Abstract
The combination of laser‐heated diamond anvil cells and synchrotron Mössbauer source spectroscopy were used to investigate high‐temperature high‐pressure chemical reactions of iron and iron nitride Fe2N with nitrogen. At pressures between 10 and 45 GPa, significant magnetic hyperfine splitting indicated compound formation after annealing at 1300 K. Subsequent in situ X‐ray diffraction reveals a new modification of FeN with NiAs‐type crystal structure, as also rationalized by first‐principles total‐energy and chemical‐bonding studies.
Keywords: high-pressure chemistry, Mössbauer spectroscopy, nitrides, structure elucidation
Since the pioneering research into binary nitrides,1 this field of chemistry has grown into a rich diversity of sub‐classes.2, 3, 4, 5, 6, 7, 8, 9 One of the families that has been investigated more intensely is that of iron nitrides. Several different phases are already known,10 which range from the most iron‐rich phases, such as α′′‐Fe16N2 or α′‐Fe8N through γ′‐Fe4N, ϵ‐Fe3N1±x and ζ‐Fe2N to γ′′′‐FeN,11 which represents the most nitrogen‐rich phase to date. However, in 2011, the existence of nitrogen‐richer FeN2 was predicted.12 Iron nitrides are used as coatings for steel‐based materials combining hardening with protection against corrosion. More recent studies have shown that iron nitrides can be used as catalysts for the production of hydrocarbons13 and as a potential treatment of cancer cells.14 Moreover, iron nitrides are of relevance for the geosciences as they may be an important constituent of the Earth's core. Since the interaction of iron and nitrogen at higher pressures has seen little attention, there remains much to be investigated with regard to properties and phase relations.15, 16, 17
In 1993, the much debated cubic FeN was reported.11, 18, 19, 20 The published data supports either the rock salt structure,21, 22, 23, 24 or the zinc blende arrangement.25, 26 An independent prediction stated that cubic FeN would actually prefer the zinc blende type, while FeNx (x=0.5–0.7) would adopt a rock salt arrangement,25 but results remain controversial. Single‐phase thin films of γ′′′‐FeN adopt a zinc blende pattern.20 Herein, we report on synthesis, magnetic properties, and electronic structure of NiAs‐type bulk FeN. For synthesis at elevated temperatures and pressures, we chose the diamond anvil cell technique combined with laser heating. Phase transformations were probed with synchrotron source Mössbauer spectroscopy which serves as an ideal tool to investigate electronic configuration and chemical state of iron.27, 28, 29
Two different types of experiments were carried out, one using 57Fe and the other using ζ‐57Fe2N, both with N2 as reactant and pressure medium. Samples heated up to 1300 K at pressures below 10 GPa form an iron nitride with a composition close to ζ‐Fe2N/ϵ‐Fe3N1.4.30 At pressures higher than 10 GPa, a new phase was observed after annealing, which gave moderate magnetic hyperfine splitting, as shown in Figure 1 (green curve). The magnetic splitting remains essentially up to 45 GPa, suggesting a quite robust magnetic order. Upon heating at a pressure below 10 GPa, the new phase transforms back into ζ‐Fe2N/ϵ‐Fe3N1.4.
Figure 1.
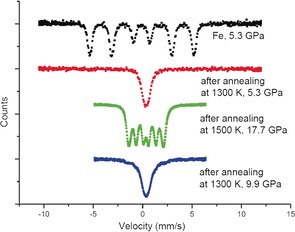
57Fe‐Mössbauer spectra at various pressures before and after heat treatment of iron–nitrogen mixtures. Black: Initial 57Fe in N2 pressure medium at 5.3 GPa prior to laser heating. Red: After laser heating at 5.3 GPa the spectrum indicates formation of ζ‐57Fe2N/ϵ‐57Fe3N1+x 30 Green: Multiplet of the new phase after laser heating. Blue: Spectrum indicating the reformation of ζ‐57Fe2N/ϵ‐57Fe3N1+x after laser heating at pressures below 10 GPa.
