Abstract
Endothelin is a potent mammalian vasoconstrictive peptide with structural homology to cation channel-binding insect toxins. We tested the proposal that this peptide directly activates dihydropyridine-sensitive Ca2+ channels in cultured vascular smooth muscle (VSM) cells. First, we found that cell Ca2+ can be altered in VSM by activation of voltage-operated Ca2+ channels. KCl-induced depolarization and the dihydropyridine Ca2+ channel agonist (-) Bay K 8644 (10 microM) both raised cell Ca2+ more than twofold; the effect of KCl was blocked by the inhibitory enantiomer, (+) Bay K 8644 (40 microM). Similar responses were observed in Chinese hamster ovary (CHO) cells. Synthetic endothelin (4 x 10(-8) M) raised Ca2+ in VSM but not CHO cells from 100 +/- 17 to 561 +/- 34 nM within 12 s. Ca2+ subsequently fell to basal levels after 30 min. Half maximal Ca2+ response was at 4 x 10(-9) M endothelin. Unlike endothelin, thrombin raised Ca2+ in both VSM and CHO cells. The Ca2+ responses to endothelin and thrombin were not affected by nicardipine (1 microM), (+) Bay K 8644, or Ca2+-free solutions. Lastly, both hormones caused release of inositol phosphates in VSM cells. However, the response to thrombin was more than 10-fold larger and was more rapid than the response to endothelin; the thrombin response was sensitive to pertussis toxin, while the response to endothelin was not. Thus endothelin, like thrombin, raises cell Ca2+ in VSM by mobilization of intracellular stores and not by activation of dihydropyridine-sensitive Ca2+ channels. However, their receptors are distinct and they exhibit important differences in signal transduction.
Full text
PDF

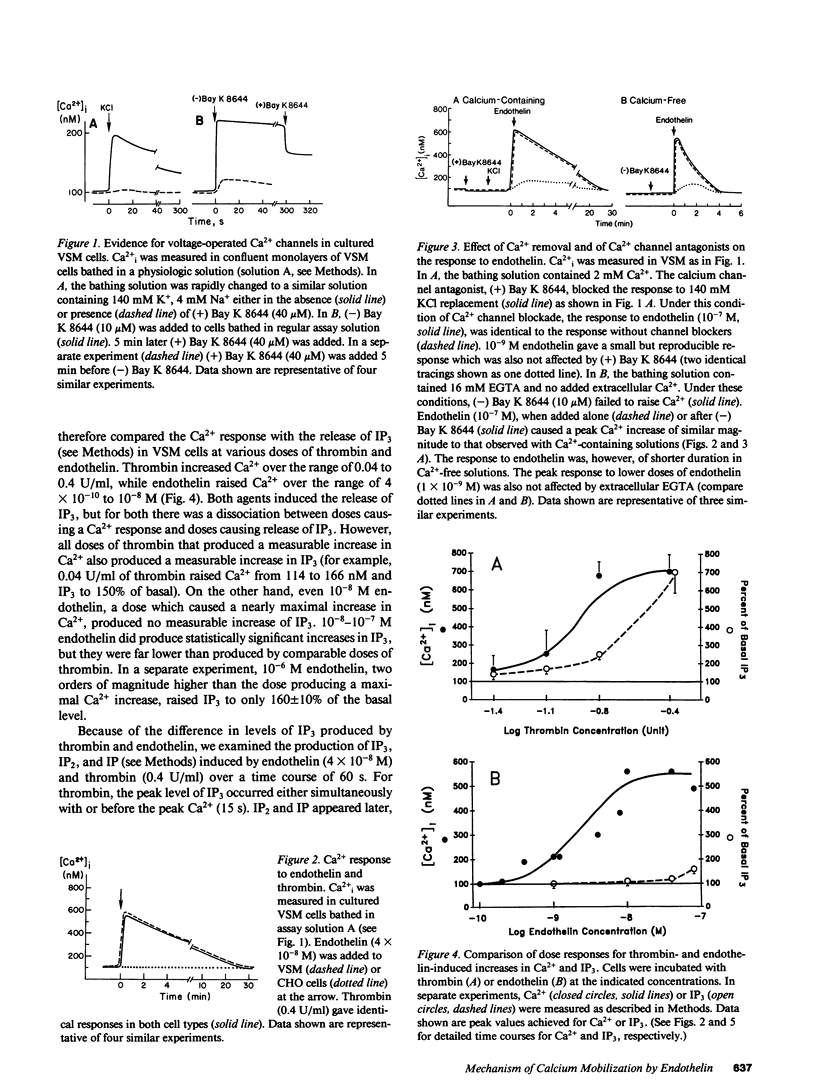
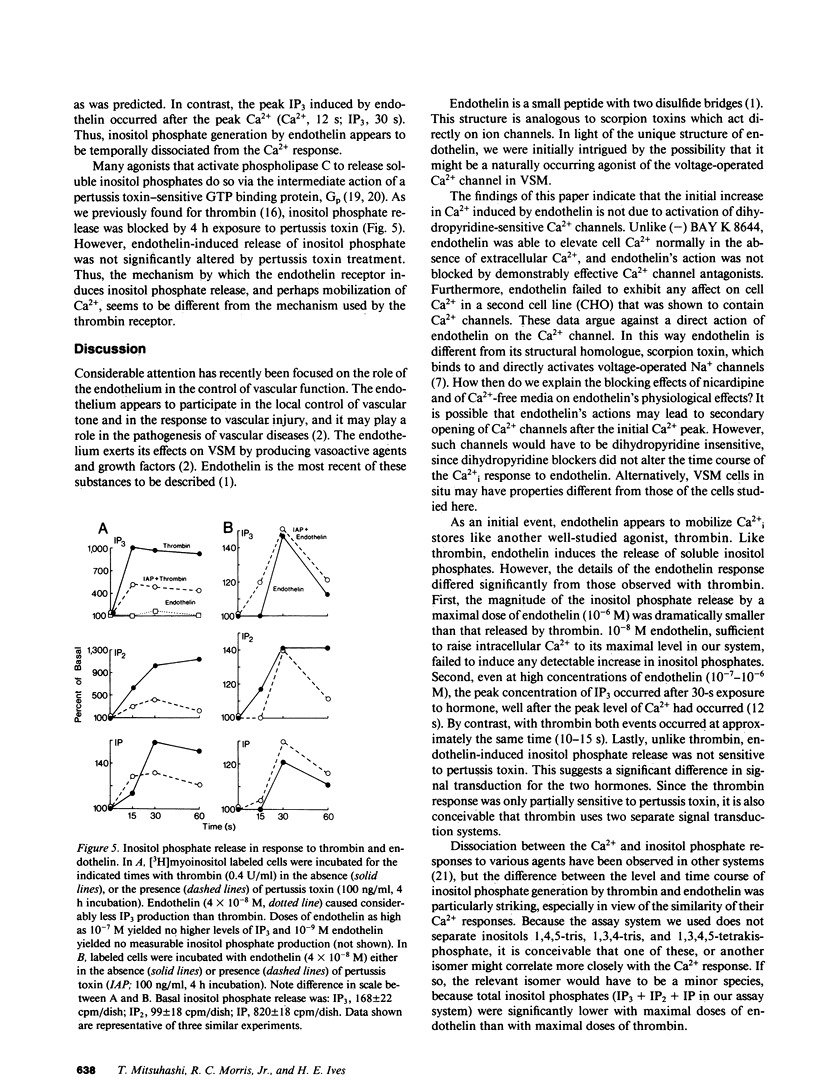

