Abstract
Background
It is probable that the great majority of human cataract results from the spontaneous decomposition of long-lived macromolecules in the human lens. Breakdown/reaction of long-lived proteins is of primary importance and recent proteomic analysis has enabled the identification of the particular crystallins, and their exact sites of amino acid modification.
Scope of review
Analysis of proteins from cataractous lenses revealed that there are sites on some structural proteins that show a consistently greater degree of deterioration than age-matched normal lenses.
Major conclusions
The most abundant posttranslational modification of aged lens proteins is racemization. Deamidation, truncation and crosslinking, each arising from the spontaneous breakdown of susceptible amino acids within proteins, are also present. Fundamental to an understanding of nuclear cataract etiology, it is proposed that once a certain degree of modification at key sites occurs, that protein-protein interactions are disrupted and lens opacification ensues.
General Significance
Since long-lived proteins are now recognized to be present in many other sites of the body, such as the brain, the information gleaned from detailed analyses of degraded proteins from aged lenses will apply more widely to other age-related human diseases.
Brief Background on Human Cataract
Cataract is an opacification of the lens that interferes significantly with vision. In humans, by far the major risk factor is age. There are three types: age-related nuclear cataract (ARNC), posterior subcapsular and cortical cataract. ARNC is typically colored, affects primarily the center of the lens, and is the main subject of this review.
Until very recently, the cause of ARNC remained a mystery. Clearly aging played a key role since almost all human cataract occurs in elderly people. Animal experiments were generally not particularly instructive. Cataract can be induced readily in experimental animals, by many agents [1] but, with few exceptions, it was unclear if the routes to lens opacification in the laboratory had parallels in the human population. These experimental data on animals, together with the range of clinical presentations of human cataract, lead to a pervasive view that human cataract was ‘multifactorial’. Recent data and insights challenge this view, at least with respect to ARNC. There may indeed be an underlying mechanism responsible for the majority of human age-related cataract!
The lack of a unifying mechanism that could explain most human cataract remained until very recently. Several key findings relating to cataract were published prior to the advent of proteomics and these are listed briefly, below. Of particular importance for understanding human ARNC, were studies that revealed the importance of glutathione for maintaining lens transparency [2], the fact that the abundant lens protein, α-crystallin, is a chaperone [3] and that the amount of this small heat shock protein decreases with age until none of the active form remains in the center of the human lens by age 40 [4]. Oxidation was found to be a characteristic feature of cataractous lens proteins [5] and importantly the degree of protein oxidation correlated with the grade of cataract [6].
Understanding lens aging and its role in cataract
Ultimately two realisations were fundamental to understanding the etiology of human ARNC. Firstly, that proteins in the lens do not turnover. They are present for life, and this has been confirmed using 14-C techniques [7]. Secondly, that lens polypeptides break down over time. Once these two factors were recognized, some important questions arose.
Do all lens proteins degrade? Which sites are most susceptible and to what extent, and over what time frame? What are the major posttranslational modifications (PTMs) and are they consistent from lens to lens? Are there specific PTMs in cataractous lenses that are less abundant, or absent, in normal age-matched controls? Could these changes alone be responsible for converting the normal transparent lens into a cloudy opaque one? Answers to some of these questions have now emerged.
The human lens as a chemical laboratory
Because of the unique growth of the lens, and thermal denaturation over many years, the interior of the human adult lens contains very few, if any, active enzymes. As a consequence, the lens nucleus can usefully be regarded as a flask. In this container are proteins, membrane lipids and the small molecules typical of cells, but no enzymes. Only the metabolites are able to diffuse through the walls of the flask; the lipids and proteins are confined to the container. Over time at 35°C, the macromolecules change to varying degrees. Since the major component is protein, instability of polypeptides is likely to have the most effect on the overall properties of the lens.
There are two major categories of protein PTMs
One type of PTM arises from covalent modification by reactive small molecules (a), the other from the intrinsic instability of certain amino acids (b).
a) Protein modification by cellular metabolites
A number of classes of biomolecules contain chemicals that can covalently attach to proteins. One well-known example involves the formation of advanced glycation end products (AGEs). These are formed by the nucleophilic attack of amino acid residues on carbonyl compounds [8] and these aldehydes and ketones are typically produced in the body by the breakdown of carbohydrates. Long-lived proteins in the body can thus be modified by sugars, and/or their metabolites (e.g. methylglyoxal). The decomposition of other biomolecules, for example ascorbate and fatty acids, can also result in formation of similar reactive carbonyls.
In the human lens the major PTM due to reaction with biochemicals appears to be methylation [9, 10] and the source is S-adenosyl methionine [10]. Given the sustained high levels of glucose in the lens, the levels of AGEs, such as carboxymethyl or carboxyethyl Lys and Arg, are much less e.g. [11, 12] (Table 1). It is likely that glutathione which is present at very high levels in the lens, acts as a nucleophilic scavenger, and thus spares proteins from covalent modification and the formation of AGEs. The very low AGE content of proteins, even in old lenses, suggests that this type of PTM may be of less importance in causing changes to lens proteins that lead the induction of cataract.
Table 1.
