Abstract
Chronic infection with Helicobacter pylori cagA-positive strains is the strongest risk factor of gastric cancer. The cagA gene-encoded CagA protein is delivered into gastric epithelial cells via bacterial type IV secretion, where it undergoes tyrosine phosphorylation at the Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs. Delivered CagA then acts as a non-physiological scaffold/hub protein by interacting with multiple host signaling molecules, most notably the pro-oncogenic phosphatase SHP2 and the polarity-regulating kinase PAR1/MARK, in both tyrosine phosphorylation-dependent and -independent manners. CagA-mediated manipulation of intracellular signaling promotes neoplastic transformation of gastric epithelial cells. Transgenic expression of CagA in experimental animals has confirmed the oncogenic potential of the bacterial protein. Structural polymorphism of CagA influences its scaffold function, which may underlie the geographic difference in the incidence of gastric cancer. Since CagA is no longer required for the maintenance of established gastric cancer cells, studying the role of CagA during neoplastic transformation will provide an excellent opportunity to understand molecular processes underlying “Hit-and-Run” carcinogenesis.
Keywords: gastric cancer, Helicobacter pylori, CagA, EPIYA motif, CM motif, Hit-and-Run carcinogenesis
Introduction
Gastric cancer is the fifth most common malignancy, with approximately 950,000 new cases registered each year, and the third leading cause of cancer-related deaths worldwide, accounting for more than 700,000 victims every year.1) It is the most common cancer in several geographic regions of the world, most notably East Asia. More than half of total gastric cancer patients are from East Asian countries such as Japan, China and Korea, and the incidence of gastric cancer in these countries is almost ten-times higher than that in the United States. Like other cancers, gastric cancer is a male-dominant malignancy and the incidence in men is almost twice higher than that in women.
According to Lauren’s classification, gastric cancer is divided into two major types, intestinal type and diffuse type.2,3) Intestinal-type gastric cancer is well-differentiated, grows slowly and forms glands. This type of gastric cancer often arises via sequential pathological changes, starting from atrophic gastritis, and progression to intestinal metaplasia and then to dysplasia.4) Histopathologically, intestinal-type cancer shows irregular tubular structures, multiple lumens, and reduced stromal region. Diffuse-type gastric cancer spreads aggressively throughout the stomach without forming glands and rapidly metastasize to remote organs/tissues. Diffuse-type gastric cancer is comprised of poorly differentiated tumor cells and is characterized by the production of mucus. A morphological variation of diffuse-type cancer rapidly invades the muscles of the stomach wall with extensive fibrosis, making the stomach wall thick, hard, and rubbery. This subtype of diffuse gastric cancer is called scirrhous gastric cancer or linitis plastica,5) the prognosis of which is extremely poor. Diffuse-type gastric cancer tends to arise at a younger age than intestinal type does. A small percentage of diffuse-type gastric cancer is of familial origin, caused by germline mutations in the CDH1 gene encoding E-cadherin.6) In hereditary diffuse gastric cancer (HDGC) cases, the tumor often develops at an age younger than 40 years.
Helicobacter pylori is a micro-aerophilic, spiral-shaped Gram-negative bacterium discovered in the epithelial lining of the stomach by Marshall and Warren.7) Although H. pylori is not a commensal bacterium, it is estimated to infect approximately half of the entire human population. The acidic environment in the stomach does not allow the survival of viruses, bacteria and other microorganisms, but H. pylori has evolved to uniquely overcome this harsh environment. H. pylori secretes urease, an enzyme that converts urea into ammonia and bicarbonate to neutralize gastric acid, making the stomach a more hospitable place for H. pylori. Upon acquisition of the ability to survive, the stomach provides H. pylori with a special living niche. Host immune cells as well as antibodies that would normally recognize and attack invading bacteria are unable to reach H. pylori freely swimming in the mucus lining of the stomach. Instead, ineffective host immune responses continue to respond to the site of infection, where immune and epithelial cells die and release nutrients that feed the gastric pathogen. H. pylori infection is primarily acquired during childhood and the transmission occurs through an oral-oral or fecal-oral route primarily within families, particularly in the setting of poor sanitation and hygiene. In the majority of cases, colonized H. pylori persists in the stomach over the lifetime of the individual host unless eradicated with antibiotics. Just after its discovery, H. pylori was revealed to play a major etiological role in chronic atrophic gastritis and peptic ulcers. Subsequent clinico-epidemiological studies also indicated a close relationship between H. pylori infection and gastric cancer.8–10) Based on these, the International Agency for Research on Cancer, World Health Organization (IARC/WHO) classified H. pylori as a group I carcinogen, a definite cause of cancer in humans, in 1994. Additional large-scale prospective cohort studies confirmed the association.11,12) Studies on infection in rodents with H. pylori independently provided evidence for the role of the bacterial pathogen in the development of gastric cancer.13–16) Both intestinal- and diffuse-types of gastric cancers are associated with H. pylori infection.17) It is now well accepted that H. pylori is the single greatest risk factor for the development of gastric cancer, and the stomach pathogen is estimated to be the cause of ∼75% of all gastric cancers.18) The attributable risk, however, may be substantially underestimated in East Asian countries given that the frequency of gastric cancer without a history of H. pylori infection is incredibly low (1–3%) in this region.19,20) Consistent with the etiologic role of H. pylori in the development of gastric cancer, large-scale intervention studies carried out in East Asian countries provided evidence that eradication of H. pylori by antibiotics is beneficial for preventing gastric cancer.21–23) This review summarizes recent advances in the study of H. pylori-associated gastric cancer, particularly focusing on the role of the H. pylori CagA (Cytotoxin-associated gene A) protein, the first identified bacterial protein playing an active role in the development of human caner.
Type IV secretion system and CagA
Based on the presence or absence of cagA, a gene encoding the CagA protein, in its bacterial genome, H. pylori can be divided into cagA-positive and cagA-negative strains. The cagA-encoded CagA protein varies in size from 130 to 145 kilo Daltons (kDa) because of structural polymorphism in the carboxy-terminal (C-terminal) region (Fig. 1).24,25) As expected from its name, CagA was originally considered to act as a bacterial cytotoxin.26) However, subsequent study revealed that the acute cytotoxic activity of H. pylori was due to another bacterial toxin termed VacA (vacuolating toxin A).27) The cagA gene is localized at one end of the cag pathogenicity island (cag PAI), a 40-kilobase genomic DNA segment that was acquired by a horizontal DNA transfer from an unknown donor.28,29) Approximately 30–40% of H. pylori strains isolated in Western countries do not carry the cag PAI and thus are cagA-negative, whereas almost all of the H. pylori isolates from East Asian countries contain the cag PAI and are thus cagA-positive.30,31) Depending on strains, the cag PAI contains 27–31 putative genes, including cagA. Among them, at least 18 genes encode proteins serving as building blocks of a type IV secretion system (TFSS),32) which form a syringe-like structure that is capable of delivering macromolecules across two bacterial membranes (outer and inner membranes).32) An association of H. pylori CagA with increased risk of gastric cancer was first reported in 1995.33) The importance of infection with cagA-positive H. pylori in the development of gastric cancer was subsequently consolidated by a number of epidemiological studies34,35) as well as animal infection experiments.16,36)
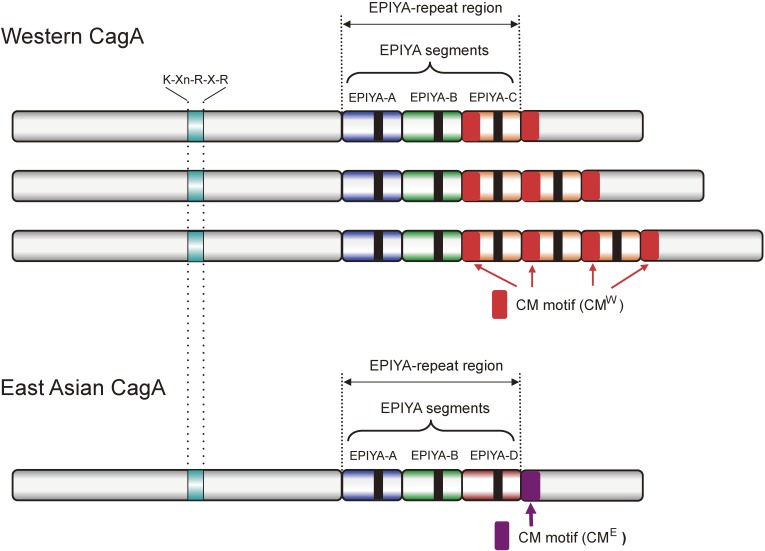
Figure 1.
Structural diversity of H. pylori CagA. The C-terminal EPIYA-repeat region of Western CagA comprises the EPIYA-A, EPIYA-B and a variable number (mostly 1–3) of EPIYA-C segments. The C-terminal EPIYA-repeat region of East Asian CagA comprises the EPIYA-A, EPIYA-B and EPIYA-D segments. Each of the EPIYA segments contains a single EPIYA tyrosine phosphorylation motif (shown as a black box), which is phosphorylated by kinases such as SFKs and c-Abl. East Asian CagA has a single CM (CME) motif immediately downstream of the EPIYA-D segment. Western CagA possesses at least two CM motifs, one in the EPIYA-C segment, which is unique to Western CagA (CMW) and the other located distal to the last EPIYA-C segment (either CMW or CME). The K-Xn-R-X-R motif in the central region is required for the binding of CagA with the membrane phospholipid, phosphatidylserine (PS).
Mechanism of CagA delivery
Adhesion of H. pylori on the surface of gastric epithelial cells, mediated via numerous adhesins such as BabA/B, SabA, OipA, HopZ, and AlpA/B, initiates and facilitates the formation of the pilus-like TFSS syringe.37) Once stably attached to gastric epithelial cells, H. pylori cagA-positive strains initiate delivery of CagA into the cytoplasm of the host cells via the TFSS.38–42) Formation of a conduit that connects the bacterium and host cytoplasm to deliver CagA is triggered by the interaction of CagL, a pilus component of the TFSS, with the host membrane β1-integrin in a manner that is dependent of the Arg-Gly-Asp (RGD) motif, the sequence motif that recognizes β1-integrin, present in CagL.43) Through complex formation, CagL activates integrin signaling that stimulates Src family kinase (SFK) activity, which in turn phosphorylates delivered CagA. β1-Integrin also interacts with other TFSS components such as CagI and CagY as well as CagA independently of the RGD motif.44) These Cag proteins may cooperatively bind to integrin molecules to stabilize TFSS-host cell interaction. Additionally, CagA presented on top of the TFSS pilus interacts with the membrane phospholipid phosphatidylserine (PS), which is usually enriched in the inner membrane leaflet but is aberrantly exposed to the outer membrane leaflet upon direct contact with H. pylori, to facilitate delivery of CagA into the cells.45) Since translocation of CagA is attenuated by treatment of host cells with hydroxymethylglutaryl (HMG)-CoA reductase inhibitors, statins, cholesterol enriched in membrane lipid rafts may also be involved in TFSS-mediated CagA delivery.46,47) The requirement of multiple proteins as well as membrane lipids in the translocalization of CagA across the plasma membrane indicates that the process may not be a simple physical penetration of the TFSS pilus through the membrane but rather require complicated biophysical/biochemical events, molecular details of which warrant further investigation.
