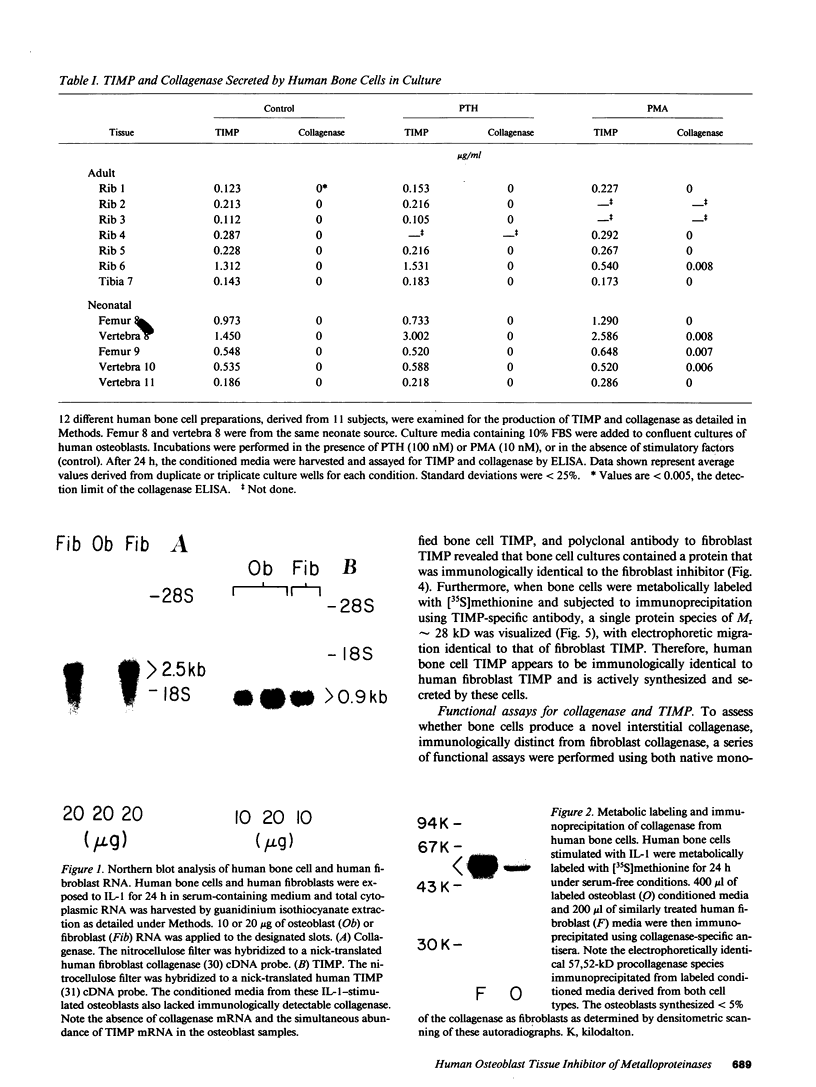
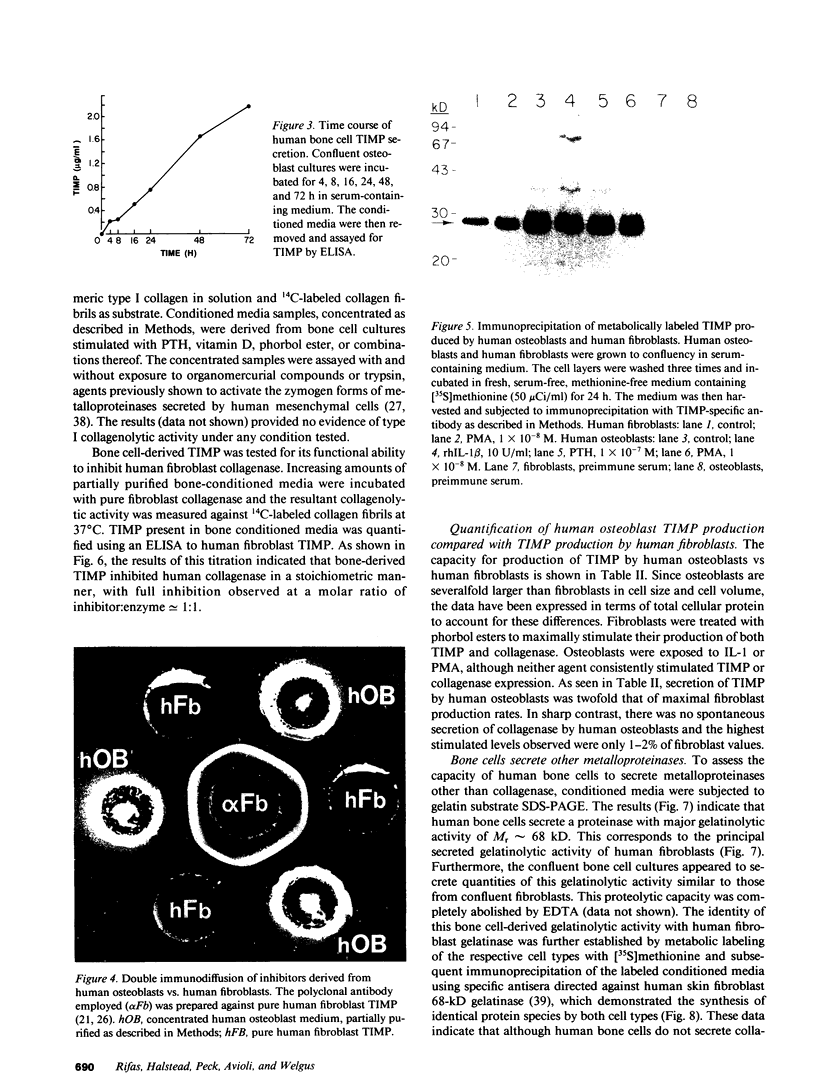
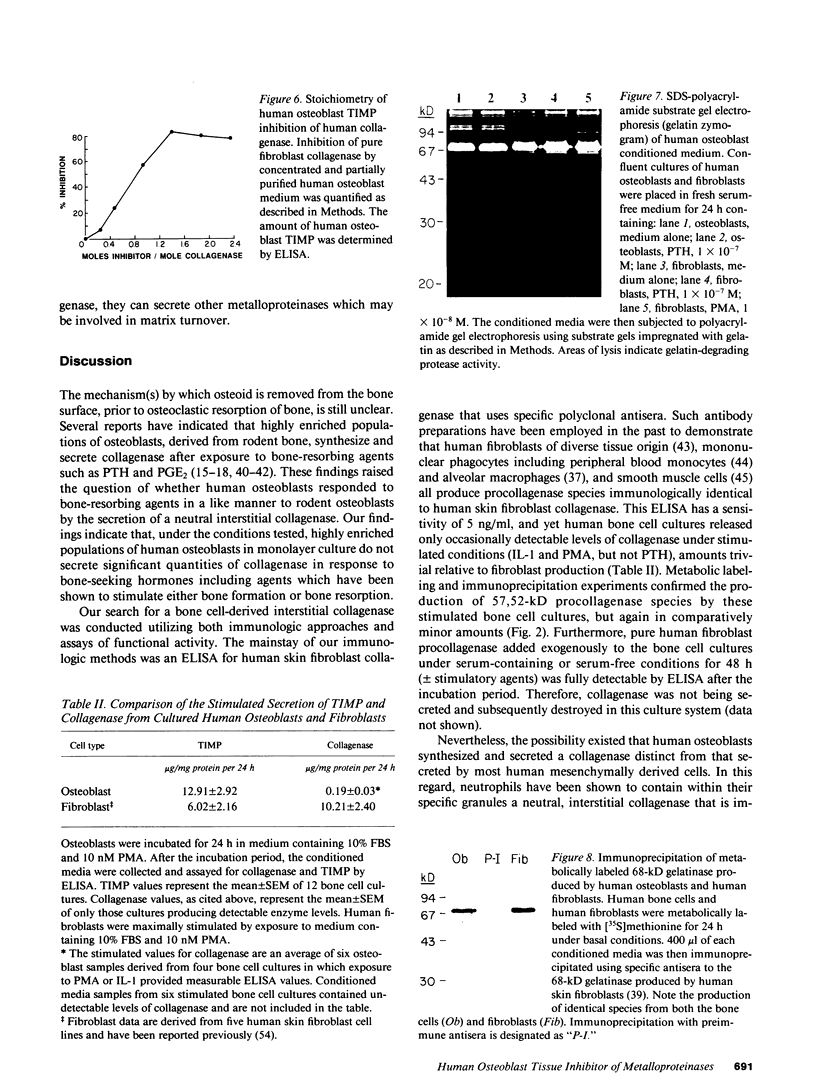
Abstract
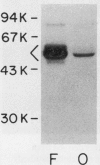
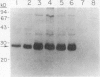
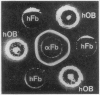
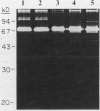
Human osteoblast cultures (hOB) were examined for the production of interstitial collagenase, tissue inhibitor of metalloproteinases (TIMP), and gelatinolytic enzymes. Cells were isolated by bacterial collagenase digestion of trabecular bone (vertebra, rib, tibia, and femur) from 11 subjects (neonatal to adult). Confluent cultures were exposed to phorbol 12-myristate 13-acetate, PTH, PGE2, epidermal growth factor, 1,25(OH)2 vitamin D3, recombinant human IL-1 beta, and dexamethasone. Collagenase and TIMP were assayed immunologically and also by measurements of functional activity. Collagenase was not secreted in significant quantities by human bone cells under any tested condition. Furthermore, collagenase mRNA could not be detected in hOB. However, hOB spontaneously secreted large amounts of TIMP for at least 72 h in culture. hOB TIMP was found to be identical to human fibroblast TIMP by double immunodiffusion, metabolic labeling and immunoprecipitation, Northern blot analysis, and stoichiometry of collagenase inhibition. SDS-substrate gel electrophoresis of hOB-conditioned media revealed a prominent band of gelatinolytic activity at 68 kD, and specific polyclonal antisera established its identity with the major gelatinolytic protease of human fibroblasts. Abundant secretion of gelatinolytic, but not collagenolytic, enzymes by hOB may indicate that human osteoblasts do not initiate and direct the cleavage of osteoid collagen on the bone surface, but may participate in the preparation of the bone surface for osteoclast attachment by removal of denatured collagen peptides. The constitutive secretion of TIMP may function to regulate metalloproteinase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Gross R. H., Nagase H., Sheldon L., Jackson R. C., Harris E. D., Jr Increased level of translatable collagenase messenger ribonucleic acid in rabbit synovial fibroblasts treated with phorbol myristate acetate or crystals of monosodium urate monohydrate. Biochemistry. 