distribution, using the R package APE (Paradis et al. 2004).
Results
(i) DIFFERENT REALIZATIONS of C4 LEAF ANATOMY IN A. CIMICINA AND A. SEMIALATA/A. angusta
Grasses ancestrally possess two concentric rings of bundle sheath cells and either can be co‐opted for C4 photosynthesis (Brown 1975; Lundgren et al. 2014). The closely related C4 A. cimicina and A. paniculata co‐opted the outer bundle sheath for Rubisco segregation, as evidenced by the proliferation of chloroplasts in this tissue (Fig. S1; Table S2). In these species, the overall proportion of outer bundle sheath tissue within the leaf is increased via enlarged outer bundle sheath cells. Indeed, the outer sheath is 7.8‐fold larger than the inner sheath in C4 A. cimicina and A. paniculata, compared to a 1.2‐ to 0.6‐fold differences in C4 A. semialata and A. angusta (Table S2). This contrasts strongly with the anatomy of the C4 A. semialata and A. angusta (Fig. S1). Both of these species use the inner bundle sheath for Rubisco segregation and increase the overall proportion of this tissue via the proliferation of minor veins, and enlargement of the inner sheath cell size (Fig. S1; Table S2).
Staining by Toluidine Blue O indicates some starch production occurs in the inner bundle sheaths of both the C3 and C3+C4 A. semialata (Fig. S1), which implies some Rubisco activity in these cells, confirming previous reports (Ueno and Sentoku 2006; Lundgren et al. 2016). The absence of minor veins in the C3 and C3+C4 A. semialata results in a larger proportion of mesophyll compared to C4 A. semialata (Table S2; Fig. S1). In the C3 and C3+C4 A. semialata, the outer bundle sheath is slightly larger than the inner one (1.2‐ to 1.8‐fold; Table S2), while the C3 outgroup species P. pygmaeum and E. marginata have outer bundle sheaths that are considerably larger than their small inner sheaths (4.5‐ and 5.3‐fold; Fig. S1; Table S2).
In summary, our comparative studies of leaf anatomy indicate that the C4 A. cimicina and A. semialata/A. angusta use different tissues for Rubisco segregation and achieve high bundle sheath proportions via distinct modifications, supporting independent origins of C4 anatomical components in these two groups. Some Rubisco activity is suggested in the inner sheath of the C3 A. semialata, which supports an early origin migration of chloroplasts to this tissue (Fig. 2). In addition, a slight enlargement of the inner sheath, absent in the C3 outgroup, is common to all non‐C4 A. semialata.
Figure 2.
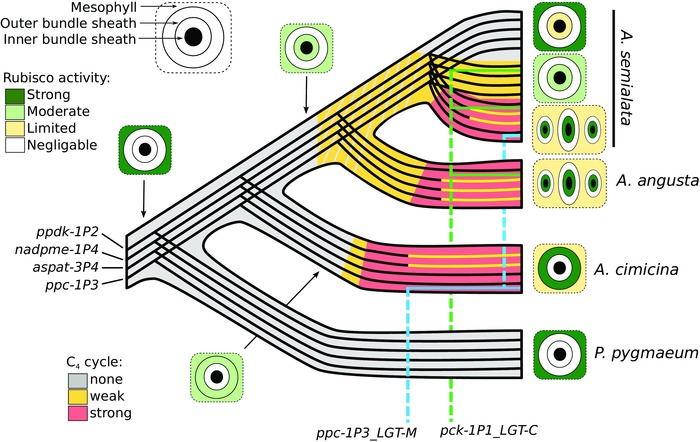
Inferred transitions among C4 components.
A schematic phylogenetic tree is presented, based on previous genome‐wide analyses (Lundgren et al. 2015; Olofsson et al. 2016). Individual lines represent the transmission of individual genes within the species complex. For each of the four genes subject to C4‐related selection, episodes of positive selection are indicated by changes to yellow. Other lines track the spread of two genes that were originally laterally‐acquired from distant relatives, and have subsequently been introgressed among Alloteropsis species. The inferred phenotype is represented by the background colour, in grey for C3, in yellow for C3+C4, and in red for C4. The grey hatching indicates uncertainty about the ancestral state. A simplified version of leaf anatomy is represented, for extant taxa and some hypothetical ancestors (see Fig. S1 for details of leaf anatomy of extant accessions).
(ii) A.CIMICINA USES DIFFERENT ENZYMES AND GENES FOR C4 BIOCHEMISTRY THAN A. SEMIALATA/A. ANGUSTA
All Alloteropsis C3+C4 and C4 accessions have high expression abundance in their leaves of co‐orthologs encoding phosphoenolpyruvate carboxylase (PEPC), the enzyme used for the initial fixation of atmospheric carbon into organic compounds in C4 plants. However, the gene lineage most highly expressed varies among accessions (Figs. 3 and 4). The close relationships between some of the genes for PEPC and one for phosphoenolpyruvate carboxykinase (PCK) isolated from Alloteropsis and those of distantly related C4 species was confirmed by our phylogenetic analyses (Figs. S3 and S4), supporting the previous conclusion that these genes were acquired by Alloteropsis via lateral gene transfer (LGT; Christin et al. 2012). Based on the read abundance, A. cimicina uses ppc‐1P3_LGT‐M, while A. angusta uses ppc‐1P3 (Fig. 4). There is variation within A. semialata, with C3+C4 and C4 accessions using either one or a combination of several gene lineages several gene lineages (Fig. 4).
Figure 3.
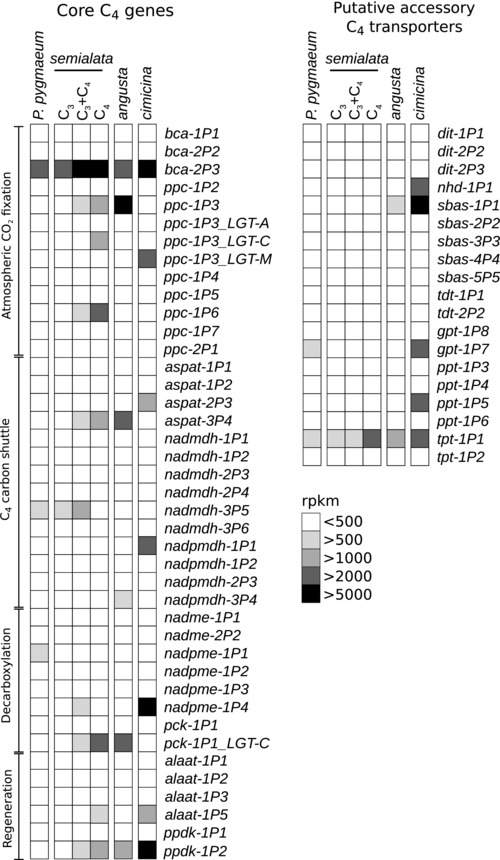
Expression of C4‐related enzymes in Alloteropsis.