EPIYA motif and CM motif
Upon delivery into gastric epithelial cells, CagA is tethered to the inner leaflet of the plasma membrane via two distinct mechanisms depending on the status of cell polarity. In polarized epithelial cells, interaction of the central region of CagA, which contains multiple basic amino acids including those constituting the PS-binding K-Xn-R-X-R motif, plays an important role for the membrane association of CagA (Fig. 1).45) In non-polarized cells, however, the C-terminal region is primarily responsible for the membrane tethering of CagA.48) Membrane-localized CagA then undergoes tyrosine phosphorylation at the Glu-Pro-Ile-Tyr-Ala (EPIYA) tyrosine-phosphorylation motif, present in multiple numbers in the C-terminal polymorphic region (EPIYA-repeat region) of the protein,49) by SFK members such as c-Src, Yes, Fyn, and/or Lyn,50,51) which are expressed in gastric epithelial cells at variable levels, and by c-Abl.52,53) The structural diversity in the C-terminal region of CagA is due to complicated recombination events within the genomic region encoding the C-terminal CagA.54) Based on the sequences flanking the EPIYA motifs, four distinct EPIYA segments, EPIYA-A, -B, -C and -D, each of which contains a single EPIYA motif, have been identified (Fig. 1).48,55,56) The EPIYA-repeat region of CagA from H. pylori circulating all over the world except East Asian countries is in an arrangement of EPIYA-A, EPIYA-B and EPIYA-C segments (termed Western CagA or ABC-type CagA). The EPIYA-C segment, composed of 34 amino-acid residues, variably multiplies (in most cases 1–3 times) in tandem among distinct Western CagA species. CagA from East Asian H. pylori isolates also possesses EPIYA-A and EPIYA-B segments but not the repeatable EPIYA-C segment. Instead, it has a distinct EPIYA-containing segment, termed EPIYA-D. Accordingly, the EPIYA-repeat region of East Asian H. pylori-specific CagA is in an arrangement of EPIYA-A, EPIYA-B and EPIYA-D segments (termed East Asian CagA or ABD-type CagA). Since a single CagA protein never carries both EPIYA-C and EPIYA-D segments, the presence of EPIYA-C is the hallmark of Western CagA, whereas the presence of EPIYA-D is the hallmark of East Asian CagA. In addition to these two major CagA isoforms, variable alignments of these EPIYA segments create further diversity among CagA isolates.57,58) Tyrosine phosphorylation of these EPIYA segments by host kinases does not seem to be a simple stochastic process because SFK members selectively phosphorylate the EPIYA-C and EPIYA-D segments whereas c-Abl phosphorylates all of the EPIYA segments. Interestingly, in the delivered host cells, a single CagA protein appears to undergo tyrosine phosphorylation on 1 or 2 EPIYA segments but never on 3 or more EPIYA segments, with the preferred combination of phosphorylated EPIYA segments being EPIYA-A and EPIYA-C in Western CagA and EPIYA-B and EPIYA-D in East Asian CagA. Phosphorylation of CagA in the host cells may therefore be a sequential event, starting with EPIYA-C or EPIYA-D phosphorylation by SFKs at an initial stage of infection, followed by phosphorylation of EPIYA-A or EPIYA-B by c-Abl at a later stage of infection.59)
In addition to the EPIYA motif, the C-terminal CagA region contains another repeatable sequence motif, originally designated as the CagA-multimerization (CM) motif as CagA can multimerize (dimerize) through the motif.60) The CM motif, comprising 16 amino-acid residues, is located immediately distal to the last EPIYA segment (Fig. 1). Whereas the CM motif is highly conserved, there are 5 amino-acid alterations between East Asian and Western CagA species. From this variation, the CM motif of Western CagA is termed as CMW and that of East Asian CagA is termed CME.61) Remarkably, the amino-terminal (N-terminal) 16-amino-acid stretch that constitutes the 34-amino-aid EPIYA-C segment is identical to CMW.60) Accordingly, multiplication of the EPIYA-C segment also increases the number of CM motifs in Western CagA (Fig. 1).
EPIYA-dependent CagA functions
Expression of CagA in cultured gastric epithelial cells such as AGS cells, either by infection, transfection, or transduction, induces a unique cell-morphological change termed the “hummingbird phenotype”, which is characterized by an elongated cell shape with elevated cell motility and scattering (Fig. 2).38) Induction of the hummingbird phenotype requires tyrosine phosphorylation of CagA in the cells.49) Since the morphological change is reminiscent of the cellular change caused by treatment with hepatocyte growth factor (HGF), CagA was suggested to aberrantly activate intracellular signaling that is otherwise triggered by growth factor stimuli.38) Such a role of CagA in signal perturbation was first provided by the finding that CagA specifically interacts with the Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 2 (SHP2) (Fig. 3 and Table 1).49) The CagA-SHP2 complex formation is mediated via the interaction between tyrosine-phosphorylated EPIYA-C or EPIYA-D segment and the SH2 domains of SHP2.55)
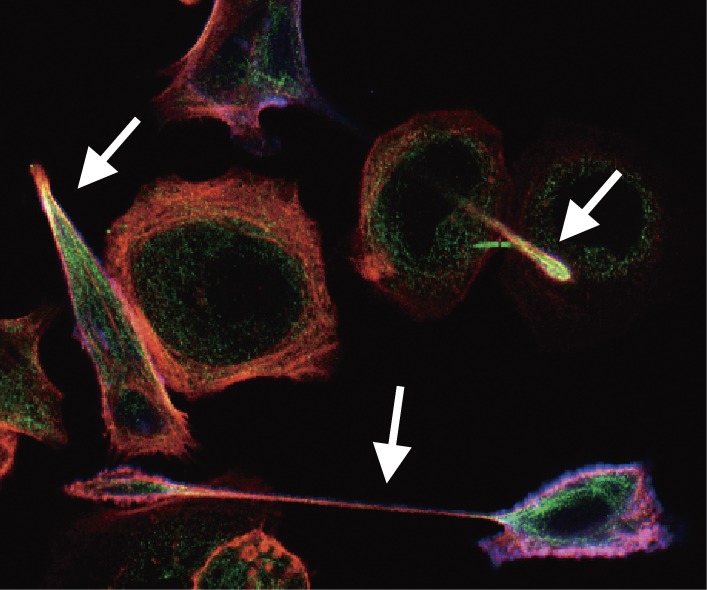
Figure 2.
The hummingbird phenotype induced by H. pylori CagA. Upon delivery into gastric epithelial cells, CagA induces an extremely elongated cell-shape known as the hummingbird phenotype (white arrows), which is concomitantly associated with elevated cell motility. Induction of the hummingbird phenotype requires tyrosine phosphorylation of CagA by host cell kinases.
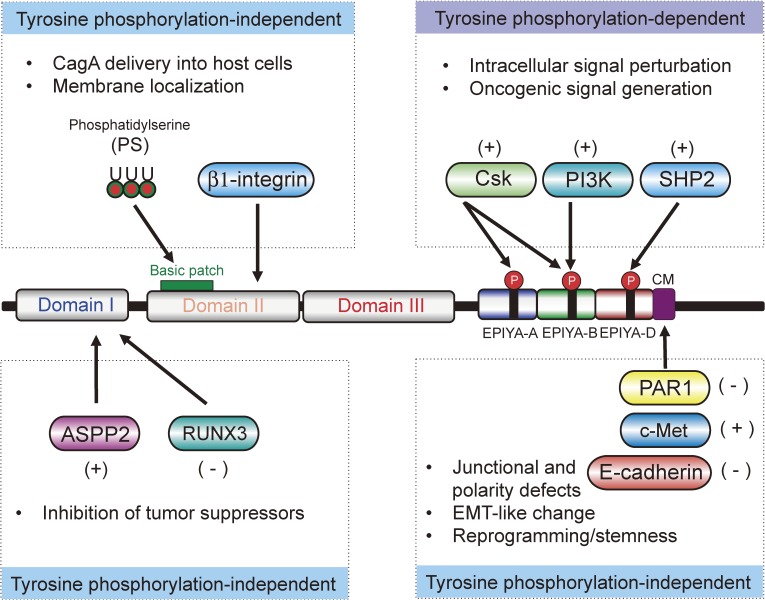
Figure 3.
Pathogenic scaffold function of CagA. CagA interacts with and thereby perturbs a number of host signal transducing molecules via tyrosine phosphorylated EPIYA motifs in its disordered C-terminal tail. CagA also binds to polarity regulating molecules via the C-terminal CM motifs, causing junctional and polarity defects. Domain I of the structured N-terminal CagA interacts with tumor suppressors, ASPP2 and RUNX1, resulting in inactivation of tumor suppressor functions. Domain II and/or Domain III of CagA are involved in CagA delivery into host cells and subsequent membrane localization. Through the interaction, CagA activates [indicated by (+)] or inactivates [indicated by (−)] the target proteins.
Table 1.
Host cell molecules that interact with CagA
| Name of Molecule | Binding site on CagA | Pathobiological effect of the interaction |
|---|---|---|
| ASPP2 | Domain I | Degradation and inactivation of p53 |
| RUNX3 | Domain I | Degradation and inactivation of RUNX3 |
| Phosphatidylserine (PS) | Domain II (basic patch) | CagA delivery, Membrane tethering of CagA |
| β1-Integrin | Domain II | CagA delivery, Stimulation of SFKs and FAK |
| C-terminal Src kinase (CSK) | EPIpYA-A | Aberrant activation of CSK, Inhibition of SFK activity |
| EPIpYA-B | ||
| PI3K | EPIpYA-B | Deregulation of the PI3K-AKT pathway |
| SHP2 | EPIpYA-C | Deregulation of the Ras-ERK pathway, Inhibition of FAK |
| EPIpYA-D | ||
| Grb2 | EPIYA? | Deregulation of the Ras-ERK pathway |
| Crk | EPIpYA? | Loss of gastric epithelial cell adhesion |
| PAR1/MARK | CM | Disruption of tight junctions |
| Loss of epithelial cell polarity | ||
| c-Met | CM | Deregulation of the Wnt pathway |
| Enhanced cell motility | ||
| E-cadherin | CM | Disruption of adherens junctions |
| Deregulation of the Wnt pathway | ||
| SHP1 | N-terminal and C-terminal regions | Tyrosine dephosphorylation of CagA |
| TAK1 | N-terminal and C-terminal regions | Activation of NF-κB activity |
“EPIpYA” indicates a tyrosine-phosphorylated EPIYA segment.
In a physiological setting, SHP2 is required for full activation of the Ras-ERK mitogen-activated protein kinase (MAPK) pathway and is also involved in cell morphogenesis as well as cell motility.62) SHP2 is catalytically activated by binding with membrane-associated scaffold/adaptors such as Grb2-associated binding (GAB) proteins. As in the case of CagA, SHP2 binding requires tyrosine phosphorylation of GAB, which is primarily mediated by receptor tyrosine kinases. This in turn raises the idea that CagA acts as a non-physiological scaffold/hub protein that mimics the function of GAB in mammalian cells.63) This notion is supported by the results of an experiment showing that transgenically expressed CagA in photoreceptor cells in the Drosophila eye can substitute for the Drosophila Gab, Daughter of Sevenless (Dos), and this CagA activity requires the Drosophila SHP2, Corkscrew.64) Expectedly, expression of CagA in gastric epithelial cells induces sustained ERK activation, which provokes pro-mitogenic cellular response while morphologically inducing the hummingbird phenotype. Inhibition of CagA tyrosine phosphorylation, disruption of the CagA-SHP2 complex, knockdown of SHP2 expression by siRNA, or inhibition of ERK signaling abolishes the hummingbird phenotype by CagA.49,65,66) In addition, CagA-deregulated SHP2 down-regulates the kinase activity of focal adhesion kinase (FAK) by dephosphorylating the activating phosphotyrosine residues (Tyr-369, -574, and -575), resulting in impaired focal adhesion turnover.67) This CagA activity dampens cell-matrix interaction, which also promotes induction of the hummingbird phenotype. Deregulation of SHP2 by CagA may play an important role in terms of carcinogenesis because activating mutation of PTPN11, the gene encoding SHP2, has been found in a variety of human malignancies such as juvenile myelomonocytic leukemia (JMML), childhood myelodysplastic syndrome, B-cell acute lymphoblastic leukemia and acute myelocytic leukemia as well as several solid tumors.68,69) Most of the reported PTPN11 mutations are missense mutations in exons 3 and 8, which encode the N-SH2 domain and the phosphatase domain, respectively. Crystal structure analysis of SHP2 suggests that such mutations weaken the autoinhibitory intramolecular interaction that occurs between the N-SH2 domain and the C-terminal phosphatase domain and thereby constitutively activate the catalytic function of SHP2.70) Binding of tyrosine-phosphorylated CagA to the SH2 domains also induces a conformational change in SHP2 that relieves the autoinhibitory composition, resulting in the aberrant activation of the SHP2 phosphatase activity.
