Abstract
Oxidative stress, generated by excessive reactive oxygen species (ROS) or decrease in antioxidant defences (and possibly both), is associated with male infertility. A consequence of oxidative stress is the generation of redox dependent protein modifications, such as tyrosine nitration and S-glutathionylation. Normozoospermic sperm samples from healthy individuals were included in this study. Samples were incubated with increasing concentrations (0 to 5 mM) of exogenous hydrogen peroxide, tert-buthyl hydroperoxide or diethylamine NONOate (DA-NONOate; a nitric oxide (NO•) donor) added to the medium. Spermatozoa treated with or without ROS were incubated under capacitating conditions and then, levels of tyrosine phosphorylation and percentage of acrosome reaction (AR) induced by lysophosphatidylcholine (LPC) were determined. Modified sperm proteins from cytosolic, Triton-soluble and – insoluble fractions were analysed by SDS-PAGE immunoblotting and immunocytochemistry with anti-glutathione and anti-nitro tyrosine antibodies. Levels of S-glutathionylation increased dose dependently after exposure to hydroperoxides (p<0.05), and were localised mainly in the cytosolic and Triton-soluble fractions of the spermatozoa. Levels of tyrosine nitrated proteins increased dose dependently after exposure to DA-NONOate (p<0.05), and were mainly localized in the Triton-insoluble fraction. ROS-treated spermatozoa showed impaired motility without affecting viability (hypoosmotic swelling test). These treated spermatozoa had tyrosine phosphorylation and AR levels similarly to that of non-capacitated spermatozoa following incubation under capacitating conditions, suggesting an impairment of sperm capacitation by oxidative stress. In conclusion, oxidative stress promotes a dose dependent increase of tyrosine nitration and S-glutathionylation and alters motility and the ability of spermatozoa to undergo capacitation.
Keywords: reactive oxygen species, oxidative stress, redox protein modifications
INTRODUCTION
Human infertility is an important health and a social concern which affects 15% of couples in the reproductive age (WHO 2010; de Kretser 1997). Of these cases of infertility, 50% can be attributed to the male factor (Abid et al. 2008). Male infertility is a multifactorial disorder which is presented clinically as low or absent sperm counts, or the presence of mutated or nonfunctional sperm cells due to abnormal spermatogenesis (Tournaye & Cohlen 2012). This defective spermatogenesis can be linked to medical conditions such as varicocele, cryptorchidism, infections, nutritional deficiencies, or trauma. It can also be caused by exposure to environmental agents, chemotherapeutic agents, smoking or even diseases (Anderson & Williamson 1988; Brennemann et al. 1997; Hasegawa et al. 1997; Smith et al. 2006). Interestingly, all the above mentioned conditions have the oxidative stress as an important component of their pathophysiological mechanisms (Agarwal et al. 2008; Turner 2001; Anderson & Williamson 1988; Smith et al. 2006; Brennemann et al. 1997; Hasegawa et al. 1997).
Oxidative stress, is the result of an excessive production of reactive oxygen species (ROS) and/or a decrease in the antioxidant defenses (Halliwell 2006; Halliwell & Gutteridge 2007) and targets all cell components decreasing sperm motility and mitochondrial activity (Griveau & Le Lannou 1997; Sikka et al. 1995), promoting peroxidation of membrane lipids (Storey 1997) and DNA fragmentation and oxidation (Aitken et al. 1998; Barroso et al. 2000). The ROS-mediated damage to sperm is a significant contributing factor in 30–80% of infertile men (Agarwal et al. 2006; Aitken 2006; Gagnon et al. 1991; de Lamirande & Gagnon 1995; Tremellen 2008). Low levels of antioxidant enzymes in both seminal plasma and spermatozoa are associated with impairment of sperm function, DNA integrity and men infertility (Gong et al. 2012; Aitken & Curry 2011).
Paradoxically, the spermatozoon requires of low and controlled amounts of ROS to acquire fertilizing ability during capacitation (de Lamirande & O’Flaherty 2012). ROS trigger most of the recognized events associated with capacitation: activation of adenylyl cyclase, increase of intracellular calcium and phosphorylation events (protein kinases A, C, ERK and PI3K/Akt pathways) culminating with the late tyrosine phosphorylation (de Lamirande & O’Flaherty 2012; Leclerc et al. 1996; Visconti et al. 1995; O’Flaherty et al. 2006a). Noteworthy, failure to undergo tyrosine phosphorylation were observed in spermatozoa from infertile patients (Buffone et al. 2005). Hydrogen peroxide (H2O2) affected motility (de Lamirande & Gagnon C 1992) and sperm hemi-zona binding (Oehninger et al. 1995) at 0.5 or 0.2 mM, respectively. The incubation of human spermatozoa with sodium nitropruside (a NO• donor) promoted similar results but at higher concentrations (1 mM) (Wu et al. 2004).
S-glutathionylation of proteins is a post translational modification that occurs under normal conditions as well as under conditions of oxidative stress. This modification occurs by the addition of glutathione (GSH) to cysteine residues of certain target proteins; the disulfide linkage between the glutathione and the protein is reversible affecting the functionality of enzymes, receptors and structural proteins, thus altering normal cell biology (Halliwell & Gutteridge 2007).
Nitrotyrosine is formed by the reaction of peroxynitrite or donors of NO• with tyrosine residues (Halliwell & Gutteridge 2007). It can be produced by the sperm cell by the reaction of superoxide anion (O2·−) and NO• (Herrero et al. 2001). The nitrotyrosine protein modification can result in alteration of protein function or structure and thus may affect sperm motility (Vignini et al. 2006), but is required at low amounts in the spermatozoon in order to undergo capacitation (Herrero et al. 2001).
Although it is known that high levels of ROS are detrimental for sperm motility and zona-binding ability (de Lamirande & Gagnon C 1992; Oehninger et al. 1995) there are no studies in the literature specifically elucidating the effects of ROS on the ability of human spermatozoa to undergo capacitation. Moreover, little is known regarding the promotion of redox-dependent protein modifications in human spermatozoa, thus, the objectives of this work were to determine the effect of different ROS on the production of tyrosine nitration and S-glutathionylation and their subcellular localization and whether an increase in modified proteins is associated with an impairment of function of human spermatozoa.
