Abstract
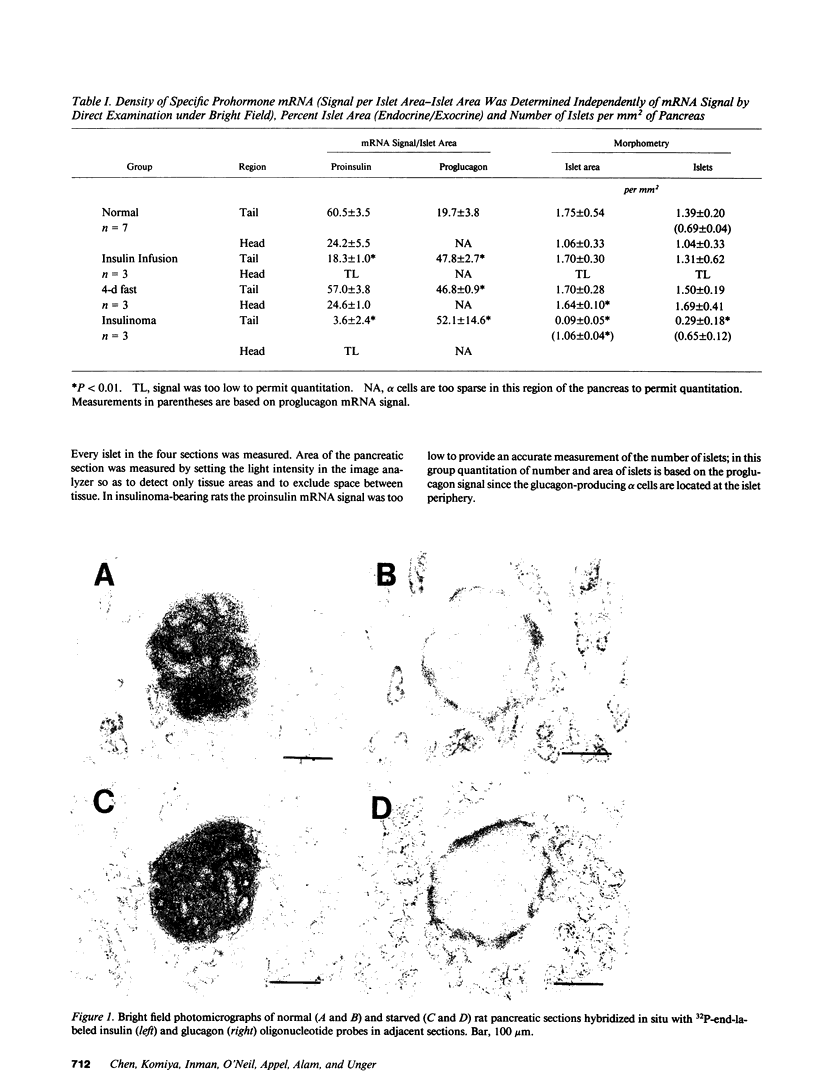
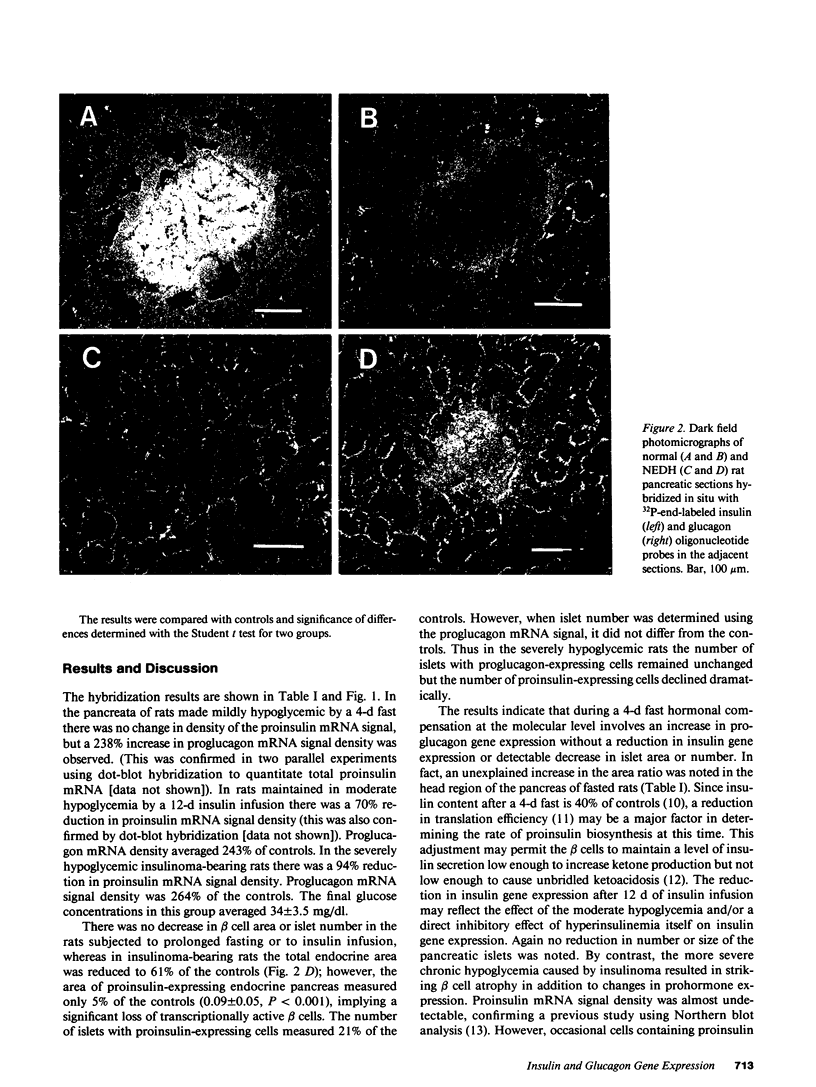
In situ hybridization of proinsulin and proglucagon mRNA was performed in rat pancreas to assess prohormone gene expression during various glucopenic conditions. During a 4-d fast mean blood glucose declined by 48 mg/dl; proinsulin mRNA signal density remained normal while proglucagon mRNA signal density more than doubled. At the end of a continuous 12-d insulin infusion blood glucose averaged 53 +/- 12 mg/dl; proinsulin mRNA signal density declined to 30% of controls while proglucagon mRNA signal density more than doubled. In insulinoma-bearing NEDH rats blood glucose averaged 34 +/- 3.5 mg/dl; the proinsulin mRNA signal was virtually undetectable and proglucagon mRNA signal density was more than twice the controls. There was no detectable change in either beta-cell area or islet number in rats subjected to fasting or insulin infusion, but in insulinoma-bearing rats beta cell area was markedly reduced. Thus compensation during 4 d of starvation involves an increase in glucagon gene expression without change in insulin gene expression or beta cell mass. In moderate insulin-induced hypoglycemia glucagon gene expression is increased and insulin gene expression decreased. In more profound insulinoma-induced hypoglycemia, in addition to the foregoing changes in hormone gene expression, there is a profound reduction in the number of insulin-expressing cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Chen L., Komiya I., Inman L., McCorkle K., Alam T., Unger R. H. Molecular and cellular responses of islets during perturbations of glucose homeostasis determined by in situ hybridization histochemistry. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1367–1371. doi: 10.1073/pnas.86.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. The effects of fasting and feeding on preproinsulin messenger RNA in rats. J Clin Invest. 1981 Apr;67(4):952–960. doi: 10.1172/JCI110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V. K., Hynes M. A., Jin C., Towle A. C., Lauder J. M., Lund P. K. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16(1):97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- Itoh N., Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980 Jan 3;283(5742):100–102. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Horwitz D. L., Jennings A. S., Chiasson J. L., Keller U., Rubenstein A. H. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978 May;27(5):563–570. doi: 10.2337/diab.27.5.563. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., MEBANE D., UNGER R. H., LOCHNER A. THE HYPOGLYCEMIC ACTION OF KETONES. II. EVIDENCE FOR A STIMULATORY FEEDBACK OF KETONES ON THE PANCREATIC BETA CELLS. J Clin Invest. 1964 Mar;43:408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., MADISON L. L. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest. 1963 Jul;42:1031–1039. doi: 10.1172/JCI104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med. 1981 Jun 18;304(25):1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]