Investigation of a new SHP2 substrate(s) potentially involved in CagA-mediated oncogenesis revealed parafibromin/Cdc73, a core component of the RNA polymerase II-associated factor (PAF) complex.71) Upon tyrosine dephosphorylation by SHP2, parafibromin stably interacts with β-catenin, which activates Wnt target genes. Together with the Hedgehog (Hh) and Notch pathways, the Wnt pathway plays an essential role in the development, homeostasis, and pathogenesis of multicellular organisms. Parafibromin competitively interacts with β-catenin and Gli1, the transcriptional effector of the Hh pathway, thereby potentiating transactivation of Wnt- and Hh-target genes in a mutually exclusive manner.72) Parafibromin also binds to the Notch intracellular domain (NICD), the transcriptional effector of the Notch pathway, enabling concerted activation of Wnt- and Notch-target genes. The transcriptional platform function of parafibromin is potentiated by tyrosine dephosphorylation, mediated by SHP2, while it is attenuated by tyrosine phosphorylation, mediated by protein tyrosine kinase 6 (PTK6) that is also known as breast tumor kinase (Brk). Thus, parafibromin integrates and converts signals conveyed by these morphogen pathways into appropriate transcriptional outputs in a tyrosine phosphorylation/dephosphorylation-regulated manner.73) The role of the CagA/SHP2/parafibromin axis in gastric carcinogenesis warrants further investigation. Also notably, SHP2 forms a physical complex with transcriptional coactivators Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), targets of the cell density-sensing Hippo signal. Through the interaction, YAP/TAZ mediate nuclear translocalization of SHP2, which in turn dephosphorylates and activates parafibromin in the nucleus.73)
The tyrosine-phosphorylated EPIYA-A or EPIYA-B segment serves as a docking site for the C-terminal Src kinase (CSK), a negative regulator of SFKs (Fig. 3 and Table 1).74) The tyrosine-phosphorylated EPIYA-B segment also binds to the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and activates the lipid kinase activity. Many of the Western CagA proteins carry a single nucleotide polymorphism (SNP) in EPIYA-B that reduces CagA binding to PI3K.75) SILAC (Stable Isotope Labeling by Amino Acids in Cell Culture)-based phosphoproteomics study showed that, in addition to the above-described SH2-containing proteins, the EPIYA-A peptide can bind to SHP1, the EPIYA-B peptide can bind to SHP1, Grb2, and SHP2, and the EPIYA-C peptide can bind to Grb2, Grb7, SHP1, and Ras-GTPase activating protein (GAP) in a manner that is dependent on EPIYA tyrosine phosphorylation, indicating the potential of tyrosine-phosphorylated EPIYA segments in promiscuously binding with numerous SH2 domains.76) CagA is also capable of binding with Crk adaptor proteins (Crk-I, Crk-II, and Crk-L) in a tyrosine phosphorylation-dependent manner, though the responsible EPIYA segment remains unknown. Through the interaction, CagA disrupts adherens junctions by perturbing the adaptor function of Crk in cell signaling.77) CagA binds to Grb2 via the EPIYA-containing region and thus aberrantly activates the Ras signaling pathway to stimulate abnormal cell proliferation.78) However, the CagA-Grb2 interaction is independent of CagA tyrosine phosphorylation.
SHP1, the one and the only SHP2 homologue in mammals, is primarily expressed in hematopoietic cells, where it negatively regulates immune cell activation. Although less abundant, it is also present in the gastrointestinal linings. Like SHP2, SHP1 forms a physical complex with CagA in gastric epithelial cells. However, the interaction is independent of EPIYA tyrosine phosphorylation and potentiates the SHP1 phosphatase activity that dampens CagA action by dephosphorylating the EPIYA motifs. Accordingly, SHP1 is the long-sought host cell phosphatase that counteracts phosphorylation-dependent H. pylori CagA actions.79)
EPIYA-independent CagA functions
The function of CagA as a pathogenic scaffold/hub in mammalian cells does not always require the EPIYA motif. The CM motif serves as a binding site for the polarity-regulating serine/threonine kinase Partitioning defective-1 (PAR1) (Fig. 3 and Table 1).80,81) PAR1 was originally discovered as one of the PAR proteins (PAR1 to PAR6), which play essential roles in the establishment of cell polarity during C. elegans development. In mammals, PAR1 was independently identified as microtubule affinity-regulating kinase (MARK), which regulates the stability of microtubules by phosphorylating microtubule-associated proteins (MAPs) such as MAP1, MAP2, and tau.82) Mammalian PAR1/MARK comprises four isoforms, PAR1a/MARK3, PAR1b/MARK2, PAR1c/MARK1, and PAR1d/MARK4. Of these, PAR1b is a predominant isoform in epithelial cells. PAR1b distributes to the basolateral membrane of polarized epithelial monolayers, where it plays crucial roles in the establishment and maintenance of apical-basal polarity.83,84) Moreover, PAR1b inhibits a RhoA-specific guanine nucleotide exchange factor (GEF), GEF-H1, by phosphorylation to down-regulate RhoA and thereby inhibit actin stress fiber formation.85) Thus, the role of PAR1b in the cytoskeleton is not confined just to microtubule dynamics but also extends to filamentous actin (F-actin) rearrangements. CagA is capable of binding with all of the PAR1 isoforms,86) with the highest affinity to PAR1b, and the 15-amino-acid stretch within the 16-amino-acid CM motif, termed the MARK Kinase Inhibitor (MKI) peptide, directly occupies the kinase catalytic center of PAR1b to inhibit the kinase activity by mimicking host PAR1b substrates.80,87) Accordingly, the CagA-PAR1b interaction dampens the PAR1b kinase activity,80,81) which underlies junctional and polarity defects caused by delivery of CagA in the polarized epithelial layer,88) thereby making cells susceptible to oncogenic insults.89) For bacteria, the polarity defect might have an advantage as it enables them to replicate and grow in a larger area of epithelial cells.90,91) CagA is capable of homodimerization via the CM motif in cells.60,92) Given that PAR1 can exist as a homodimer in cells, CM-mediated CagA dimerization may represent juxtaposition of two CagA proteins by a PAR1 dimer. The CM motif also interacts with the activated HGF receptor c-Met, thereby potentiating c-Met-dependent motogenic response and enhancing c-Met-mediated activation of PI3K/Akt signaling (Fig. 3 and Table 1).93,94) This in turn leads to the stimulation of pro-oncogenic Wnt signaling and nuclear factor κB (NF-κB) signaling, which mediates cancer-promoting inflammatory responses.95) Additionally, CagA associates with the cytoplasmic domain of E-cadherin via the CM motif and thereby disrupts the E-cadherin/β-catenin complex, leading to aberrant activation of Wnt signaling (Fig. 3 and Table 1).96) Moreover, CagA is capable of forming a multimeric protein complex comprising E-cadherin, c-Met, and p120 catenin, though the functional relevance of the complex formation remains unknown.97) CagA also associates with glycogen synthase kinase (GSK)-3β via the C-terminal region containing the EPIYA and CM motifs. Through the complex formation, CagA sequesters GSK-3β to the insoluble fraction and thus potentiates Wnt activation by inhibiting the GSK-3β kinase activity.98)
In addition to the C-terminal region, the N-terminal region of CagA also interacts with several cellular proteins, which may additionally contribute to oncogenesis (Fig. 3 and Table 1). The N-terminal CagA region binds to the gastric tumor suppressor RUNX3 to promote proteasome-mediated degradation of RUNX3 by recruiting an unidentified E3 ubiquitin ligase.99) N-terminal CagA also enhances proteasome-dependent degradation of a p53 tumor suppressor by physically associating with apoptosis-stimulating protein of p53 2 (ASPP2).100) Also notably, CagA-activated Akt phosphorylates and activates human double minute 2 (HDM2) and ARF-binding protein 1 (ARF-BP1) E3 ligases that promote p53 degradation in conjunction with ASPP2. Since p14ARF is a negative regulator of both HDM2 and ARF-BP1, loss of the ARF gene locus in gastric epithelial cells may potentiate CagA-dependent p53 degradation.101,102) These findings collectively indicate that N-terminal CagA promotes survival of CagA-delivered cells by subverting pro-apoptotic signaling.
Structural basis of CagA action
CagA has a unique tertiary structure, consisting of a solid N-terminal region (70% of the entire CagA) and an intrinsically disordered C-terminal tail (30% of the entire CagA).103,104) The crystal structure of the N-terminal CagA region predicts a square plate-like shape with approximate dimensions of 110 × 80 × 55 Å3, which comprises three discrete domains (Fig. 4). Domain I is the extreme N-terminal domain of CagA, having a small interacting surface area with Domain II but not with Domain III. As a result, Domain I is highly mobile and flexible. The intrinsically disordered proline-rich region of ASPP2 binds to the pocket formed by the three-helix bundle present in Domain I of CagA with high affinity that suffices co-crystallization.105,106) The P-P-X-Y motif of RUNX3 has been reported to interact with the WW domain of CagA.107) Although the presumed WW domain should be located in Domain I, there is no structural evidence for the presence of a functional WW domain in this CagA region. Domain II and Domain III comprise a protease-resistant structural CagA core. Domain II also contains a large anti-parallel β-sheet, with which CagA binds to β1-integrin for its delivery into the host cell.104) Domain II contains a basic patch, a cluster of basic residues containing those constituting the PS-binding K-Xn-R-X-R motif.45) The basic patch plays an important role in the interaction of CagA with PS. The N-terminal binding sequence (NBS) located within Domain III forms a four-helix bundle with the C-terminal binding sequence (CBS) located within the disordered C-terminal tail, creating a C-terminal lariat loop that strengthens interaction of CagA with PAR1 and SHP2 (Fig. 5).103) Accordingly, the degree of the scaffold/hub function of CagA is fine-tuned by the intramolecular interaction of CagA. The highly diverged structure in the C-terminal CagA region may be explained by the intrinsically disordered nature of the EPIYA segments, which can be aligned in variable numbers and orders through homologous recombination at the DNA level with minimal structural constraints that hamper the CagA function.108)
Figure 4.
Ribbon diagram of the crystal structure of the N-terminal structured region of CagA (residues 1-876 of CagA from H. pylori strain 26695). The CagA protein consists of structured N-terminal and disordered C-terminal regions. Folded N-terminal CagA displays a heretofore-unidentified structure with three distinct domains, Domain I (blue), Domain II (yellow), and Domain III (red). Domain I constitutes the N-terminus, while Domain II tethers CagA to the inner plasma membrane through electrostatic interaction between the basic patch and the acidic phosphatidylserine (PS) that is primarily distributed to the inner leaflet of the plasma membrane.
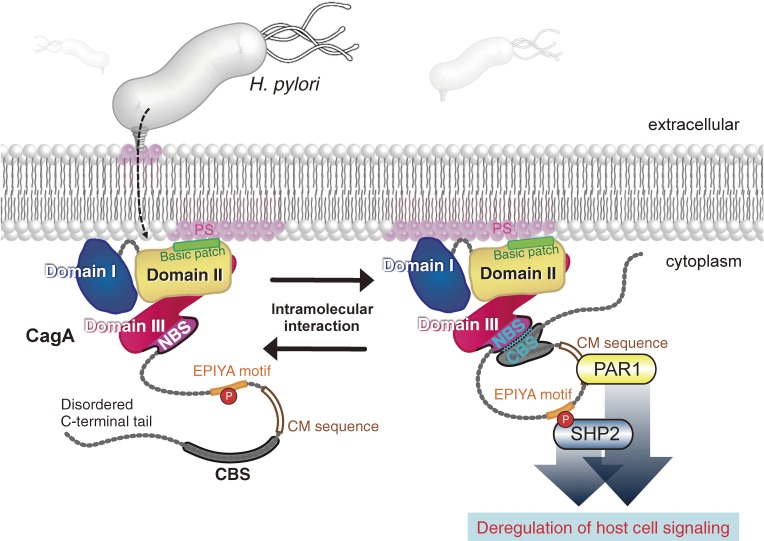
Figure 5.
Regulation of CagA activity by intramolecular interaction. CagA is delivered into gastric epithelial cells via the H. pylori type IV secretion system, where it acts as an oncogenic scaffold/hub. Intramolecular interaction, mediated between the N-terminal binding sequence (NBS) in Domain III and the C-terminal binding sequence (CBS) in the disordered C-terminal tail, potentiates binding capability of the tail with SHP2 and PAR1 via the EPIYA motif and CM motif, respectively, thereby strengthening the pro-oncogenic scaffold function of CagA.