Levels of various posttranslational modifications in normal aged human lens proteins. Values are for normal human lenses aged 60–70.
| Modification | Amount (mmol/mol protein)# |
|---|---|
| Racemisation | |
| D-Asp | 1100–1800[13] |
| D-Ser | 500–1100[13] |
| D-Thr | 100–200[13] |
| Deamidation | 50–500[26] |
| AGEs | |
| Carboxymethyl Lys | 14–35, 0–0.1[12, 66] |
| Carboxyethyl Lys | 7–28[12] |
| GOLD | 0.14–1.4[67] |
| MOLD | 0.7–5.6[67] |
| OP-Lys | 0.01[68, 69] |
| K2P | 0.01[70] |
| Ornithine | 0–0.3[71] |
| Methylglyoxal-Derived Hydroimidazolones (MG-H1+MG-H2) | 40–197[72] |
| Reactive molecule addition | |
| Methylation | 10–250[10] |
| Oxidation markers | |
| Ortho-Tyrosine | 1.8–5.4[57] |
| Di-Tyrosine | 6–18[57] |
| Methionine sulfoxide | ~0[56] |
AGEs, Advanced Glycation Endproducts.
It should be noted that for proper comparison, analyses of the different types of PTMs should be performed on the same lenses. Since this Table reports the values from different authors, this information was not available. AGEs are also likely to be comprised of a heterogeneous mix of products.
b) Spontaneous decomposition of amino acids in proteins
This category of PTM, arising from intrinsic amino acid instability, is the most abundant. Indeed the levels found in human lenses typically exceed those in section (a) by orders of magnitude (Table 1).
The amino acid residues that are particularly susceptible to age-related decomposition are Ser, Asp, Asn and Ser phosphate. Lesser roles in protein denaturation may be ascribed to modifications of Thr, Thr phosphate and Cys.
Aging of proteins involves cleavage of peptide bonds, isomerisation, crosslinking and deamidation
Lens proteins become progressively more insoluble with age and the properties of a particular polypeptide from a young lens will not be the same as those of the modified protein from an old normal human lens, or a cataractous lens. This factor can lead to ‘isolation artefacts’ where a crystallin peak from a young lens will not contain the same components as the peak from an old lens. This being so, rather than attempting to track, over time, changes to one purified protein from the lens, the optimal approach is to examine whole lens tissue without prior protein purification. The high resolving power of modern proteomics allows unfractionated lens samples to be investigated. Isomerisation, crosslinking, deamidation and cleavage of peptide bonds have been found to be major PTMs of human lens proteins. These will be discussed individually.
Isomerisation/Racemization
The three main amino acids involved in age-dependent isomerization/racemization are Asn, Asp (Figure 1) and Ser. Thr and Phe are implicated to a lesser extent.
Figure 1.
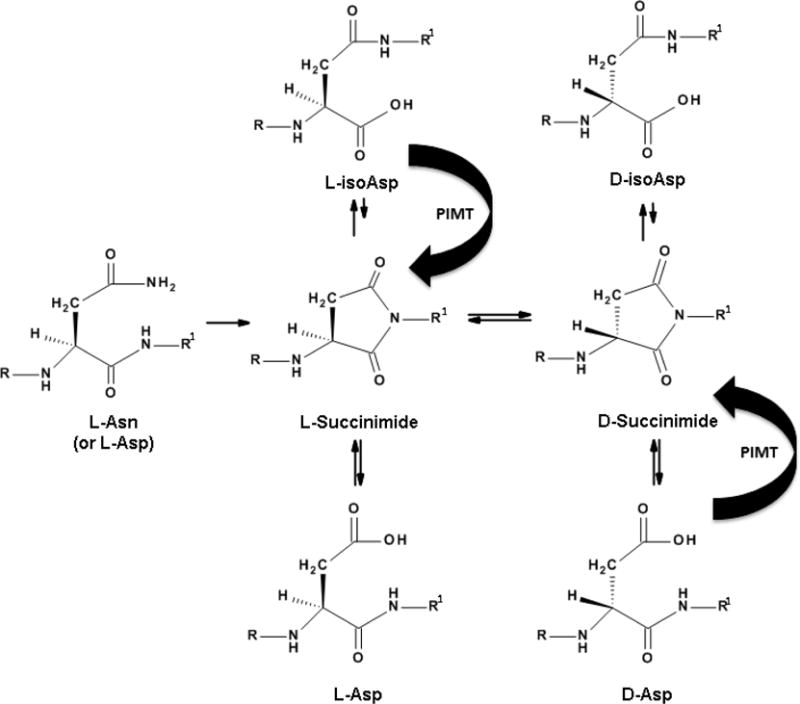
Long-lived proteins decompose in the body. In lens proteins, and other long-lived proteins, major degradative processes involve racemisation. These are spontaneous events that particularly affect aspartate, asparagine and serine residues in unstructured regions of the protein. In lens proteins the major end product of L-Asn and L-Asp breakdown is D-isoAsp, which arises via succinimide intermediates. isoAsp peptides appear to be stable and undergo little interconversion [20]. In most cells (but not the human lens nucleus) protein isoaspartate methyl transferase (PIMT) can partially ameliorate Asp racemization in long-lived proteins.
The racemization of these amino acids as a function of age and cataract was first established by the use of acid digestion of dissected lens regions coupled with HPLC separation of the resulting D- and L-amino acids [13]. This technique showed that Asp and Asn were the major amino acids involved in racemization (Figure 2), with Ser the next most abundant. D-Thr and D-Phe levels increased to a lesser extent as a function of age.
Figure 2.
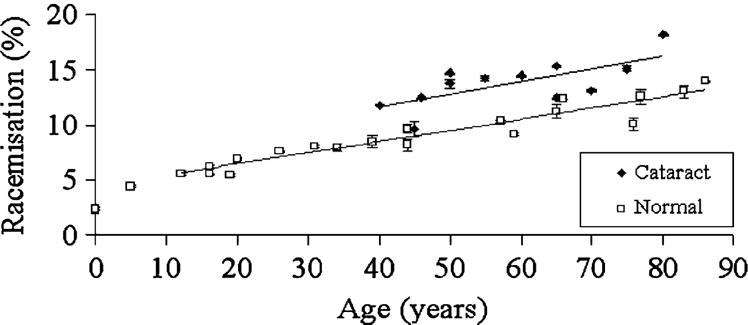
Overall racemisation of proteins is greater in cataractous lenses than in normal lenses. Racemisation of Asx (i.e. Asp + Asn) as a function of age, in normal and cataractous lens proteins. Racemisation expressed as a % of D/(D+L). From ref [13] and used with permission.