For each gene encoding a C4‐related enzyme, the shade indicates the category of transcript abundance, using averages per group. For raw values, see Table S4. Note that ppc abundance varies among C4 accessions of A. semialata (Fig. 4). The enzymes involved in core C4 reactions (left column) are grouped by functional property, and gene names are written in italics on the right of the expression values.
Figure 4.
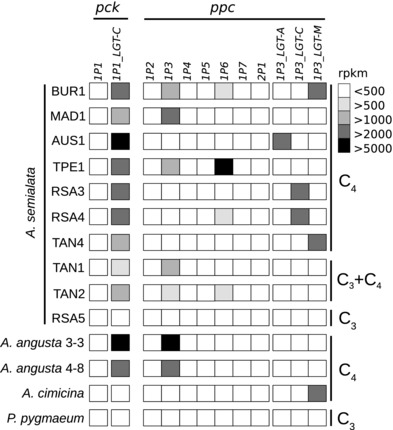
Leaf abundance of pck and ppc genes in the different accessions.
The shade indicates the relative expression (in rpkm) in the different accessions. For each accession, the averages are used. For raw values, see Table S4.
From the expression profiles (Fig. 3), the carbon shuttle of A. cimicina relies on enzymes and transporters associated with the most common form of C4 photosynthesis (NADP‐malic enzyme type; Gowik et al. 2011; Bräutigam et al. 2014; Mallman et al. 2014). This expression profile differs markedly from that observed in the C4 A. semialata and A. angusta accessions. These two species mainly use the PCK decarboxylating enzyme, through the high expression of the same gene (pck‐1P1_LGT‐C; Fig. 4). There is little evidence in these species for an involvement of the auxiliary transporters observed in A. cimicina (Fig. 3; Table S4), and some of the core enzymes are not shared by A. cimicina and A. semialata/A. angusta (Fig. 3). Furthermore, even when the same enzyme family is used, it is not necessarily encoded by the same locus (e.g., A. cimicina expresses aspat‐2P3 and A. semialata/A. angusta express aspat‐3P4; Fig. 3).
The transcriptomes of the C3+C4 A. semialata show elevated levels of some of the genes used by the C4 A. semialata, with a slightly higher abundance of those encoding the NADP‐malic enzyme (nadpme‐1P4; Fig. 3; Table S4). In terms of the expression levels of genes encoding C4‐related enzymes, the transcriptome of the C3 A. semialata is not markedly different from that of the C3 outgroup P. pygmaeum (Fig. 3; Table S4).
Our comparative transcriptomics therefore indicate that A. cimicina uses different genes and different enzymes for the C4 pathway than A. semialata/A. angusta, suggesting multiple origins of the C4 cycle (Fig. 2). The only C4‐related genes used by some C4 Alloteropsis that are abundant in the C3 A. semialata (bca‐2P3 and tpt‐1P1) are also highly expressed in the C3 outgroup and in other distantly related C3 taxa (Fig. 3; Külahoglu et al. 2014; Ding et al. 2015), indicating that high levels in leaves is not specific to our group of species. For the C4‐related genes used by the C4 Alloteropsis, but not abundant in the outgroup, there is no evidence for high expression or pseudogenization in the C3 A. semialata. Evidence is thus lacking that the C3 A. semialata represent a reversal from an ancestor with a C4 cycle.
(iii) INDEPENDENT EPISODES OF C4‐RELATED POSITIVE SELECTION IN EACH C4 SPECIES
The codon models do not support positive selection on any genes involved in C4 photosynthesis at the base of Alloteropsis or along the branch leading to the A. angusta/A. semialata group (Table 1). In two cases (nadpme‐1P4 and ppdk‐1P2), analyses including all Alloteropsis accessions clearly point to changes in selective pressures specifically in the branch leading to A. cimicina (Table 1; Fig. S5). No evidence of positive selection was found for the two other genes analyzed on the three Alloteropsis species (aspat‐2P3 and alaat‐1P5; Table 1). When testing for selection only in the A. angusta/A. semialata clade, no positive selection was found on ppdk‐1P2, while positive selection on ppc‐1P3 was identified only on the branch leading to A. angusta (Table 2). For the two other genes (nadpme‐1P4 and aspat‐3P4), the model that assumes positive selection after the split of the two species was favored (Table 2). A majority of the amino acid sites identified as under positive selection by the Bayes Empirical Bayes analysis overlapped with those previously identified in other C4 taxa (e.g., site 241 in nadpme‐1P4; Fig. 5; Christin et al. 2009), or were shared with other C4 species in our phylogenies (e.g., Fig. 5), supporting their link to C4 photosynthesis. For aspat‐3P4, more amino acid substitutions were fixed in A. angusta than in A. semialata. This variation among A. semialata C4 accessions indicates repeated bouts of positive selection during the diversification of this species (Fig. S6). Conclusions based on the selection tests were also supported using only codons with fixed nucleotides within a lineage (i.e., photosynthetic types in A. semialata, and A. angusta), with the exception of nadpme‐1P4 for which no positive selection was inferred after removing the unfixed codons (Tables S5 and S6). Furthermore, gene tree topology had no effect on our conclusions, since all bootstrap replicates supported the same model, with the exception of 2% of nadpme‐1P4 bootstrap replicates (Tables S7 and S8).
Table 1.
Results of positive selection analyses inferring the episodes of enzymatic adaptation in Alloteropsis 1
| One origin | Two origins | Three origins | Only A. cimicina | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Number of sequences | Site model M1a | BSA | BSA1 | BSA | BSA1 | BSA | BSA1 | BSA | BSA1 |
| aspat‐2P3 | 14 | 0.00* | 4.02 | 4.02 | 4.02 | 4.02 | 3.94 | 4.02 | 4.00 | 4.00 |
| nadpme‐1P4 | 15 | 35.07 | 30.44 | 27.26 | 26.34 | 24.28 | 19.52 | 13.31 | 3.34 | 0.00* |
| ppdk‐1P2 | 15 | 29.45 | 32.00 | 26.35 | 32.31 | 27.17 | 26.37 | 23.37 | 3.55 | 0.00* |
| alaat‐1P5 | 14 | 0.00* | 1.74 | 1.74 | 2.03 | 2.03 | 0.69 | 0.69 | 4.02 | 4.02 |
1The ΔAICc values compared to the best‐fit model for that gene are shown. The most appropriate model is indicated with an asterisk, with the null model (M1a) only rejected if the ΔAICc was at least 5.22 (equivalent to a P‐value of 0.01 with a likelihood ratio test with df = 2). Two branch‐site models were used to test for a relaxation of purifying selection (BSA), and potential positive selection (BSA1).
Table 2.