CagA as a bacterial protein that can promote carcinogenesis in metazoans
Despite accumulating in vitro evidence for the pro-oncogenic action of CagA, animal infection studies using mice or Mongolian gerbils have so far failed to convincingly demonstrate a causal role of CagA in oncogenesis. To challenge this important question, transgenic mice that systemically express wild-type or phosphorylation-resistant CagA, which does not have functional EPIYA motifs, were generated.109) Mice expressing wild-type CagA showed gastric epithelial hyperplasia and some of them spontaneously developed gastric polyps as well as adenocarcinomas of the stomach and small intestine. The mice also developed leukocytosis that was associated with hypersensitivity to hematopoietic cytokines such as interleukin 3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in bone-marrow cells, which may be due to hyperactivation of SHP2. Furthermore, a fraction of the transgenic mice spontaneously developed myeloid leukemias and B-cell lymphomas, hematological malignancies that are also caused by gain-of-function SHP2 mutations. In contrast, no pathological abnormalities were observed in transgenic mice expressing phosphorylation-resistant CagA. These results provide the first direct evidence for the role of CagA as a bacterium-derived protein that can stimulate carcinogenesis in mammals. The results also point to the importance of CagA tyrosine phosphorylation, which enables CagA to bind to and thereby deregulate SHP2, in the development of neoplasias. Likewise, transgenic expression of CagA in zebrafish increased the incidence of intestinal neoplasias caused by a mutation in the tumor suppressor gene p53,110) providing additional in vivo evidence for the carcinogenic potential of CagA in animals. At the same time, the results of these transgenic experiments indicate the requirement of oncogenic collaboration between CagA and host cancer-associated genes in in vivo tumorigenesis.
It is well recognized that chronic inflammation promotes oncogenesis.111) Infection with H. pylori cagA-positive strains provokes strong inflammatory responses in the stomach mucosa. However, there is no evidence for the presence of chronic inflammation in the gastrointestinal tract of CagA-transgenic mice.109) Thus, to investigate the contribution of chronic inflammation in CagA-driven oncogenesis, CagA-transgenic mice were cyclically treated with a colitis inducer, dextran sulfate sodium (DSS).112) Compared with control mice, DSS-induced colitis was markedly deteriorated in CagA-transgenic mice due to a substantial decrease in the cellular pool of IκB, which binds and sequesters NF-κB in the cytoplasm. Although the CagA-mediated IκB reduction did not automatically activate NF-κB, it lowered the threshold of NF-κB activation by inflammogenic insults. The incidence of colonic dysplasia was also increased in CagA-transgenic mice treated with DSS. Thus, CagA exacerbates inflammation, whereas inflammation strengthens the pro-oncogenic potential of CagA. Through this functional interplay, CagA and inflammation reinforce each other in creating a downward spiral that instigates neoplastic transformation of epithelial cells.
H. pylori induces inflammation by utilizing multiple distinct mechanisms. Bacterial peptidoglycan can enter gastric epithelial cells via the TFSS, and it then stimulates the cytoplasmic pathogen recognition receptor nucleotide-binding oligomerization domain 1 (Nod1) to activate NF-κB for induction of inflammatory cytokines such as IL-1, IL-6, IL-8, and/or tumor necrosis factor α (TNFα).113) H. pylori lipopolysaccharide (LPS), recognized by toll-like receptor 2 (TLR2) or TLR4, also activates NF-κB. Whereas CagA on its own does not seem to be a potent inflammogen, CagA delivered into host cells is capable of stimulating NF-κB-dependent transcription through multiple distinct mechanisms in addition to lowering the cellular pool of IκB. CagA triggers the PI3K/Akt/NF-κB signaling pathway by forming a complex with c-Met.94) CagA also associates with TNF receptor associated factor 6 (TRAF6) and TGF-β-activated kinase 1 (TAK1) to induce TAK1 polyubiquitination, which in turn activates TAK1.114) CagA-deregulated TAK1 is responsible for phosphorylation and stimulation of the IκB kinase (IKK) complex, leading to the activation of NF-κB and subsequent inflammatory response. However, it remains to be determined what extent of which CagA per se is involved in the activation of NF-κB in H. pylori-induced gastritis. H. pylori cagA-positive strains also induce hyperactivation of another proinflammatory transcription factor, signal transducer and activator of transcription 3 (STAT3). In the stomach mucosa, STAT3 is activated in response to stimuli by the IL-6 family of cytokines such as IL-6 and IL-11, resulting in induction of Janus kinase (JAK)/STAT3 signaling via the gp130 subunit of the IL-6 receptor. The gp130 subunit of the IL-6 receptor modulates the balance between SHP2/ERK signaling and JAK/STAT3 signaling. Expression of a gp130 mutant that lacks the SHP2 binding site in mice causes signal imbalance and thereby induces gastric inflammation and subsequent gastric cancer with high penetration.115) CagA may also perturb the balance between SHP2/ERK signaling and JAK/STAT3 signaling by sequestrating SHP2 from gp130 by complex formation in gastric epithelial cells, resulting in the promotion of neoplastic transformation.116)
Approximately 10% of gastric cancer cases are not only infected by H. pylori but also carry Epstein-Barr virus (EBV) in the tumor cells.117) EBV-positive gastric cancer is noted for genome-wide CpG hypermethylation.118) In vitro infection of gastric epithelial cells with EBV induces promoter hypermethylation of the SHP1-encoding PTPN6 gene, which potentiates tyrosine phosphorylation-dependent CagA action via epigenetic downregulation of SHP1, the CagA phosphatase.79) Clinical specimens of EBV-positive gastric cancers also exhibit PTPN6 hypermethylation with reduced SHP1 expression. Augmented H. pylori CagA activity, via SHP1 inhibition, may therefore contribute to the development of EBV-positive gastric cancer.
In addition to gastric cancer, cagA-positive H. pylori is associated with the development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma of B-cell origin. Most of the patients with MALT lymphoma are infected with H. pylori cagA-positive strains.119) Eradication of H. pylori by antibiotics leads to regression of gastric MALT lymphoma in more than 75% of patients.120) This clinical observation provides compelling evidence for a causal link between cagA-positive H. pylori and MALT lymphoma and raises the possibility that CagA is injected not only into gastric epithelial cells but also into B cells that have migrated to the gastric mucosa in response to H. pylori infection. Consistent with this, CagA is detectable in B cells that have infiltrated the stomach epithelial linings and also in tumor cells of MALT lymphoma.121,122) CagA expressed in B cells by transfection gives rise to ERK activation, which results in the phosphorylation of Bcl2-associated death promoter (BAD) and thereby prevention of apoptosis of CagA-expressing cells.123) CagA also inhibits apoptosis of delivered B cells by simultaneously perturbing the function of p53 and the JAK/STAT signaling pathway.124) This CagA activity should promote accumulation of preneoplastic B cells by subverting their elimination though apoptosis, which may contribute to the development of MALT lymphoma.
CagA polymorphism in pro-oncogenic action
The CagA-SHP2 interaction, mediated by tyrosine-phosphorylated EPIYA-C or EPIYA-D, is one of key interactions through which CagA exerts its pro-oncogenic action. The sequence flanking the phosphotyrosine (pY) residue of the EPIYA-D segment perfectly matches the consensus high-affinity binding sequence for the SHP2 SH2 domains, whereas the sequence flanking the pY residue of the EPIYA-C segment differs from the consensus sequence by a single amino acid at the pY+5 position.55,56) This finding indicates that East Asian CagA is more potent than Western CagA in terms of SHP2 perturbation. Indeed, clinical studies have revealed that gastric cancer is more closely associated with East Asian CagA-producing H. pylori strains than with Western CagA-producing H. pylori strains in geographical regions where two distinct strains co-circulate.125–127) The notion is also supported by the observation that transgenic mice carrying East Asian CagA developed neoplastic lesions more frequently than did transgenic mice carrying Western CagA.128)
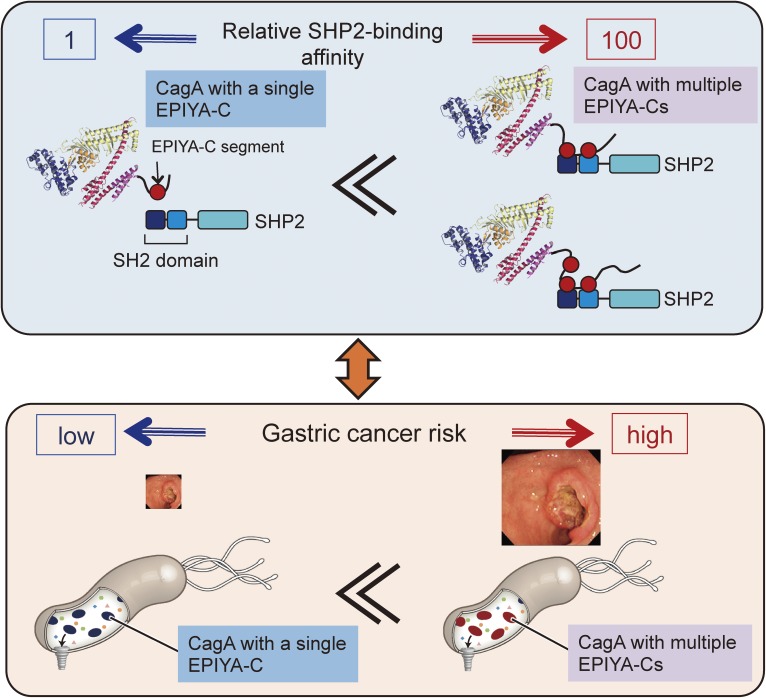
A unique feature associated with Western CagA is that the EPIYA-C segment tandem-duplicates with a variable number, mostly from one to three but possibly extending up to six.129) The proportions of Western CagA species containing one, two, and three EPIYA-C segments are approximately 60–70%, 20–30%, and <5%, respectively.58) In contrast to EPIYA-C, the EPIYA-D segment rarely duplicates in East Asian CagA species. As a result, East Asian CagA containing a single EPIYA-D segment binds to SHP2 more strongly than does Western CagA carrying a single EPIYA-C segment.55) Within Western CagA species, those having a greater number of EPIYA-C segments exhibit stronger ability to interact with SHP2.57,130) The results of a number of recent clinico-epidemiological studies have shown that infection with H. pylori strains carrying Western CagA with two or more EPIYA-C segments is more closely associated with the development of gastric cancer than is infection with H. pylori carrying CagA with a single EPIYA-C segment.131–136) Since SHP2 binding is the only known CagA activity for which the magnitude is correlated with the number of EPIYA-C segments,55,56) the degree of CagA-SHP2 interaction may link the number of EPIYA-C segments with gastric cancer risk. Indeed, a quantitative study revealed that the strength of CagA-SHP2 binding is dramatically elevated by more than a hundredfold upon duplication of the CagA EPIYA-C segment from one to two (Fig. 6).137) This is most probably due to monomeric interaction vs. dimeric interaction of CagA with SHP2, which possesses two CagA-binding SH2 domains. The robust increase in the SHP2 binding activity of CagA by EPIYA-C duplication is also correlated with markedly enhanced cell invasion into the extracellular matrix, a malignant cellular trait caused by SHP2 deregulation.138,139) These observations provide a molecular basis for the role of multiple EPIYA-C segments as a distinct risk factor of gastric cancer.
Figure 6.
EPIYA-C repeats and gastric cancer risk. Western CagA containing two or more EPIYA-C repeats (Type-II Western CagA) binds to both the N-SH2 and C-SH2 domain of SHP2. This bivalent interaction makes CagA-SHP2 complex formation extremely stable. On the other hand, interaction of CagA containing a single EPIYA-C segment (Type I Western CagA) with SHP2 is monovalent, making the CagA-SHP2 complex unstable. The decisive difference in SHP2-binding activity between CagA with a single EPIYA-C and CagA with multiple EPIYA-C repeats provides a molecular basis underlying an increased risk of gastric cancer in individuals infected with H. pylori carrying CagA with multiple EPIYA-C segments (Type-II Western CagA).