MATERIALS AND METHODS
Materials
Percoll was obtained from GE Healthcare (Baie d’Urfe, QC, Canada). Mouse monoclonal anti-glutathione antibody (clone G8) was purchased from Virogen (Watertown, MA, USA). Mouse monoclonal anti-phosphotyrosine (clone 4G10) and mouse monoclonal anti-nitro-tyrosine antibodies were obtained from Upstate Biotechnology, Inc (Lake Placid, NY, USA) and from Abcam (Toronto, ON, Canada), respectively. Donkey anti-rabbit immunoglobulin IgG and goat anti-mouse IgG antibodies (both conjugated with horseradish peroxidase) were provided by Cederlane Laboratories Ltd (Hornby, Canada). Nitrocellulose membranes (pore size, 0.22 um) were purchased from Osmonics Inc (Westborough, Massachusetts, USA) and the enhanced chemiluminescence kit Lumi-Light from Roche Molecular Biochemicals (Laval, QC Canada). Radiographic films (obtained brom Fuji; Minami-Ashigara, Japan) were used for immunodetection of blotted proteins. For immunocytochemistry studies, both biotinylated goat anti-rabbit IgG (H+L) and biotinylated horse anti-mouse IgG (H+L) were purchased from Vector Laboratories Inc (Burlingame, CA, USA) and Alexa Fluor 555 conjugate of streptavidin and Prolong Antifade were purchased from Life Technologies Inc (Burlington, ON, Canada). Diethylamine NONOate (DA-NONOate) was obtained from Calbiochem (San Diego, CA, USA). bis(dimethyl acetal), 1,4-diazabicyclo-[2.2.2.] octane (DABCO), and Pisum satvium agglutinin conjugated to fluorescein isothiocyanate (PSA-FITC) were purchased from Sigma- Aldrich Chemical Co (Milwaukee, WI, USA). Other chemicals used were of at least reagent grade.
Subjects
Semen samples were obtained from healthy volunteers (n=21) after three days of sexual abstinence. This study was approved by the Ethics Board of the Royal Victoria Hospital-McGill University Health Centre, and all participants signed an informed consent form prior to participating. Following collection, samples were incubated at 37°C for 30 minutes to induce liquefaction. The liquefied semen was then analyzed by computer assisted semen analysis system (CASA) (Sperm vision HR software v1.01, Penetrating Innovations, Ingersoll, ON, Canada) and the quality of the sample was determined according to the parameters set out by the World Health Organization guidelines (WHO 2010)(Supplementary Table 1).
Sperm sample preparation
Liquefied semen samples were centrifuged for 30 min at 2300×g at 20°C over a four-layer Percoll gradient (95-65-40–20%, made with isotonic HEPES balanced saline (HBS)). This step was used to separate the abnormal sperm cells, seminal plasma, white blood cells, from the sperm cells with the best motility and morphology (Kovalski et al. 1992) without increasing ROS levels (Zini et al. 1993; Plante et al. 1994; Iwasaki & Gagnon 1992). Highly motile spermatozoa recovered from the 95% layer and the 65–95% interface were diluted to 50 × 106 cell/ml in Biggers, Whitten and Whittingham medium (BWW, pH 8.0) (Biggers et al. 1971), and used for experimentation.
Induction of in vitro oxidative stress in spermatozoa
Spermatozoa were incubated during 30 min incubation at 37°C with increasing concentrations of either hydrogen peroxide (H2O2), tert-buthyl hydroperxide (tert-BHP; a synthetic organic hydroperoxides that can produce alkoxyl radical, O2·− and H2O2), or Da-NONOate (NO• donor) in BWW. Concentrations were selected to mimic both mild (0.1–0.25 mM) and strong (0.5–5 mM) oxidative stress. After treatment, electrophoresis sample buffer (Tris-HCl, pH 6.8, containing 2 SDS, 10% glycerol, 0.0025% bromophenol, vanadate 0.1 mM, sodium floride 5 mM and glycerol phosphate 20 mM) supplemented or not with 100 mM diothiothreitol (DTT) was added to an 50-μl aliquot of the sperm suspension (100 ×106/ml) of each sample. The absence of DTT in the sample buffer is indispensable to be able to see the S-glutathionilated proteins; as a reducing agent, DTT will cleave the glutathione from the protein and thus eliminating the signal. The rest of the aliquot was used to determine motility, viability and ability to undergo capacitation and acrosome reaction.
Sperm motility and viability analysis
Spermatozoa were subjected to oxidative stress as previously described, washed and resuspened in fresh BWW medium and an aliquot of 10×106 cells/ml was smeared onto collodion-coated slides. Sperm motility was analyzed using the CASA system (Sperm Vision HR software v1.01, Penetrating Innovations, Ingersoll, ON, Canada) according to WHO guidelines (WHO 2010) (Supplementary Table 1). This was achieved by averaging the motility parameters for rapid progressive, slow progressive, non-progressive and immotile sperm, obtained from 10 different fields.
Both ROS-treated and control samples were centrifuged for 5 min at 600×g at 20°C. The supernatant was removed and replaced with a hypo-osmotic solution (HOS) at 37°C (WHO 2010). Samples were incubated for 30 minutes at 37°C. Following this, sperm samples were gently centrifuged for 5 minutes at 1,000×g. The supernatant was removed, and the pellet was resuspended in ethanol; the fixed cells were smeared onto superfrost plus slides, and viability was assessed by using bright field microscopy to observe the presence or absence of tail curl (WHO 2010). Only sperm with a visible tail curl were considered viable and at least 200 cells were counted for each treatment.
Induction of sperm capacitation
ROS-treated spermatozoa were centrifuged for 5 minutes at 600×g at 20°C. The supernatant was then discarded and replaced with fresh BWW containing either 10% fetal chord serum ultrafiltrate (FCSu), 3mg/ml bovine serum albumin (BSA) or 10 μM progesterone in order to induce capacitation and spermatozoa were then incubated for 3.5 hours at 37°C (O’Flaherty et al. 2004). Then, an aliquot was taken from each sample, supplemented with reducing sample buffer containing DTT as explained above, and used for immunoblotting in order to determine capacitation-associated tyrosine phosphorylation using an anti-phosphotyrosine antibody (1:10,000 dilution) (O’Flaherty et al. 2006b). The remaining sample was centrifuged at 2,000×g for 5 min at 20°C. The resulting pellet was recuperated and resuspended in fresh BWW containing 2.5 μM lysophosphatidylcholine (LPC) and incubated for 30 minutes at 37°C in order to induce the acrosome reaction (de Lamirande et al. 1997). In these samples, the percentage of capacitated spermatozoa was determined by the fluorescein isothiocyanate-conjugated Pissum sativum agglutinin staining (FITC-PSA) (de Lamirande et al. 1997). Sperm samples were centrifuged for 5 minutes at 600×g at 20°C, and fixed with ethanol. An aliquot of 10×106 cells was then smeared onto superfrost plus slides (Fischer Scientific, Montreal, QC, Canada) and air dried. Following this, slides were incubated for 5 min with PSA-FITC. Slides were then washed with water. A 1,4-Diazabicyclo[2.2.2]octane (DABCO) solution was then applied to each slide, and they were sealed with a coverslip. Slides were then observed under a Carl Zeiss (Oberkochen, Germany) Axiophot microscope (exciter filter BP450- 490) at 1,000 magnifications. Two hundred cells per duplicate were counted for presence or absence of an intact acrosome.
