Abstract
The metabolic syndrome (MetS) is a cluster of clinical disorders including an unhealthy body habitus with a large waistline, dyslipidemia, glucose intolerance and hypertension. It is known that these disorders not only increase the chances of developing type 2 diabetes mellitus (T2DM), but also cardiovascular disease (CVD). Furthermore, the co-occurrence of all these risk factors known as the MetS is linked to pathways sharing common underlying mediators and mechanisms. Though insulin resistance has been considered as the root of the problem to explain the conglomerate of metabolic abnormalities within this syndrome; new evidence points to several pro-inflammatory cytokines, reactive oxygen species and free fatty acid intermediates might play an even greater role in regulating a series of intracellular signaling pathways sustain as well as perpetuate the development of the MetS and its CVD complications. Since having a diagnosis of MetS confers not only a 5-fold increase in the risk of T2DM, but also a 2-fold risk of developing CVD over a period of 5 to 10 years; it is vital to better recognize the mechanisms by which the MetS is associated with such adverse outcomes. Therefore, it is the purpose of this review to address (1) how inflammation modifies insulin sensitivity, (2) known factors believed to contribute to this process, and (3) new concepts of inflammatory markers in regulating the development of MetS and its individual components.
Keywords: Chronic inflammation, Metabolic syndrome, Cardiovascular disease
INTRODUCTION
Despite great efforts to improve public awareness and modify perception and behavior tendencies urging both children and adults to adopt healthier choices and to make lifestyle modifications, most Americans fail and do not follow any of these recommended guidelines to maintain a healthy lifestyle [1]. Specifically, data from the Behavioral Risk Factor Surveillance System including more than 153,000 U.S. adults showed that only 3% of the participants followed a healthy lifestyle. Moreover, only 1 in 10 adults followed no weight, dietary, or smoking recommendations [1]. In addition, overweight and obesity have now reached epidemic proportions in the U.S. and trail only smoking as preventable causes of death [2]. Therefore, weight related issues have not only become increasingly important as an epidemic health concern, but also an essential component of the Metabolic Syndrome (MetS) and a modifiable risk factor for type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).
Despite all available data regarding the atherogenic damage associated to MetS, there is substantial confusion regarding the conceptual definition of the MetS, particularly clinical screening, cut-off values to define MetS and triggers that initiate and perpetuate MetS. Additionally, preventive management strategies and implementation protocols are scarce and poorly applied by primary care physicians in U.S.
Since the last century, inflammation has been implicated as a potential mediator for the development of T2DM [3]. Although all molecular mechanisms have not been clearly defined, the role of pro-inflammatory cytokines, reactive oxygen species and free fatty acids intermediaries have been suggested as key elements in modulating specific intracellular signaling pathways that appear to regulate insulin sensitivity at least in certain animal models [4]. In order to advance our understanding of the MetS, it is important to link these same pathways to each individual component of the MetS and their potential role in the development of CVD complications.
With this in mind, this review will address: (1) how inflammation modifies insulin sensitivity, (2) known factors believed to contribute to this process, and (3) new concepts of inflammatory markers in regulating the development of MetS and its individual components.
METABOLIC SYNDROME
Even though there has been controversy in defining the MetS and its clinical utility, it is now conclusively apparent that it encompasses a collection of unhealthy body habitus, dyslipidemia, hypertension, glucose intolerance, a proinflammatory state, and a prothrombotic state. The most commonly used criteria at present comes from the World Health Organization (WHO), the European Group for the study of Insulin Resistance (EGIR), the National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III), American Association of Clinical Endocrinologists (AACE), and most recently from the International Diabetes Federation (IDF) [5–10]. Clinical recognition of MetS becomes increasingly important, as this syndrome has been shown to confer a 5-fold increase in the risk of T2DM and 2-fold the risk of developing CVD over a period of 5 to 10 years [11]. Furthermore, patients with the MetS are at 2- to 4-fold increased risk of stroke, a 3- to 4-fold increased risk of myocardial infarction (MI), and 2-fold the risk of dying from such an event compared with those without the syndrome regardless of a previous history of cardiovascular events [12,13].
It has been estimated that the worldwide prevalence of MetS ranges from <10% to as much as 84%, depending on the region, urban or rural environment, sex, age, race, and ethnicity studied, as well as definition used to classify patients [14,15]; hence it is estimated that one-quarter of the world’s adult population has MetS [9]. These staggering statistics become even more significant when considering the association between MetS and the elevated CVD risk involving subclinical target organ damage [16]; particularly when most physicians cannot measure indices of insulin sensitivity in the context of their clinical practice. This difficulty persists despite efforts by some organizations such as the WHO, NCEP-ATP III, EGIR, AACE, and IDF in proposing the use of simple clinical parameters with cut-off values to identify individuals who would probably be insulin resistant and who would also show the atherogenic and diabetogenic abnormalities associated with MetS. In this setting, glucose tolerance testing, the homeostatic model assessment (HOMA) and more recently the quantitative insulin sensitivity check index (QUICKI) have demonstrated to be useful tools for determining insulin resistance. However, no direct marker of insulin resistance to diagnose the MetS is currently recommended in medical guidelines.
Inclusion of abdominal obesity as a clinically measurable variable to identify MetS was a key conceptual step in the right direction. Waist circumference, though imperfect, correlates fairly well with total abdominal fat and is also the most prevalent manifestation of the MetS [17–20]. Nonetheless, it cannot distinguish visceral adiposity from the amount of subcutaneous abdominal fat [5,21,22]. This is crucially important since increased visceral adiposity, along with increased macrophage type 1 infiltration into omental adipose tissue, are critical to the development of insulin resistance in obese patients independent of body mass index and total body fat mass [23–26].
