Abstract
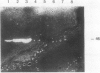
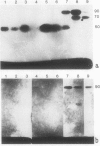
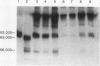
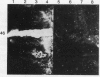
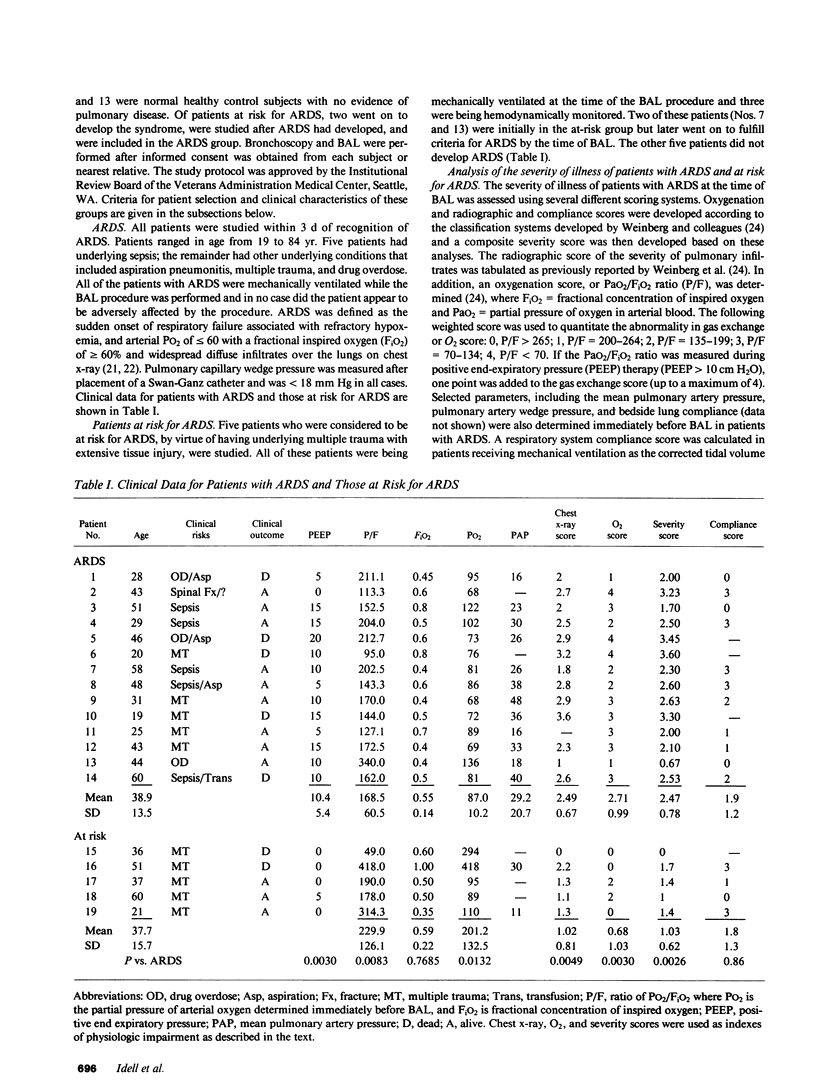
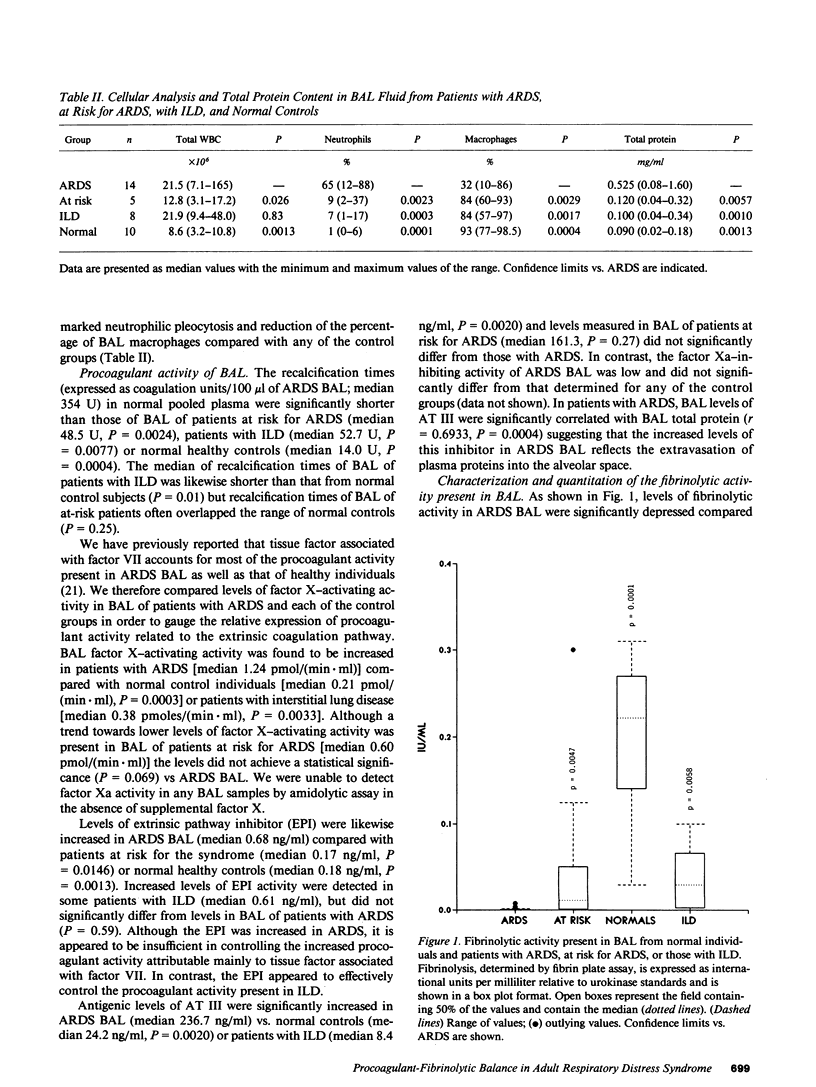
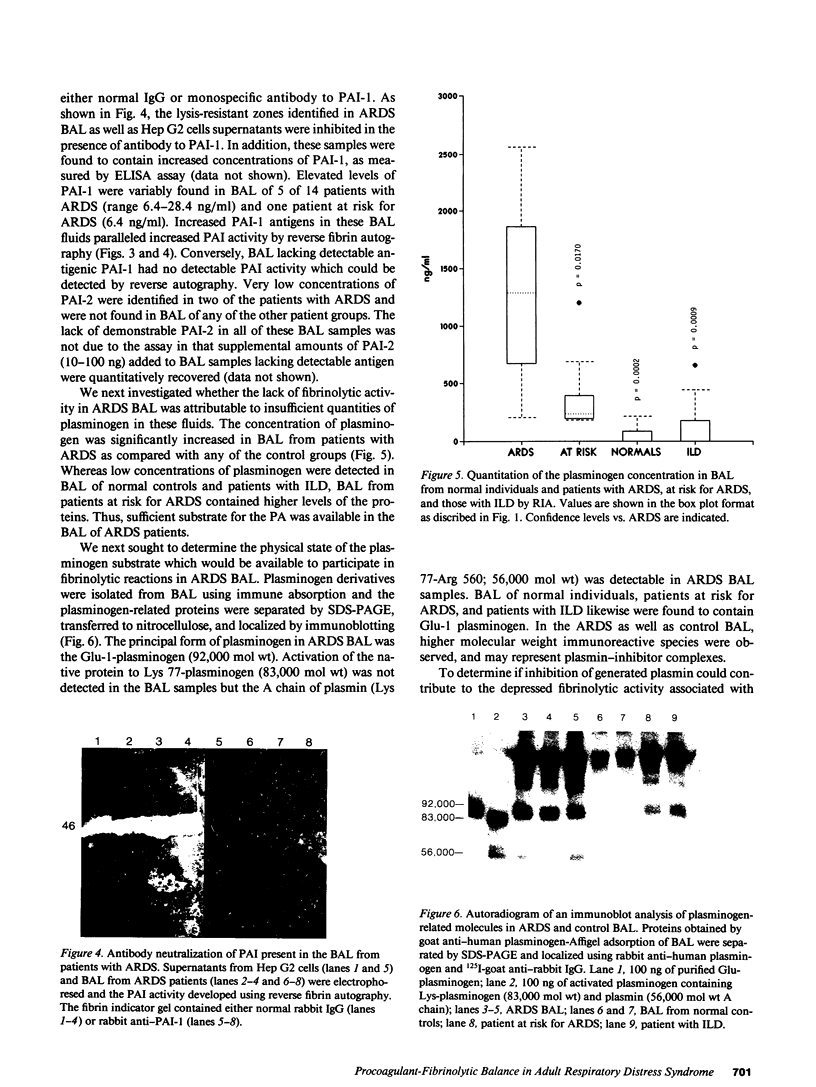
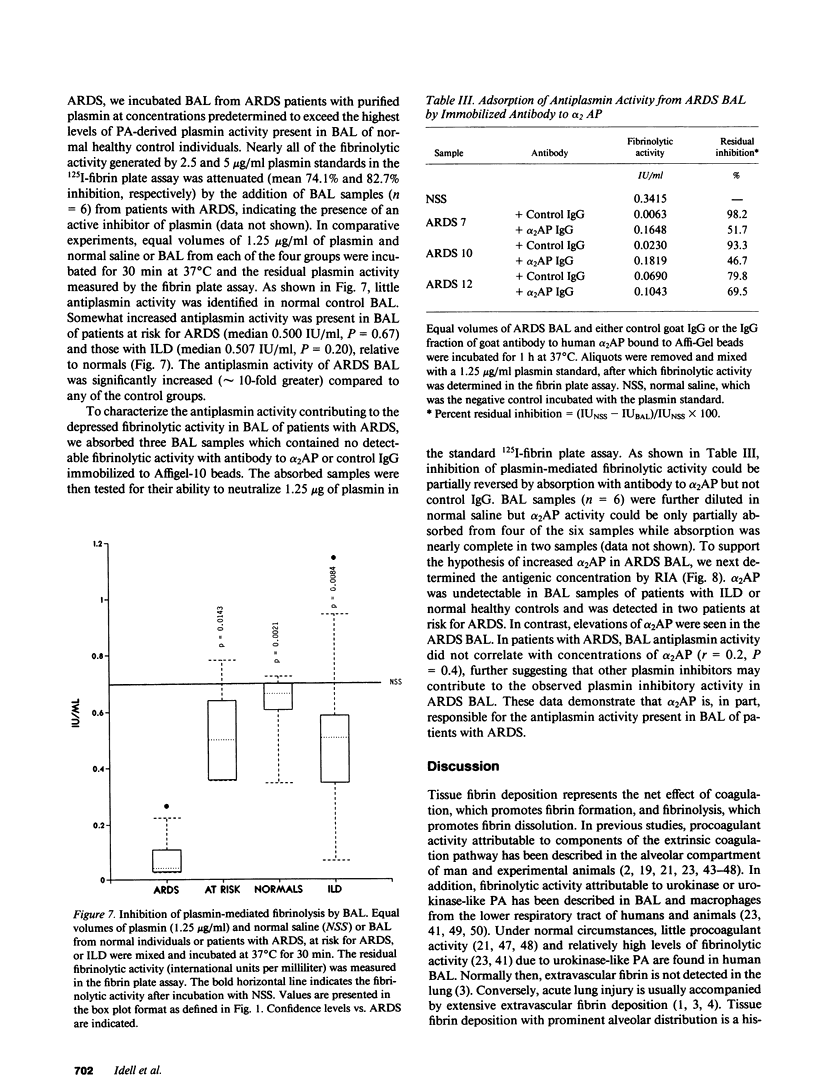
To determine the possible mechanism(s) promoting alveolar fibrin deposition in the adult respiratory distress syndrome (ARDS), we investigated the initiation and regulation of both fibrinolysis and coagulation from patients with ARDS (n = 14), at risk for ARDS (n = 5), and with interstitial lung diseases (ILD) (n = 8), and normal healthy individuals (n = 13). Bronchoalveolar lavage (BAL) extrinsic pathway inhibitor activity was increased in ARDS BAL compared with patients at risk for ARDS (P = 0.0146) or normal controls (P = 0.0013) but tissue factor-factor VII procoagulant activity was significantly increased in ARDS BAL compared with all other groups (P less than 0.001). Fibrinolytic activity was not detectable in BAL of 10 of the 14 patients with ARDS and low levels of activity were found in BAL of the other four ARDS patients. Depressed fibrinolysis in ARDS BAL was not due to local insufficiency of plasminogen; rather, there was inhibition of both plasmin and plasminogen activator. Plasminogen activator inhibitor 1 was variably detected and low levels of plasminogen activator inhibitor 2 were found in two ARDS BAL samples, but plasminogen activator inhibitor 2 was otherwise undetectable. ARDS BAL antiplasmin activity was, in part, due to alpha 2-antiplasmin. We conclude that abnormalities that result in enhanced coagulation and depressed fibrinolysis, thereby predisposing to alveolar fibrin deposition, occur in the alveolar lining fluids from patients with ARDS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Busch C., Jakobson S., Lindquist O., Malmberg P., Rammer L., Saldeen T. Pulmonary insufficiency following intravenous infusion of thrombin and AMCA (tranexamic acid) in the dog. Acta Chir Scand. 