Identifying the sites of racemization within individual crystallins involved more complex experiments. Some sites, such as Asp 58 and Asp 151 in αA-crystallin had already been identified [14–16].
MS/MS spectra alone do not generally permit identification of the site of L- to D-racemization in a protein, therefore LC/MS/MS is necessary. If more than one tryptic peptide peak with the same MS/MS spectrum was found in LC chromatograms of lens digests, then these were investigated in more detail as potential sites for racemization. To confirm the sites of racemization, peptides with the same amino acid sequences, but differing in one (or more) site by the replacement of an L- by a D-amino acid, were synthesized commercially and each subjected to the same LC/MS/MS protocol. Such procedures can be done readily now, since peptide synthesis is rapid and inexpensive. A novel method for analyzing Asp/Asn racemization has recently been published [17].
There are two major types of isomerization/racemization of amino acids in proteins. The simplest one involves transformation of L- to D-amino acids via removal of the hydrogen atom attached to the α-carbon atom. Re-attachment of a proton can produce a D-amino acid. This seems to be the predominant route of racemization for amino acids such as Ser and Thr and Phe. Recently D-Ser has been found to accumulate linearly with age at two sites in αA-crystallin and to be higher in cataractous lenses [18].
In the case of Asn and Asp, the situation is more complex but this complexity assists in the identification of peptides that have been isomerized. The main process of racemization of Asn and Asp involves intramolecular cyclisation (see figure 1) [19]. A succinimde ring forms and this permits facile racemization. Once the ring opens, a D-amino acid can result. Since there are two potential sites of hydrolysis of the succinimide, more than one isomer can result and typically four distinct isoforms are produced: L-Asp, D-Asp, L-isoAsp and D-isoAsp (Figure 3). The identification of the isoAsp forms, provides strong evidence that cyclisation has taken place [19] rather than simple abstraction of the α-proton from the amino acid.
Figure 3.
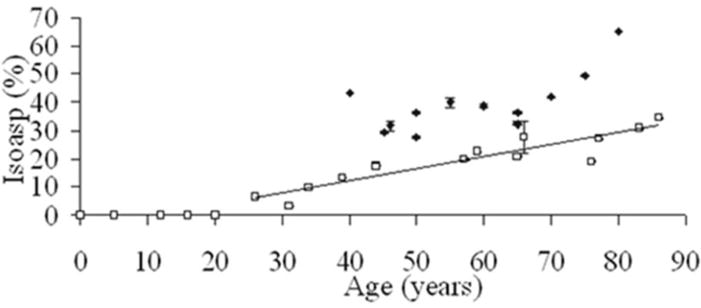
Racemisation at specific sites on crystallins may be cataractogenic. Deamidation of Asn 76 in γS crystallin as a function of age, in normal and cataractous lens proteins. The content of (L+D)-isoaspartic acid (isoAsp) at residue 76 following tryptic digestion of whole nuclear lens protein and LC/MS/MS. If deamidation of Asn occurs via a cyclic intermediate, four Asp isoforms are produced, two of which are isoAsp peptides – see Fig 1. (Symbols: □ Normal ◆ Cataract). From ref [28] and used with permission.
MS/MS spectra of the isoAsp forms are different from those of the L- or D-Asp peptides [20] so this is a useful indicator that isomerization of Asp or Asn has taken place. Asn racemises more readily than Asp and the products will be the four Asp isomers referred to above [21]. Once an Asn residue is cyclized and converted to an Asp isomer, it cannot be regenerated.
The four isoforms of Asp are often depicted in the literature as being in equilibrium. This simple view is unlikely to be correct. One peptide has been investigated in detail. In this α-crystallin peptide, the L- and D-Asp forms interconverted readily, whereas the two isoAsp peptides were very stable and showed essentially no reversion to the L- and D-Asp versions despite prolonged incubation [20]. In practical terms, this means that once D-isoAsp forms in a protein, this PTM may be irreversible. This feature, and the part played by adjacent amino acids, needs further investigation. Within cells (but not cells in the lens nucleus) protein isoaspartate methyl transferase (PIMT) can catalyse the reversion of L-isoAsp to L-Asp. PIMT is however inactive on D-isoAsp.
The extent of racemization of proteins in an adult lens is remarkable. It can be calculated that in a 60 year-old normal lens that, on average, every crystallin contains between 2 and 3 racemized amino acids [13]. This extent of racemization will almost certainly result in destabilization of the proteins. This is especially so since isoAsp residues are abundant, and each site of isoAsp disrupts the regular peptide bond sequence by insertion of a methylene group.
Deamidation
Deamidation of Asn and Gln has been widely studied [24–26]. Asn deamidates more readily than Gln [27]. The major process of deamidation in aged proteins involves the succinimide intermediates. For this reason, deamidation and isomerization are intimately linked, at least for the two amino acids with amide side chains. Mass spectrometry can differentiate tryptic peptides where an amide side chain has been converted to a carboxylic acid, although the mass change is only one Dalton.
A survey of deamidation in all human crystallins from older lenses revealed that some proteins were more susceptible to deamidation than others. For example, αA- and βB1-crystallin contained a number of deamidation sites whereas the Gln and Asn residues of βB2-crystallin appeared to remain largely intact [25, 26].