Cellular fractionation and localization of tyrosine nitration and S-glutathionylation in spermatozoa
ROS-treated spermatozoa were fractionated into cytosolic, Triton-soluble and Triton-insoluble fractions as previously described (O’Flaherty & de Souza 2011). Briefly, cells were frozen at −80°C for 15 minutes, and then thawed at 37°C in order to disrupt sperm membranes and allow the release of the cytosolic content. Sperm suspensions (50 × 106 cells/ml) were then centrifuged for 5 min at 12,000×g, and the supernatant was collected. The remaining pellet was resuspended (100 × 106/ml final concentration) in BWW containing 0.2% Triton-x100 (BWW-T) and incubated for 10 minutes on ice. This chilled sample was then centrifuged for 5 minutes at 12,000×g at 5°C, and the supernatant, containing the Triton-soluble fraction, was collected. The remaining pellet (Triton-insoluble fraction) was resuspended (100 × 106/ml final concentration) in BWW-T, and sonicated (three cycles of 5 min at 30% output) with a Sonic Vibracell (Sonics and Materials Inc, Newtown, CT, USA) with net power output: 10 watts and 20 kHz. The cytosolic, Triton-soluble and – insoluble fractions were supplemented with sample buffer with (reducing conditions) or without (non-reducing conditions) 100 mM dithiothreitol (DTT) and boiled for 5 min at 96°C.
SDS-PAGE and Immunoblotting
Aliquots of supernatant containing sperm proteins from entire spermatozoa or subcelullar fractions (under non- or reducing conditions) were loaded in 12% polyacrylamide gels, electrophoresed and electrotransferred on to nitrocellulose membranes using transfer buffer (192 mM glycine and 25 mM Tris, pH 8.3) containing 20% methanol. The membranes were then blocked via a 30 minutes incubation in 5% skim milk dissolved in 2 mM Tris (pH 7.8)- buffered saline and 0.1% tween 20 (TTBS). Membranes containing proteins under non- or reducing conditions were then washed with TTBS and incubated overnight with anti-Nitro-tyrosine 1:10,000 or anti-glutathione 1:2,000 dilution prepared in 1% skim milk in TTBS, respectively. Following incubation, membranes were washed using 10 min incubations in fresh TTBS. This was followed by 1 hour incubation at room temperature with a horseradish peroxidase conjugated secondary antibody (1:5,000 dilution). Positive immunoreactive bands were detected using chemiluminescence (Lumi-light; Roche Molecular Biochemicals). After detection, membranes were washed with distilled water and silver stained (Jacobson & Karsnas 1990) to assure for the equal loading for each sample.
Relative intensity of proteins bands were done as previously reported (O’Flaherty et al. 2005; Gong et al. 2012). Briefly, films with the same time of exposure were scanned using a Hewlett Packard scanjet G4010 (Hewlett Pakcard, Mississauga, ON, Canada) and the resulted images were analyzed using the Un-Scan-It gel software version 5.1 (Silk Scientific Corporation, Orem, Utah). Each band’s intensity was obtained and normalized to the respective intensity of the 55 kDa band present in the membrane after staining with colloidal silver as explained above. Then, the total value of all the normalized intensity bands were obtained and again normalized with that of the control sample. Therefore, the intensity of each sample is a proportion of the intensity of the respective control for each experiment. This last normalization allowed us to determine the relative increases or decreases in intensities obtained under various experimental conditions.
Immunocytochemistry
Sperm suspensions were treated with either 5mM H2O2 or 500μM DA-NONOate and incubated for 30 min at 37°C to induce oxidation. Aliquots containing 10×106 cells were then smeared onto superfrost plus slides (Fisher Scientific, Montreal, QC, Canada), and allowed to dry at room temperature. Dried cells were permeabilized with methanol as done before (O’Flaherty & de Souza 2011). Cells were rehydrated with PBS supplemented with Triton-X100 (PBS-T), and blocked with 5% goat serum in PBS-T for 30 min at 20°C. Slides were washed in PBS-T and incubated overnight at 4°C with anti-nitro-tyrosine or anti-glutathione antibodies. Cells were then washed, and incubated for 1 hour at 20°C with their respective biotinylated anti-IgG antibody (dilution 3:1000). Following this, strepavidin conjugated to alexa fluor 555 (1:500) was applied to slides. Smears were mounted with prolong antifade, and sealed with a coverslip. Negative controls were prepared in the same way, except samples were incubated solely with the respective biotinylated anti-IgG antibody.
Statistical analysis
Percentages of capacitation, motility and viability were transformed as arcsin square root of the proportion value and analyzed using ANOVA and the Bonferroni’s test. Normal distribution was confirmed by using Anderson-Darling test. A difference was considered to be significant when the p value was equal to or less than 0.05. Systat 13 for Windows (Systat software inc.) was used for all statistical analyses.
RESULTS
Impact of oxidative stress on sperm motility and viability
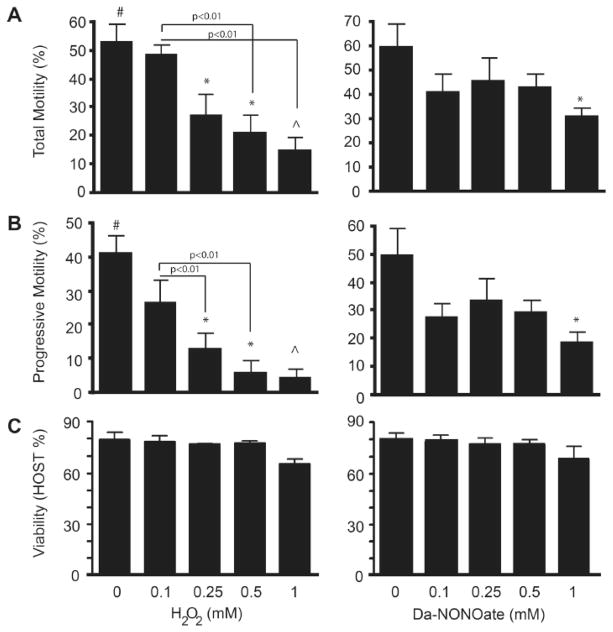
We generated an in vitro mild to strong oxidative stress with exogenous sources of ROS, generating H2O2, alkoxyl radical, O2·−, NO• and peroxynitrite to mimic what is happening to spermatozoa of infertile men affected by high levels of ROS. A dose dependent decrease (p<0.05) in both total and progressive motility was observed in spermatozoa treated with H2O2 and a significant reduction in DA-NONOate-treated spermatozoa (Figure 1A and 1B). A significant decrease total motility was documented in spermatozoa treated with 0.5 and 1mM of H2O2 as compared to non-oxidized controls. In the case of sperm treated with DA-NONOate, a significant decrease was noted only after incubation with 1mM DA-NONOate (Figure 1A and 1B). Noteworthy, H2O2 showed a stronger negative effect on sperm motility at higher doses than DA-NONOate.
Figure 1. Total and progressive motility decreases dose dependently in sperm treated with H2O2 and DA-NONOate without affecting sperm viability.
The motility of sperm cells was analyzed using the CASA sperm analysis system. Total motility (A), progressive motility (B) and sperm viability (C) were recorder from 6 different donors. # or ^means the highest and lowest values, respectively. * means lower than control (0 mM).
Sperm viability was then assessed using a hypo-osmotic swelling test in order to determine whether the loss of motility is due to cell death. Our results confirmed that sperm viability was not affected following oxidation with both H2O2 and DA-NONOate (Figure 1C).