Even though the role of fat cells in metabolic dysfunction has long been considered, their potential role in an inflammatory process is a relatively new concept as data has now shown that adipocytes and immune cells share certain properties such as complement activation and pro-inflammatory cytokine production [27,28]. In addition, fat cell precursors also share features with macrophages, such as the capacity for phagocytosis in response to several stimuli, as well as numerous genes that code for transcription factors, cytokines, inflammatory signaling molecules, and fatty acid transporters [29–33].
Furthermore, adipose tissue has been characterized as a heterogeneous mix of adipocytes, stromal preadipocytes, immune cells, and endothelium that respond rapidly and dynamically to alterations in nutrient excess through adipocyte hypertrophy and hyperplasia [34]. It has been suggested that progressive adipocyte enlargement results in visceral obesity, causing a state of hypoxia that has been considered the main trigger of necrosis and macrophage infiltration into adipose tissue leading to an overproduction of biologically active metabolites known as adipocytokines [35,36]. See a list of these adipocytokines and corresponding proposed biologic contributions in Table 1.
Table 1.
| Adipocytokine | Biological Action |
|---|---|
| Free fatty acids (FFA) |
|
| Tumor necrosis factor alpha (TNF α) |
|
| C-reactive protein (CRP) |
|
| Interleukin 6 (IL-6) |
|
| Plasminogen activator inhibitor-1 (PAI-1) |
|
| Adiponectin |
|
| Leptin |
|
IR = insulin resistance; RS = receptor substrate; BW = body weight; WC = waist circumference; TGs = triglycerides; HDL-C = high-density lipoprotein–cholesterol; BMI = body mass index; HG = hyperglycemia; MetS = Metabolic syndrome; CVD = cardiovascular disease; T2DM = type 2 diabetes mellitus; LDL-C = low-density lipoprotein cholesterol; BP = blood pressure; NO = nitric oxide; and PVR = peripheral vascular resistance.
In three recent studies, the association between MetS and inflammation has been highlighted. First, data from Ebron et al, showed that a larger body mass index was associated not only with an increased atherogenic dyslipidemia and insulin resistance in individuals with MetS, but also with a low-grade level of inflammation; particularly shown by the positive association between BMI and the proinflammatory C-reactive protein and interleukin-6 [37]. Second, data from Slagter and associates demonstrated the impact obesity has on quality of life and found that quality of life is enhanced by grade of obesity, T2D, MetS and inflammation, and that these are mainly related to reduced physical health [38]. Third, data from Marques-Rocha et al, showed that the use of a Mediterranean-based nutritional diet induced changes in the expression of let-7b and miR-155-3p in white blood cells from patients with MetS after an 8-week intervention [39]. Moreover, the quality of this Mediterranean-based diet had an important effect on the expression of inflammation-related micro RNAs (miRNAs). The latter is critically important since expression of these miRNAs not only has been associated with regulation of inflammatory genes, but also important in the development of human diseases.
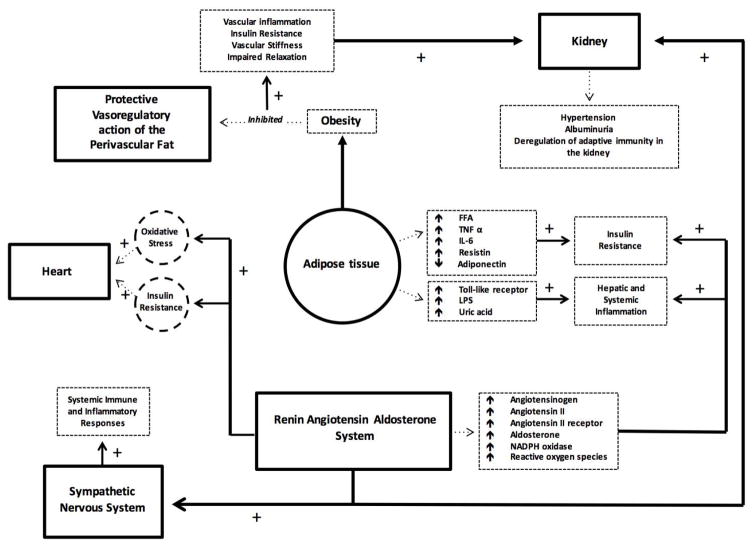
Figure 1 represents the current model that will integrate the maladaptive immune and inflammatory responses leading to insulin resistance and CVD.
Figure 1.
Current model integrating maladaptive immune and inflammatory responses leading to insulin resistance and CVD.
DIABETES MELLITUS
The combination of T2DM and obesity is rapidly growing and has become a major public health issue associated with inordinate morbidity and mortality. In fact, the incidence of this metabolic combination rises steeply as HbA1c increases from the 5.0% to 6.5% [40]. It has been estimated that approximately 79 million adults in the U.S. 20 years or older have prediabetes [41]. This staggering number of patients clearly indicates the presence of CVD related complications of epidemic proportions [42–46].
A low-level chronic inflammation state not only has been shown to be clearly associated with the development on insulin resistance and T2DM [47–49]; but also current data seems to suggest that elevation of certain cytokines vary according to ethnicity [50–56]. In the Multi-Ethnic Study of Atherosclerosis (MESA), inflammation markers differed significantly by race/ethnicity. IL-6, CRP, and fibrinogen were lower among Chinese patients while Hispanic and black subjects had higher levels when compared with white subjects [50].