1982 May 25;21(11):2674–2679. doi: 10.1021/bi00540a015. [DOI] [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Lazarus C. J., Welgus H. G. Monocyte procollagenase and tissue inhibitor of metalloproteinases. Identification, characterization, and regulation of secretion. J Biol Chem. 1987 Nov 25;262(33):15862–15868. [PubMed] [Google Scholar]
- Carmichael D. F., Sommer A., Thompson R. C., Anderson D. C., Smith C. G., Welgus H. G., Stricklin G. P. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2407–2411. doi: 10.1073/pnas.83.8.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Darby J. A., Fuller K. Mammalian collagenase predisposes bone surfaces to osteoclastic resorption. Cell Tissue Res. 1985;241(3):671–675. doi: 10.1007/BF00214590. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Fuller K. Bone cells predispose bone surfaces to resorption by exposure of mineral to osteoclastic contact. J Cell Sci. 1985 Jun;76:155–165. doi: 10.1242/jcs.76.1.155. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Eisen A. Z., Stricklin G. P., Welgus H. G. Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc Natl Acad Sci U S A. 1985 May;82(9):2779–2783. doi: 10.1073/pnas.82.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury J. D., Campbell E. J., Lazarus C. J., Albin R. J., Welgus H. G. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol. 1988 Dec 15;141(12):4306–4312. [PubMed] [Google Scholar]
- GROSS J. [Studies on the formation of collagen. III. Time-dependent solubility changes of collagen in vitro]. J Exp Med. 1958 Aug 1;108(2):215–226. doi: 10.1084/jem.108.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Heterogeneity among human collagenases demonstrated by monoclonal antibody that selectively recognizes and inhibits human neutrophil collagenase. J Exp Med. 1984 May 1;159(5):1455–1463. doi: 10.1084/jem.159.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986 Apr 25;261(12):5645–5650. [PubMed] [Google Scholar]