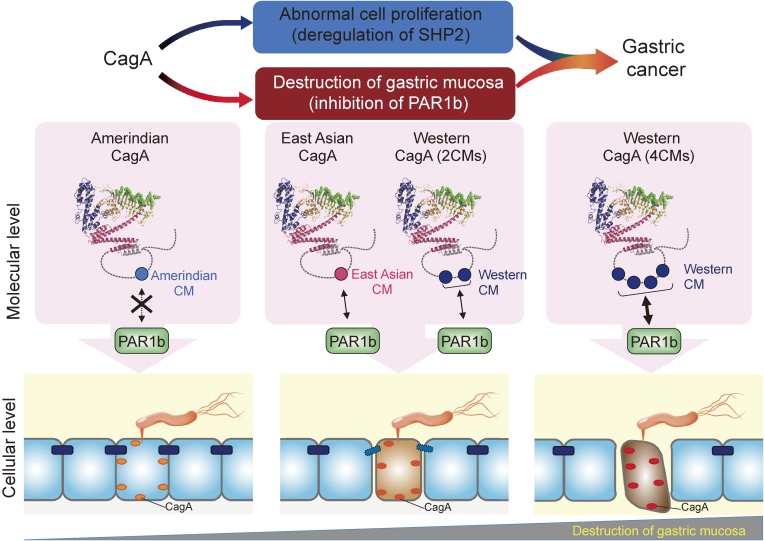
As mentioned above, sequence polymorphism exists in the CM motif between Western CagA and East Asian CagA. Western CagA typically possesses 2–4 repeats of the CMW motif as it usually contains 1–3 tandem repeats of the EPIYA-C segment that is followed by a single CMW immediately distal to the last EPIYA-C repeat. East Asian CagA, in contrast, does not contain a CM motif in its EPIYA-D segment but carries a single CME immediately downstream of EPIYA-D.60,61) Interestingly, a fraction of Western CagA species carry CME rather than CMW at the most distal CM motif.140) The CM motif, either CMW or CME, serves as a binding site for PAR1. Non-canonical CM motifs have also been identified from other parts of the world such as Amerindian type-I CM (CMAmI) and Amerindian type-II CM (CMAmII) from indigenous tribes of the Amazon rainforest.141–144) An increase in the number of CMW motif in a single Western CagA synergistically augments its PAR1b binding, which is in proportional to the degrees of stress fiber formation and disruption of tight junction function (Fig. 7).145) For instance, a Western CagA containing 4 CMW motifs exhibits a PAR1b-binding affinity that is more than 30-fold higher than that of a Western CagA containing a single CMW. Also, a single CME shows a binding affinity to PAR1b that is comparable to that of two tandem CMW motifs. In contrast, neither CMAmI nor CMAmII motifs bound to PAR1b, confirming that the binding of PAR1b to the Amerindian CagA proteins is extremely weak, if any.144) Accordingly, the CM polymorphism is a determinant for the magnitude of CagA-mediated perturbation of cytoskeletal systems, both microtubule-mediated and actin-mediated, which may influence clinical outcome of infection with cagA-positive H. pylori as recently described.140)
Figure 7.
Role of CM polymorphism in CagA-PAR1b interaction. CagA binds to PAR1b as well as other PAR1 family members via the C-terminal CM motif. Sequence diversity within the CM motif of CagA influences the strength of CagA-PAR1b interaction. PAR1b-binding affinity of East Asian CagA possessing one CME motif is equivalent to Western CagA containing two CMW motifs. Western CagA possessing four CMW motifs, though very rare (<5% of all Western CagA), exhibits an extremely strong binding to PAR1b.
CagA, reprogramming, and genetic instability
H. pylori primarily targets mucus pit cells, terminally differentiated cells with a short life span of at most several days, for CagA injection. This raises a concern as to how such differentiated cells could be transformed by H. pylori CagA. One possible answer to this question is that CagA is capable of reprogramming and thereby de-differentiating fully terminated cells into tissue stem-like precursor cells. Indeed, CagA induces cell morphological change that resembles epithelial-to-mesenchymal transition (EMT) with properties in common with cancer stem cells.146–148) Induction of EMT by CagA involves activation of Wnt signaling, triggered by disruption of E-cadherin/β-catenin interaction and/or c-Met activation.94,96) CagA also promotes EMT by inhibiting GSK-3β, which results in stabilization of Snail, a transcriptional repressor of E-cadherin.98) Mechanistically, CagA-mediated deregulation of Wnt signaling in gastric epithelial cells induces ectopic expression of the Caudal-related homeobox transcription factor Cdx1, which in turn transactivates reprogramming factors such as Sal-like protein 4 (SALL4) and Krüppel-like factor 5 (KLF5) to confer a stem-cell property in gastric epithelial cells. This CagA-mediated cell-fate reprogramming may underlie intestinal metaplasia, abnormal transdifferentiation of gastric epithelial cells to intestinal-like cells, that occurs in gastric mucosa that has been chronically infected with cagA-positive H. pylori.149) Although detailed mechanisms remain unknown, CagA expressed in gastric epithelial cells also provokes translocation of the nuclear factor of activated T cells (NFAT) transcription factor from the cytoplasm to the nucleus, thereby eliciting aberrant transactivation of NFAT-dependent genes.150) Recent studies demonstrated that activation of NFAT is required for successful reprogramming of somatic cells.151) Thus, CagA-mediated activation of NFAT may also be involved in the reprogramming process of gastric epithelial cells. In the meantime, a subpopulation of H. pylori colonizes the gastric gland base and directly interacts with the progenitor and stem cell compartments, giving rise to glandular hyperplasia with the expansion of Lgr5+ gastric stem cells in the antral glands in a CagA-dependent manner.152) Thus, CagA may also be delivered into stem/progenitor cells in the stomach mucosa. Furthermore, CagA resists autophagic degradation in gastric epithelial cells expressing CD44v, a cell surface marker associated with cancer stem cells. This is because the autophagy pathway is suppressed in CD44v-positive stem-like cells due to their resistance to reactive oxygen species (ROS) through elevated levels of intracellular glutathione by CD44v.153)
H. pylori CagA is an exogenous cancer-promoting protein that is injected intermittently into gastric epithelial cells by H. pylori. Once established, however, gastric cancer cells no longer require H. pylori and CagA for maintaining their transformed phenotype. Thus, gastric carcinogenesis should go through a “Hit-and-Run” process where dedifferentiated stem-like cells, led by CagA, eventually lose their CagA dependency.154) Such a “Hit-and-Run” mechanism was originally proposed by Skinner for certain types of virus-associated cancers.155) During the course of gastric cancer development, pro-oncogenic functions of CagA should be replaced or compensated by genetic and/or epigenetic alterations of the host genome. Obviously, this Hit-and-Run process will be accelerated in cells displaying genetic instability. Several studies have indicated that CagA on its own contributes to the disruption of genetic stability. CagA-mediated inactivation of PAR1, which perturbs microtubule stability, causes microtubule-based spindle dysfunction, leading to prophase/metaphase delay and subsequent misorientation of spindle formation.156) As a result, chronic exposure of epithelial cells to CagA induces chromosomal instability leading to aneuploidy. Sustained activation of NF-κB in gastric epithelial cells infected with H. pylori cagA-positive strains induces aberrant expression of activation-induced cytidine deaminase (AID), a DNA editing enzyme that physiologically introduces somatic hypermutation in immunoglobulin variable regions in B cells. Ectopically expressed AID enhances accumulation of mutations in cancer-related genes such as p53 in gastric epithelial cells.157) Infection with H. pylori cagA-positive strains is also associated with an increased level of H2O2 production, which causes oxidative DNA damage.158) Additionally, CagA down-regulates the expression of Heme oxygenase-1 (HO-1), a potent anti-oxidant and anti-inflammatory molecule that cleaves the heme ring at the α methene bridge to form biliverdin, thereby enhancing genotoxic stress. A recent study demonstrated that H. pylori infection induces DNA double-strand breaks (DSBs).159,160) However, since H. pylori mutants possessing functional TFSS but lacking the cagA gene efficiently induced DSBs in gastric epithelial cells, CagA is unlikely to be a major inducer of this type of DNA damage.
Pragmin, a host protein containing a functional EPIYA motif
A versatile EPIYA-dependent interaction of the bacterial CagA protein with a variety of SH2-containing mammalian proteins suggests that there might be a host EPIYA-containing protein(s) that functions as a scaffold molecule by utilizing a tyrosine-phosphorylated EPIYA motif. Evolutionally, CagA might have acquired the EPIYA motif by mimicking the function of such a host cell protein. Pragmin, also known as Sgk223, was originally identified as a downstream effector of Rnd2, a Rho family small GTPase, in neuronal cells.161) Pragmin associates with Rnd2 and thereby stimulates RhoA to induce cell contraction, which then inhibits neurite outgrowth by nerve growth factor. Also, Pragmin possesses a pseudokinase domain in the C-terminal region. Pragmin is one of the few human proteins that contain the EPIYA motif, which undergoes tyrosine phosphorylation by SFKs or CSK.162) In addition, CSK directly interacts with Pragmin via the tyrosine-phosphorylated EPIYA motif and this interaction potentiates the kinase activity of CSK. The phospho-EPIYA-dependent Pragmin-CSK interaction generates a positive feedback loop of CSK activation at focal adhesions and thereby induces cell-morphological transformation with elevated cell motility, deregulation of which provokes aberrant cell migration and invasion that contribute to oncogenesis.163) Indeed, several studies have shown a functional link between Pragmin and oncogenesis. An increase in the level of Pragmin promotes invasion of advanced colon carcinoma cells.164) Also, Pragmin is overexpressed in pancreatic ductal adenocarcinoma (PDAC) cells.165) The pro-oncogenic activity of CagA might therefore involve deregulation of Pragmin. There are bacterial effectors other than CagA that also contain EPIYA or EPIYA-like tyrosine phosphorylation motifs. Eukaryotic proteins containing the EPIYA motif such as Pragmin may provide a clue to the evolution of such effector proteins in pathogenic bacteria.108)
CagA and extragastric disorders
Recent epidemiological studies have suggested that infection with H. pylori cagA-positive strains is associated with coronary heart diseases166,167) and ischemic stroke.168) Preeclampsia, a pregnancy-associated disorder characterized by hypertension and proteinuria, is another vascular disease related to cagA-positive H. pylori infection.169) These studies indicated that CagA may also contribute to the development of extragastric diseases. Many types of cells are known to release extracellular vesicles with unique biophysical and biochemical properties.170) These vesicles are classified on the basis of their biogenesis; vesicles formed by exocytosis of multivesicular bodies are denoted as exosomes (with diameters ranging from 30 to 200 nm), while those budded directly from the plasma membrane are referred to as microvesicles (with diameters ranging from 100 to 1000 nm).171) Extracellular vesicles are found in various biological fluids, including blood, urine, saliva, and breast milk, and they have been shown to play an important role in cell-to-cell communication through transport of informative constituents, including proteins, lipids, and microRNAs. CagA is detectable in serum-derived exosomes in patients infected with cagA-positive H. pylori.172) Gastric epithelial cells expressing CagA also secrete exosomes containing CagA. The addition of purified CagA-containing exosomes to gastric epithelial cells induced the hummingbird phenotype (Fig. 2), indicating that the exosomes deliver functional CagA into cells. These observations raise the idea that exosomes secreted from CagA-injected gastric epithelial cells may enter into general circulation, delivering CagA to distant organs and tissues. CagA-containing exosomes might thus be involved in the development of extragastric disorders associated with cagA-positive H. pylori infection.
Conclusion and perspectives
Rapid progress in our understanding of H. pylori CagA has provided substantial insights into the molecular pathogenesis underlying the development of gastric cancer. CagA is the first identified bacterial protein functioning as a pro-oncogenic scaffold/hub protein in animal cells. Given the Hit-and-Run mechanism of carcinogenesis conducted by the bacterial protein, a key question is the molecular event that converts cancer-predisposed gastric epithelial cells from CagA-dependent to CagA-independent. Oncogenic insults triggered by injected CagA in cultured epithelial cells provoke premature cell senescence and/or programmed cell death (apoptosis), primarily through the induction of cell cycle inhibitors such as p21Cip1/WAF1.65,96,148,150) If such a cellular response, which serves as a fail-safe system against uncontrolled cell proliferation, is genetically or epigenetically perturbed in normal epithelial cells constituting the stomach mucosa, then evasion of host cells from oncogenic stress would become a key event that makes cells pass a “point of no return”, after which cells acquire CagA independence. A molecular understanding of the transition process through the point of no return would require comprehensive genome-wide investigation of genetic/epigenetic alterations in gastric epithelial cells that have been exposed to CagA for variable periods with the use of next-generation sequencing technologies. Recently developed techniques for gastric organoids (gasteroids)173) as well as embryonic stem (ES) cell-derived stomach tissue174) should also provide a power tool for elucidating the molecular mechanisms underpinning acquisition of CagA independency. Assuming that established gastric cancer cells are still addicted to signaling pathways deregulated by CagA even after cells become CagA-independent, intracellular components conveying such signals will become critical molecular targets in the prevention and treatment of gastric cancer.