It is now apparent that a series of intracellular signaling pathways activated by a state of chronic, low-grade inflammation, particularly within the white adipose tissue, participate in the regulation of insulin signaling that in turn regulates a series of downstream signaling events [57,58]. Moreover, inhibition of these signaling steps is known to be a primary mechanism through which inflammatory signaling leads to insulin resistance [59–61]. Specific to these pathways are several serine/threonine kinases activated by inflammatory or stressful stimuli that contribute to inhibition of insulin signaling, including JNK, inhibitor of NF-κB kinase (IKK), and PKC-θ [62]. Furthermore, activation of these kinases, at least in obesity, not only has been shown to highlight the overlap that exists between metabolic and immune pathways; but also these same kinases, particularly IKK and JNK, are activated in the innate immune response by Toll-like receptor (TLR) signaling in response to LPS, peptidoglycan, double-stranded RNA, and other microbial products [63].
The inflammatory activation mediated by inflammasomes through IL-1β activation may contribute to insulin resistance and T2DM [64]. In obesity, levels of palmitate and ceramide have been found to be elevated and these lipids are known to activate inflammasomes [65]. In the mice mode, insulin sensitivity improves when mice deficient in central inflammasome molecules are fed high-fat diets and this improvement is accompanied by suppression of immune and inflammatory responses [66, 67].
Data from both genetic and pharmacological manipulations on different effectors of the inflammatory response have shown modulation of insulin sensitivity in different animal models [68]. Tissue-specific over-expression of IK kinase in liver and adipose tissue, but not in skeletal muscle, leads to systemic insulin resistance. In contrast, selective inhibition of the nuclear factor-KB function in liver and adipose tissue protects against insulin resistance in nutritional and genetic animal models of obesity [69].
HYPERTENSION
Hypertension is one of the most frequently encountered conditions in clinical practice [70]. The most recent JNC-8 addressed the need for treatment in order to lower systemic blood pressure readings to 150/90 mmHg in those aged 60 and older, and to 140/90 for adults less than 60 years of age [71]. In the population age 18 and older with diabetes, the guidelines recommend initiating drug treatment to a goal of systolic BP < 140mmHg, and diastolic blood pressure goals of < 90mmHg. These guidelines also apply to patients with chronic kidney disease. Establishment of hypertension as a primary component of MetS not only has allowed for earlier detection and proper management [72]; but has also allowed for better understanding of the multifactorial etiology of this condition.
As previously mentioned, the metabolically active visceral fat linked to insulin sensitivity through the production of adipocytokines including leptin, tumor necrosis factor-α (TNF-α), angiotensinogen, interleukin-6 (Il-6), and non-esterified fatty acids (NEFA), interact in a diversity of metabolic pathways culminating in the activation of the renin-angiotensin-aldosterone system (RAAS) pathway and the development of insulin resistance [73].
Studies in experimental animals have provided mechanistic insights into CVD, as well as renal changes associated with obesity. Specifically, reproducible increases in systemic blood pressure have been identified in both dogs and rabbits fed fat diets that result in excess weight gain [74–77]. In fact, the metabolic, endocrine, cardiovascular, and renal changes caused by dietary-induced obesity in these experimental animals have closely mimicked changes observed in obese humans.
Nowadays, there is overwhelming evidence that excess weight gain and visceral obesity are major causes of hypertension, perhaps accounting for as much as 65–75% of the risk for human essential hypertension [73]. Although the mechanisms of obesity-induced hypertension are still being intensively studied, research in experimental animals and humans suggest important roles for impaired renal-pressure natriuresis due to physical compression of the kidneys and activation of RAAS and sympathetic nervous system [78]. As obesity and its metabolic and hemodynamic consequences are sustained over many years, renal injury gradually makes the hypertension more severe and more resistance to therapy.
In addition to the potential mechanical compression caused by obesity that may mediate the development of hypertension, several other mediators of sympathetic nervous system activation have been proposed. These include the presence of impaired baroreceptor reflexes; activation of chemoreceptor-mediated reflexes secondary to the development of sleep apnea with intermittent hypoxia; hyperinsulinemia; Angiotensin II (Ang II); and cytokines released from adipocytes such as leptin, tumor necrosis factor-α and interleukin-6 [79–82].
Surely adipose tissue is known to widely express angiotensinogen, angiotensin converting enzyme (ACE), and type 1 angiotensin receptor (AT1) gene, with the potential of increasing the overall production of Ang II and thus activate RAAS [83]. While RAAS plays a key role in the modulation of many key cardiovascular functions, it is known that patients with Mets have an altered up regulation of RAAS resulting in chronic activation of inflammatory responses [84].
Over the past 10 years, several studies have presented evidence for the existence of a new arm of the RAAS, namely the ACE (angiotensin-converting enzyme) 2/Ang-(1-7) [angiotensin-(1-7)]/Mas axis [85]. Angiotensin-(1-7) can be produced from Ang I or Ang II via endo- or carboxy-peptidases; therefore, ACE2 appears to play a central role in Ang-(1-7) formation. Recent studies have shown that the Ang-(1-7)/Mas axis not only modulate both lipid and glucose metabolism, but also counter regulate the effects of Ang II [86].
Furthermore, for over two decades, different subsets of Th1 interferon-γ-producing and Th2 interleukin-4 producing lymphocytes, as well as Th17 producing interleukin-17 and T-suppressor lymphocytes that participate as pro- and anti-inflammatory cells have been shown to participate in the process of vascular remodeling that occurs with hypertension [87]. In addition, the role of pro-inflammatory T-lymphocytes has also been shown to mediate the effects of Ang II and mineralocorticoids in both Dahl-salt sensitive and spontaneously hypertensive rats [87,88].
Though the specific mechanism mediating this activation of immunity remains largely unknown, it has been proposed that formation of neo-antigens could be generated by elevated blood pressure through damage-associated molecular pathways. Moreover, Th1 cells once activated may contribute to increases in systemic blood pressure through the interaction of cytokines produced or through their effects on perivascular fat [88].