1972;138(5):445–452. [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982 Jan;3(1):35–56. [PubMed] [Google Scholar]
- Baker S. P., O'Neill B., Haddon W., Jr, Long W. B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974 Mar;14(3):187–196. [PubMed] [Google Scholar]
- Bowen R. M., Hoidal J. R., Estensen R. D. Urokinase-type plasminogen activator in alveolar macrophages and bronchoalveolar lavage fluid from normal and smoke-exposed hamsters and humans. J Lab Clin Med. 1985 Dec;106(6):667–673. [PubMed] [Google Scholar]
- Broze G. J., Jr, Miletich J. P. Characterization of the inhibition of tissue factor in serum. Blood. 1987 Jan;69(1):150–155. [PubMed] [Google Scholar]
- Chapman H. A., Allen C. L., Stone O. L. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986 Mar;133(3):437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Allen C. L., Stone O. L., Fair D. S. Human alveolar macrophages synthesize factor VII in vitro. Possible role in interstitial lung disease. J Clin Invest. 1985 Jun;75(6):2030–2037. doi: 10.1172/JCI111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Stahl M., Allen C. L., Yee R., Fair D. S. Regulation of the procoagulant activity within the bronchoalveolar compartment of normal human lung. Am Rev Respir Dis. 1988 Jun;137(6):1417–1425. doi: 10.1164/ajrccm/137.6.1417. [DOI] [PubMed] [Google Scholar]
- Ciano P. S., Colvin R. B., Dvorak A. M., McDonagh J., Dvorak H. F. Macrophage migration in fibrin gel matrices. Lab Invest. 1986 Jan;54(1):62–70. [PubMed] [Google Scholar]
- Colvin R. B., Gardner P. I., Roblin R. O., Verderber E. L., Lanigan J. M., Mosesson M. W. Cell surface fibrinogen-fibrin receptors on cultured human fibroblasts. Association with fibronectin (cold insoluble globulin, LETS protein) and loss in SV40 transformed cells. Lab Invest. 1979 Nov;41(5):464–473. [PubMed] [Google Scholar]