There are a distinct major and a minor component observed by comparing the 57Fe‐Mössbauer spectra from all samples annealed above 1000 K and 10 GPa (Figure 2 and Figure S1 in the Supporting Information). The major component of all spectra consists of a magnetic sextet, while the minor component represents a non‐magnetic singlet (Table 1). After annealing, magnetic components have similar hyperfine parameters: a small magnetic hyperfine field (ca. 11 T) and a moderate center shift (ca. 0.35 mm s−1), suggesting the presence of a new phase. The minor component singlet with a center shift of 0.4 mm s−1 corresponds to the center shift of ζ‐Fe2N.30
Figure 2.
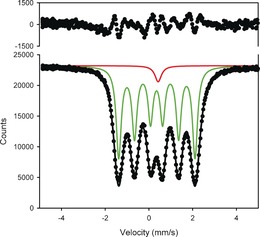
57Fe‐Mössbauer spectra of ζ‐57Fe2N in N2 pressure medium, after annealing, at 17.7 GPa.
Table 1.
Refined 57Fe‐Mössbauer spectra parameters before annealing and of the major component after annealing.[a]
| Sample | δ [mm s−1] | ΔE Q [mm s−1] | ΔE M (T) |
|---|---|---|---|
| 57Fe in N2 (0.1 MPa, not annealed) | −0.073±0.005 | 0.011±0.009 | 32.645±0.037 |
| 57Fe in N2 (11.9 GPa, annealed) | 0.305±0.012 | 0.015±0.019 | 11.553±0.063 |
| ζ‐57Fe2N in N2 (0.1 MPa, not annealed) | 0.441±0.023 | – | – |
| ζ‐57Fe2N in N2 (17.7 GPa, annealed) | 0.356±0.002 | 0.012±0.003 | 10.814±0.014 |
[a] δ represents the center shift, ΔEQ the quadrupole splitting, and ΔE M the hyperfine field. Spectra were fit using MossA software.32
X‐ray diffraction patterns were obtained at pressures of 13.3 GPa, 4.4 GPa, and 0.1 MPa using synchrotron radiation. Patterns of the new phase were indexed on basis of a hexagonal unit cell, and Rietveld refinements indicate a NiAs‐type arrangement (Figure 3 a, b and Table S1).31 Most patterns contain extra reflections, which are mainly attributed to N2 or residual ζ‐Fe2N. Despite the small number of experiments for pressure–volume correlation, a least squares fit of a Murnaghan‐type equation of state to the experimental data results in the reasonable values V 0=33.78(3) Å3 and B 0=198(8) GPa with B0′ fixed to 4.
Figure 3.
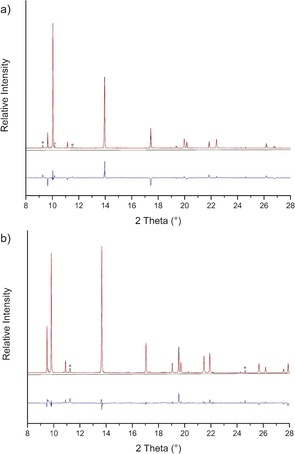
Crystal‐structure refinements of NiAs‐type FeN based on full diffraction profiles at a) 13.3 GPa and b) 0.1 MPa. The observed pattern is shown in black, the calculated one in red, and the difference in blue. The marked reflections (*) are attributed to secondary phases (see text).
Crystal structure refinements based on full diffraction profiles measured at various pressures and sample positions indicate a broad homogeneity range for the new phase FexN with x in the estimated range of between 0.60(5) and 1.0(1). In the following we will denote the phase as FeN.