Obviously, confirmation of these mechanisms in humans might provide new therapeutic venues not only to change our current approach to managing hypertension, but also how we can improve CVD outcomes.
DYSLIPIDEMIA
Overproduction of very-low-density lipoprotein remnants with apolipoproteins B-100, small low-density lipoprotein particles, and reduced levels of high-density lipoprotein cholesterol are the primary dyslipidemic abnormalities of most MetS patients [89]. The triglyceride portion in very-low-density lipoprotein remnants is initially hydrolyzed by lipoprotein lipase to intermediate density lipoproteins, and these in turn are further hydrolyzed into low-density lipoprotein particles [90]. The cholesterol esters in these low-density lipoproteins are then exchanged for triglycerides in very-low density lipoproteins by cholesterol ester transfer proteins, followed by hydrolysis of triglycerides in low-density lipoproteins by hepatic lipase, which produces small, dense low-density lipoproteins.
In adipocytes, reduced fatty acid trapping and retention by adipose tissue may result from a primary defect in the incorporation of free fatty acids into triglycerides. Alternatively, insulin resistance may promote reduced retention of free fatty acids by adipocytes. Both of these abnormalities lead to increased levels of free fatty acids in plasma, increased flux of free fatty acids back to the liver, enhanced production of triglycerides, decreased proteolysis of Apo B-100, and increased VLDL production [90].
Regardless of their fundamental causes, small, dense low-density lipoproteins particles remain in circulation for longer periods of time; hence, are more susceptible to oxidation. Consequently, these oxidized particles are more prone to enter more easily the arterial wall and thus retained more readily. These trapped small and dense low-density lipoproteins not only promote endothelial dysfunction and enhanced production of procoagulants by endothelial cells, but also appear more atherogenic than normal low-dense lipoproteins.
In addition to the abnormalities described with regards to low-density lipoproteins, we also need to be reminded of the long-standing association existing between elevated triglycerides and CVD [91,92]. This elevated level of triglycerides is known to occur as a result of several clinical conditions [93]; however, for the purpose of this review, isolated elevation of triglycerides should prompt physicians to exclude T2DM or the MetS [94–101]. In addition, high triglycerides are also regularly found in obese individuals [102–105].
In diabetic patients, high triglyceride levels and low HDL concentrations are not only proinflammatory [106], but also elevated levels of triglycerides rather than hyperglycemia results in large release of pro-inflammatory proteins by adipose tissue contributing to CVD [107]. While dyslipidemia has been studied as a component of the Mets, further studies regarding the chronic inflammatory state derived from this pathologic process are still lacking. Specifically, dyslipidemic abnormalities have been mostly studied in conjunction to T2DM and obesity, but their sole contributory effect has not yet been thoroughly elucidated.
NORMAL AGING
Though normal aging is not part of the MetS is an inevitable universal truth for every living organism associated with the ultimate exposure to various chronic ailments and diseases; hence somewhat related to changes in inflammation and the body response to these changes.
The normal process of aging is known to be associated with increased total body fat, particularly central obesity, that unfortunately contributes to a number of important health problems such as insulin resistance, cardiovascular disease, sarcopenia and disability [108–112]. With regards to the particular kind of adipose tissue that contributes to such problems, white adipose tissue has been proposed to be a key regulator of lifespan. In several model organisms, genetic manipulations that modify fat mass also impact on life expectancy, in part through sirtuin 1 (SIRT1) and suppression of the nuclear receptor protein peroxisome proliferator-activated receptors gamma (PPARγ) [113–115].
While the complete underlying mechanism for the association between age-related obesity and disease is not completely understood, adipose tissue inflammation has been identified as a critical regulator of the overall low-level systemic inflammatory milieu in diet-induced and genetic obesity models [116,117].
Adipose tissue is composed of a heterogeneous cell population that becomes evident after fat is further purified through collagenase digestion. This process culminates with the dissociation of adipocytes and stromal vascular fraction. In the latter, the dominant cell components are the leukocytes, mainly macrophages and lymphocytes, and adipose tissue stromal cells, including preadipocytes and fibroblasts [118].
The normal process of aging is associated with profound alterations in the innate immune system. First, there are significant alterations in the T and B cell compartments, involution of the thymus gland, functional decline in the monocytes and macrophages, low expression of Toll-like receptors from activated splenic and peritoneal macrophages, and an altered secretion of several chemokines and cytokines [119]. Second, aging decreases both humoral and cellular immune responses [120,121]. Third, residential macrophages impair the proliferative response of activated peripheral T-lymphocytes, and paired to neutrophils they can sometimes exhibit inappropriate respiratory bursts with concomitant release of reactive nitrogen and oxygen intermediates which may decrease the ability to destroy pathogens [122]. Furthermore, aged dendritic cells have been reportedly found to be less efficient in activating T and B cell populations and aged natural killer cells exhibit a reduced ability and efficiency in killing tumor cells. Fourth, mitogen activated peripheral blood mononuclear cells isolated from elderly population not only show a higher production of pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α ex-vivo, compared to young people; but also there is an up-regulation of COX-2 expression leading to an increase in the production of prostaglandin E2, a critical regulator, of age-related inflammatory changes [123]. Fifth, reduced efficacy of vaccine-induced protection against infections/diseases and poor response to new pathogens, mainly due to defective T, suggest that naive CD4 cells are defective in generating efficient memory and are found to produce less IL-2 and exhibit poor proliferation and differentiation upon antigen stimulation in older mice [124–126]. Finally, alterations in B cells have also been recognized in age-related changes in immune system. Specifically, available antibody repertoires to specific antigens and pathogens are markedly different in old vs. young splenic or peripheral blood B cells [121,127]. In addition, peripheral B cell lymphocyte percentages and numbers significantly decrease with age. Antibodies generated in old mice (20 months or older) and in humans (65 years or older) are less protective compared with the antibodies generated in the young individuals [128,129].