Crosslinking
Another major PTM in the human lens involves covalent crosslinking of polypeptides. This can be most clearly illustrated using techniques such as Western blotting [22]. Until very recently the reason for this crosslinking was unknown. It is now apparent that spontaneous processes are again responsible; in this case the susceptible amino acids are phosphoserine (PSer) and phosphothreonine (PThr). Over time these residues decompose via an elimination reaction to yield dehydroalanine (from PSer) or dehydrobutyrine (from PThr) [23]. The dehydroalanine or dehydrobutyrine formed becomes a site for nucleophilic attack by the thiol group of Cys, or the amino side chain of Lys. Once formed, there is no known way of breaking these covalent bonds, i.e. the linkage is permanent. Other possible crosslinking processes may also occur in aged lenses and these are currently being investigated.
Spontaneous PTMs occur in unstructured regions within proteins
The structures of the major lens crytallins are known [28, 29]. Once the major sites of deamidation/isomerization obtained from proteomic experiments were mapped onto the structures, a consistent picture emerged. Sites of racemization and deamidation were localized almost exclusively within unstructured regions [24, 30]. This finding is consistent with other data [31, 32]. One conclusion is that the region of the protein appears to be of more importance than the nature of the adjoining or adjacent amino acids. Although the amino acid sequence is important, since peptide studies have clearly demonstrated that having small residues, such as Gly, next to an Asp or Asn residue facilitates formation of the cyclic intermediate [33], however it would appear that conformational flexibility is a pre-requisite for deamidation/isomerization within proteins.
Spontaneous cleavage of peptide bonds
Asn residues can be sites of spontaneous peptide bond cleavage [34]. The mechanism appears to involve nucleophilic attack by the side chain amide nitrogen atom on the peptide bond on the C-terminal side of the Asn residue [21] (Figure 4). Cleavage sites adjacent to Asn residues in the membrane water channel, aquaporin 0, from older human lenses conform to this pattern [35]. Intriguingly major sites of cleavage in older lens crystallins were often on the N-terminal side of Ser residues [36, 37]. Peptide incubations [38] suggest that the hydroxyl group of Ser is implicated in a process [39] (Figure 4) in a manner analogous to that of intein cleavage [40].
Figure 4.
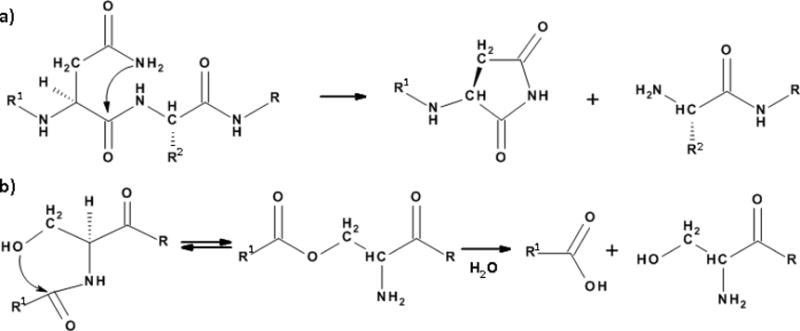
Old proteins undergo peptide bond cleavage. Mechanisms to account for spontaneous cleavage; a) on the C-terminal side of Asn, and b) on the N-terminal side of Ser residues.
Certainly the aged human lens contains a wide variety of peptides, [36, 41] the sequences of which have pointed the way to understanding the crystallin degradation mechanisms involved in their formation. In addition, some modified crystallin-derived peptides have enabled the part played by certain amino acid sequences in PTM and protease resistance to be deduced [42].
Intriguingly, two of the major amino acids responsible for isomerization (Ser and Asn) are also those implicated in spontaneous peptide bond cleavage. In the case of Ser, the mechanism of truncation is different from that involved in racemisaton [39]. It is not yet clear to what extent protein truncation is involved in changes to the lens as a whole. There are two aspects to this. Firstly, cleavage of the original protein will disrupt its tertiary structure and packing with other proteins in the cell. Secondly the peptides produced by spontaneous hydrolysis may themselves have biological activity [36, 43]. For example, peptides derived from breakdown of α-crystallin may promote protein aggregation – a key process in lens aging and cataract. Peptides from age-related cleavage of γS-crystallin lodge tightly into fibre cell membranes and potentially alter water dynamics [43] in a way that is consistent with that associated with older lens membranes [44]. Impaired water flow will presumably also affect solute transport (e.g. antioxidants) and this may influence cataract etiology.
The time scale of protein deterioration
Over what time period can a life-long protein exist within a cell before it undergoes spontaneous PTM? The lens provides a beautiful system for investigating this question. Prior to the commencement of proteomic studies, a number of hypotheses were feasible. One hypothesis was that extensive protein degradation may take place just prior to lens opacification: typically after about 60 years. Before that time, lens crystallins could be relatively stable.
An alternative scenario was that the structural proteins could deteriorate gradually over decades and that once a certain extent of breakdown had taken place, the lens became opaque. If the latter view were correct, did some crystallins decompose more rapidly than others? Was the time course of each process linear?
The proteomic data were clear. Age-related deterioration of crystallins begins early in life [30, 43, 45]. A remarkable discovery was that a large degree of change often occurred in the first 10–15 years of life (see Fig 2).