NiAs‐type FeN consists of a hexagonal closed packing of nitrogen, with the iron occupying all octahedral voids for x=1. These FeN6 octahedra form infinite chains by face sharing along the c direction (Figure 4). To our knowledge, this is not only the first face‐sharing arrangement of FeN6 octahedra in a nitride, but also the single NiAs‐type 3d transition‐metal nitride. Although some have been predicted, such as VN33 and MoN,34 they remain elusive and only heavier homologues, such as δ‐NbN35 and TaN,36 have been successfully synthesized. Comparison of the interatomic iron distances of NiAs‐type FeN and ϵ‐iron with the same iron arrangement reveals37 that d(Fe–Fe) along the c axis varies only slightly (2.467 Å versus 2.442 Å) whereas along the a axis there is a larger difference: In the nitride, the Fe–Fe distance is significantly larger than in ϵ‐iron (2.737 Å versus 2.473 Å). Typical Fe–N distances range from 1.8 Å to 2.0 Å, but in NiAs‐type FeN this distance is at the upper limit of 2.0 Å and hardly changes with pressure. FeN shows a large c/a ratio of about 1.80 (Table 2) which is typical for NiAs‐type compounds of transition metals with Group V or VI elements.
Figure 4.
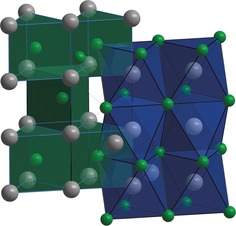
Crystal structure of NiAs‐type FeN, showing the face‐sharing condensation of the iron‐centered octahedra (blue), as well as the trigonal‐prismatic coordination environment of the nitride ions (green).
Table 2.
The c/a ratios of NiAs‐type FeN, under pressure and ambient pressure, with related NiAs‐type compounds.
An insight into the electronic structure of NiAs‐type FexN for x=1 was gained by examining band structure and density of states (DOS), while a bonding analysis used the projected crystal orbital Hamilton populations (−pCOHP), a variant of the COHP technique38 and their integrated values (−IpCOHP; Computational Details are provided in the Supporting Information). Features at the Fermi level, E F, in the non‐spin‐polarized DOS and −pCOHP curves (Figure 5 c,d) indicate an electronically unfavorable situation as E F falls into a maximum of the DOS curves39 with strongly antibonding Fe–N interactions; such attributes at E F, however, may also suggest that the material alleviates this unfavorable situation by approaching a magnetic state,40 as indicated by Mössbauer spectroscopy.
Figure 5.
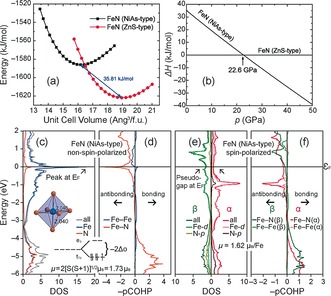
a) Energy–volume curves and b) relative enthalpies as functions of pressure for NiAs‐type as well as ZnS‐type FeN at 0 K; c), d) non‐spin‐polarized DOS and −pCOHP curves; e), f): spin‐polarized DOS and −pCOHP curves of NiAs‐type FeN.
A comparison of the total energies between various magnetic models reveals that the antiferromagnetic NiAs‐type FeN model is just 0.6 kJ mol−1 higher in energy than the ferromagnetic model, which, accordingly, tends to be preferred. Because the Fermi level falls into a pseudogap of the spin‐polarized DOS curves (Figure 5 e and Figure S2), an electronically favorable situation is inferred for magnetic NiAs‐type FeN. This outcome is in good agreement with the Mössbauer spectra in which the magnetic sextet clearly denotes the presence of a magnetic ground state (Figure 2). The theoretical magnetic moment of 1.62 μB/Fe follows a spin‐only rule‐derived value for low‐spin Fe3+ ( configuration), for which the ligand‐field stabilization energy is maximized (Figure 5 c). The presence of such a low‐spin‐configuration for the iron atoms corresponds well to the observed Fe–N distances of approximately 2.01 Å, which is expected based on the covalent radii of nitrogen (0.71 Å) and low‐spin iron (1.32 Å).44 An alternative calculus using Shannon's ionic radii of N3− (1.54 Å) and low‐spin Fe3+ (0.55 Å) yields a similar Fe–N distance of 2.09 Å.45 The states near and below the Fermi level stem primarily from Fe d as well as N p atomic orbitals (Figure 5 e). Although antibonding Fe–N interactions are evident below E F in the spin‐polarized −pCOHP curves of both magnetic models (Figure 5 f and Figure S3), the integrated values (Table S2) of the Fe–N −pCOHP (−IpCOHP) are indicative of a net bonding character for these contacts.