Acknowledgements
I express my sincere thanks to past and present members of Hatakeyama Lab, who have contributed to the H. pylori CagA research. I also thank Drs. Takeru Hayashi and Hiroko Nishikawa for help in preparing figures. Our works presented in this review article were supported by Grants-in-Aid for Scientific Research (A) and (S) and Grant-in Aid on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, by CREST, Japan Science and Technology Agency, and by the Max-Planck Society, Germany.
Abbreviations
- AID
activation-induced cytidine deaminase
- ASPP2
apoptosis-stimulating protein of p53 2
- CagA
Cytotoxin-associated gene A
- CBS
C-terminal binding sequence
- CM
CagA multimerization
- CMAmI
Amerindian type-I CM
- CMAmII
Amerindian type-II CM
- CME
East Asian CM
- CMW
Western CM
- CSK
C-terminal Src kinase
- EBV
Epstein-Barr virus
- EMT
epithelial-to-mesenchymal transition
- EPIYA
Glu-Pro-Ile-Tyr-Ala
- GAB
Grb2-associated binder
- H. pylori
Helicobacter pylori
- IκB
Inhibitor of NF-κB
- MALT
mucosa-associated lymphoid tissue
- MARK
microtubule affinity-regulating kinase
- NBS
N-terminal binding sequence
- PAI
pathogenicity island
- PAR1
Partitioning defective-1
- PS
phosphatidylserine
- RGD
Arg-Gly-Asp
- SFK
Src family kinase
- SHP1/2
SH2 domain-containing protein tyrosine phosphatase 1/2
- TFSS
typeIV secretion system
Profile
Masanori Hatakeyama was born in 1956 in Hokkaido, Japan. He graduated from Hokkaido University in 1981 (bachelor of medicine) and passed the National Examination for Medical Practitioners Japan (medical license) in the same year. After clinical practice in hematology and gastroenterology at the Hokkaido University hospital (1981–1982), he went to the Graduate School of Medicine, Hokkaido University (The Third Department of Internal Medicine, Prof. Tamotsu Miyazaki) in 1982 and obtained his Ph.D. from the Hokkaido University in 1986. In the same year, he became a research associate at the Institute for Molecular and cellular Biology, Osaka University (Prof. Tadatsugu Taniguchi), where he performed a series of pioneering works on the interleukin-2 receptor (IL-2R), including the world-first molecular cloning of the gene encoding the IL-2R β-chain in 1989. In 1991, he went to study at the Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology (MIT), Cambridge, Massachusetts, U.S.A., where he worked as a postdoctoral fellow on the retinoblastoma tumor suppressor gene under the supervision of Prof. Robert A. Weinberg. In 1995, he returned to Japan and was appointed a member and chief at the Division of Viral Oncology, The Cancer Institute of The Japanese Foundation for Cancer Research (Ganken). In 1999, He became a professor, Division of Chemistry, Institute of Immunological Science, Hokkaido University. Due to reorganization of the Institute in 2000, he became a professor at the Division of Molecular Oncology, Institute for Genetic Medicine, Hokkaido University. In 2009, he moved to the Graduate School of Medicine, The University of Tokyo and became a professor at the Department of Microbiology (up to the present). In 2014, he was also appointed Deputy Director, Max Planck-The University of Tokyo Center for Integrative Inflammology (up to the present). His research interest is the infection-associated cancers, especially the molecular mechanism underlying Helicobacter pylori-associated gastric cancer. He is the Director of the Japanese Cancer Association (JCA). He is serving as the Associate Editor of Cancer Science and the Editorial Board Member of the International Journal of Cancer. He received Incitement Award of the Japanese cancer Association (1991), Human Frontier Science Program (HSFP) Long-term Fellowship (1991), JCA-Mauvernay Award (2006), Sagawa Special Award (2011), Medical Award of the Japan Medical Association (2014), and Hideyo Noguchi Memorial Medical Prize (2016).
References
- 1).Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 2).Lauren P. (1965) The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 64, 31–49. [DOI] [PubMed] [Google Scholar]
- 3).Qiu M.Z., Cai M.Y., Zhang D.S., Wang Z.Q., Wang D.S., Li Y.H., Xu R.H. (2013) Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J. Transl. Med. 11, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Correa P. (1992) Human gastric carcinogenesis: a multistep and multifactorial process — first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 52, 6735–6740. [PubMed] [Google Scholar]
- 5).Takemura S., Yashiro M., Sunami T., Tendo M., Hirakawa K. (2004) Novel models for human scirrhous gastric carcinoma in vivo. Cancer Sci. 95, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Guilford P., Hopkins J., Harraway J., McLeod N., Harawira P., Taite H., Scoular R., Miller A., Reeve A.E. (1998) E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405. [DOI] [PubMed] [Google Scholar]
- 7).Warren J.R., Marshall B. (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 321, 1273–1275. [PubMed] [Google Scholar]
- 8).Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131. [DOI] [PubMed] [Google Scholar]
- 9).Nomura A., Stemmermann G.N., Chyou P.H., Kato I., Perez-Perez G.I., Blaser M.J. (1991) Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 325, 1132–1136. [DOI] [PubMed] [Google Scholar]
- 10).Forman D., Newell D.G., Fullerton F., Yarnell J.W., Stacey A.R., Wald N., Sitas F. (1991) Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 302, 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. (2001) Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345, 784–789. [DOI] [PubMed] [Google Scholar]
- 12).Hsu P.I., Lai K.H., Hsu P.N., Lo G.H., Yu H.C., Chen W.C., Tsay F.W., Lin H.C., Tseng H.H., Ger L.P., Chen H.C. (2007) Helicobacter pylori infection and the risk of gastric malignancy. Am. J. Gastroenterol. 102, 725–730. [DOI] [PubMed] [Google Scholar]
- 13).Hirayama F., Takagi S., Kusuhara H., Iwao E., Yokoyama Y., Ikeda Y. (1996) Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J. Gastroenterol. 31, 755–757. [DOI] [PubMed] [Google Scholar]
- 14).Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. (1998) Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology 115, 642–648. [DOI] [PubMed] [Google Scholar]
- 15).Honda S., Fujioka T., Tokieda M., Satoh R., Nishizono A., Nasu M. (1998) Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 58, 4255–4259. [PubMed] [Google Scholar]
- 16).Franco A.T., Israel D.A., Washington M.K., Krishna U., Fox J.G., Rogers A.B., Neish A.S., Collier-Hyams L., Perez-Perez G.I., Hatakeyama M., Whitehead R., Gaus K., O’Brien D.P., Romero-Gallo J., Peek R.M., Jr. (2005) Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 102, 10646–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hansson L.R., Engstrand L., Nyrén O., Lindgren A. (1995) Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology 109, 885–888. [DOI] [PubMed] [Google Scholar]
- 18).Parkin D.M. (2006) The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044. [DOI] [PubMed] [Google Scholar]
- 19).Matsuo T., Ito M., Takata S., Tanaka S., Yoshihara M., Chayama K. (2011) Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 16, 415–419. [DOI] [PubMed] [Google Scholar]
- 20).Ono S., Kato M., Suzuki M., Ishigaki S., Takahashi M., Haneda M., Mabe K., Shimizu Y. (2012) Frequency of Helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 86, 59–65. [DOI] [PubMed] [Google Scholar]
- 21).Wong B.C., Lam S.K., Wong W.M., Chen J.S., Zheng T.T., Feng R.E., Lai K.C., Hu W.H., Yuen S.T., Leung S.Y., Fong D.Y., Ho J., Ching C.K., Chen J.S., China Gastric Cancer Study Group (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291, 187–194. [DOI] [PubMed] [Google Scholar]
- 22).Wu C.Y., Kuo K.N., Wu M.S., Chen Y.J., Wang C.B., Lin J.T. (2009) Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 137, 1641–1648. [DOI] [PubMed] [Google Scholar]
- 23).Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., Terao S., Amagai K., Hayashi S., Asaka M., Japan Gast Study Group (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372, 392–397. [DOI] [PubMed] [Google Scholar]
- 24).Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Rappuoli R. (1993) Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. U.S.A. 90, 5791–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Tummuru M.K., Cover T.L., Blaser M.J. (1993) Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun. 61, 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Cover T.L., Dooley C.P., Blaser M.J. (1990) Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 58, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Tummuru M.K., Cover T.L., Blaser M.J. (1994) Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect. Immun. 62, 2609–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Censini S., Lange C., Xiang Z., Crabtree J.E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. U.S.A. 93, 14648–14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Akopyants N.S., Clifton S.W., Kersulyte D., Crabtree J.E., Youree B.E., Reece C.A., Bukanov N.O., Drazek E.S., Roe B.A., Berg D.E. (1998) Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28, 37–53. [DOI] [PubMed] [Google Scholar]
- 30).Ito Y., Azuma T., Ito S., Miyaji H., Hirai M., Yamazaki Y., Sato F., Kato T., Kohli Y., Kuriyama M. (1997) Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J. Clin. Microbiol. 35, 1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Azuma T., Yamakawa A., Yamazaki S., Fukuta K., Ohtani M., Ito Y., Dojo M., Yamazaki Y., Kuriyama M. (2002) Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 186, 1621–1630. [DOI] [PubMed] [Google Scholar]
- 32).Backert S., Tegtmeyer N., Fischer W. (2015) Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Future Microbiol. 10, 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Blaser M.J., Perez-Perez G.I., Kleanthous H., Cover T.L., Peek R.M., Chyou P.H., Stemmermann G.N., Nomura A. (1995) Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55, 2111–2115. [PubMed] [Google Scholar]
- 34).Parsonnet J., Friedman G.D., Orentreich N., Vogelman H. (1997) Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Huang J.Q., Zheng G.F., Sumanac K., Irvine E.J., Hunt R.H. (2003) Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 125, 1636–1644. [DOI] [PubMed] [Google Scholar]
- 36).Rieder G., Merchant J.L., Haas R. (2005) Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242. [DOI] [PubMed] [Google Scholar]
- 37).Backert S., Clyne M., Tegtmeyer N. (2011) Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun. Signal. 9, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Segal E.D., Cha J., Lo J., Falkow S., Tompkins L.S. (1999) Altered states: Involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 96, 14559–14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Asahi M., Azuma T., Ito S., Ito Y., Suto H., Nagai Y., Tsubokawa M., Tohyama Y., Maeda S., Omata M., Suzuki T., Sasakawa C. (2000) Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Backert S., Ziska E., Brinkmann V., Zimmy-Arndt U., Fauconnier A., Jungblut P.R., Naumann M., Meyer T. (2000) Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2, 155–164. [DOI] [PubMed] [Google Scholar]
- 41).Odenbreit S., Puls J., Sedlmaier B., Gerland E., Fischer W., Haas R. (2000) Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500. [DOI] [PubMed] [Google Scholar]
- 42).Stein M., Rappuoli R., Covacci A. (2000) Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. U.S.A. 97, 1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Kwok T., Zabler D., Urman S., Rohde M., Hartig R., Wessler S., Misselwitz R., Berger J., Sewald N., König W., Backert S. (2007) Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449, 862–866. [DOI] [PubMed] [Google Scholar]