As noted earlier, some proteins degrade more rapidly than others. Typically α-crystallins were subject to a large degree of PTM, particularly αA-crystallin. This may well be significant since α-crystallins are molecular chaperones. They are small heat shock proteins that bind to proteins as they unfold and prevent them from precipitating [46]. There is a certain allocation of this chaperone protein at birth [47] and once it has been consumed, the centre of the lens is presumably left relatively defenseless. This disappearance has typically taken place by age 50. Within a cell that is now bereft of protective chaperones, the outcome of inexorable protein denaturation is likely to be quite different from that of a younger lens cell where the unfolding protein would be sequestered by α-crystallin.
Despite the time course of modification for each crystallin being different, there was a surprising concordance of the data sets for both normal and cataractous lenses. This is noteworthy, since experience with human biological data shows that, as a species, we have evolved to encompass a large degree of diversity: much greater than, for example, is found in laboratory rats. It seems that most spontaneous protein PTMs have a defined, almost unrelenting, time frame and that there is a substantial component of inevitability associated with the decline.
Why do proteins deteriorate with age?
The concept that our bodies contain numerous proteins that do not turnover, and furthermore, that they degrade over time, is a relatively new one for human biology [48, 49]. The agents responsible appear quite simple: just heat and time. Evidence for this conclusion comes from the fact that essentially all of the characteristic signatures of aged proteins can be replicated in the test tube, using peptides exposed to heat (e.g. 60°C) at neutral pH. Although these spontaneous processes may be relatively straightforward, the cellular protection mechanisms, their integration, individual variation and how these alter with age are not likely to be so straightforward.
Is protein deterioration responsible for human cataract?
It is now clear that massive changes take place to lens proteins over our lifespan. It would be surprising if alterations of such magnitude were not accompanied by detectable changes to the properties of the tissue. Physical properties of the human lens do change steadily with age. Stiffening of the lens is recognized as the basis for presbyopia [50].
Although crystallins undergo quantitatively major modifications over our lifespan, the vast majority of lenses remain transparent. If spontaneous PTMs were implicated in cataract, then we should expect to observe either consistently higher levels of modification at the same sites than in age-matched normals, or new sites in cataractous lenses.
So far, two proteins γS and αA crystallin, have been found with a consistently greater degree of PTM in cataractous lenses than in age-matched normal lenses [18, 30]. The specific sites are deamidation/racemization of Asn 14 and 76 in γS-crystallin and isomerization of Ser 59 and 62 as well as racemization of Asp 58 in αA-crystallin. These sites are located in unstructured regions of the crystallins and, in the case of γS-crystallin, may also be involved in crystallin-crystallin interactions within the cells. It may not be possible to maintain lens transparency beyond a certain level of disruption of this crystallin packing. Modifications to αA-crystallin could affect its chaperone ability and/or its membrane-binding properties. It is probable that other crystallin sites remain to be discovered.
Is it pertinent to investigate the mechanism of human cataract using animal models?
It is difficult, if not impossible, to prove that these age-related crystallin modifications are truly responsible for human cataract. Animal models are of little help. Human lenses are quite different from most other animal lenses, particularly rodent lenses [51] and the major crystallins are not identical. Membrane phospholipids also differ [51].
Another problem is that even if it were possible to obtain lenses that resembled human lenses, it is not feasible to reproduce experimentally, the suite of changes that are characteristic of crystallin aging? For example, it is not yet possible to selectively insert D-amino acids into mammalian proteins in vivo, at specific sites. Sites of individual Gln and Asn can be mutated into Glu and Asp residues however this is an all, or nothing, phenomenon. In the human lens the extent of deamidation is rarely 100% and typically ranges from 10–70% [26]. In addition, multiple different PTMs occur within the one protein and each lens cell contains a number of crystallins that each display their own particular time courses for degradation. For example, in addition to deamidation and racemization, covalent crosslinking and peptide bond cleavage are common in aged and cataractous crystallins. The problem of establishing any relevant animal model for human cataract may well be impossible to solve.
In the absence of any appropriate animal model, we must be satisfied with a “smoking gun”. The hypothesis that cumulative age-related modifications to proteins leads to human lens opacification is bolstered by genetic data. Numerous inborn errors of metabolism involve single amino acid substitutions in lens crystallins and these single changes can be enough to cause cataract e.g. [52–54]. Such findings serve to emphasize a key point: small changes to just one crystallin can be sufficient to induce human cataract. Some of the cataract-inducing single amino acid substitutions are conservative and have been detected in crystallins (e.g. γS-crystallin [55]) that have subsequently been found to be modified in proteomic investigations of aged human lenses.
Despite these caveats, animal lenses can still be useful in some specific cases e.g. in the case of ARNC, for examining the effect of oxygen/UV light on glutathione in lenses or on macromolecular degradation.
Nuclear cataract PTMs
It should be noted that some PTMs are specific for nuclear cataractous lenses. Oxidized versions of Cys and Met residues increase as cataract worsens and thus oxidation plays a key role in the progression of age-related nuclear cataract [5]. Somewhat surprisingly, the levels of oxidation of Cys [5], Met [56] and Tyr [57] in normal lens proteins that have been resident in the lens for decades are very small. It is likely that the lack of oxidation of proteins in the normal lens, and the minimal levels of chemically-induced PTMs by agents such carbonyls, are linked. One factor is the very low oxygen tension in the lens interior [58]. The other is glutathione. Glutathione is the main cellular antioxidant and it can also efficiently intercept reactive molecules. By contrast it is likely to have little, or no, effect on racemization, deamidation or spontaneous peptide bond cleavage.
Of special note, the levels of D-Asp and D-Ser from cataractous lenses were consistently higher than from age-matched normal lenses (Figure 1). [13, 18]. This finding was in agreement with earlier studies [59, 60]. Consistent with these data, there were locations where the degree of deamidation was greater in cataractous lenses (Figure 2) [25, 30]. This finding suggested, for the first time, that some sites within some crystallins, such as Asn 76 γS-crystallin, could possibly be implicated in the genesis of cataract (Figure 3).