Results of positive selection analyses inferring the episodes of enzymatic adaption in the A. angusta/A. semialata clade1
| One origin | Two origins | Only A. angusta | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Number of sequences | Site model M1a | BSA | BSA1 | BSA | BSA1 | BSA | BSA1 |
| aspat‐3P4 | 13 | 12.33 | 10.20 | 6.70 | 6.37 | 0.00* | 5.45 | 5.29 |
| nadpme‐1P4 | 14 | 10.19 | 14.19 | 9.66 | 13.52 | 0.00* | 14.18 | 14.18 |
| ppc‐1P3 | 9 | 72.43 | 66.62 | 66.58 | 11.70 | 9.85 | 5.66 | 0.00* |
| ppdk‐1P2 | 14 | 0.00* | 4.01 | 4.01 | 4.01 | 4.01 | 3.91 | 3.91 |
1The ΔAICc values compared to the best‐fit model for that gene are shown. The most appropriate model is indicated with an asterisk, with the null model (M1a) only rejected if the ΔAICc was at least 5.22 (equivalent to a P‐value of 0.01, with a likelihood ratio test with df = 2). Two branch‐site models were used to test for a relaxation of purifying selection (BSA), and potential positive selection (BSA1).
Figure 5.
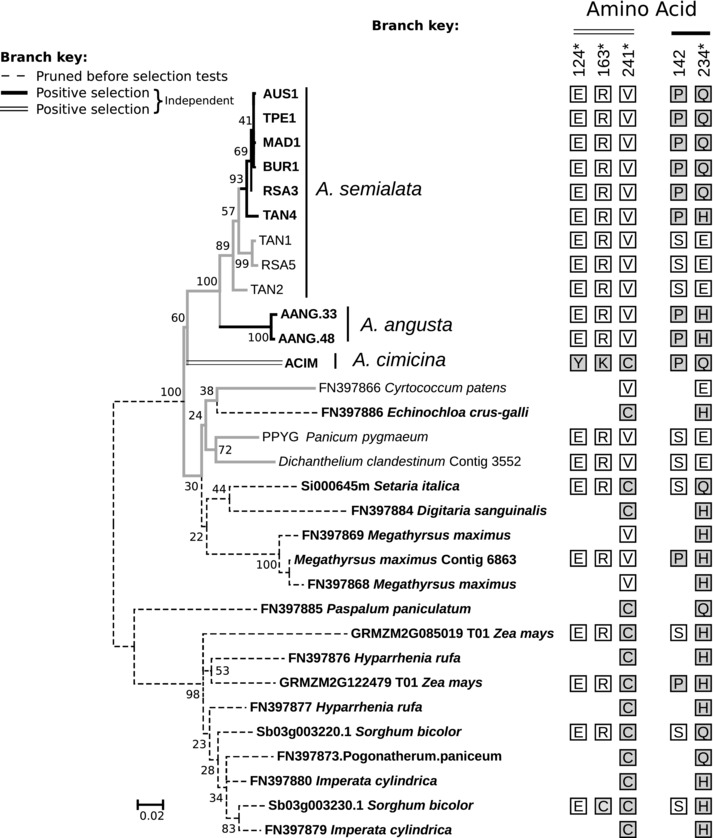
Evolution of nadpme‐1P4 genes in Alloteropsis and other Panicoideae.
This phylogenetic tree was inferred on 3rd positions of codons of nadpme‐1P4 genes of Panicoideae. Bootstrap values are indicated near branches. Names of C4 accessions are in bold. Amino acid at positions under positive selection are indicated on the right, with those associated with C4 accessions in gray. Positions are indicated on the top, based on Sorghum gene Sb03g003220.1. Amino acid positions with a posterior probability >0.90 of being under positive selection are indicated on the right, asterisks indicate positions with a posterior probability >0.95.
Overall, our positive selection tests point to independent episodes of enzyme adaptation for the C4 context in each of the C4 groups (Fig. 2). None of the models that included adaptive evolution on branches leading to C3 and/or C3+C4 A. semialata were favored, suggesting no evolutionary loss of a full C4 cycle.
(iv) GENES FOR PEPC AND PCK WERE SPREAD ACROSS SPECIES BOUNDARIES
The 2,797 groups of orthologs extracted from genomes and transcriptomes led to a wide range of estimated divergence times, with 95% of the medians falling between 6.51 and 17.92 Ma for the crown of Alloteropsis, and between 4.17 and 11.27 Ma for the split of A. semialata and A. angusta (Fig. 6). The peak of values (i.e., 50% of the points) ranged between 9.38 and 13.07 Ma for the crown of Alloteropsis and 5.93 and 8.18 Ma for the split of A. semialata and A. angusta (Fig. 6). Finally, 95% of the markers estimated the crown of A. semialata between 1.88 and 7.77 Ma, with a peak between 3.12 and 5.07 Ma (Fig. 6). Note that monophyly of the groups was not enforced, and various combinations of A. semialata accessions were included across markers, contributing to the observed variation.
Figure 6.
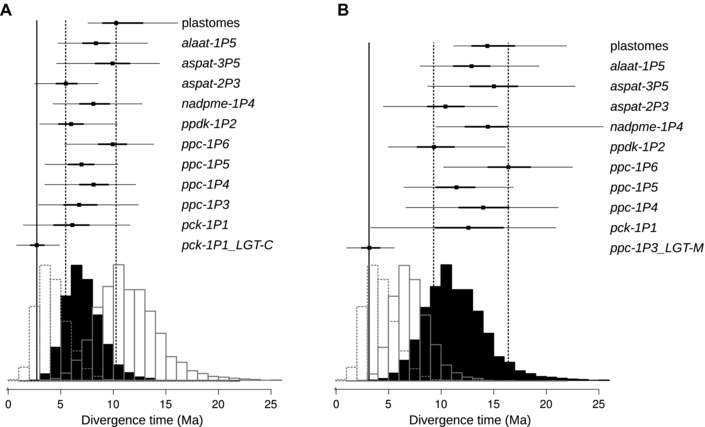
Estimates of divergence times.
On the top, divergence times are shown for selected nuclear genes and plastomes for A) the split of A. angusta and A. semialata and B) the crown of Alloteropsis. For each marker, the median of the estimates is indicated by a square, with thick bars connecting the 25 and 75 percentiles and thin bars connect the 2.5 and 97.5 percentiles. The distribution of medians for the crown of A. semialata (left), the split of A. angusta and A. semialata (middle), and the crown of Alloteropsis (right) over 2,797 markers extracted from the transcriptomes is shown at the bottom. The scale is given in million years ago (Ma).
Most of the C4‐related genes, as well as the plastomes, provided age estimates ranging from 5.54 to 10.32 Ma for the split of A. semialata and A. angusta, which matches the distribution of estimates from the transcriptome‐wide data (Fig. 6A), and indicates their transmission followed the species tree. The only exception is the gene pck‐1P1_LGT‐C, for which the last common ancestor of A. semialata and A. angusta was estimated at 2.77 Ma (Fig. 6A), which is smaller than all but four of the 2,797 estimates from the transcriptome‐wide markers. While the confidence intervals of the estimate for this gene do overlap with those of almost all other markers, this estimate matches more closely the diversification of A. semialata accessions (Fig. 6A).