- Heath J. K., Atkinson S. J., Meikle M. C., Reynolds J. J. Mouse osteoblasts synthesize collagenase in response to bone resorbing agents. Biochim Biophys Acta. 1984 Nov 6;802(1):151–154. doi: 10.1016/0304-4165(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Roodman G. D., McManus L. M., Mundy G. R. Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol. 1984 Aug;99(2):471–480. doi: 10.1083/jcb.99.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986 Oct;119(4):1654–1659. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- McSheehy P. M., Chambers T. J. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986 Feb;118(2):824–828. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Wolf A. M., Arnaud C. D. Bone cells in culture: morphologic transformation by hormones. Science. 1976 Jun 25;192(4246):1340–1343. doi: 10.1126/science.1273593. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Otsuka K., Sodek J., Limeback H. Synthesis of collagenase and collagenase inhibitors by osteoblast-like cells in culture. Eur J Biochem. 1984 Nov 15;145(1):123–129. doi: 10.1111/j.1432-1033.1984.tb08530.x. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., Teitelbaum S., Slatopolsky E., McCracken R., Bergfeld M., Lee W., Avioli L. V., Peck W. A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge N. C., Jeffrey J. J., Ehlich L. S., Teitelbaum S. L., Fliszar C., Welgus H. G., Kahn A. J. Hormonal regulation of the production of collagenase and a collagenase inhibitor activity by rat osteogenic sarcoma cells. Endocrinology. 1987 May;120(5):1956–1962. doi: 10.1210/endo-120-5-1956. [DOI] [PubMed] [Google Scholar]
- Pun K. K., Arnaud C. D., Nissenson R. A. Parathyroid hormone receptors in human dermal fibroblasts: structural and functional characterization. J Bone Miner Res. 1988 Aug;3(4):453–460. doi: 10.1002/jbmr.5650030413. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Studies on bone formation and resorption in vitro. Horm Res. 1984;20(1):22–27. doi: 10.1159/000179971. [DOI] [PubMed] [Google Scholar]