Oxidative stress impairs sperm capacitation
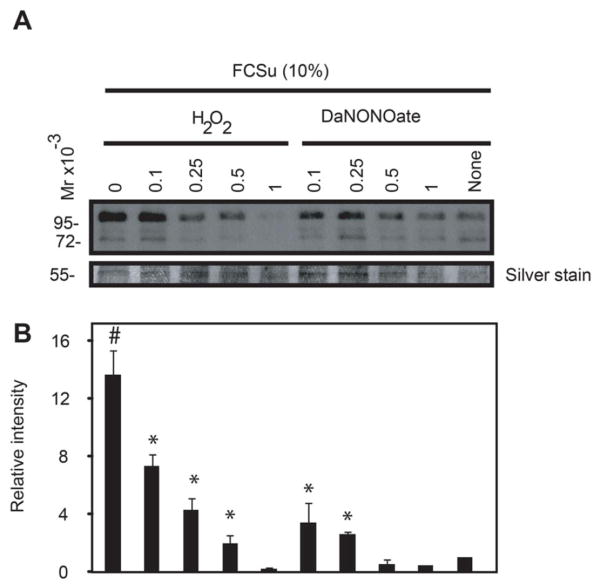
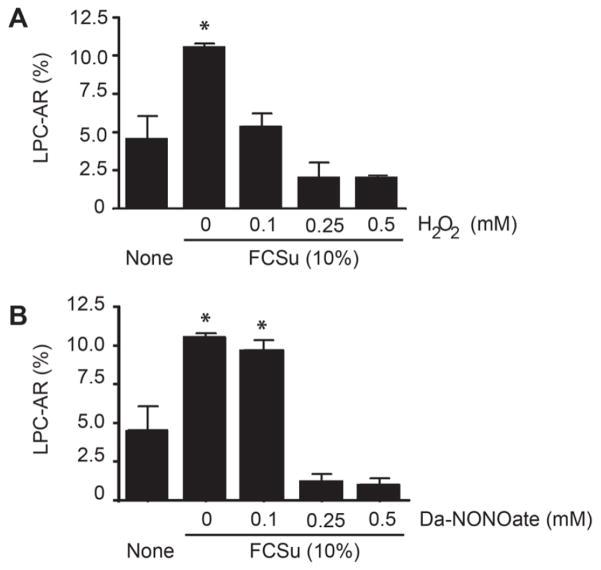
Spermatozoa, treated with H2O2 or DA-NONOate prior to capacitation with FCSu01, had similar levels of tyrosine phosphorylation compared to non-capacitated controls (Figure 2). Hydrogen peroxide produced a greater decrease in tyrosine phosphorylation than DA-NONOate, particularly at concentrations of 1 mM. There was a dose dependent decrease in LPC-induced acrosome reaction levels in spermatozoa previously treated with H2O2 (Figure 3A). However, spermatozoa treated with DA-NONOate showed no change in the percentage of acrosome reaction following incubation with 0.1 mM (Figure 3B), while this percentage drastically dropped in spermatozoa treated with 0.25 or 0.5 mM. Similar results were obtained with BSA or progesterone as capacitation inducers (data not shown).
Figure 2. Spermatozoa treated with H2O2 or DA-NONOate showed lower levels of protein tyrosine phosphorylation after capacitation than non-oxidized controls.
A) Treated spermatozoa were capacitated for 3.5h in BWW, pH 8.0, 37°C supplemented without (None) or with the capacitation inducer FCSu (10%). Sperm proteins from 0.1×106 spermatozoa were loaded in each well, electrophoresed in SDS polyacrylamyde gel under reducing conditions and immunoblotted with anti-phosphotyrosine antibody. Silver stain was used as loading control (Band at 55 kDa is shown in the bottom panel). B) Relative intensity of protein tyrosine phosphorylation. The density value of bands from sample incubated under non-capacitating conditions (None) was used to normalize the values obtained with the other samples. Relative intensity of bands is presented as the mean ± SEM. Results are representative of 3 others done with different healthy donors (n=4). #means the highest value, *means higher than non-capacitating control (None).
Figure 3. Oxidized spermatozoa displayed lower level of capacitation compared to untreated controls under capacitation conditions.
Spermatozoa previously treated with H2O2 or DA-NONOate were capacitated with FCSu for 3.5h and then, with LPC for 30 minutes to induce the acrosome reaction. Sperm cells were stained with PSA-FITC to visualize the acrosome, and two hundred spermatozoa were observed under fluorescent microscope. Representative blot from 4 other experiments done with different donors (n=5). *Means higher than non-capacitating control (None).
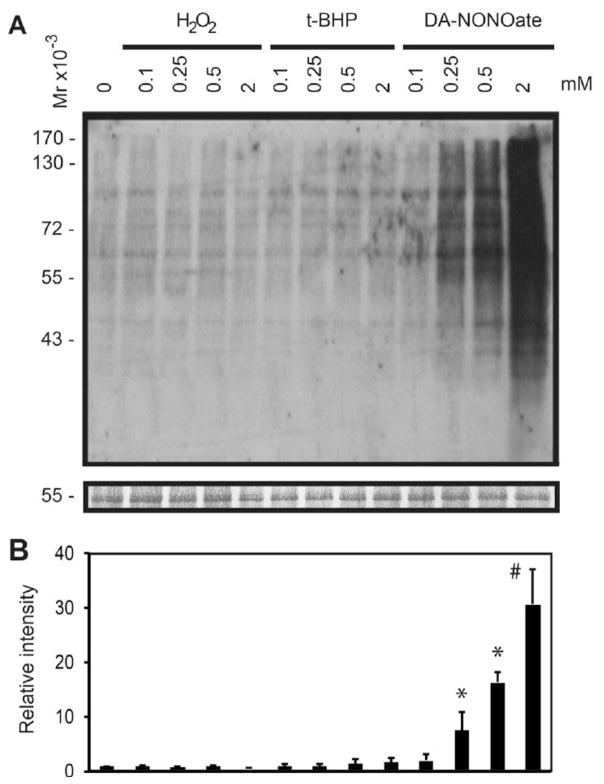
Reactive oxygen species promote tyrosine nitration and S-glutathionylation of sperm proteins
Percoll-washed spermatozoa were exposed to increasing concentrations of DA-NONOate, H2O2, or tert-BHP (Figure 4). DA-NONOate promoted a dose dependent increase of the tyrosine nitration in spermatozoa (Figure 4). Treatment with peroxides (H2O2 or tert-BHP) generated a dose dependent increase in S-glutathionylation in spermatozoa (Figure 5), showing a minimum or no effect on inducing the tyrosine nitration modification (Figure 4). The levels of S-glutathionylation in spermatozoa treated with DA-NONOate (0.1 to 1 mM) were similar to those of the control and never exceeding those generated with 0.25 mM H2O2 (Figure 6), suggesting that NO• is not a major inducer of S-glutathionylation in human spermatozoa.