The different markers selected for detailed analyses similarly yielded estimates for the crown of Alloteropsis matching those obtained from transcriptome‐wide data, between 9.38 and 16.46 Ma (Fig. 6B). The only exception is the gene ppc‐1P3_LGT‐M, for which the last common ancestor of A. cimicina and A. semialata is estimated at 3.25 Ma (Fig. 6B), which is smaller than all estimates based on markers extracted from the transcriptomes. The 95% CI of the divergence estimate based on this gene does not overlap with many of those based on other markers, and again matches closely with the diversification of A. semialata accessions (Fig. 6B).
Overall, our dating analyses support an introgression of these two genes among Alloteropsis species after their divergence, while the other genes were transmitted following the species tree (Fig. 2).
Discussion
TWO INDEPENDENT TRANSITIONS FROM C3 TO C4
The earliest split in Alloteropsis separates the lineage containing A. cimicina from A. angusta and A. semialata (Fig. 2). These two lineages co‐opted different tissues for the segregation of Rubisco activity and achieved a large proportion of bundle sheath tissue via different modifications (Fig. S1). The evidence therefore strongly supports two independent origins of C4 anatomical properties, which is generally accepted as the first step during the C3 to C4 transition (Fig. 1; Sage et al. 2012; Heckmann et al. 2013). Gene expression analyses show that the two clades use different enzymes for parts of the C4 cycle, express different genes encoding the same enzyme family when there is an overlap (Fig. 3), and positive selection analyses show that the enzymes were independently adapted for their C4 function (Table 1). We therefore conclude that the different transitions to C4 biochemistry occurred independently after the split of these two lineages (Fig. 2). The only exception to the distinctiveness of A. cimicina and the two other C4 species is the gene ppc‐1P3_LGT‐M, used by both A. cimicina and some C4 A. semialata accessions (Fig. 4). This gene is absent from other accessions (Olofsson et al. 2016) and, as such, we previously concluded that it was acquired early during the diversification of the group and then recurrently lost (Christin et al. 2012). This hypothesis is falsified by our dating analyses here, which show that this gene was only recently transferred among species boundaries, likely as a result of a rare hybridization event (Fig. 6).
ONE INDEPENDENT C3 TO C4 TRANSITION INCLUDES TWO SEPARATE C3+C4 TO C4 SHIFTS
The C4 phenotype is realized in A. angusta and A. semialata via identical anatomical modifications, using the same enzymes, and the same genes encode these enzymes. Chloroplasts are present in the inner sheaths of all A. semialata and A. angusta accessions, independent of their photosynthetic type, which suggests that this characteristic represents the ancestral condition for the clade (Fig. 2). The C4 cycle is realized using the same set of genes in A. angusta and A. semialata, which can be explained by convergent evolution (e.g., as indicated for other C4 grasses; Christin et al. 2013b) or a single origin of a weak C4 cycle (C3+C4), followed by a reversal to expression levels that resemble the ancestral condition in the C3 accessions (Fig. 2). Differentiating these two scenarios would require retracing the origin of the mutations responsible for the increased expression of C4 enzymes to identify where they occurred on the phylogeny. Unfortunately, the molecular mechanisms controlling C4 gene expression are poorly known, and can involve both cis‐ and transacting elements (Gowik et al. 2004; Brown et al. 2011; Williams et al. 2016).
The positive selection analyses indicate that enzyme adaptation happened independently in A. angusta and A. semialata (Table 2). Together with the variation observed within the C4 A. semialata (Fig. 4, S6), this evidence strongly suggests that the biochemical adaptation allowing the transition to a full C4 cycle happened recently, and independently in the two species (Fig. 2). The dramatic increase in the proportion of the inner bundle sheath tissue via the proliferation of minor veins is limited to the C4 A. semialata and A. angusta (Fig. S1). The genetic control of these features is unknown, preventing a comparison of the causal mutations. However, the distribution of anatomical characters among grasses indicates that the vast majority of C4 lineages that co‐opted the inner bundle sheath increased its proportion via the addition of minor veins (Renvoize 1987; Christin et al. 2013a).
With the current state of knowledge, we hypothesize that the common ancestor of A. semialata and A. angusta had chloroplasts in the inner bundle sheath, and that this facilitated the emergence of a weak C4 cycle via the upregulation of some enzymes. Following their split, A. angusta strengthened its C4 anatomy via the proliferation of minor veins, and enzyme adaptations led to a strong C4 cycle (Fig. 2). In the A. semialata lineage, some isolated populations acquired mutations that added minor veins and adapted the enzymes, leading to a C4 cycle. Other populations, potentially under pressures linked to the colonization of colder environments (Lundgren et al. 2015), might have lost the weak C4 cycle by downregulating the genes (Fig. 2). However, the details of the changes leading to C3 photosynthesis in some A. semialata will need to be confirmed by comparative genomics, when mutations regulating expression of C4 enzymes and anatomy are identified.
INTROGRESSION OF C4 COMPONENTS AMONG SPECIES
Our dating analyses suggest that the gene pck‐1P1_LGT‐C that encodes the decarboxylating enzyme PCK was introgressed among some members of A. semialata and A. angusta (Figs. 2 and 6). The C4 cycle carried out before this event was likely based on NADP‐malic enzyme, an enzyme still abundant in the C3+C4 A. semialata and some C4 accessions (Fig. 4; Frean et al. 1983). The acquisition of pck‐1P1_LGT‐C, a gene already adapted for the C4 context, probably added a PCK shuttle, which alters the stoichiometry of the pathway and the spatial distribution of its energy requirements, increasing its efficiency under some conditions (Bellasio and Griffiths 2014; Wang et al. 2014). This important component of the C4 cycles of extant A. semialata and A. angusta populations first evolved its C4‐specific properties in the distantly related Cenchrus (Fig. S3; Christin et al. 2012), and therefore never evolved within Alloteropsis. Instead, it represents the spread of a component of a complex physiology across multiple species boundaries. Therefore, in addition to the possibility that the sequential steps generating a complex physiology can happen on different branches of a species phylogeny (Fig. 2), introgression among close relatives can disconnect the origins of key components from the species tree.