Previous research on the electronic and vibrational properties revealed that the zinc blende‐type structure of FeN maintains dynamically stable.25, 46 To evaluate the stability of NiAs‐type iron nitride, we determined the energy–volume curves and pressure‐dependent relative enthalpies at absolute zero (Figure 5 a,b). Because crystal structure data, Mössbauer spectra and electronic structure calculations suggest a ferromagnetic ground state for NiAs‐type FeN while an antiferromagnetic ground state is proposed for ZnS‐type iron nitride,11 spin‐polarized band structure computations were used. A comparison reveals that the total energy of the ZnS modification is lower by almost 36 kJ mol−1. At the atomic scale, the preference to adopt the ZnS‐type rather than NiAs‐type modification originates from shorter Fe–N contacts corresponding to larger −IpCOHP values in ZnS‐type FeN, that is, the modification is favored owing to a larger number of strong heteroatomic contacts. However, the values of the pressure‐dependent relative enthalpies imply a phase transition from the ZnS‐type to the NiAs‐type for pressures ≥22.6 GPa. Additionally, investigations of the vibrational properties for NiAs‐type FeN (Figure S4) point to dynamic stability.
In summary, a new high‐pressure phase has been discovered in the Fe–N system by combining diamond anvil cell laser heating techniques with synchrotron‐source Mössbauer spectroscopy and in situ X‐ray diffraction. Theoretical investigations confirm that NiAs‐type FeN exhibits a magnetic ground state. Although the modification is less stable than the ZnS‐type form at ambient conditions, the application of pressure provides access to this modification at elevated temperatures.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities. We thank Rudolf Rüffer and the staff members for using beamline ID18 under experiment CH‐4383. The allocation of CPU time through the IT Center of RWTH Aachen University is gratefully acknowledged. S.S. is grateful for a Liebig Stipend from the Fonds der Chemischen Industrie e.V. (FCI).
W. P. Clark, S. Steinberg, R. Dronskowski, C. McCammon, I. Kupenko, M. Bykov, L. Dubrovinsky, L. G. Akselrud, U. Schwarz, R. Niewa, Angew. Chem. Int. Ed. 2017, 56, 7302.
References
- 1.
- 1a. Juza R., Hahn H., Z. Anorg. Allg. Chem. 1938, 239, 282; [Google Scholar]
- 1b. Juza R., Langer K., Von Benda K., Angew. Chem. Int. Ed. Engl. 1968, 7, 360; [Google Scholar]; Angew. Chem. 1968, 80, 373. [Google Scholar]
- 2. Schnick W., Angew. Chem. Int. Ed. Engl. 1993, 32, 806; [Google Scholar]; Angew. Chem. 1993, 105, 846. [Google Scholar]
- 3. Vajenine G. V., Auffermann G., Prots Y., Schnelle W., Kremer R. K., Simon A., Inorg. Chem. 2001, 40, 4866. [DOI] [PubMed] [Google Scholar]
- 4. Auffermann G., Prots Y., Kniep R., Angew. Chem. Int. Ed. 2001, 40, 547; [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 565. [Google Scholar]
- 5. von Appen J., Lumey M. W., Dronskowski R., Angew. Chem. Int. Ed. 2006, 45, 4365; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 4472. [Google Scholar]
- 6. Young A. F., Sanloup C., Gregoryanz E., Scandolo S., Hemley R. J., Mao H. K., Phys. Rev. Lett. 2006, 96, 1. [DOI] [PubMed] [Google Scholar]
- 7. Crowhurst J. C., Goncharov A. F., Sadigh B., Zaug J. M., Aberg D., Meng Y., Prakapenka V. B., J. Mater. Res. 2008, 23, 1. [Google Scholar]
- 8. Niwa K., Dzivenko D., Suzuki K., Riedel R., Troyan I., Eremets M., Hasegawa M., Inorg. Chem. 2014, 53, 697. [DOI] [PubMed] [Google Scholar]
- 9. Bhadram V. S., Kim D. Y., Strobel T. A., Chem. Mater. 2016, 28, 1616. [Google Scholar]
- 10. Wriedt H. A., Gokcen N. A., Nafziger R. H., Bull. Alloy Phase Diagr. 1987, 8, 355. [Google Scholar]
- 11. Suzuki K., Morita H., Kaneko T., Yoshida H., Fujimori H., J. Alloys Compd. 1993, 201, 11. [Google Scholar]
- 12. Wessel M., Dronskowski R., Chem. Eur. J. 2011, 17, 2598. [DOI] [PubMed] [Google Scholar]
- 13. Yang Z., Guo S., Pan X., Wang J., Bao X., Energy Environ. Sci. 2011, 4, 4500. [Google Scholar]
- 14.Y. Namiki, S. Matsunuma, T. Inoue, S. Koido, A. Tsubota, Y. Kuse, N. T, in Nanocrystal, InTech, 2011, p. 349.