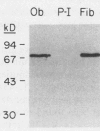
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Roswit W. T., Rifas L., Gast M. J., Welgus H. G., Jeffrey J. J. Purification and characterization of human myometrial smooth muscle collagenase. Arch Biochem Biophys. 1988 Apr;262(1):67–75. doi: 10.1016/0003-9861(88)90169-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M., Goldhaber P., Glimcher M. Collagenase and bone resorption: isolation of collagenase from culture medium containing serum after stimulation of bone resorption by addition of parathyroid hormone extract. Biochem Biophys Res Commun. 1975 Mar 3;63(1):172–178. doi: 10.1016/s0006-291x(75)80026-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M. Osteoblast collagenase: collagenase synthesis by clonally derived mouse osteogenic (MC3T3-E1) cells. Biochem Int. 1984 Jul;9(1):51–58. [PubMed] [Google Scholar]
- Shen V., Rifas L., Kohler G., Peck W. A. Prostaglandins change cell shape and increase intercellular gap junctions in osteoblasts cultured from rat fetal calvaria. J Bone Miner Res. 1986 Jun;1(3):243–249. doi: 10.1002/jbmr.5650010302. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Welgus H. G. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem. 1983 Oct 25;258(20):12252–12258. [PubMed] [Google Scholar]
- Thomson B. M., Atkinson S. J., Reynolds J. J., Meikle M. C. Degradation of type I collagen films by mouse osteoblasts is stimulated by 1,25 dihydroxyvitamin D3 and inhibited by human recombinant TIMP (tissue inhibitor of metalloproteinases). Biochem Biophys Res Commun. 1987 Oct 29;148(2):596–602. doi: 10.1016/0006-291x(87)90918-1. [DOI] [PubMed] [Google Scholar]
- Thomson B. M., Saklatvala J., Chambers T. J. Osteoblasts mediate interleukin 1 stimulation of bone resorption by rat osteoclasts. J Exp Med. 1986 Jul 1;164(1):104–112. doi: 10.1084/jem.164.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Wilhelm S. M., Eisen A. Z., Teter M., Clark S. D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]