- Dang C. V., Bell W. R., Kaiser D., Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985 Mar 22;227(4693):1487–1490. doi: 10.1126/science.4038818. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Curtiss L. K., Plow E. F. A linkage between the hemostatic and immune systems embodied in the fibrinolytic release of lymphocyte suppressive peptides. J Immunol. 1985 Jan;134(1):471–477. [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Fair D. S., Bahnak B. R. Human hepatoma cells secrete single chain factor X, prothrombin, and antithrombin III. Blood. 1984 Jul;64(1):194–204. [PubMed] [Google Scholar]
- Fair D. S., Plow E. F., Edgington T. S. Combined functional and immunochemical analysis of normal and abnormal human factor X. J Clin Invest. 1979 Oct;64(4):884–894. doi: 10.1172/JCI109554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M., Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980 Feb;19(2):517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Idell S., Gonzalez K. K., MacArthur C. K., Gillies C., Walsh P. N., McLarty J., Thrall R. S. Bronchoalveolar lavage procoagulant activity in bleomycin-induced lung injury in marmosets. Characterization and relationship to fibrin deposition and fibrosis. Am Rev Respir Dis. 1987 Jul;136(1):124–133. doi: 10.1164/ajrccm/136.1.124. [DOI] [PubMed] [Google Scholar]
- Idell S., Gonzalez K., Bradford H., MacArthur C. K., Fein A. M., Maunder R. J., Garcia J. G., Griffith D. E., Weiland J., Martin T. R. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis. 1987 Dec;136(6):1466–1474. doi: 10.1164/ajrccm/136.6.1466. [DOI] [PubMed] [Google Scholar]