How could the increased structural PTMs in crystallins lead to lens opacification? One theory for nuclear cataract is as follows: after middle age, once the PTM load becomes sufficient and crystallins unfold, large-scale binding of protein aggregates takes place to fibre cell membranes [61, 62]. This membrane binding may be responsible for the occlusion of membrane pores and consequently the creation of a permeability barrier [62]. A reduction in the rate of transport of reduced glutathione into the centre of the middle-aged lens may well be the principal factor in initiating ARNC [63]. ARNC is characterized by massive oxidation of proteins in the centre of the lens, as well as extensive covalent cross-linking, colouration and insolubilisation of crystallins [6].
Is cataract just one of a number of age-related human diseases that are due to the breakdown of long-lived proteins?
The list of organs and tissues within the body contain long-lived, or life-long, proteins is expanding and some long-lived proteins are abundant [31, 64, 65]. Due to its simple architecture and composition, the lens is an ideal tissue for characterizing the many PTMs that arise over a period of decades due to retention of proteins within the human body. As outlined in this article, several age-related PTMs have been characterized but we know little about details of some of the processes and, in particular, what agents act within the body to minimize these changes. There is a great deal to learn. The most likely causative agents for human protein denaturation are heat and time [50]. We know much less about factors that ameliorate these processes. Chaperones will most likely be important. Overall, the work outlined in this review leads to a conclusion that there is little prospect for preventing age-related cataract and that our best option is to investigate ways of impeding the processes involved.
If the degradation of long-lived proteins does indeed play a widespread role in human aging, then the same conundrums and barriers that apply to understanding human cataract will emerge in a more general manner. For example, animal models will be of limited use because of the multifaceted nature of the age-related protein modifications. Correlation may have to suffice. Such outcomes are yet to be proven, but if this scenario eventuates, scientists may need to re-assess their experimental approaches when investigating the many of the conditions associated with old age in humans.
Highlights.
Spontaneous breakdown of long-lived macromolecules is a probable cause of cataract
The most common PTM of aged lens proteins is racemization
PTMs occurring in the lens may also occur in other long-lived proteins
Acknowledgments
Aspects of this work were funded by NHMRC and NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Harding J. Cataract biochemistry, Epidemiology and Pharmacology. Chapman and Hall; London: 1991. [Google Scholar]
- 2.Giblin FJ, McCready JP, Reddy VN. The role of glutathione metabolism in the detoxification of H2O2 in rabbit lens. Investigative Ophthalmology & Visual Science. 1982;22:330–335. [PubMed] [Google Scholar]
- 3.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Experimental Eye Research. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- 5.Truscott RJW. Age-related nuclear cataract—oxidation is the key. Experimental Eye Research. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Truscott RJW, Augusteyn RC. Changes in human lens proteins during nuclear cataract formation. Experimental Eye Research. 1977;24:159–170. doi: 10.1016/0014-4835(77)90256-1. [DOI] [PubMed] [Google Scholar]
- 7.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life. PLoS ONE. 2008;3:e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 9.Lapko VN, Smith DL, Smith JB. S-methylated cysteines in human lens gamma S-crystallins. Biochemistry. 2002;41:14645–14651. doi: 10.1021/bi0267700. [DOI] [PubMed] [Google Scholar]
- 10.Truscott RJW, Mizdrak J, Friedrich MG, Hooi MY, Lyons B, Jamie JF, Davies MJ, Wilmarth PA, David LL. Is protein methylation in the human lens a result of non-enzymatic methylation by S-adenosylmethionine? Experimental Eye Research. 2012;99:48–54. doi: 10.1016/j.exer.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: age-dependent accumulation of N.epsilon.-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989;28:9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed MU, F Brinkmann E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324:565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooi M, Truscott RW. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. AGE. 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii N, Takemoto LJ, Momose Y, Matsumoto S, Hiroki K, Akaboshi M. Formation of Four Isomers at the Asp-151 Residue of Aged Human αA-Crystallin by Natural Aging. Biochemical and Biophysical Research Communications. 1999;265:746–751. doi: 10.1006/bbrc.1999.1748. [DOI] [PubMed] [Google Scholar]
- 15.Groenen P, van den Ijssel P, Voorter C, Bloemendal H, de Jong W. Site-specific racemization in aging alpha A-crystallin. FEBS Lett. 1990;20:109–112. doi: 10.1016/0014-5793(90)81131-7. [DOI] [PubMed] [Google Scholar]
- 16.Fujii N, Satoh K, Harada K, Ishibashi Y. Simultaneous Stereoinversion and Isomerization at Specific Aspartic Acid Residues in αA-Crystallin from Human Lens. Journal of Biochemistry. 1994;116:663–669. doi: 10.1093/oxfordjournals.jbchem.a124577. [DOI] [PubMed] [Google Scholar]
- 17.Maeda H, Takata T, Fujii N, Sakaue H, Nirasawa S, Takahashi S, Sasaki H, Fujii N. Rapid Survey of Four Asp Isomers in Disease-Related Proteins by LC-MS combined with Commercial Enzymes. Analytical Chemistry. 2015;87:561–568. doi: 10.1021/ac504413e. [DOI] [PubMed] [Google Scholar]
- 18.Hooi MY, Raftery MJ, Truscott RJ. Age-dependent racemization of serine residues in a human chaperone protein. Protein Sci. 2013;22:93–100. doi: 10.1002/pro.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiger T, Clark S. Deamidation, Isomerization, and Racemization at Asparaginyl and Aspartyl Residues in Peptides. Journal of Biological Chemistry. 1987;262:785–794. [PubMed] [Google Scholar]