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Peter-Riesch B., Schlegel W., Wollheim C. B. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987 Mar 15;262(8):3567–3571. [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Forder J., Scriabine A., Rasmussen H. Plasma membrane calcium flux, protein kinase C activation and smooth muscle contraction. J Pharmacol Exp Ther. 1985 Nov;235(2):267–273. [PubMed] [Google Scholar]
- Franckowiak G., Bechem M., Schramm M., Thomas G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol. 1985 Aug 15;114(2):223–226. doi: 10.1016/0014-2999(85)90631-4. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Hirata Y., Yoshimi H., Kojima T., Kobayashi Y., Yanagisawa M., Masaki T. Endothelin is a potent secretagogue for atrial natriuretic peptide in cultured rat atrial myocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):167–172. doi: 10.1016/s0006-291x(88)81064-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takata S., Watanabe T. X., Kumagai S., Nakajima K., Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Cogan M. G., Cragoe E. J., Jr, Ives H. E. Thrombin activation of the Na+/H+ exchanger in vascular smooth muscle cells. Evidence for a kinase C-independent pathway which is Ca2+-dependent and pertussis toxin-sensitive. J Biol Chem. 1987 Oct 15;262(29):14134–14140. [PubMed] [Google Scholar]
- Jones P. A., Scott-Burden T., Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y., Ambar I., Sokolovsky M., Kochva E., Wollberg Z., Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988 Oct 14;242(4876):268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Lynch C. J., Blackmore P. F., Charest R., Exton J. H. The relationships between receptor binding capacity for norepinephrine, angiotensin II, and vasopressin and release of inositol trisphosphate, Ca2+ mobilization, and phosphorylase activation in rat liver. Mol Pharmacol. 1985 Aug;28(2):93–99. [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Vascular physiology: the end of the quest? Nature. 1987 Jun 11;327(6122):459–460. doi: 10.1038/327459a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