- 15. Schwarz U., Wosylus A., Wessel M., Dronskowski R., Hanfland M., Rau D., Niewa R., Eur. J. Inorg. Chem. 2009, 1634. [Google Scholar]
- 16. Niewa R., Rau D., Wosylus A., Meier K., Wessel M., Hanfland M., Dronskowski R., Schwarz U., J. Alloys Compd. 2009, 480, 76. [Google Scholar]
- 17. Niewa R., Rau D., Wosylus A., Meier K., Hanfland M., Wessel M., Dronskowski R., Dzivenko D. A., Riedel R., Schwarz U., Chem. Mater. 2009, 21, 392. [Google Scholar]
- 18. Borsa D. M., Boerma D. O., Hyperfine Interact. 2003, 151, 31. [Google Scholar]
- 19. Andrzejewska E., Gonzalez-Arrabal R., Borsa D., Boerma D. O., Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 249, 838. [Google Scholar]
- 20. Jouanny I., Weisbecker P., Demange V., Grafoute M., Pena O., Bauer-Grosse E., Thin Solid Films 2010, 518, 1883. [Google Scholar]
- 21. Shimizu H., Shirai M., Suzuki N., J. Phys. Soc. Jpn. 1998, 67, 922. [Google Scholar]
- 22. Filippetti A., Pickett W. E., Phys. Rev. B 1999, 59, 8397. [Google Scholar]
- 23. Houari A., Matar S. F., Belkhir M. A., Nakhl M., Phys. Rev. B 2007, 75, 1. [Google Scholar]
- 24. Kong Y., J. Phys. Condens. Matter 2000, 12, 4161. [Google Scholar]
- 25. Eck B., Dronskowski R., Takahashi M., Kikkawa S., J. Mater. Chem. 1999, 9, 1527. [Google Scholar]
- 26. Lukashev P., Lambrecht W. R. L., Phys. Rev. B 2004, 70, 1. [Google Scholar]
- 27. Ming L. C., Bassett W. A., Rev. Sci. Instrum. 1974, 45, 1115. [Google Scholar]
- 28. Kupenko I., Dubrovinsky L., Dubrovinskaia N., McCammon C., Glazyrin K., Bykova E., Ballaran T. B., Sinmyo R., Chumakov A. I., Potapkin V., Kantor A., Rüffer R., Hanfland M., Crichton W., Merlini M., Rev. Sci. Instrum. 2012, 83, 124501. [DOI] [PubMed] [Google Scholar]
- 29. Potapkin V., Chumakov A. I., Smirnov G. V., Celse J. P., Rüffer R., McCammon C., Dubrovinsky L., J. Synchrotron Radiat. 2012, 19, 559. [DOI] [PubMed] [Google Scholar]
- 30. Ksenofontov V., Reiman S., Waldeck M., Niewa R., Kniep R., Gütlich P., Z. Anorg. Allg. Chem. 2003, 629, 1787. [Google Scholar]
- 31.Crystal structure for FeN from Rietveld refinement: hexagonal, space group P63/mmc (194); Fe in 2a; N in 2c: at 13.3 GPa a=2.737(3) Å; c=4.933(5) Å; V=32.01 Å3; at 4.4 GPa a=2.775(7) Å; c=4.982(1) Å; V=33.24 Å3; at 0.0001 GPa a=2.800(2) Å; c=5.015(5) Å; V=34.05 Å3. Further details on the crystal structure investigation(s) may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49) 7247-808-666; e-mail: crysdata@fiz-karlsruhe.de), on quoting the depository numbers CSD-432891 (0.1 MPa), CSD-432893 (4.4 GPa), CSD-432892 (13.3 GPa).