ON THE INFERENCE OF TRANSITIONS AMONG CHARACTER STATES
Inferences of transitions among character states are a key component of numerous macroevolutionary studies (e.g., Cantalapiedra et al. 2017; Cooney et al. 2017). However, species trees per se are not always able to disentangle the complex scenarios underlying the appearance or losses of multicomponent adaptations, especially when complex phenotypes are modeled as different states of a single character (e.g., Goldberg and Igic 2008; Pardo‐Diaz et al. 2012; Niemiller et al. 2013; Igic and Busch 2013; King and Lee 2015). In the case of photosynthetic transitions within Alloteropsis depicted here, considering the photosynthetic type as a binary character would lead to a single C4 origin as the most plausible scenario (Ibrahim et al. 2009), and modeling photosynthetic types based on their category of C4 cycle does not improve the inference (Washburn et al. 2015). For traits assumed to evolve via sequential stages, the accepted sequence of changes can be incorporated in the model (e.g., Marazzi et al. 2012). However, the power of character modeling remains inherently limited by the small number of informative characters. Decomposing the phenotype into its components can solve this problem, especially when the underlying genetic determinism is considered (Oliver et al. 2012; Niemiller et al. 2013; Glover et al. 2015; Meier et al. 2017), and good mechanistic models exist for the evolution of DNA sequences (Liberles et al. 2013). Violation of model assumptions can still mislead the conclusions, but the multiplication of sources of information, coupled with the possibility to track the history of specific genes independently of the species tree, limits the risks of systematic errors. We therefore suggest that efforts to reconstruct the transitions leading to important traits should integrate as many underlying components as possible. As progresses in genome biology increase data availability and improve our understanding of causal mutations, modeling phenotypes as the results of cumulative changes in genomes will be able to solve the problems raised by the paucity of informative characters.
Conclusions
In this study, we dissect the genetic and anatomical components of C4 photosynthesis in Alloteropsis, a genus of grasses with multiple photosynthetic types. Our comparative efforts strongly support at least two independent origins of C4 photosynthesis within this genus. The C4 phenotype within these separate origins is realized via divergent anatomical modifications, the upregulation of distinct sets of genes, and independent enzyme adaptations. One of these lineages includes a range of photosynthetic types, and based on our analyses, we suggest that some C4 components in this group evolved in the shared common ancestor, while others were acquired independently after the lineages diverged. The history of photosynthetic transitions within Alloteropsis is furthermore complicated by the introgression of C4 genes across species boundaries. This disconnects the spread of C4 components from the species tree, and means that the number of origins varies among the different components of the complex C4 trait. This scenario is unlikely to have been inferred from traditional macroevolutionary approaches based on species trees alone. We suggest that integrating genomic data and phenotypic details in future studies of character transitions might resolve similarly complicated scenarios in other groups, enabling a better understanding of the trajectories followed during the evolution of novel adaptations.
Supporting information
Figure S1. Comparisons of leaf anatomy in Alloteropsis and relatives.
Figure S2. Phylogenetic trees for genes encoding three C4‐related enzymes.
Figure S3. Phylogeny of pck‐1P1 genes in Panicoideae.
Figure S4. Evolution of ppc‐1P3 genes in Alloteropsis and other Panicoideae.
Figure S5. Evolution of ppdk‐1P2 genes in Alloteropsis and other Panicoideae.
Figure S6. Evolution of aspat‐3P4 genes in Alloteropsis and other Panicoideae.
Table S1. Alloteropsis semialata accessions used in this study.
Table S2. Leaf anatomical data for the study species and accessions.
Table S3. RNA‐Seq data, NCBI SRA accession numbers, and growth conditions.
Table S4. Transcript abundance (in rpkm) for each C4‐related gene and sample.
Table S5. Results of positive selection analyses inferring the episodes of enzymatic adaptation in Alloteropsis using only fixed differences.
Table S6. Results of positive selection analyses inferring the episodes of enzymatic adaptation in the A. angusta/A. semialata clade using only fixed differences.
Methods 1.1. Plant growth conditions.
Methods 1.2. RNA‐Seq protocol.
Methods 1.3. Positive selection analysis.
Methods 1.4. Alignment and filtering
Supplementary Material
AUTHOR CONTRIBUTIONS
PAC, CPO, PN, and EJE designed the study, MRL, MN, and PAC secured plant material, MRL generated the anatomical data, JJMV generated the transcriptome data, LTD, MRL, JJMV, and PAC analysed the data, LTD and PAC wrote the paper with the help of all authors.
ACKNOWLEDGMENTS
This work was funded by a Royal Society Research Grant (grant number RG130448) to PAC. PAC and PN are supported Royal Society University Research Fellowships (grant numbers URF120119 and URF130423, respectively), LTD is supported by a NERC grant (grant number NE/M00208X/1), and MRL is supported by an ERC grant (grant number ERC‐2014‐STG‐638333).
DATA ARCHIVING
All raw RNA‐Seq data have been deposited in the NCBI Sequence Read Archive (project identifier SRP072730), and transcriptome assemblies are deposited in the NCBI Transcriptome Shotgun Assembly repository (Bioproject PRJNA310121).
LITERATURE CITED
Associate Editor: M. Streisfeld
Handling Editor: P. Tiffin
- Atkinson, R. R. , Mockford E. J., Bennett C., Christin P. A., Spriggs E. L., Freckleton R. P., Thompson K., Rees M., and Osborne C. P.. 2016. C4 photosynthesis boosts growth by altering physiology, allocation and size. Nat. Plants 2:16038. [DOI] [PubMed] [Google Scholar]
- Beaulieu, J. M. , O'Meara B. C., and Donoghue M. J.. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst. Biol. 62:725–737. [DOI] [PubMed] [Google Scholar]
- Bellasio, C. , and Griffiths H.. 2014. The operation of two decarboxylases, transamination, and partitioning of C4 metabolic processes between mesophyll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiol. 164:466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard, G. , Muasya A. M., Russier F., Roalson E. H., Salamin N., and Christin P. A.. 2009. Phylogenomics of C4 photosynthesis in sedges (Cyperaceae): multiple appearances and genetic convergence. Mol. Biol. Evol. 26:1909–1919. [DOI] [PubMed] [Google Scholar]
- Bläsing, O. E. , Westhoff P., and Svensson P. 2000. Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria, a conserved Serine residue in the carboxyl‐terminal part of the enzyme is a major determinant for C4‐specific characteristics. J. Biol. Chem. 275:27917–27923. [DOI] [PubMed] [Google Scholar]