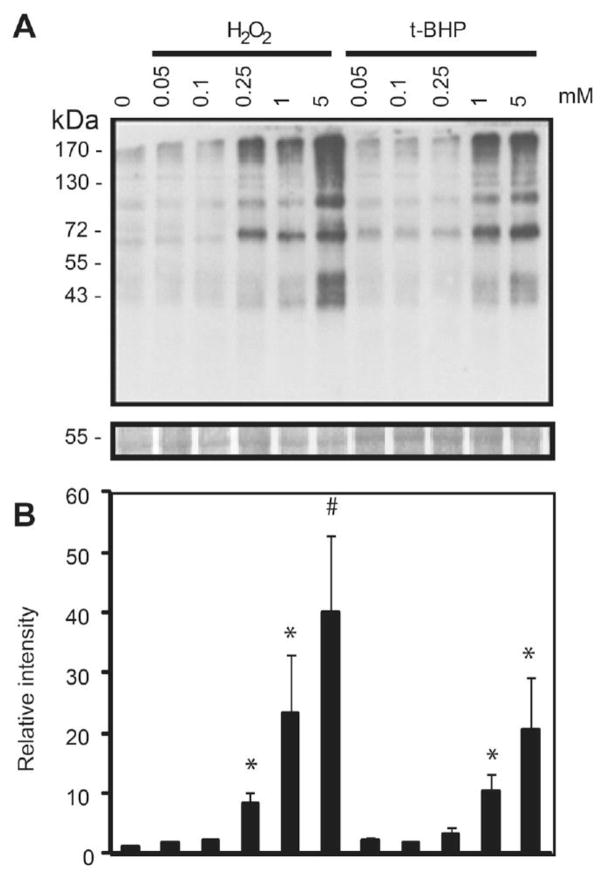
Figure 4. Dose dependent increase of tyrosine nitration in human spermatozoa following treatment with ROS.
A) Spermatozoa were treated with increasing concentrations of H2O2, tert-BHP, or DA-NONOate and immunoblotted with an anti-nitrotyrosine antibody. B) Relative intensity of tyrosine nitrated proteins. The density value of bands from sample incubated without ROS (0 mM) was used to normalize the values obtained with the other samples. Relative intensity of bands is presented as the mean ± SEM. Membranes were silver stained to confirm equal loading between lanes (band at 55kDa is shown in the bottom panel). Representative blot from 4 other experiments done with different donors (n=5). #means the highest value, *means higher than control (0 mM).
Figure 5. Dose dependent increase of S-glutathionylation modifications in human spermatozoa following treatment with ROS.
A) Spermatozoa treated with increasing concentrations of H2O2 or t-BHP and immunoblotted with an anti-glutathione antibody. B) Relative intensity of S-gluthathionylated proteins. The density value of bands from sample incubated without ROS (0 mM) was used to normalize the values obtained with the other samples. Relative intensity of bands is presented as the mean ± SEM. #means the highest value, *means higher than control (0 mM).
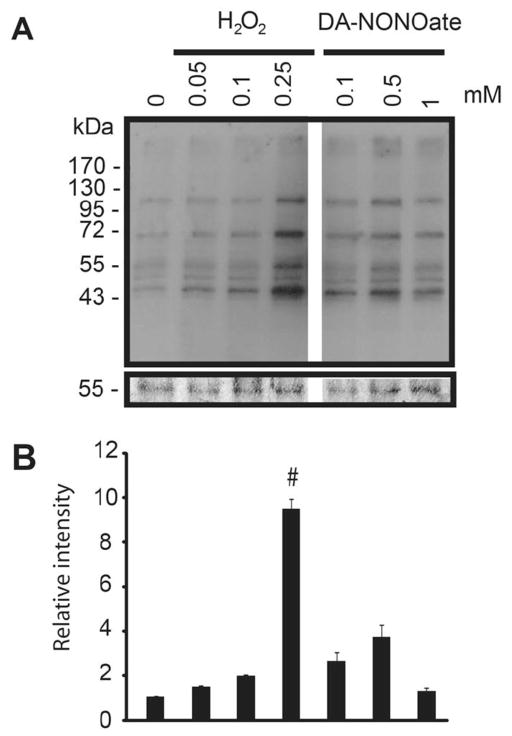
Figure 6. DA-NONOate does not increase the levels of S-glutathionylation in spermatozoa.
A) Spermatozoa were treated with increasing concentrations of DA-NONOate or H2O2 and immunoblotted with anti-glutathione antibody. Membranes were silver stained to confirm equal loading between lanes (band at 55kDa is shown in the bottom panel). The density value of bands from sample incubated without ROS (0 mM) was used to normalize the values obtained with the other samples. Relative intensity of bands is presented as the mean ± SEM. All samples were loaded in the same gel. Representative blot from 2 other experiments done with different donors (n=3). #means the highest value.
Tyrosine nitration and S-glutathionylation modified proteins are differentially localized in human spermatozoa
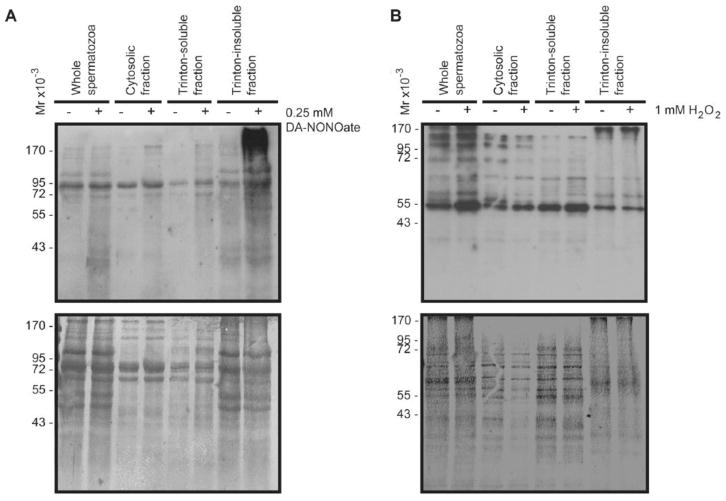
Since exposure to high concentrations of different ROS induces an increase in the tyrosine nitration and S-glutathionylation (Figures 4 and 5), the next step was to determine the localization of these modified proteins within the compartments of the sperm cell. Following fractionation, we found an increase of tyrosine nitrated proteins in all of the cytosolic, Triton-soluble and -insoluble fractions following treatment with DA-NONOate (Figure 7A). The highest levels were found in the Triton-insoluble fraction. Immunocytochemistry experiments revealed a strong labeling within the tail region (Figure 8A). Moreover, permeabilized DA-NONOate-treated spermatozoa, displayed a complete labeling of the head and tail (Figure 8, lower panel).
Figure 7. Tyrosine nitration and S-glutathionylation are differentially localized within the sperm cell, and most abundantly found in Triton-insoluble proteins of the tail and fibrous sheath.
A) Sperm proteins treated with 0.25mM of DA-NONOate under reducing conditions, and immunoblotted with an anti-nitro tyrosine antibody (upper panel; 0.5×106 cells were loaded for the whole and cytosolic lanes, while 1 x106 cells were loaded for the triton-soluble and insoluble lanes). B) Sperm proteins treated with 1mM H2O2 under non-reducing conditions, and immunoblotted with anti-GSS-R antibody (upper panel; 0.5×106 cells were loaded for the whole and cytosolic lanes, while 1 x106 cells were loaded for the triton-soluble and insoluble lanes). Lower panel in A and B represent the respective membrane stained with colloidal gold as described in material methods. Representative blots from 3 other experiments done with different donors (n=4).