- 32. Prescher C., McCammon C., Dubrovinsky L., J. Appl. Crystallogr. 2012, 45, 329. [Google Scholar]
- 33. Ravi C., Calphad 2009, 33, 469. [Google Scholar]
- 34. Zhao E., Wang J., Wu Z., Phys. Status Solidi B 2010, 247, 1207. [Google Scholar]
- 35. Terao N., J. Less-Common Met. 1971, 23, 159. [Google Scholar]
- 36. Fontbonne J. C., Gilles A., Rev. Int. Hautes Temp. 1969, 181. [Google Scholar]
- 37. Takahashi T., Bassett W. A., Mao H.-K., J. Geophys. Res. 1968, 73, 4717. [Google Scholar]
- 38.
- 38a. Dronskowski R., Blöchl P. E., J. Phys. Chem. 1993, 97, 8617; [Google Scholar]
- 38b. Deringer V. L., Tchougréeff A. L., Dronskowski R., J. Phys. Chem. A 2011, 115, 5461; [DOI] [PubMed] [Google Scholar]
- 38c. Maintz S., Deringer V. L., Tchougréeff A. L., Dronskowski R., J. Comput. Chem. 2013, 34, 2557; [DOI] [PubMed] [Google Scholar]
- 38d. Maintz S., Deringer V. L., Tchougréeff A. L., Dronskowski R., J. Comput. Chem. 2016, 37, 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.
- 39a. Deringer V. L., Lumeij M., Stoffel R. P., Dronskowski R., Chem. Mater. 2013, 25, 2220; [DOI] [PubMed] [Google Scholar]
- 39b. Steinberg S., Brgoch J., Miller G. J., Meyer G., Inorg. Chem. 2012, 51, 11356. [DOI] [PubMed] [Google Scholar]
- 40. Landrum G. A., Dronskowski R., Angew. Chem. Int. Ed. 2000, 39, 1560; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 1598. [Google Scholar]
- 41. Alsén N., Geol. Foeren. Stockholm Foerh. 1925, 47, 19. [Google Scholar]
- 42. Onoda M., Wada H., J. Less-Common Met. 1987, 132, 195. [Google Scholar]
- 43. Schönberg N., Overend W. G., Munthe-Kaas A., Sörensen N. A., Acta Chem. Scand. 1954, 8, 226. [Google Scholar]
- 44. Cordero B., Gómez V., Platero-Prats A. E., Revés M., Echeverría J., Cremades E., Barragán F., Alvarez S., Dalton Trans. 2008, 2832. [DOI] [PubMed] [Google Scholar]
- 45. Shannon R. D., Acta Crystallogr. Sect. A 1976, 32, 751 Because no Shannon radius r is provided for a sixfold coordinated N3− anion, its ionic radius has been approximated based on a fourfold coordinated one ([4] r) using the expression [4] r=[6] r (4/6)1/8. Further details are provided in: [Google Scholar]; Dronskowski R., Computational Chemistry of Solid State Materials, Wiley-VCH Publishers, Weinheim, 2005. [Google Scholar]
- 46. Soni H. R., Mankad V., Gupta S. K., Jha P. K., J. Alloys Compd. 2012, 522, 106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