- Blount, Z. D. , Borland C. Z., and Lenski R. E.. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli . Proc. Natl. Acad. Sci. U. S. A. 105:7899–7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount, Z. D. , Barrick J. E., Davidson C. J., and Lenski R. E.. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489:13–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohley, K. , Joos O., Hartmann H., Sage R. F., Liede‐Schumann S., and Kadereit G.. 2015. Phylogeny of Sesuvioideae (Aizoaceae)–Biogeography, leaf anatomy and the evolution of C4 photosynthesis. Perspect. Plant Ecol. Evol. Syst. 17:116–130. [Google Scholar]
- Bräutigam, A. , and Gowik U.. 2016. Photorespiration connects C3 and C4 photosynthesis. J. Exp. Bot. 67:2953–2962. [DOI] [PubMed] [Google Scholar]
- Bräutigam, A. , Schliesky S., Külahoglu C., Osborne C. P., and Weber A. P.. 2014. Towards an integrative model of C4 photosynthetic subtypes: insights from comparative transcriptome analysis of NAD‐ME, NADP‐ME, and PEP‐CK C4 species. J. Exp. Bot. 65:3579–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, W. V. 1975. Variations in anatomy, associations, and origins of Kranz tissue. Am. J. Bot. 62:395–402. [Google Scholar]
- Brown, N. J. , Newell C. A., Stanley S., Chen J. E., Perrin A. J., Kajala K., and Hibberd J. M.. 2011. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331:1436–1439. [DOI] [PubMed] [Google Scholar]
- Cantalapiedra, J. L. , Prado J. L., Fernández M. H., and Alberdi M. T.. 2017. Decoupled ecomorphological evolution and diversification in Neogene‐Quaternary horses. Science 355:627–630. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , and Besnard G.. 2009. Two independent C4 origins in Aristidoideae (Poaceae) revealed by the recruitment of distinct phosphoenolpyruvate carboxylase genes. Am. J. Bot. 96:2234–2239. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. and Osborne C. P.. 2014. The evolutionary ecology of C4 plants. New Phytol. 204:765–781. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Salamin N., Savolainen V., Duvall M. R., and Besnard G.. 2007. C4 photosynthesis evolved in grasses via parallel adaptive genetic changes. Curr. Biol. 17:1241–1247. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Samaritani E., Petitpierre B., Salamin N., and Besnard G.. 2009. Evolutionary insights on C4 photosynthetic subtypes in grasses from genomics and phylogenetics. Genome Biol. Evol. 1:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin, P. A. , Freckleton R. P., and Osborne C. P.. 2010. Can phylogenetics identify C4 origins and reversals? Trends Ecol. Evol. 25:403–409. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Sage T. L., Edwards E. J., Ogburn R. M., Khoshravesh R., and Sage R. F.. 2011. Complex evolutionary transitions and the significance of C3–C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65:643–660. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Edwards E. J., Besnard G., Boxall S. F., Gregory R., Kellogg E. A., Hartwell J., and Osborne C. P.. 2012. Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Curr. Biol. 22:445–449. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Osborne C. P., Chatelet D. A., Columbus J. T., Besnard G., Hodkinson T. R., Garrison L. M., Vorontsova M. S., and Edwards E. J.. 2013a. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc. Natl. Acad. Sci. U. S. A. 110:1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin, P. A. , Boxall S. F., Gregory R., Edwards E. J., Hartwell J., and Osborne C. P.. 2013b. Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biol. Evol. 5:2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin, P. A. , Spriggs E., Osborne C. P., Strömberg C. A., Salamin N., and Edwards E. J.. 2014. Molecular dating, evolutionary rates, and the age of the grasses. Syst. Biol. 63:153–165. [DOI] [PubMed] [Google Scholar]
- Christin, P. A. , Arakaki M., Osborne C. P., and Edwards E. J.. 2015. Genetic enablers underlying the clustered evolutionary origins of C4 photosynthesis in angiosperms. Mol. Biol. Evol. 32:846–858. [DOI] [PubMed] [Google Scholar]
- Cooney, C. R. , Bright J. A., Capp E. J., Chira A. M., Hughes E. C., Moody C. J., Nouri L. O., Varley Z. K. and Thomas G. H.. 2017. Mega‐evolutionary dynamics of the adaptive radiation of birds. Nature 542:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth, B. N. , Conway L., and Ji S.. 2003. Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Syst. Biol. 52:23–36. [DOI] [PubMed] [Google Scholar]
- Danforth, B. N. , Cardinal S., Praz C., Almeida E. A. B., and Michez D.. 2013. The impact of molecular data on our understanding of bee phylogeny and evolution. Annu. Rev. Entomol. 58:57–78. [DOI] [PubMed] [Google Scholar]
- Ding, Z. , Weissmann S., Wang M., Du B., Huang L., Wang L., Tu X., Zhong S., Myers C., Brutnell T. P., et al. 2015. Identification of photosynthesis‐associated C4 candidate genes through comparative leaf gradient transcriptome in multiple lineages of C3 and C4 species. Plos One 10:e0140629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, A. J. and Rambaut A.. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, E. J. , Osborne C. P., Strömberg C. A. E., Smith S. A., and C4 Grasses Consortium . 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328:587–591 [DOI] [PubMed] [Google Scholar]
- Ellis, R. P. 1974. The significance of the occurrence of both Kranz and non‐Kranz leaf anatomy in the grass species Alloteropsis semialata. S. Afr. J. Sci. 70:169–173. [Google Scholar]
- Fisher, A. E. , McDade L. A., Kiel C. A., Khoshravesh R., Johnson M. A., Stata M., Sage T. L., and Sage R. F.. 2015. Evolutionary history of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium . Int. J. Plant Sci. 176:770–790. [Google Scholar]
- Fisher, A. E. , Hasenstab K. M., Bell H. L., Blaine E., Ingram A. L., and Columbus J. T.. 2016. Evolutionary history of chloridoid grasses estimated from 122 nuclear loci. Mol. Phylogenet. Evol. 105:1–14. [DOI] [PubMed] [Google Scholar]
- Frean, M. L. , Ariovich D., and Cresswell C. F.. 1983. C3 and C4 photosynthetic and anatomical forms of Alloteropsis semialata (R. Br.) Hitchcock 2. A comparative investigation of leaf ultrastructure and distribution of Chlorenchyma in the two forms. Ann. Bot. 51:811–821. [Google Scholar]
- Gamble, T. , Greenbaum E., Jackman T. R., Russell A. P., and Bauer A. M.. 2012. Repeated origin and loss of adhesive toepads in Geckos. PLoS One 7:e39429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, B. J. , Airoldi C. A., Brockington S. F., Fernandez‐Mazuecos M., Martinez‐Perez C., Mellers G., Moyroud E., and Taylor L.. 2015. How have advances in comparative floral development influenced our understanding of floral evolution? Int. J. Plant Sci. 176:307–323. [Google Scholar]
- Goldberg, E. E. , and Igić B.. 2008. On phylogenetic tests of irreversible evolution. Evolution 62:2727–2741. [DOI] [PubMed] [Google Scholar]