- 20.Hooi MYS, Raftery MJ, Truscott RJW. Interconversion of the peptide isoforms of aspartate: Stability of isoaspartates. Mechanisms of Ageing and Development. 2013;134:103–109. doi: 10.1016/j.mad.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson RC, Clarke S. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. Journal of Biological Chemistry. 1989;264:6164–6170. [PubMed] [Google Scholar]
- 22.Friedrich MG, Lam J, Truscott RJW. Degradation of an old human protein. Age-dependent cleavage of γS crystallin generates a peptide that binds to cell membranes. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.391565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Lyons B, Truscott RJW, Schey KL. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 2014;13:226–234. doi: 10.1111/acel.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooi MYS, Raftery MJ, Truscott RJW. Age-dependent deamidation of glutamine residues in human γS crystallin: Deamidation and unstructured regions. Protein Science. 2012;21:1074–1079. doi: 10.1002/pro.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hains PG, Truscott RJW. Age-Dependent Deamidation of Lifelong Proteins in the Human Lens. Investigative Ophthalmology & Visual Science. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-Related Changes in Human Crystallins Determined from Comparative Analysis of Post-translational Modifications in Young and Aged Lens: Does Deamidation Contribute to Crystallin Insolubility? Journal of Proteome Research. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson NE, Robinson AB. Deamidation of human proteins. Proceedings of the National Academy of Sciences. 2001;98:12409–12413. doi: 10.1073/pnas.221463198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagnéris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal Structures of α-Crystallin Domain Dimers of αB-Crystallin and Hsp20. Journal of Molecular Biology. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 29.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in Biophysics and Molecular Biology. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Hooi MYS, Raftery MJ, Truscott RJW. Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age Range. Investigative Ophthalmology & Visual Science. 2012;53:3554–3561. doi: 10.1167/iovs.11-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloos PC, Christgau S. Post-Translational Modifications of Proteins: Implications for Aging, Antigen Recognition, and Autoimmunity. Biogerontology. 2004;5:139–158. doi: 10.1023/B:BGEN.0000031152.31352.8b. [DOI] [PubMed] [Google Scholar]
- 32.Gsponer J, Babu M Madan. The rules of disorder or why disorder rules. Progress in Biophysics and Molecular Biology. 2009;99:94–103. doi: 10.1016/j.pbiomolbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Robinson NE, Robinson ZW, Robinson BR, Robinson AL, Robinson JA, Robinson ML, Robinson AB. Structure-dependent nonenzymatic deamidation of glutaminyl and asparaginyl pentapeptides. The Journal of Peptide Research. 2004;63:426–436. doi: 10.1111/j.1399-3011.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 34.Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. Journal of Biological Chemistry. 1988;263:19020–19023. [PubMed] [Google Scholar]
- 35.Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational Modifications of Aquaporin 0 (AQP0) in the Normal Human Lens: Spatial and Temporal Occurrence†. Biochemistry. 2004;43:9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- 36.Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of Interactions of Low Molecular Weight Crystallin Fragments in Lens Aging and Cataract Formation. Journal of Biological Chemistry. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemoto L. Increased cleavage of the c-terminal serine from alpha-A crystallin present in the high molecular weight aggregate fraction from human and bovine lenses. Current Eye Research. 1999;19:450–455. doi: 10.1076/ceyr.19.5.450.5295. [DOI] [PubMed] [Google Scholar]
- 38.Lyons B, Jamie J, Truscott R. Spontaneous Cleavage of Proteins at Serine Residues. International Journal of Peptide Research and Therapeutics. 2011;17:131–135. [Google Scholar]
- 39.Lyons B, Jamie J, Truscott RW. Separate mechanisms for age-related truncation and racemisation of peptide-bound serine. Amino Acids. 2014;46:199–207. doi: 10.1007/s00726-013-1619-5. [DOI] [PubMed] [Google Scholar]
- 40.Noren CJ, Wang J, Perler FB. Dissecting the Chemistry of Protein Splicing and Its Applications. Angewandte Chemie International Edition. 2000;39:450–466. [PubMed] [Google Scholar]
- 41.Su SP, Lyons B, Friedrich M, McArthur JD, Song X, Xavier D, Truscott RJW, Aquilina JA. Molecular signatures of long-lived proteins: autolytic cleavage adjacent to serine residues. Aging Cell. 2012;11:1125–1127. doi: 10.1111/j.1474-9726.2012.00860.x. [DOI] [PubMed] [Google Scholar]
- 42.Lyons B, Kwan AH, Truscott R. Spontaneous cyclization of polypeptides with a penultimate Asp, Asn or isoAsp at the N-terminus and implications for cleavage by aminopeptidase. FEBS Journal. 2014;281:2945–2955. doi: 10.1111/febs.12833. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich MG, Lam J, Truscott RJW. Degradation of an Old Human Protein: Age-Dependent Cleavage of γS-Crystallin Genterates a Peptide that Binds to Cell Membranes. Journal of Biological Chemistry. 2012;287:39012–39020. doi: 10.1074/jbc.M112.391565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Gaus K, Lu Y, Magenau A, Truscott RJW, Mitchell TW. α-and β-Crystallins Modulate the Head Group Order of Human Lens Membranes during Aging. Investigative Ophthalmology & Visual Science. 2010;51:5162–5167. doi: 10.1167/iovs.09-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grey AC, Schey KL. Age-Related Changes in the Spatial Distribution of Human Lens α-Crystallin Products by MALDI Imaging Mass Spectrometry. Investigative Ophthalmology & Visual Science. 