- 44).Jiménez-Soto L.F., Kutter S., Sewald X., Ertl C., Weiss E., Kapp U., Rohde M., Pirch T., Jung K., Retta S.F., Terradot L., Fischer W., Haas R. (2009) Helicobacter pylori type IV secretion apparatus exploits β1 integrin in a novel RGD-independent manner. PLoS Pathog. 5, e1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Murata-Kamiya N., Kikuchi K., Hayashi T., Higashi H., Hatakeyama M. (2010) Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization and pathophysiological action of the CagA oncoprotein. Cell Host Microbe 7, 399–411. [DOI] [PubMed] [Google Scholar]
- 46).Lai C.H., Chang Y.C., Du S.Y., Wang H.J., Kuo C.H., Fang S.H., Fu H.W., Lin H.H., Chiang A.S., Wang W.C. (2008) Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 76, 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Lai C.H., Wang H.J., Chang Y.C., Hsieh W.C., Lin H.J., Tang C.H., Sheu J.J., Lin C.J., Yang M.S., Tseng S.F., Wang W.C. (2011) Helicobacter pylori CagA-mediated IL-8 induction in gastric epithelial cells is cholesterol-dependent and requires the C-terminal tyrosine phosphorylation-containing domain. FEMS Microbiol. Lett. 323, 155–163. [DOI] [PubMed] [Google Scholar]
- 48).Higashi H., Yokoyama K., Fujii Y., Ren S., Yuasa H., Saadat I., Murata-Kamiya N., Azuma T., Hatakeyama M. (2005) EPIYA motif is a membrane targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J. Biol. Chem. 280, 23130–23137. [DOI] [PubMed] [Google Scholar]
- 49).Higashi H., Tsutsumi R., Muto S., Sugiyama T., Azuma T., Asaka M., Hatakeyama M. (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295, 683–686. [DOI] [PubMed] [Google Scholar]
- 50).Selbach M., Moese S., Hauck C.R., Meyer T.F., Backert S. (2002) Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277, 6775–6778. [DOI] [PubMed] [Google Scholar]
- 51).Stein M., Bagnoli F., Halenbeck R., Rappuoli R., Fantl W.J., Covacci A. (2002) c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43, 971–980. [DOI] [PubMed] [Google Scholar]
- 52).Poppe M., Feller S.M., Römer G., Wessler S. (2007) Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene 26, 3462–3472. [DOI] [PubMed] [Google Scholar]
- 53).Tammer I., Brandt S., Hartig R., König W., Backert S. (2007) Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology 132, 1309–1319. [DOI] [PubMed] [Google Scholar]
- 54).Furuta Y., Yahara K., Hatakeyama M., Kobayashi I. (2011) Evolution of cagA oncogene of Helicobacter pylori through recombination. PLoS One 6, e23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Higashi H., Tsutsumi R., Fujita A., Yamazaki S., Asaka M., Azuma T., Hatakeyama M. (2002) Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. U.S.A. 99, 14428–14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Hatakeyama M. (2004) Oncogenic mechanisms of Helicobacter pylori CagA protein. Nat. Rev. Cancer 4, 688–694. [DOI] [PubMed] [Google Scholar]
- 57).Naito M., Yamazaki T., Tsutsumi R., Higashi H., Onoe K., Yamazaki S., Azuma T., Hatakeyama M. (2006) Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology 130, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 58).Xia Y., Yamaoka Y., Zhu Q., Matha I., Gao X. (2009) A comprehensive sequence and disease correlation analyses for the C-terminal region of CagA protein of Helicobacter pylori. PLoS One 4, e7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Mueller D., Tegtmeyer N., Brandt S., Yamaoka Y., De Poire E., Sgouras D., Wessler S., Torres J., Smolka A., Backert S. (2012) c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Invest. 122, 1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Ren S., Higashi H., Lu H., Azuma T., Hatakeyama M. (2006) Structural basis and functional consequence of Helicobacter pylori CagA multimerization in Cells. J. Biol. Chem. 281, 32344–32352. [DOI] [PubMed] [Google Scholar]
- 61).Lu H., Saito Y., Umeda M., Murata-Kamiya N., Zhang H., Higashi H., Hatakeyama M. (2008) Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA. Cancer Sci. 99, 2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Neel B.G., Gu H., Pao L. (2003) The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284–293. [DOI] [PubMed] [Google Scholar]
- 63).Hatakeyama M. (2003) Helicobacter pylori CagA — a potential bacterial oncoprotein that functionally mimics the mammalian Gab family of adaptor proteins. Microbes Infect. 5, 143–150. [DOI] [PubMed] [Google Scholar]
- 64).Botham C.M., Wandler A.M., Guillemin K. (2008) A transgenic Drosophila model demonstrates that the Helicobacter pylori CagA protein functions as a eukaryotic Gab adaptor. PLoS Pathog. 4, e1000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Higashi H., Nakaya A., Tsutsumi R., Yokoyama K., Fujii Y., Higuchi M., Takahashi A., Kurashima Y., Ishikawa S., Tanaka S., Azuma T., Hatakeyama M. (2004) Helicobacter pylori CagA provokes Ras-independent morphogenetic response through targeting SHP-2. J. Biol. Chem. 279, 17205–17216. [DOI] [PubMed] [Google Scholar]
- 66).Higuchi M., Tsutsumi R., Higashi H., Hatakeyama M. (2004) Conditional gene silencing utilizing the lac repressor reveals a role of SHP-2 in cagA-positive Helicobacter pylori pathogenicity. Cancer Sci. 95, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Tsutsumi R., Takahashi A., Azuma T., Higashi H., Hatakeyama M. (2006) Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Tartaglia M., Niemeyer C.M., Fragale A., Song X., Buechner J., Jung A., Hählen K., Hasle H., Licht J.D., Gelb B.D. (2003) Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34, 148–150. [DOI] [PubMed] [Google Scholar]
- 69).Bentires-Alj M., Paez J.G., David F.S., Keilhack H., Halmos B., Naoki K., Maris J.M., Richardson A., Bardelli A., Sugarbaker D.J., Richards W.G., Du J., Girard L., Minna J.D., Loh M.L., Fisher D.E., Velculescu V.E., Vogelstein B., Meyerson M., Sellers W.R., Neel B.G. (2004) Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 64, 8816–8820. [DOI] [PubMed] [Google Scholar]
- 70).Hof P., Pluskey S., Dhe-Paganon S., Eck M.J., Shoelson S.E. (1998) Crystal structure of the tyrosine phosphatase SHP-2. Cell 92, 441–450. [DOI] [PubMed] [Google Scholar]
- 71).Takahashi A., Tsutsumi R., Kikuchi I., Obuse C., Saito Y., Seidi A., Karisch R., Fernandez M., Cho T., Ohnishi N., Rozenblatt-Rosen O., Meyerson M., Neel B.G., Hatakeyama M. (2011) SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol. Cell 43, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Kikuchi I., Takahashi-Kanemitsu A., Sakiyama N., Tang C., Tang P.J., Noda S., Nakao K., Kassai H., Sato T., Aiba A., Hatakeyama M. (2016) Dephosphorylated parafibromin is a transcriptional coactivator of the Wnt/Hedgehog/Notch pathways. Nat. Commun. 7, 12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Tsutsumi R., Masoudi M., Takahashi A., Fujii Y., Hayashi T., Kikuchi I., Sato Y., Taira M., Hatakeyama M. (2013) YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev. Cell 26, 658–665. [DOI] [PubMed] [Google Scholar]
- 74).Tsutsumi R., Higashi H., Higuchi M., Okada M., Hatakeyama M. (2003) Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src Kinase. J. Biol. Chem. 278, 3664–3670. [DOI] [PubMed] [Google Scholar]
- 75).Zhang X.S., Tegtmeyer N., Traube L., Jindal S., Perez-Perez G., Sticht H., Backert S., Blaser M.J. (2015) A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions. PLoS Pathog. 11, e1004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Selbach M., Paul F.E., Brandt S., Guye P., Daumke O., Backert S., Dehio C., Mann M. (2009) Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe 5, 397–403. [DOI] [PubMed] [Google Scholar]
- 77).Suzuki M., Mimuro H., Suzuki T., Park M., Yamamoto T., Sasakawa C. (2005) Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 202, 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Mimuro H., Suzuki T., Tanaka J., Asahi M., Haas R., Sasakawa C. (2002) Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol. Cell 10, 745–755. [DOI] [PubMed] [Google Scholar]
- 79).Saju P., Murata-Kamiya N., Hayashi T., Senda Y., Nagase L., Noda S., Matsusaka K., Funata S., Kunita A., Urabe M., Seto Y., Fukayama M., Kaneda A., Hatakeyama M. (2016) Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat. Microbiol. 1, 16026. [DOI] [PubMed] [Google Scholar]
- 80).Saadat I., Higashi H., Obuse C., Umeda M., Murata-Kamiya N., Saito Y., Lu H., Ohnishi N., Azuma T., Suzuki A., Ohno S., Hatakeyama M. (2007) Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333. [DOI] [PubMed] [Google Scholar]
- 81).Zeaiter Z., Cohen D., Müsch A., Bagnoli F., Covacci A., Stein M. (2008) Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cell. Microbiol. 10, 781–794. [DOI] [PubMed] [Google Scholar]
- 82).Matenia D., Mandelkow E.M. (2009) The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem. Sci. 34, 332–342. [DOI] [PubMed] [Google Scholar]
- 83).Suzuki A., Ohno S. (2006) The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979–987. [DOI] [PubMed] [Google Scholar]
- 84).Hayashi K., Suzuki A., Ohno S. (2012) PAR-1/MARK: a kinase essential for maintaining the dynamic state of microtubules. Cell Struct. Funct. 37, 21–25. [DOI] [PubMed] [Google Scholar]
- 85).Yamahashi Y., Saito Y., Murata-Kamiya N., Hatakeyama M. (2011) Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J. Biol. Chem. 286, 44576–44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Lu H., Murata-Kamiya N., Saito Y., Hatakeyama M. (2009) Role of partitioning-defective 1/microtubule affinity-regulating kinases in the morphogenetic activity of Helicobacter pylori CagA. J. Biol. Chem. 284, 23024–23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Nesić D., Miller M.C., Quinkert Z.T., Stein M., Chait B.T., Stebbins C.E. (2010) Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat. Struct. Mol. Biol. 17, 130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Amieva M.R., Vogelmann R., Covacci A., Tompkins L.S., Nelson W.J., Falkow S. (2003) Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Halaoui R., McCaffrey L. (2015) Rewiring cell polarity signaling in cancer. Oncogene 34, 939–950. [DOI] [PubMed] [Google Scholar]
- 90).Tan S., Tompkins L.S., Amieva M.R. (2009) Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 5, e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Tan S., Noto J.M., Romero-Gallo J., Peek R.M., Jr., Amieva M.R. (2011) Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 7, e1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Nagase L., Murata-Kamiya N., Hatakeyama M. (2011) Potentiation of Helicobacter pylori CagA protein virulence through homodimerization. J. Biol. Chem. 286, 33622–33631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Churin Y., Al-Ghoul L., Kepp O., Meyer T.F., Birchmeier W., Naumann M. (2003) Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Suzuki M., Mimuro H., Kiga K., Fukumatsu M., Ishijima N., Morikawa H., Nagai S., Koyasu S., Gilman R.H., Kersulyte D., Berg D.E., Sasakawa C. (2009) Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe 5, 23–34. [DOI] [PubMed] [Google Scholar]
- 95).DiDonato J.A., Mercurio F., Karin M. (2012) NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400. [DOI] [PubMed] [Google Scholar]
- 96).Murata-Kamiya N., Kurashima Y., Teishikata Y., Yamahashi Y., Saito Y., Higashi H., Aburatani H., Akiyama T., Peek R.M., Jr., Azuma T., Hatakeyama M. (2007) Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26, 4617–4626. [DOI] [PubMed] [Google Scholar]
- 97).Oliveira M.J., Costa A.M., Costa A.C., Ferreira R.M., Sampaio P., Machado J.C., Seruca R., Mareel M., Figueiredo C. (2009) CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J. Infect. Dis. 200, 745–755. [DOI] [PubMed] [Google Scholar]
- 98).Lee D.G., Kim H.S., Lee Y.S., Kim S., Cha S.Y., Ota I., Kim N.H., Cha Y.H., Yang D.H., Lee Y., Park G.J., Yook J.I., Lee Y.C. (2014) Helicobacter pylori CagA promotes Snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat. Commun. 5, 4423. [DOI] [PubMed] [Google Scholar]