It has been widely conceptualized that chronic antigenic stress throughout life causes the accumulation of molecular and cellular scars, which act as potential triggers in mounting the inflammatory response associated with the pathogenesis of all age related diseases [130]. Thus, an underlying low grade inflammatory activity seen in the elderly, coupled with a decreased overall concentration of sex steroids; changes in life style patterns including smoking history and obesity; as well as a low grade of cytokine production caused by sub-clinical disorders due to asymptomatic infection with bacterium; leads to increased levels of circulating TNF-α, IL-6, soluble IL-2 receptors, C reactive protein and cholesterol, which act as inflammatory mediators [130–133]. Similarly, previous exposure to past infections is an additional risk factor that leads to a rise in the levels of chronic inflammatory markers and subsequent development of an increased susceptibility to risk of heart attack, stroke, and cancer [134]. Current evidence seems to point out that this chronic, low-grade inflammatory process, characterized by increased levels of cytokines, might develop in otherwise healthy individuals possibly as early as the age of 55 years old [135].
For example, in the heart muscle, this chronic inflammatory process leading to mitochondrial damage results in an increased free radical production that further activates the chronic inflammatory vicious cycle. Without a proper defense mechanism, this positive feedback loop exacerbates oxidative damage, reduction in ATP production, loss of cardiomyocytes, and formation of fibrotic tissue [135–138]. Furthermore, while aging and inflammation had differential effects on the expression levels of AT1R, recent experimental data using the mice model suggest an increase in AT1R:AT2R under these conditions and supports the notion that it may contribute to the pathogenesis of cardiomyopathies [139].
Similarly, neuro-inflammation and cytokine production have been acknowledged as potential triggers of the functional changes occurring in the brain during “normal” and “pathological” aging. In particular, the aged brain seems to be characterized by increased levels of pro-inflammatory cytokines [140]. A growing number of reports have now shown that cytokines may specifically interact with neuronal channels regulating neuronal excitability, synaptic plasticity and responses to injury.
Based on currently available data, a common pathway linking normal aging with known associated abnormalities is associated with a general decline in mitochondrial function [141–142]; which in turn not only reduces ATP production [143], but also affects the overall oxidative stress as the mitochondria regulates reactive oxygen species [140]. Even though the specific role of reactive oxygen species as the sole cause of aging remains controversial, it is known that accumulation of reactive oxygen species as a result of a dysfunctional mitochondria due to normal aging results in activation of protein 3 inflammasome in macrophages, which produce proinflammatory cytokines [144]. Accumulation of this protein impairs cellular housekeeping and expose the cell to higher risk in many age-related diseases such as atherosclerosis and T2DM. Obviously, a change in the endogenous concentration of reactive oxygen species would then alter the activity of antioxidants. Since an increased antioxidant activity can decrease the potential for CVD development by regulating reactive oxygen species and nitric oxide production [145–146]; subsequent attenuation of shear stress can then reduce the activation of endothelial nitric oxide synthase activity. This would result in the reduction of nitric oxide that will ultimately affect vasodilatory responses, leading to consequential endothelial dysfunction and vascular injury [147].
Therefore, better recognition of the physiologic and pathologic changes that occur with normal aging would definitively improve our understanding of the complexities involving the well-recognized process of chronic inflammation that occurs with normal aging, placing patients at an inordinate risk of CVD.
ARE BIOMARKERS READY FOR PRIME TIME?
It is quite evident that significant advances have occurred in our understanding of CVD. Nonetheless, additional breakthroughs will undoubtedly occur as we gather more data regarding alterations in inflammatory markers and how these changes cause vascular injury and accelerated atherosclerosis. Though considerable controversy still exists in how to diagnose individuals at a higher risk of developing cardiovascular disease, it is important to acknowledge the utility of C-reactive protein (CRP). Results from multiple large-scale prospective studies have demonstrated the utility of CRP in predicting adverse cardiovascular events such as myocardial infarction, ischemic stroke, and sudden cardiac death [148,149]. In fact, the addition of high sensitive CRP (hsCRP) information has shown to add prognostic information beyond that available from the well-known Framingham Risk Score [150].
Furthermore, the initial result from the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) trial showing that 20 mg of rosuvastatin significantly reduced the primary composite endpoint by 44% when compared to placebo, clearly suggests that patients with elevated CRP stand to benefit from statin therapy, regardless of their LDL-C level [151]. Though this study was mainly focused on secondary outcomes and not on primary prevention.
On the last publication by the American College of Cardiology/American Heart Association task force on practice guidelines, it was recommended that hs-CRP might be used to inform treatment decision making if after quantitative risk assessment a treatment decision remains uncertain [152].
In addition, the new venues of inflammatory markers open new opportunities. For example, we wait with great enthusiasm for the results of ongoing trials such as CANTOS in which Interleukin-1β inhibition using cankinumab is used; CIRT that uses low-dose methotrexate; and COLCOT evaluating if long-term use of colchicine reduces CVD events in patients post myocardial infarction. Each of these trials is driven by protocols that utilizes anti-inflammatory measures in an attempt to reduce CVD events among stable coronary disease patients who remain at risk of another events due to a persistent pro-inflammatory response [153,154]. Moreover, some other targets such as the IL-6 pathway appear also promising.
Therefore, continued vigilance of the results of ongoing trials is required, not only to determine which markers would be indeed critical in identifying individuals at a higher risk of cardiovascular disease, but also to identify which therapies might be useful on improving cardiovascular health.