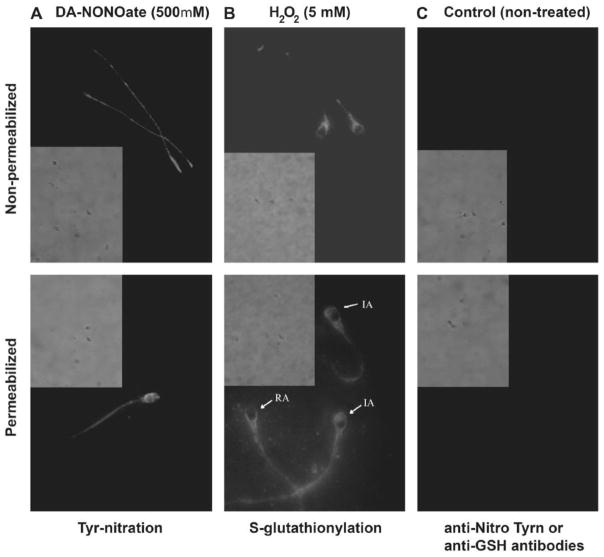
Figure 8. S-glutathionylated proteins were differentially localized within the sperm cell and preferentially found within the Triton-soluble and cytosolic fractions.
Immunocytochemistry images were taken with fluorescence and phase contrast microscopy at 1000x magnification for sperm treated with 500μM DA-NONOate (A) or with 5mM of H2O2 (B). Treated and untreated sperm were photographed at the same time of exposure. Absence of unspecific labeling by the secondary antibody was also confirmed (data not shown)(n=4).
The majority of S-glutathionylated proteins were found in the cytosolic and Triton-soluble fractions (Figure 7B). Noteworthy, a strong signal was observed in proteins of high molecular mass (~170 kDa). The H2O2 treatment promoted the highest levels of S-glutathionylation in sperm proteins found in the Triton-soluble fraction and with a less extent in the cytosolic fraction. The S-glutathionylated sperm proteins found at 170 kDa were present in the Triton-insoluble fraction. Non-permeabilized spermatozoa displayed a strong labeling throughout the midpiece and in the post acrosomal region (Figure 8B). After permeabilization, a strong labeling was visible within the acrosome, and throughout the tail region (Figure 8B, lower panel). It was also noted that treatment with high concentrations of H2O2 caused the acrosome labeling to disappear in some cells, which may be indicative of a spontaneous acrosome reaction.
DISCUSSION
In this study we showed evidence that oxidative stress impacts differently on sperm function depending on the type of ROS involved and promotes redox dependent protein modifications that display differential localization in subcellular compartments of human spermatozoa. To our knowledge this is the first report to extensively studied tyrosine nitration and S-glutathionylation protein modifications in light of impairment of sperm motility and capacitation.
Based on the motility and viability analysis, we confirmed that the impairment of sperm motility is not due to cell death, thus, there is a direct effect of ROS on motility machinery. We examined the effect of oxidative stress on capacitation by determining the percentage of capacitated spermatozoa and the levels of tyrosine phosphorylation (O’Flaherty et al. 2006b; Leclerc et al. 1996). It was found that spermatozoa, treated with H2O2 prior to capacitation, displayed levels of tyrosine phosphorylation (Figure 2) and of LPC-iduced AR (Figure 3A) that were similar to non-capacitated controls. In the case of DA-NONOate, only concentrations higher or equal to 0.25 mM were able to prevent both tyrosine phosphorylation and capacitation (Figures 2 and 3B). These results suggests that oxidative stress negatively impact on the capability of spermatozoa to acquired fertilizing ability and thus, explaining why men with high levels of ROS in semen are infertile. The inhibition of motility and capacitation was less severe with DA-NONOate than with H2O2; therefore, it is important to determine which type of ROS is driven the oxidative stress at the time to establish an antioxidant therapy. These results emphasize the importance of seeking ROS-targeted therapy to treat male infertility.
Peroxides (H2O2 or tert-BHP) and DA-NONOate (NO• donor) generated a dose dependent increase of S-glutathionylation and of tyrosine nitration in spermatozoa (Figure 4), suggesting that human spermatozoa actively produce significant levels of redox-dependent protein modifications when they are challenged with an oxidative stress. These results suggest that the oxidative stress impairs motility without affecting sperm viability when high levels of tyrosine nitration and S-glutathionylation are present in human spermatozoa.
The majority of tyrosine nitrated-modified proteins were localized in the midpiece (weak labeling) and principal piece (strong labeling) of the sperm tail (Figure 8A). We can suggest that some of these modified proteins maybe present in the fiber sheath, as proteins present in these sperm structure can be found in the Triton-insoluble fraction where the tyrosine nitrated-modified are present at high levels (Figure 7). The decreased total and progressive motility and the evident labeling of the tail (Figures 1 and 8A) suggest that an increase of tyrosine nitrated-modified proteins may disrupt the function of proteins that are important for the motility machinery. While future experiments would be required to determine the identity of the modified proteins, our results suggest that glycolytic enzymes such as glyceraldehyde 3-P dehydrogenase, enolase, enzymes involved in the Krebs cycle such as aconitase, α-ketoglutarate dehydrogenase, malate dehydrogenase and dihydro lipoamide dehydrogenase (present in the pyruvate dehydrogenase that converts pyruvate into acetylCoA) may be targets since they can be altered by this modification (Shi et al. 2011; Gokulrangan et al. 2007; Lind et al. 2002)(Supplementary Table 2). The evidence that the size of bands obtained during western blot experiments for tyrosine nitrated-modified proteins (Figure 4 and 7A) that are similar to the molecular mass of the mentioned enzymes accounts for the possibility that these energy production-related enzymes may be affected by tyrosine nitration in spermatozoa affected by oxidative stress.
We found in the head of permeabilized spermatozoa, high levels of tyrosine nitration after treatment with DA-NONOate (Figure 8). Tyrosine nitration activates metalloproteinase-9 (MMP-9) promoting astrocyte migration and subsequent inflammation of the brain under oxidative stress (Wang et al. 2011). The localization of tyrosine nitration in the sperm head is similar to that of metalloproteinases MMP-2 and MMP-9 found human spermatozoa (Buchman-Shaked et al. 2002) and suggested as important proteins for sperm zona penetration (Ferrer et al. 2012). These proteins form the extracellular coat on the inner acrosomal membrane that is exposed after acrosome reaction (Ferrer et al. 2012). It is possible that a premature activation of MMPs by tyrosine nitration (Wang et al. 2011), decreases the capability of the spermatozoon to penetrate the zona pellucida.