- Gowik, U. , Burscheidt J., Akyildiz M., Schlue U., Koczor M., Streubel M., and Westhoff P.. 2004. cis‐Regulatory elements for mesophyll‐specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell 16:1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik, U. , Bräutigam A., Weber K. L., Weber A. P. M., and Westhoff P.. 2011. Evolution of C4 photosynthesis in the genus Flaveria: how many and which genes does it take to make C4 ? Plant Cell 23:2087–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II . 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 193:304–312. [DOI] [PubMed] [Google Scholar]
- Guindon, S. , and Gascuel O.. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704. [DOI] [PubMed] [Google Scholar]
- Halliday, T. J. D. , Upchurch P., and Goswami A.. 2016. Eutherians experienced elevated evolutionary rates in the immediate aftermath of the Cretaceous–Palaeogene mass extinction. Proc. R. Soc. B 283:20153026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, L. , and Edwards E. J., 2014. Phylogeny and the inference of evolutionary trajectories. J. Exp. Bot. 65:3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch, M. D. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta 895:81–106. [Google Scholar]
- Heckmann, D. , Schulze S., Denton A., Gowik U., Westhoff P., Weber A. P. M., and Lercher M. J.. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153:1579–1588. [DOI] [PubMed] [Google Scholar]
- Huang, P. , Studer A. J., Schnable J. C., Kellogg E. A., and Brutnell T. P.. 2017. Cross species selection scans identify components of C4 photosynthesis in the grasses. J. Exp. Botany 68:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton, C. M. , Rawsthorne S., Smith A. M., Jones D. A., and Woolhouse H. W.. 1988. Glycine decarboxylase is confined to the bundle‐sheath cells of leaves of C3‐C4 intermediate species. Planta 175:452–459. [DOI] [PubMed] [Google Scholar]
- Ibrahim, D. G. , Burke T., Ripley B. S., and Osborne C. P. 2009. A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion from C4 to C3 photosynthesis. Ann. Bot. 103:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic, B. , and Busch J. W.. 2013. Is self‐fertilization an evolutionary dead end? New Phytol. 198:386–397. [DOI] [PubMed] [Google Scholar]
- Igic, B. , Bohs L., and Kohn J. R.. 2006. Ancient polymorphism reveals unidirectional breeding system shifts. Proc. Natl. Acad. Sci. U. S. A. 103:1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit, G. , Lauterbach M., Pirie M. D., Rami A., and Helmut F.. 2014. When do different C4 leaf anatomies indicate independent C4 origins? Parallel evolution of C4 leaf types in Camphorosmeae (Chenopodiaceae). J. Exp. Bot. 65:3499–3511. [DOI] [PubMed] [Google Scholar]
- Kellogg, E. A. 1999. Phylogenetic aspects of the evolution of C4 photosynthesis Pp. 411–444 in Sage R. F. and Monson R. K., eds. C4 plant biology. Academic Press, San Diego, CA: [Google Scholar]
- King, B. , and Lee M. S. Y.. 2015. Ancestral state reconstruction, rate heterogeneity, and the evolution of reptile viviparity. Syst. Biol. 64:532–544. [DOI] [PubMed] [Google Scholar]
- Külahoglu, C. , Denton A. K., Sommer M., Mass J., Schliesky S., Wrobel T. J., Berckmans B., Gongora‐Castillo E., Buell C. R., Simon R., et al. 2014. Comparative transcriptome atlases reveal altered gene expression modules between two Cleomaceae C3 and C4 plant species. Plant Cell 26:3243–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. W. , Villarreal J. C., Kelly S., Rothfels C. J., Melkonian M., Frangedakis E., Ruhsam M., Sigel E. M., Der J. P., Pittermann J., and Burge D. O.. 2014. Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. PNAS 111:6672–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Davis B. W., Eizirik E., and Murphy W. J.. 2016. Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae). Genome Res. 26:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Ma X., Zhao J., Xu J., Shi J., Zhu X. G., Zhao Y., and Zhang H.. 2015. Developmental genetic mechanisms of C4 syndrome based on transcriptome analysis of C3 cotyledons and C4 assimilating shoots in Haloxylon ammodendron . Plos One 10:e0117175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles, D. A. , Teufel A. I., Liu L., and Stadler T.. 2013. On the need for mechanistic models in computational genomics and metagenomics. Genome Biol. Evol. 5:2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, M. R. , Osborne C. P., and Christin P. A.. 2014. Deconstructing Kranz anatomy to understand C4 evolution. J. Exp. Bot. 65:3357–3369. [DOI] [PubMed] [Google Scholar]
- Lundgren, M. R. , Besnard G., Ripley B. S., Lehmann C. E. R., Chatelet D. S., Kynast R. G., Namaganda M., Vorontsova M. S., Hall R. C., Elia J., et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecol. Lett. 18:1021–1029. [DOI] [PubMed] [Google Scholar]
- Lundgren, M. R. , Christin P. A., Gonzalez Escobar E., Ripley B. S., Besnard G., Long C. M., Hattersley P. W., Ellis R. P., Leegood R. C., and Osborne C. P.. 2016. Evolutionary implications of C3‐C4 intermediates in the grass Alloteropsis semialata . Plant Cell Environ. 39:1874–85 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. 2006. Confounding asymmetries in evolutionary diversification and character change. Evolution 60:1743–1746. [PubMed] [Google Scholar]
- Mallmann, J. , Heckmann D., Bräutigam A., Lercher M. J., Weber A. P. M., Westhoff P., and Gowik U.. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria . Elife 3:e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazzi, B. , Ané C., Simon M. F., Delgado‐Salinas A., Luckow M., and Sanderson M. J.. 2012. Locating evolutionary precursors on a phylogenetic tree. Evolution 66:3918–3930. [DOI] [PubMed] [Google Scholar]
- Marcussen, T. , Sandve S. R., Heirer L., Spannagl M., Pfeifer M., The International What Genome Sequencing Consortium , Jakobsen K. S., Wulff B. B. H., Steuernagel B., Mayer K. F. X., Olsen O. A.. 2014. Ancient hybridizations among the ancestral genomes of bread wheat. Science 345:1250092. [DOI] [PubMed] [Google Scholar]
- McGuire, J. A. , Witt C. C., Remsen J. V., Corl A., Rabosky D. L., Altshuler D. L., and Dudley R.. 2014. Molecular phylogenetics and the diversification of hummingbirds. Curr. Biol. 24:910–916. [DOI] [PubMed] [Google Scholar]
- McKown, A. D. , and Dengler N. G.. 2007. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am. J. Bot. 94:382–399. [DOI] [PubMed] [Google Scholar]
- Meier, J. I. , Marques D. A., Mwaiko S., Wagner C. E., Excoffier L., and Seehausen O.. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8:14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, C. S. , and Bell C. D.. 2013. Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution 67:2240–2257. [DOI] [PubMed] [Google Scholar]
- Niemiller, M. L. , Fitzpatrick B. M., Shah P., Schmitz L., Near T. J.. 2013. Evidence for repeated loss of selective constraint in rhodopsin in amblyopsid cavefishes (Teleostei: Amblyopsidae). Evolution 67:732–748. [DOI] [PubMed] [Google Scholar]