CONCLUSION
While several therapeutic modalities have been identified to target inflammation in the basis of MetS, ongoing studies continue to surface new molecular and cellular pathways that could be potential links to the pathogenesis of cardiovascular comorbidities in MetS. These findings could aid in the premature identification of high-risk individuals and in the development of goal-targeted treatment. Also, additional studies are warranted to determine whether anti-inflammatory medications such as NSAIDs and colchicine can be effective in preventing the complications of obesity.
Acknowledgments
This publication was partially supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health Award Numbers CCTRECD-R25MD007607 and HiREC-S21MD001830. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: No conflicts declared.
References
- 1.Reeves MJ, Rafferty AP. Healthy lifestyle characteristics among adults in the United States, 2000. Arch Intern Med. 2005;165:854–857. doi: 10.1001/archinte.165.8.854. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics: 2005 Update. American Heart Association; Dallas, TX: 2005. [Google Scholar]
- 3.Ebstein W. Zur therapie des diabetes mellitus, insbesondere über die Answendung des salicylsauren natron bei demselben. Berlin Klin Wochenschrift. 1876;13:337. [Google Scholar]
- 4.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006 Mar;17(1):4–12. [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation: European Group for the Study of Insulin Resistance (EGIR) Diabetic Medicine. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 7.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Journal of the American Medical Association. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocrine Practice. 2003;9:237–252. [PubMed] [Google Scholar]
- 9.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. http://www.idf.org/metabolic-syndrome.
- 10.Kaur J. A Comprehensive Review on Metabolic Syndrome. Cardiology Research and Practice. 2014:1–21. doi: 10.1155/2014/943162. Article ID 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; And international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KGMM, Zimmet P. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 13.Olijhoek JK, Van Der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FLJ. The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. European Heart Journal. 2004;25:342–348. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Desroches S, Lamarche B. The evolving definitions and increasing prevalence of the metabolic syndrome. Applied Physiology, Nutrition and Metabolism. 2007;32:23–32. doi: 10.1139/h06-095. [DOI] [PubMed] [Google Scholar]
- 15.Kolovou GD, Anagnostopoulou KK, Salpea KD, Mikhailidis DP. The prevalence of metabolic syndrome in various populations. The American Journal of the Medical Sciences. 2007;333:362–371. doi: 10.1097/MAJ.0b013e318065c3a1. [DOI] [PubMed] [Google Scholar]
- 16.Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi D, et al. Metabolic syndrome is associated with early signs of organ damage in nondiabetic, hypertensive patients. J Intern Med. 2005;257:454–460. doi: 10.1111/j.1365-2796.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 19.Despre’s JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 21.Reaven GM. The individual components of the metabolic syndrome: is there a raison d’etre? J Am Coll Nutr. 2007;26:191–195. doi: 10.1080/07315724.2007.10719601. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 23.Klőting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 24.de Luis DA, Sagrado MG, Conde R, et al. Relation of resistin levels with cardiovascular risk factors and insulin resistance in non-diabetes obese patients. Diab Res Clin Pract. 2009;84:174–178. doi: 10.1016/j.diabres.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Karayannis G, Giamouzis G, Tziolas N, et al. Association between epicardial fat thickness and weight homeostasis hormones in patients with noncachectic heart failure. Angiology. 2013;64:173–180. doi: 10.1177/0003319712447978. [DOI] [PubMed] [Google Scholar]
- 26.Gluba A, Mikhailidis DP, Lip GY, Hannam S, Rysz J, Banach M. Metabolic syndrome and renal disease. Int J Cardiol. 2013;164:141–150. doi: 10.1016/j.ijcard.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- 29.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrrague P, Casteilla L, Penicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 31.Totonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 32.Nagy L, Totonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 33.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinology and Metabolism Clinics of North America. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of Lipid Research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. The American Journal of Physiology—Heart and Circulatory Physiology. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 37.Ebron K, Andersen CJ, Aguilar D, Blesso CN, Barona J, Dugan CE, Jones JL, Al-Sarraj T, Fernandez ML. A Larger Body Mass Index is Associated with Increased Atherogenic Dyslipidemia, Insulin Resistance, and Low-Grade Inflammation in Individuals with Metabolic Syndrome. Metab Syndr Relat Disord. 2015 Oct 2; doi: 10.1089/met.2015.0053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Slagter SN, van Vliet-Ostaptchouk JV, van Beek AP, Keers JC, Lutgers HL, van der Klauw MM, Wolffenbuttel BH. Health-Related Quality of Life in Relation to Obesity Grade, Type 2 Diabetes, Metabolic Syndrome and Inflammation. PLoS One. 2015 Oct 16;10(10):e0140599. doi: 10.1371/journal.pone.0140599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques-Rocha JL, Milagro FI, Mansego ML, Zulet MA, Bressan J, Martínez JA. Expression of inflammation-related miRNAs in white blood cells from subjects with metabolic syndrome after 8 week of following a Mediterranean diet-based weight loss program. Nutrition. 2015 Jul 17; doi: 10.1016/j.nut.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33:1665–73. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 42.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23:1113–8. doi: 10.2337/diacare.23.8.1113. [DOI] [PubMed] [Google Scholar]
- 43.Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933–46. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 44.Haffner SM, Stern MP, Gruber MK, Hazuda HP, Mitchell BD, Patterson JK. Microalbuminuria. Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis. 1990;10:727–31. doi: 10.1161/01.atv.10.5.727. [DOI] [PubMed] [Google Scholar]