- Idell S., Kucich U., Fein A., Kueppers F., James H. L., Walsh P. N., Weinbaum G., Colman R. W., Cohen A. B. Neutrophil elastase-releasing factors in bronchoalveolar lavage from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 Nov;132(5):1098–1105. doi: 10.1164/arrd.1985.132.5.1098. [DOI] [PubMed] [Google Scholar]
- Idell S., Peterson B. T., Gonzalez K. K., Gray L. D., Bach R., McLarty J., Fair D. S. Local abnormalities of coagulation and fibrinolysis and alveolar fibrin deposition in sheep with oleic acid-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1282–1294. doi: 10.1164/ajrccm/138.5.1282. [DOI] [PubMed] [Google Scholar]
- Jackson L. K. Idiopathic pulmonary fibrosis. Clin Chest Med. 1982 Sep;3(3):579–592. [PubMed] [Google Scholar]
- Johanson W. G., Jr, Holcomb J. R., Coalson J. J. Experimental diffuse alveolar damage in baboons. Am Rev Respir Dis. 1982 Jul;126(1):142–151. doi: 10.1164/arrd.1982.126.1.142. [DOI] [PubMed] [Google Scholar]
- Johnson A., Tahamont M. V., Malik A. B. Thrombin-induced lung vascular injury. Roles of fibrinogen and fibrinolysis. Am Rev Respir Dis. 1983 Jul;128(1):38–44. doi: 10.1164/arrd.1983.128.1.38. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. Activation and control mechanisms of Hageman factor-dependent pathways of coagulation, fibrinolysis, and kinin generation and their contribution to the inflammatory response. J Allergy Clin Immunol. 1975 Dec;56(6):491–506. doi: 10.1016/0091-6749(75)90067-6. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Silverberg M., Dunn J. T., Ghebrehiwet B. Interaction of the clotting, kinin-forming, complement, and fibrinolytic pathways in inflammation. Ann N Y Acad Sci. 1982;389:25–38. doi: 10.1111/j.1749-6632.1982.tb22123.x. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Gudinchet A., Hauert J., Nicoloso G., Genton C., Welti H., Bachmann F. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987 Feb;69(2):460–466. [PubMed] [Google Scholar]