2009;50:4319–4329. doi: 10.1167/iovs.09-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwitz J. Alpha-crystallin. Experimental Eye Research. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 47.Roy D, Spector A. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proceedings of the National Academy of Sciences. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truscott RJW. Macromolecular deterioration as the ultimate constraint on human lifespan. Ageing Research Reviews. 2011;10:397–403. doi: 10.1016/j.arr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Truscott RJW. Are Ancient Proteins Responsible for the Age-Related Decline in Health and Fitness? Rejuvenation Research. 2010;13:83–89. doi: 10.1089/rej.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, α-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 51.Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJW. Human lens lipids differ markedly from those of commonly used experimental animals. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2008;1781:288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Li L, Chang B, Cheng C, Chang D, Hawes NL, Xia C-h, Gong X. Dense Nuclear Cataract Caused by the γB-Crystallin S11R Point Mutation. Investigative Ophthalmology & Visual Science. 2008;49:304–309. doi: 10.1167/iovs.07-0942. [DOI] [PubMed] [Google Scholar]
- 53.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal Dominant Congenital Cataract Associated with a Missense Mutation in the Human Alpha Crystallin Gene CRYAA. Human Molecular Genetics. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A Novel αB-Crystallin Mutation Associated with Autosomal Dominant Congenital Lamellar Cataract. Investigative Ophthalmology & Visual Science. 2006;47:1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. Journal of Medical Genetics. 2005;42:706–710. doi: 10.1136/jmg.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sochaski MA, Jenkins AJ, Lyons TJ, Thorpe SR, Baynes JW. Isotope Dilution Gas Chromatography/Mass Spectrometry Method for the Determination of Methionine Sulfoxide in Protein. Analytical Chemistry. 2001;73:4662–4667. doi: 10.1021/ac010228k. [DOI] [PubMed] [Google Scholar]
- 57.Wells-Knecht MC, Huggins TG, Dyer DG, Thorpe SR, Baynes JW. Oxidized amino acids in lens protein with age. Measurement of o-tyrosine and dityrosine in the aging human lens. Journal of Biological Chemistry. 1993;268:12348–12352. [PubMed] [Google Scholar]
- 58.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJW, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. The Journal of Physiology. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujii N, Takemoto LJ, Matsumoto S, Hiroki K, Boyle D, Akaboshi M. Comparison of d-Aspartic Acid Contents in α A-Crystallin from Normal and Age-Matched Cataractous Human Lenses. Biochemical and Biophysical Research Communications. 2000;278:408–413. doi: 10.1006/bbrc.2000.3778. [DOI] [PubMed] [Google Scholar]
- 60.Masters PM, Bada JL, Zigler JS. Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proceedings of the National Academy of Sciences. 1978;75:1204–1208. doi: 10.1073/pnas.75.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedrich MG, Truscott RJW. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investigative Ophthalmology & Visual Science. 2009;50:4786–4793. doi: 10.1167/iovs.09-3588. [DOI] [PubMed] [Google Scholar]
- 62.Friedrich MG, Truscott RJW. Large-Scale Binding of α-Crystallin to Cell Membranes of Aged Normal Human Lenses: A Phenomenon That Can Be Induced by Mild Thermal Stress. Investigative Ophthalmology & Visual Science. 2010;51:5145–5152. doi: 10.1167/iovs.10-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweeney MHJ, Truscott RJW. An Impediment to Glutathione Diffusion in Older Normal Human Lenses: a Possible Precondition for Nuclear Cataract. Experimental Eye Research. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- 64.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. The Journal of Clinical Investigation. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapira R, Wilkinson K, Shapira G. Racemization of individual aspartate residues in human myelin basic protein. J Neurochem. 1988;50:649–654. doi: 10.1111/j.1471-4159.1988.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 66.Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G. Increased levels of advanced glycation end products in human cataractous lenses. Journal of Cataract & Refractive Surgery. 2003;29:998–1004. doi: 10.1016/s0886-3350(02)01841-2. [DOI] [PubMed] [Google Scholar]
- 67.Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard Reaction in Aging of Tissue Proteins: Advanced Glycation End Products-Dependent Increase In Imidazolium Cross-Links in Human Lens Proteins. Journal of Biological Chemistry. 1998;273:18714–18719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- 68.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. Structure Elucidation of a Novel Yellow Chromophore from Human Lens Protein. Journal of Biological Chemistry. 2004;279:45441–45449. doi: 10.1074/jbc.M405664200. [DOI] [PubMed] [Google Scholar]
- 69.Argirov OK, Lin B, Ortwerth BJ. 2-Ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) Is a Newly Identified Advanced Glycation End Product in Cataractous and Aged Human Lenses. Journal of Biological Chemistry. 2004;279:6487–6495. doi: 10.1074/jbc.M309090200. [DOI] [PubMed] [Google Scholar]
- 70.Cheng R, Feng QI, Argirov OK, Ortwerth BJ. K2P—A Novel Cross-Link from Human Lens Protein. Annals of the New York Academy of Sciences. 2005;1043:184–194. doi: 10.1196/annals.1333.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sell DR, Monnier VM. Conversion of Arginine into Ornithine by Advanced Glycation in Senescent Human Collagen and Lens Crystallins. Journal of Biological Chemistry. 2004;279:54173–54184. doi: 10.1074/jbc.M408946200. [DOI] [PubMed] [Google Scholar]
- 72.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-Derived Hydroimidazolone Advanced Glycation End-Products of Human Lens Proteins. Investigative Ophthalmology & Visual Science. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]