Most of the S-glutathionylated-modified proteins were found in the cytosolic and triton-soluble fractions (Figure 7B). Immunocytochemistry studies revealed a strong labeling throughout the midpiece and in the post acrosomal region (Figure 8B). Following permeabilization, a strong labeling was visible within the acrosome, and the tail region. Within the head, components of the cytoskeleton play a role during the activation of spermatozoon; during capacitation, there is polymerization of the actin filaments that is necessary for the spermatozoon to undergo the acrosome reaction (Breitbart et al. 2005; Brener et al. 2003). Actin can be S-glutathionylated (Dalle-Donne et al. 2003) and its modified form has been associated with Friedreich’s Ataxia (Pastore et al. 2003). The strong labeling in the acrosome may also be indicative of S-glutathionylation of actin. This redox-dependent modification of actin promotes the impossibility of actin polymerization and thus preventing the acrosome reaction to occur upon LPC treatment (Figure 3).
Since S-glutathionylation seemed to be present over the entirety of the sperm cell, similarly to the tyrosine nitration modification, we can also suggest that S-glutathionylation may impair sperm function by impeding the energy production. It was shown that enzymes of glycolysis and of the Krebs cycle can be S-glutahionylated under oxidative stress (Supplementary Table 2) (Fratelli et al. 2004). Noteworthy, tubulin, the major component of the sperm flagellum, can be modified by tyrosine nitration and S-glutathionylation (Landino et al. 2004), thus our results suggest that sperm motility is affected also at the level of flagellar structure.
Oxidative stress affects human sperm function (Agarwal et al. 2006; de Lamirande E. & Gagnon 1994; Gagnon et al. 1991; Aitken & Baker 2006); however, little is known regarding the players and mechanisms affected by high levels of ROS and whether various sources of oxidative stress differentially impact on sperm function. Although high levels of ROS impair motility (Smith et al. 2006; Aitken et al. 1998), the ability to fuse to zona-free hamster oocytes (Aitken et al. 1998), promote increased DNA damage and inhibition of capacitation (present study), it is yet to be elucidated the molecular mechanisms associated with these functions that are impaired by high levels of ROS.
Besides the high sperm lipid peroxidation and DNA damage, it was recently suggested that the antioxidant enzymes peroxiredoxins (PRDXs) are highly oxidized and thus inactive in infertile patients (Gong et al. 2012). PRDXs are highly abundant and differentially distributed in all sub-compartments of the human spermatozoon and are considered the first line of defense against oxidative stress in human spermatozoa (O’Flaherty & de Souza 2011). Our laboratory reported that PRDX1 and PRDX6 form ~170kDa protein complexes detected in spermatozoa treated high H2O2 concentrations (0.25–5 mM) and also found in spermatozoa from infertile men (Gong et al. 2012; O’Flaherty & de Souza 2011). There is increasing evidence supporting the involvement of PRDXs in the regulation of redox signaling, especially H2O2-dependent signaling (Fourquet et al. 2008; Rhee et al. 2005) Since oxidative stress promotes S-glutathionylation of PRDXs (Lind et al. 2002; Noguera-Mazon et al. 2006) and because we found 130–170 kDa bands with high levels of S-glutathionylation, it is possible that S-glutathionylation of PRDX1 and PRDX6 promotes the impairment of sperm function observed in this study and previously (Gong et al. 2012; O’Flaherty & de Souza 2011). It is worth noting that the strong signal of tyrosine nitration and of S-glutathionylation on the sperm head (Figure 8) corresponds with the localization of PRDXs (O’Flaherty & de Souza 2011; O’Flaherty 2014). It is then plausible that the redox-dependent modifications of PRDXs are causing the increase of levels of sperm DNA damage observed in infertile men (Gong et al. 2012). We recently found that males mice lacking PRDX6 show high levels of DNA fragmentation and oxidation (Ozkosem & O’Flaherty 2012); these data support the role of PRDXs and particularly PRDX6 in the protection of paternal genome against oxidative stress.
In conclusion, tyrosine nitration and S-glutathionylation increase dose dependently in spermatozoa after treatment with ROS, and they are differentially localized within spermatozoa. The oxidative stress prevents spermatozoa from undergoing capacitation, impairs sperm motility and increases tyrosine nitration and S-glutathionylation. Excessive levels of tyrosine nitration and of S-glutathionylation of specific sperm proteins may be involved in the pathological mechanisms leading to impairment of sperm function.
Supplementary Material
Acknowledgments
FUNDING
This study was supported by The Canadian Institutes of Health Research (MOP 133661), the Fonds de Recherché en Santé Quebec (FRSQS #22151) and a Chercheur Boursier Junior 1 Salary Award from FRSQS (20482) to CO, a studentship from the Research Institute-MUHC (#4696) to TM.
We thank donors that participated in this study.
Footnotes
DECLARATION OF INTEREST
None of the authors have conflict of interest to disclosure.
References
- Abid S, Maitra A, Meherji P, Patel Z, Kadam S, Shah J, Shah R, Kulkarni V, Baburao V, Gokral J. Clinical and laboratory evaluation of idiopathic male infertility in a secondary referral center in India. Journal of Clinical Laboratory Analysis. 2008;22:29–38. [Google Scholar]
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Current Opinio in Obstetrics and Gynecology. 2006;18:325–332. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. American Journal of Reproductive Immunology. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Aitken RJ. Sperm function tests and fertility. International Journal of Andrology. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Molecular and Cellular Endocrinology. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxidant and Redox Signaling. 2011;14:367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biology of Reproduction. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- Anderson JB, Williamson RC. Testicular torsion in Bristol: a 25-year review. British Journal of Surgery. 1988;75:988–992. doi: 10.1002/bjs.1800751015. [DOI] [PubMed] [Google Scholar]
- Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Human Reproduction. 2000;15:1338–1344. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittngham DG. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian embryology. San Francisco: Freeman; 1971. pp. 86–116. [Google Scholar]
- Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263–268. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- Brener E, Rubinstein S, Cohen G, Shternall K, Rivlin J, Breitbart H. Remodeling of the Actin Cytoskeleton During Mammalian Sperm Capacitation and Acrosome Reaction. Biology of Reproduction. 2003;68:837–845. doi: 10.1095/biolreprod.102.009233. [DOI] [PubMed] [Google Scholar]
- Brennemann W, Stoffel-Wagner B, Helmers A, Mezger J, Jager N, Klingmuller D. Gonadal function of patients treated with cisplatin based chemotherapy for germ cell cancer. Journal of Urology. 1997;158:844–850. doi: 10.1097/00005392-199709000-00041. [DOI] [PubMed] [Google Scholar]
- Buchman-Shaked O, Kraiem Z, Gonen Y, Goldman S. Presence of Matrix Metalloproteinases and Tissue Inhibitor of Matrix Metalloproteinase in Human Sperm. Journal of Andrology. 2002;23:702–708. [PubMed] [Google Scholar]
- Buffone MG, Calamera JC, Verstraeten SV, Doncel GF. Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction. 2005;129:697–705. doi: 10.1530/rep.1.00584. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. Actin S-glutathionylation: evidence against a thiol-disulphide exchange mechanism. Free Radical Biology and Medicine. 2003;35:1185–1193. doi: 10.1016/s0891-5849(03)00504-5. [DOI] [PubMed] [Google Scholar]