- 45.Plantinga LC, Crews DC, Coresh J, Miller ER, 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–82. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Player MS, Diaz VA, Mainous AG, 3rd, Gregorie SH, Knoll ME, Everett CJ. Ethnic differences in the relationship of prediabetes with the presence of target-organ disease. Diabetes Metab. 2011;37:403–9. doi: 10.1016/j.diabet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 48.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 49.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 50.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33:804–10. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Maratha A, Gajanayake T, Siednienko J, Natarajan A, Hoashi S, et al. Cytokine profiling of pre-diabetic patients. Presented at Society for Endocrinology BES 2011; Birmingham, UK. 2011; Endocrine Abstracts. [Google Scholar]
- 52.Lin J, Zhang M, Song F, Qin J, Wang R, Yao P, et al. Association between C-reactive protein and pre-diabetic status in a Chinese Han clinical population. Diabetes Metab Res Rev. 2009;25:219–23. doi: 10.1002/dmrr.923. [DOI] [PubMed] [Google Scholar]
- 53.Cardellini M, Andreozzi F, Laratta E, Marini MA, Lauro R, Hribal ML, et al. Plasma interleukin-6 levels are increased in subjects with impaired glucose tolerance but not in those with impaired fasting glucose in a cohort of Italian Caucasians. Diabetes Metab Res Rev. 2007;23:141–5. doi: 10.1002/dmrr.679. [DOI] [PubMed] [Google Scholar]
- 54.Konukoglu D, Hatemi H, Bayer H, Bagriacik N. Relationship between serum concentrations of interleukin-6 and tumor necrosis factor alpha in female Turkish subjects with normal and impaired glucose tolerance. Hormone and Metabolic Research. 2006;38:34–37. doi: 10.1055/s-2006-924974. [DOI] [PubMed] [Google Scholar]
- 55.KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. Comparison of serum concentrations of C-reactive protein, TNF-alpha, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract. 2004;64:99–106. doi: 10.1016/j.diabres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Szelachowska M, Kinalska I. Plasma interleukin 8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc Diabetol. 2003;2:5. doi: 10.1186/1475-2840-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–S17. 33. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 58.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- 59.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 60.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 61.Paz K, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272:29911–29918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 62.Zick Y. Role of Ser/Thr kinases in the uncoupling of insulin signaling. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S56–S60. doi: 10.1038/sj.ijo.0802503. [DOI] [PubMed] [Google Scholar]
- 63.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 64.Stienstra R, Tack CJ, Kanneganti TD, et al. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–8. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Akasheh RT, Pang J, York JM, et al. New pathways to control inflammatory responses in adipose tissue. Curr Opin Pharmacol. 2013 May 3; doi: 10.1016/j.coph.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 68.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999–2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999–2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html.
- 71.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 72.Dunjak L, Bulum T, Metelko Z. Hypertension and the Metabolic Syndrome. Diabetologia Croatica. 2008:37–4. [Google Scholar]
- 73.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015 Mar 13;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carroll JF, Huang M, Hester RL, Cockrell K, Mizelle HL. Hemodynamic alterations in hypertensive obese rabbits. Hypertension. 1995;26:465–470. doi: 10.1161/01.hyp.26.3.465. [DOI] [PubMed] [Google Scholar]
- 75.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 76.Hall JE, Brands MW, Dixon WN, Smith MJ. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 77.Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, Frohlich ED. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981;141:81–85. doi: 10.1001/archinte.141.1.81. [DOI] [PubMed] [Google Scholar]
- 78.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999;892:91–107. doi: 10.1111/j.1749-6632.1999.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 79.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life. 2013;65:692–698. doi: 10.1002/iub.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens. 2013;22:135–140. doi: 10.1097/MNH.0b013e32835d0c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.da Silva AA, do Carmo JM, Wang Z, Hall JE. The brain melanocortin system, sympathetic control, and obesity hypertension. Physiology (Bethesda) 2014;29:196–202. doi: 10.1152/physiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. J Hypertens. 1999;17:555–560. doi: 10.1097/00004872-199917040-00014. [DOI] [PubMed] [Google Scholar]
- 84.Zhou MS, Schulman I, Zeng Q. Link between the reninangiotensin system and insulin resistance: Implications for cardiovascular disease. Vascular Medicine. 2012;17(5):330–341. doi: 10.1177/1358863X12450094. [DOI] [PubMed] [Google Scholar]
- 85.Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardiorenal actions. Clin Sci (Lond) 2013 Apr;124(7):443–56. doi: 10.1042/CS20120461. [DOI] [PubMed] [Google Scholar]
- 86.Santos SH, Andrade JM. Angiotensin 1-7: a peptide for preventing and treating metabolic syndrome. Peptides. 2014 Sep;59:34–41. doi: 10.1016/j.peptides.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014 Feb;126(4):267–74. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 88.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 2013 May;29(5):543–8. doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Grundy SM. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone. 2006;8(Suppl 1):S21–7. doi: 10.1016/s1098-3597(06)80005-0. [DOI] [PubMed] [Google Scholar]
- 90.Kwiterovich PO., Jr Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002 Oct 17;90(8A):30i–47i. doi: 10.1016/s0002-9149(02)02749-2. [DOI] [PubMed] [Google Scholar]
- 91.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 92.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 93.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011 May 24;123(20):2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 94.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 95.D’Agostino RB, Jr, Hamman RF, Karter AJ, Mykkanen L, Wagenknecht LE, Haffner SM Insulin Resistance Atherosclerosis Study Investigators. Cardiovascular disease risk factors predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:2234–2240. doi: 10.2337/diacare.27.9.2234. [DOI] [PubMed] [Google Scholar]
- 96.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 97.Betteridge DJ. Diabetes, lipoprotein metabolism and atherosclerosis. Br Med Bull. 1989;45:285–311. doi: 10.1093/oxfordjournals.bmb.a072317. [DOI] [PubMed] [Google Scholar]
- 98.Kasai T, Miyauchi K, Kurata T, Ohta H, Okazaki S, Miyazaki T, Kajimoto K, Kubota N, Daida H. Prognostic value of the metabolic syndrome for long-term outcomes in patients undergoing percutaneous coronary intervention. Circ J. 2006;70:1531–1537. doi: 10.1253/circj.70.1531. [DOI] [PubMed] [Google Scholar]