- Oliver, J. C. , Tong X. L., Gall L. F., Piel W. H., and Monteiro A.. 2012. A single origin for nymphalid butterfly eyespots followed by widespread loss of associated gene expression. PLoS Genet. 8:e1002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson, J. K. , Bianconi M., Besnard G., Dunning L. T., Lundgren M. R., Holota H., Vorontsova M. S., Hidalgo O., Leitch I. J., Nosil P., Osborne C. P., and Christin P. A.. 2016. Genome biogeography reveals the intraspecific spread of adaptive mutations for a complex traitptive mutations for a complex trait. Mol. Ecol. 25:6107–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel, M. 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. [DOI] [PubMed] [Google Scholar]
- Pagel, M. 2004. Limpets break Dollo's Law. Trends Ecol. Evol. 19:278–280. [DOI] [PubMed] [Google Scholar]
- Paradis, E. , Claude J., and Strimmer K.. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. [DOI] [PubMed] [Google Scholar]
- Pardo‐Diaz, C. , Salazar C., Baxter S. W., Merot C., Figueiredo‐Ready W., Joron M., McMillan W. O., and Jiggins C. D.. 2012. Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet. 8:e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, H. D. V. , Hattersley P. W., and Stone N. E.. 1987. New structural/biochemical associations in leaf blades of C4 grasses (Poaceae). Funct. Plant Biol. 14:403–420. [Google Scholar]
- Protas, M. E. , Hersey C., Kochanek D., Zhou Y., Wilkens H., Jeffery W. R., Zon L. I., Borowsky R., and Tabin C. J.. 2006. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genet. 38:107–111. [DOI] [PubMed] [Google Scholar]
- Pyron, R. A. , and Burbink F. T.. 2014. Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol. Lett. 17:13–21. [DOI] [PubMed] [Google Scholar]
- Rabosky, D. L. , Santini F., Eastman J., Smith S. A., Sidlauskas B., Chang J., and Alfaro M. E.. 2013. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Commun. 6:4. [DOI] [PubMed] [Google Scholar]
- Renvoize, S. A. 1987. A survey of leaf‐blade anatomy in grasses XI. Paniceae. Kew Bull. 739–768. [Google Scholar]
- Sage, R. F. , Christin P. A., and Edwards E. J.. 2011. The C4 plant lineages of planet Earth. J. Exp. Bot. 62:3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage, R. F. , Sage T. L., and Kocacinar F.. 2012. Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 63:19–47. [DOI] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, K. S. , Shimizu‐Inatsugi R., Tsuchimatsu T., and Purugganan M. D.. 2008. Independent origins of self‐incompatibility in Arabidopsis thaliana . Mol. Ecol. 17:704–714. [DOI] [PubMed] [Google Scholar]
- Sinha, N. R. , and Kellogg E. A.. 1996. Parallelism and diversity in multiple origins of C4 photosynthesis in the grass family. Am. J. Bot. 83:1458–1470. [Google Scholar]
- Smith, J. , and Kronforst M. R.. 2013. Do Heliconius butterfly species exchange mimicry alleles?. Biol. Lett. 9:20130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros, C. L. , and Dengler N. G.. 2001. Ontogenetic derivation and cell differentiation in photosynthetic tissues of C3 and C4 Cyperaceae. Am. J. Bot. 88:992–1005. [PubMed] [Google Scholar]
- Trueman, J. W. H. , Pfeil B. E., Kelchner S. A., and Yeates D. K.. 2004. Did stick insects really regain their wings?. Syst. Entomol. 29:138–139. [Google Scholar]
- Ueno, O. , and Sentoku N.. 2006. Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant Cell Environ. 29:257–268. [DOI] [PubMed] [Google Scholar]
- Vilella, A. J. , Severin J., Ureta‐Vidal A., Heng L., Durbin R., and Birney E., 2009. EnsemblCompara GeneTrees: complete, duplication‐aware phylogenetic trees in vertebrates. Genome Res. 19:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer, S. , and Furbank R. T.. 2003. The C4 pathway: an efficient CO2 pump. Photosynth. Res. 77:191–207. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Gowik U., Tang H., Bowers J. E., Westhoff P., and Paterson A. H.. 2009. Comparative genomic analysis of C4 photosynthetic pathway evolution in grasses. Genome Biol. 10:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Bräutigam A., Weber A. P. M., and Zhu X. G.. 2014. Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. J. Exp. Bot. 65:3579–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn, J. D. , Schnable J. C., Davidse G., and Pires J. C.. 2015. Phylogeny and photosynthesis of the grass tribe Paniceae. Am. J. Bot. 102:1493–1505. [DOI] [PubMed] [Google Scholar]
- Werner G. D. A., Cornwell W. K., Sprent J. I., Kattge J., and Kiers E. T.. 2014. A single evolutionary innovation drives the deep evolution of symbiotic N2‐fixation in angiosperms. Nature Commun 5:4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting, M. F. , Bradler S., and Maxwell T.. 2002. Loss and recovery of wings in stick insects. Nature 421:264–267. [DOI] [PubMed] [Google Scholar]
- Wiens, J. J. 1999. Phylogenetic evidence for multiple losses of a sexually selected character in phrynosomatid lizards. Proc. R. Soc. B 266:1529–1535. [Google Scholar]
- Wiens, J. J. , Brandley M. C., and Reeder T. W.. 2006. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution 60:123–141. [PubMed] [Google Scholar]
- Williams, B. P. , Johnston I. G., Covshoff S., and Hibberd J. M.. 2013. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. Elife 2:e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B. P. , Burgess S. J., Reyna‐Llorens I., Knerova J., Aubry S., Stanley S., and Hibberd J. M.. 2016. An untranslated cis‐element regulates the accumulation of multiple C4 enzymes in Gynandropsis gynandra mesophyll cells. Plant Cell 28:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24:1586–1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparisons of leaf anatomy in Alloteropsis and relatives.
Figure S2. Phylogenetic trees for genes encoding three C4‐related enzymes.
Figure S3. Phylogeny of pck‐1P1 genes in Panicoideae.
Figure S4. Evolution of ppc‐1P3 genes in Alloteropsis and other Panicoideae.
Figure S5. Evolution of ppdk‐1P2 genes in Alloteropsis and other Panicoideae.
Figure S6. Evolution of aspat‐3P4 genes in Alloteropsis and other Panicoideae.
Table S1. Alloteropsis semialata accessions used in this study.
Table S2. Leaf anatomical data for the study species and accessions.
Table S3. RNA‐Seq data, NCBI SRA accession numbers, and growth conditions.
Table S4. Transcript abundance (in rpkm) for each C4‐related gene and sample.
Table S5. Results of positive selection analyses inferring the episodes of enzymatic adaptation in Alloteropsis using only fixed differences.
Table S6. Results of positive selection analyses inferring the episodes of enzymatic adaptation in the A. angusta/A. semialata clade using only fixed differences.
Methods 1.1. Plant growth conditions.
Methods 1.2. RNA‐Seq protocol.
Methods 1.3. Positive selection analysis.
Methods 1.4. Alignment and filtering
Supplementary Material