- de Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. Journal of Andrology. 1992;13:368–378. [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Reactive oxygen species (ROS) and reproduction. Advances in Experimental Medicine and Biology. 1994;366:185–197. doi: 10.1007/978-1-4615-1833-4_14. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10(Suppl1):15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Molecular Human Reproduction. 1997;3:175–194. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O’Flaherty C. Sperm capacitation as an oxidative event. In: Aitken J, Alvarez J, Agawarl A, editors. Studies on men’s health and fertility, oxidative stress in applied basic research and clinical practice. Springer Science; 2012. pp. 57–94. [Google Scholar]
- Ferrer M, Rodriguez H, Zara L, Yu Y, Xu W, Oko R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell and Tissue Research. 2012;349:881–895. doi: 10.1007/s00441-012-1429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourquet S, Huang ME, D’Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxidant and Redox Signaling. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Gianazza E, Ghezzi P. Redox proteomics: identification and functional role of glutathionylated proteins. Expert Review of Proteomics. 2004;1:365–376. doi: 10.1586/14789450.1.3.365. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Iwasaki A, de Lamirande E, Kovalski N. Reactive oxygen species and human spermatozoa. Ann New York Academy of Sciences. 1991;637:436–444. doi: 10.1111/j.1749-6632.1991.tb27328.x. [DOI] [PubMed] [Google Scholar]
- Gokulrangan G, Zaidi A, Michaelis ML, Schoneich C. Proteomic analysis of protein nitration in rat cerebellum: effect of biological aging. Journal of Neurochemistry. 2007;100:1494–1504. doi: 10.1111/j.1471-4159.2006.04334.x. [DOI] [PubMed] [Google Scholar]
- Gong S, San Gabriel M, Zini A, Chan P, O’Flaherty C. Low Amounts and High Thiol Oxidation of Peroxiredoxins in Spermatozoa from Infertile Men. Journal of Andrology. 2012;33:1342–1351. doi: 10.2164/jandrol.111.016162. [DOI] [PubMed] [Google Scholar]
- Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. International Journal of Andrology. 1997;20:61–69. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? Journal of Neurochemistry. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B, Gutteridge J, editors. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. pp. 187–267. [Google Scholar]
- Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiation Research. 1997;147:457–467. [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Tyrosine nitration in human spermatozoa: a physiological function of peroxynitrite, the reaction product of nitric oxide and superoxide. Molecular Human Reproduction. 2001;7:913–921. doi: 10.1093/molehr/7.10.913. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertility and Sterility. 1992;57:409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- Jacobson G, Karsnas P. Important parameters in semi-dry electrophoretic transfer. Electrophoresis. 1990;11:46–52. doi: 10.1002/elps.1150110111. [DOI] [PubMed] [Google Scholar]
- Kovalski NN, de Lamirande E, Gagnon C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: protective effect of seminal plasma and scavengers. Fertility and Sterility. 1992;58:809–816. [PubMed] [Google Scholar]
- Landino LM, Iwig JS, Kennett KL, Moynihan KL. Repair of peroxynitrite damage to tubulin by the thioredoxin reductase system. Free Radical Biology and Medicine. 2004;36:497–506. doi: 10.1016/j.freeradbiomed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biology of Reproduction. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, von Löwenhielm HB, Holmgren A, Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Archives of Biochemistry and Biophysics. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- Noguera-Mazon V, Lemoine J, Walker O, Rouhier N, Salvador A, Jacquot JP, Lancelin JM, Krimm I. Glutathionylation Induces the Dissociation of 1-Cys D-peroxiredoxin Non-covalent Homodimer. Journal of Biological Chemistry. 2006;281:31736–31742. doi: 10.1074/jbc.M602188200. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Molecular Human Reproduction. 2004;10:355–363. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biology of Reproduction. 2005;73:94–105. doi: 10.1095/biolreprod.104.038794. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006a;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radical Biology and Medicine. 2006b;40:1045–1055. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biology of Reproduction. 2011;84:238–247. doi: 10.1095/biolreprod.110.085712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty C. Peroxiredoxins: hidden players in the antioxidant defence of human spermatozoa. Basic and Clinical Andrology. 2014;24:4. doi: 10.1186/2051-4190-24-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehninger S, Blackmore P, Mahony M, Hodgen G. Effects of hydrogen peroxide on human spermatozoa. Journal of Assisted Reproduction and Genetics. 1995;12:41–47. doi: 10.1007/BF02214128. [DOI] [PubMed] [Google Scholar]
- Ozkosem B, O’Flaherty C. Detrimental Effects of Oxidative Stress on Spermatozoa Lacking Peroxiredoxin 6. Free Radical Biology and Medicine. 2012;53:S86. [Google Scholar]
- Pastore A, Tozzi G, Gaeta LM, Bertini E, Serafini V, Cesare SD, Bonetto V, Casoni F, Carrozzo R, Federici G, Piemonte F. Actin Glutathionylation Increases in Fibroblasts of Patients with Friedreich’s Ataxia. Journal of Biological Chemistry. 2003;278:42588–42595. doi: 10.1074/jbc.M301872200. [DOI] [PubMed] [Google Scholar]
- Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertility and Sterility. 1994;62:387–393. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Shi Q, Xu H, Yu H, Zhang N, Ye Y, Estevez AG, Deng H, Gibson GE. Inactivation and reactivation of the mitochondrial alpha-ketoglutarate dehydrogenase complex. Journal of Biological Chemistry. 2011;286:17640–17648. doi: 10.1074/jbc.M110.203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. Journal of Andrology. 1995;16:464–468. [PubMed] [Google Scholar]
- Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, Castro A. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Human Reproduction. 2006;21:986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Molecular Human Reproduction. 1997;3:203–213. doi: 10.1093/molehr/3.3.203. [DOI] [PubMed] [Google Scholar]
- Tournaye HJ, Cohlen BJ. Management of male-factor infertility. Best Practice & Research Clinical Obstetrics & Gynaecology. 2012;26:769–775. doi: 10.1016/j.bpobgyn.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility: a clinical perspective. Human Reproduction Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- Turner TT. The study of varicocele through the use of animal models. Human Reproduction Update. 2001;7:78–84. doi: 10.1093/humupd/7.1.78. [DOI] [PubMed] [Google Scholar]
- Vignini A, Nanetti L, Buldreghini E, Moroni C, Ricciardo-Lamonica G, Mantero F, Boscaro M, Mazzanti L, Balercia G. The production of peroxynitrite by human spermatozoa may affect sperm motility through the formation of protein nitrotyrosine. Fertility and Sterility. 2006;85:947–953. doi: 10.1016/j.fertnstert.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. Journal of Cellular Physiology. 2011;226:2244–2256. doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. WHO Press; 2010. [Google Scholar]
- Wu TP, Huang BM, Tsai HC, Lui MC, Liu MY. Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryo development. Systems Biology in Reproductive Medicine. 2004;50:173–179. doi: 10.1080/01485010490425494. [DOI] [PubMed] [Google Scholar]
- Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. International Journal of Andrology. 1993;16:183–188. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.