- 99.Anderson JL, Horne BD, Jones HU, Reyna SP, Carlquist JF, Bair TL, Pearson RR, Lappé DL, Muhlestein JB Intermountain Heart Collaborative (IHC) Study. Which features of the metabolic syndrome predict the prevalence and clinical outcomes of angiographic coronary artery disease? Cardiology. 2004;101:185–193. doi: 10.1159/000076695. [DOI] [PubMed] [Google Scholar]
- 100.Karadag MK, Akbulut M. Low HDL levels as the most common metabolic syndrome risk factor in heart failure. Int Heart J. 2009:50. doi: 10.1536/ihj.50.571. [DOI] [PubMed] [Google Scholar]
- 101.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud’homme D, Nadeau A, Després JP. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 102.Centers for Disease Control and Prevention. Prevalence of abnormal lipid levels among youths: United States, 1999–2006 [published correction appears in MMWR Morb Mortal Wkly Rep. 2010;59:78] MMWR Morb Mortal Wkly Rep. 2010;59:29–33. [PubMed] [Google Scholar]
- 103.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 104.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–1420. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 105.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 106.Lee SH, Woo HY, Baik EJ, Moon CH. High glucose enhances IL-1β-induced cyclooxygenase-2 expression in rat vascular smooth muscle cells. Life Sci. 2000;68:57–67. doi: 10.1016/s0024-3205(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 107.Meerarani P, Badimon JJ, Zias E, Fuster V, Moreno PR. Metabolic syndrome and diabetic atherothrombosis: implications in vascular complications. Curr Mol Med. 2006;6:501–514. doi: 10.2174/156652406778018680. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar APN, Sarkar FH. Aging and Inflammation: Etiological Culprits of Cancer. Curr Aging Sci. 2009;2(3):174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 110.Heymsfield SB, Gallagher D, Poehlman ET, Wolper C, Nonas K, Nelson D, Wang ZM. Menopausal changes in body composition and energy expenditure. Exp Gerontol. 1994;29:377–389. doi: 10.1016/0531-5565(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 111.Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, Bergamo-Andreis IA, Bosello O. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 112.Pascot A, Lemieux S, Lemieux I, Prud’homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, Despres JP. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 113.Argmann C, Dobrin R, Heikkinen S, Auburtin A, Pouilly L, Cock TA, Koutnikova H, Zhu J, Schadt EE, Auwerx J. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009 doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Howroyd P, Swanson C, Dunn C, Cattley RC, Corton JC. Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) Toxicol Pathol. 2004;32:591–599. doi: 10.1080/01926230490515283. [DOI] [PubMed] [Google Scholar]
- 115.Picard F, Guarente L. Molecular links between aging and adipose tissue. Int J Obes (Lond) 2005;29(1):S36–39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- 116.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 117.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lumeng CN, Liu J, Geletka L, Delaney C, Jennifer DelProposto, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. Immunol. 2011 Dec 15;187(12):6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, et al. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–126. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 121.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180(5):2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 122.Yu BP, Chen JJ, Kang CM, Choe M, Maeng YS, Kristal BS. Mitochondrial aging and lipoperoxidative products. Ann NY Acad Sci. 1996;786:44–56. doi: 10.1111/j.1749-6632.1996.tb39050.x. [DOI] [PubMed] [Google Scholar]
- 123.Meydani SN, Wu D. Age-associated inflammatory changes: role of nutritional intervention. Nutr Rev. 2007;65(12 Pt2):S213–S216. doi: 10.1111/j.1753-4887.2007.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 124.Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- 125.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8(7):512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190(7):1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol. 2000;20(4):240–249. doi: 10.1023/a:1006659401385. [DOI] [PubMed] [Google Scholar]
- 128.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150(2):543–9. [PubMed] [Google Scholar]
- 129.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(23):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 130.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115(4):278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 132.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50(4):638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 134.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 135.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 136.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 137.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burks TN, Marx R, Powell L, Rucker J, Bedja D, Heacock E, Smith BJ, Foster DB, Kass D, O’Rourke B, Walston JD, Abadir PM. Combined effects of aging and inflammation on renin-angiotensin system mediate mitochondrial dysfunction and phenotypic changes in cardiomyopathies. Oncotarget. 2015;6:11979–11993. doi: 10.18632/oncotarget.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Viviani B, Boraso M. Cytokines and neuronal channels: a molecular basis for age-related decline of neuronal function? Exp Gerontol. 2011;46(2–3):199–206. doi: 10.1016/j.exger.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 141.Yen WL, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- 142.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chuang SY, Lin CH, Fan JY. Natural Compounds and Aging: Between Autophagy and Inflammasome. Biomed Res Int. 2014;2014:297293. doi: 10.1155/2014/297293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 146.Fukai T, Folz RJ, Landmesser U, Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 147.Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He G-W, et al. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res. 2011;92:267–275. doi: 10.1093/cvr/cvr233. [DOI] [PubMed] [Google Scholar]
- 148.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 149.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 150.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004 Jun 15;109(23):2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 151.Watson KE. The JUPITER trial: How will it change clinical practice? Rev Cardiovasc Med. 2009 Spring;10(2):91–6. [PubMed] [Google Scholar]
- 152.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep. 2013 Jan;15(1):295. doi: 10.1007/s11883-012-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nidorf SM, Eikelboom JW, Thompson PL. Colchicine for secondary prevention of cardiovascular disease. Curr Atheroscler Rep. 2014 Mar;16(3):391. doi: 10.1007/s11883-013-0391-z. [DOI] [PubMed] [Google Scholar]