- Kruithof E. K., Vassalli J. D., Schleuning W. D., Mattaliano R. J., Bachmann F. Purification and characterization of a plasminogen activator inhibitor from the histiocytic lymphoma cell line U-937. J Biol Chem. 1986 Aug 25;261(24):11207–11213. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Fair D. S., Loskutoff D. J. Human hepatoma cell line plasminogen activator. J Lab Clin Med. 1983 Oct;102(4):500–508. [PubMed] [Google Scholar]
- McGee M. P., Rothberger H. Tissue factor in bronchoalveolar lavage fluids. Evidence for an alveolar macrophage source. Am Rev Respir Dis. 1985 Mar;131(3):331–336. doi: 10.1164/arrd.1985.131.3.331. [DOI] [PubMed] [Google Scholar]
- Murano G., Aronson D., Williams L., Brown L. The inhibition of high and low molecular weight urokinase in plasma. Blood. 1980 Mar;55(3):430–436. [PubMed] [Google Scholar]
- Plow E. F. Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. J Clin Invest. 1982 Mar;69(3):564–572. doi: 10.1172/JCI110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberger H., McGee M. P., Lee T. K. Tissue factor activity. A marker of alveolar macrophage maturation in rabbits. Effects of granulomatous pneumonitis. J Clin Invest. 1984 Jun;73(6):1524–1531. doi: 10.1172/JCI111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland F. N., Donovan M. J., Picciano P. T., Wilner G. D., Kreutzer D. L. Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol. 1984 Dec;117(3):418–428. [PMC free article] [PubMed] [Google Scholar]
- Saldeen T. Trends in microvascular research. The microembolism syndrome. Microvasc Res. 1976 Mar;11(2):227–259. doi: 10.1016/0026-2862(76)90054-6. [DOI] [PubMed] [Google Scholar]
- Schwartz B. S., Monroe M. C., Bradshaw J. D. Endotoxin-induced production of plasminogen activator inhibitor by human monocytes is autonomous and can be inhibited by lipid X. Blood. 1989 Jun;73(8):2188–2195. [PubMed] [Google Scholar]
- Schwartz B. S., Monroe M. C., Levin E. G. Increased release of plasminogen activator inhibitor type 2 accompanies the human mononuclear cell tissue factor response to lipopolysaccharide. Blood. 1988 Mar;71(3):734–741. [PubMed] [Google Scholar]
- Senior R. M., Skogen W. F., Griffin G. L., Wilner G. D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986 Mar;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrin R. G., Brubaker P. G., Fantone J. C. Tissue fibrin deposition during acute lung injury in rabbits and its relationship to local expression of procoagulant and fibrinolytic activities. Am Rev Respir Dis. 1987 Apr;135(4):930–936. doi: 10.1164/arrd.1987.135.4.930. [DOI] [PubMed] [Google Scholar]
- Sitrin R. G., Kaltreider H. B., Ansfield M. J., Webster R. O. Procoagulant activity of rabbit alveolar macrophages. Am Rev Respir Dis. 1983 Aug;128(2):282–287. doi: 10.1164/arrd.1983.128.2.282. [DOI] [PubMed] [Google Scholar]
- Sprengers E. D., Princen H. M., Kooistra T., van Hinsbergh V. W. Inhibition of plasminogen activators by conditioned medium of human hepatocytes and hepatoma cell line Hep G2. J Lab Clin Med. 1985 Jun;105(6):751–758. [PubMed] [Google Scholar]
- Sueishi K., Nanno S., Tanaka K. Permeability enhancing and chemotactic activities of lower molecular weight degradation products of human fibrinogen. Thromb Haemost. 1981 Feb 23;45(1):90–94. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Kucich U., James H. L., Scott C. F., Schapira M., Zimmerman M., Cohen A. B., Colman R. W. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983 Nov;72(5):1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg P. F., Matthay M. A., Webster R. O., Roskos K. V., Goldstein I. M., Murray J. F. Biologically active products of complement and acute lung injury in patients with the sepsis syndrome. Am Rev Respir Dis. 1984 Nov;130(5):791–796. doi: 10.1164/arrd.1984.130.5.791. [DOI] [PubMed] [Google Scholar]