- 99).Tsang Y.H., Lamb A., Romero-Gallo J., Huang B., Ito K., Peek R.M., Jr., Ito Y., Chen L.F. (2010) Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene 29, 5643–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Buti L., Spooner E., Van der Veen A.G., Rappuoli R., Covacci A., Ploegh H.L. (2011) Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl. Acad. Sci. U.S.A. 108, 9238–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Wei J., Nagy T.A., Vilgelm A., Zaika E., Ogden S.R., Romero-Gallo J., Piazuelo M.B., Correa P., Washington M.K., El-Rifai W., Peek R.M., Zaika A. (2010) Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology 139, 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Wei J., Noto J.M., Zaika E., Romero-Gallo J., Piazuelo M.B., Schneider B., El-Rifai W., Correa P., Peek R.M., Zaika A.I. (2015) Bacterial CagA protein induces degradation of p53 protein in a p14ARF-dependent manner. Gut 64, 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Hayashi T., Senda M., Morohashi H., Higashi H., Horio M., Kashiba Y., Nagase L., Sasaya D., Shimizu T., Venugopalan N., Kumeta H., Noda N.N., Inagaki F., Senda T., Hatakeyama M. (2012) Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe 12, 20–33. [DOI] [PubMed] [Google Scholar]
- 104).Kaplan-Türköz B., Jiménez-Soto L.F., Dian C., Ertl C., Remaut H., Louche A., Tosi T., Haas R., Terradot L. (2012) Structural insights into Helicobacter pylori oncoprotein CagA interaction with β1 integrin. Proc. Natl. Acad. Sci. U.S.A. 109, 14640–14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Nešić D., Buti L., Lu X., Stebbins C.E. (2014) Structure of the Helicobacter pylori CagA oncoprotein bound to the human tumor suppressor ASPP2. Proc. Natl. Acad. Sci. U.S.A. 111, 1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Reingewertz T.H., Iosub-Amir A., Bonsor D.A., Mayer G., Amartely H., Friedler A., Sundberg E.J. (2015) An intrinsically disordered region in the proapoptotic ASPP2 protein binds to the Helicobacter pylori oncoprotein CagA. Biochemistry 54, 3337–3347. [DOI] [PubMed] [Google Scholar]
- 107).Tsang Y.H., Lamb A., Romero-Gallo J., Huang B., Ito K., Peek R.M., Jr., Ito Y., Chen L.F. (2010) Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene 29, 5643–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Hayashi T., Morohashi H., Hatakeyama M. (2013) Bacterial EPIYA effectors — where do they come from? What are they? Where are they going? Cell. Microbiol. 15, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Ohnishi N., Yuasa H., Tanaka S., Sawa H., Miura M., Matsui A., Higashi H., Musashi M., Iwabuchi K., Suzuki M., Yamada G., Azuma T., Hatakeyama M. (2008) Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. U.S.A. 105, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Neal J.T., Peterson T.S., Kent M.L., Guillemin K. (2013) H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis. Model. Mech. 6, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Mantovani A., Allavena P., Sica A., Balkwill F. (2008) Cancer-related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- 112).Suzuki N., Murata-Kamiya N., Yanagiya K., Suda W., Hattori M., Kanda H., Bingo A., Fujii Y., Maeda S., Koike K., Hatakeyama M. (2015) Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci. Rep. 5, 10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Viala J., Chaput C., Boneca I.G., Cardona A., Girardin S.E., Moran A.P., Athman R., Mémet S., Huerre M.R., Coyle A.J., DiStefano P.S., Sansonetti P.J., Labigne A., Bertin J., Philpott D.J., Ferrero R.L. (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5, 1166–1174. [DOI] [PubMed] [Google Scholar]
- 114).Lamb A., Yang X.D., Tsang Y.H., Li J.D., Higashi H., Hatakeyama M., Peek R.M., Blanke S.R., Chen L.F. (2009) Helicobacter pylori CagA activates NF-κB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 10, 1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Tebbutt N.C., Giraud A.S., Inglese M., Jenkins B., Waring P., Clay F.J., Malki S., Alderman B.M., Grail D., Hollande F., Heath J.K., Ernst M. (2002) Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097. [DOI] [PubMed] [Google Scholar]
- 116).Lee I.O., Kim J.H., Choi Y.J., Pillinger M.H., Kim S.Y., Blaser M.J., Lee Y.C. (2010) Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J. Biol. Chem. 285, 16042–16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Akiba S., Koriyama C., Herrera-Goepfert R., Eizuru Y. (2008) Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 99, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Matsusaka K., Funata S., Fukayama M., Kaneda A. (2014) DNA methylation in gastric cancer, related to Helicobacter pylori and Epstein-Barr virus. World J. Gastroenterol. 20, 3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119).Eck M., Schmausser B., Haas R., Greiner A., Czub S., Müller-Hermelink H.K. (1997) MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 112, 1482–1486. [DOI] [PubMed] [Google Scholar]
- 120).Ferreri A.J., Govi S., Ponzoni M. (2013) The role of Helicobacter pylori eradication in the treatment of diffuse large B-cell and marginal zone lymphomas of the stomach. Curr. Opin. Oncol. 25, 470–479. [DOI] [PubMed] [Google Scholar]
- 121).Lin W.C., Tsai H.F., Kuo S.H., Wu M.S., Lin C.W., Hsu P.I., Cheng A.L., Hsu P.N. (2010) Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 70, 5740–5748. [DOI] [PubMed] [Google Scholar]
- 122).Kuo S.H., Chen L.T., Lin C.W., Wu M.S., Hsu P.N., Tsai H.J., Chu C.Y., Tzeng Y.S., Wang H.P., Yeh K.H., Cheng A.L. (2013) Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: clinical and biological significance. Blood Cancer J. 3, e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123).Zhu Y., Wang C., Huang J., Ge Z., Dong Q., Zhong X., Su Y., Zheng S. (2007) The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell. Microbiol. 9, 952–961. [DOI] [PubMed] [Google Scholar]
- 124).Umehara S., Higashi H., Ohnishi N., Asaka M., Hatakeyama M. (2003) Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene 22, 8337–8342. [DOI] [PubMed] [Google Scholar]
- 125).Azuma T., Yamazaki S., Yamakawa A., Ohtani M., Muramatsu A., Suto H., Ito Y., Dojo M., Yamazaki Y., Kuriyama M., Keida Y., Higashi H., Hatakeyama M. (2004) Association between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J. Infect. Dis. 189, 820–827. [DOI] [PubMed] [Google Scholar]
- 126).Vilaichone R.K., Mahachai V., Tumwasorn S., Wu J.Y., Graham D.Y., Yamaoka Y. (2004) Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter 9, 453–459. [DOI] [PubMed] [Google Scholar]
- 127).Satomi S., Yamakawa A., Matsunaga S., Masaki R., Inagaki T., Okuda T., Suto H., Ito Y., Yamazaki Y., Kuriyama M., Keida Y., Kutsumi H., Azuma T. (2006) Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. J. Gastroenterol. 41, 668–673. [DOI] [PubMed] [Google Scholar]
- 128).Miura M., Ohnishi N., Tanaka S., Yanagiya K., Hatakeyama M. (2009) Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int. J. Cancer 125, 2497–2504. [DOI] [PubMed] [Google Scholar]
- 129).Karlsson A., Ryberg A., Dehnoei M.N., Borch K., Monstein H.J. (2012) Association between cagA and vacA genotypes and pathogenesis in a Helicobacter pylori infected population from South-eastern Sweden. BMC Microbiol. 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Argent R.H., Kidd M., Owen R.J., Thomas R.J., Limb M.C., Atherton J.C. (2004) Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology 127, 514–523. [DOI] [PubMed] [Google Scholar]
- 131).Basso D., Zambon C.F., Letley D.P., Stranges A., Marchet A., Rhead J.L., Schiavon S., Guariso G., Ceroti M., Nitti D., Rugge M., Plebani M., Atherton J.C. (2008) Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 135, 91–99. [DOI] [PubMed] [Google Scholar]
- 132).Batista S.A., Rocha G.A., Rocha A.M., Saraiva I.E., Cabral M.M., Oliveira R.C., Queiroz D.M. (2011) Higher number of Helicobacter pylori CagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol. 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Beltrán-Anaya F.O., Poblete T.M., Román-Román A., Reyes S., de Sampedro J., Peralta-Zaragoza O., Rodríguez M.Á., del Moral-Hernández O., Illades-Aguiar B., Fernández-Tilapa G. (2014) The EPIYA-ABCC motif pattern in CagA of Helicobacter pylori is associated with peptic ulcer and gastric cancer in Mexican population. BMC Gastroenterol. 14, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Ferreira Júnior M., Batista S.A., Vidigal P.V., Cordeiro A.A., Oliveira F.M., Prata L.O., Diniz A.E., Barral C.M., Barbuto R.C., Gomes A.D., Araújo I.D., Queiroz D.M., Caliari M.V. (2015) Infection with CagA-positive Helicobacter pylori strain containing three EPIYA C phosphorylation sites is associated with more severe gastric lesions in experimentally infected Mongolian gerbils (Meriones unguiculatus). Eur. J. Histochem. 59, 2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).González I., Romero J., Rodríguez B., Llanos J., Morales E., Figueroa H., Perez-Castro R., Valdés E., Cofre C., Rojas A. (2011) High prevalence of virulence-associated genotypes in Helicobacter pylori clinical isolates in the Region del Maule, Chile. Scand. J. Infect. Dis. 43, 652–655. [DOI] [PubMed] [Google Scholar]
- 136).Sicinschi L.A., Correa P., Peek R.M., Camargo M.C., Piazuelo M.B., Romero-Gallo J., Hobbs S.S., Krishna U., Delgado A., Mera R., Bravo L.E., Schneider B.G. (2010) CagA C-terminal variations in Helicobacter pylori strains from Colombian patients with gastric precancerous lesions. Clin. Microbiol. Infect. 16, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137).Nagase L., Hayashi T., Senda T., Hatakeyama M. (2015) Dramatic increase in SHP2 binding activity of Helicobacter pylori Western CagA by EPIYA-C duplication: its implications in gastric carcinogenesis. Sci. Rep. 5, 15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Aceto N., Sausgruber N., Brinkhaus H., Gaidatzis D., Martiny-Baron G., Mazzarol G., Confalonieri S., Quarto M., Hu G., Balwierz P.J., Pachkov M., Elledge S.J., van Nimwegen E., Stadler M.B., Bentires-Alj M. (2012) Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med. 18, 529–537. [DOI] [PubMed] [Google Scholar]
- 139).Sausgruber N., Coissieux M.M., Britschgi A., Wyckoff J., Aceto N., Leroy C., Stadler M.B., Voshol H., Bonenfant D., Bentires-Alj M. (2015) Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene 34, 2272–2278. [DOI] [PubMed] [Google Scholar]
- 140).Ogorodnik E., Raffaniello R.D. (2013) Analysis of the 3′-variable region of the cagA gene from Helicobacter pylori strains infecting patients at New York City hospitals. Microb. Pathog. 56, 29–34. [DOI] [PubMed] [Google Scholar]
- 141).Kersulyte D., Kalia A., Gilman R.H., Mendez M., Herrera P., Cabrera L., Velapatiño B., Balqui J., Paredes Puente de la Vega F., Rodriguez Ulloa C.A., Cok J., Hooper C.C., Dailide G., Tamma S., Berg D.E. (2010) Helicobacter pylori from Peruvian amerindians: traces of human migrations in strains from remote Amazon, and genome sequence of an Amerind strain. PLoS One 5, e15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Mane S.P., Dominguez-Bello M.G., Blaser M.J., Sobral B.W., Hontecillas R., Skoneczka J., Mohapatra S.K., Crasta O.R., Evans C., Modise T., Shallom S., Shukla M., Varon C., Mégraud F., Maldonado-Contreras A.L., Williams K.P., Bassaganya-Riera J. (2010) Host-interactive genes in Amerindian Helicobacter pylori diverge from their Old World homologs and mediate inflammatory responses. J. Bacteriol. 192, 3078–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Suzuki M., Kiga K., Kersulyte D., Cok J., Hooper C.C., Mimuro H., Sanada T., Suzuki S., Oyama M., Kozuka-Hata H., Kamiya S., Zou Q.M., Gilman R.H., Berg D.E., Sasakawa C. (2011) Attenuated CagA oncoprotein in Helicobacter pylori from Amerindians in Peruvian Amazon. J. Biol. Chem. 286, 29964–29972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144).Hashi K., Murata-Kamiya N., Varon C., Mégraud F., Dominguez-Bello M.G., Hatakeyama M. (2014) Natural variant of the Helicobacter pylori CagA oncoprotein that lost the ability to interact with PAR1. Cancer Sci. 105, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145).Nishikawa H., Hayashi T., Arisaka F., Senda T., Hatakeyama M. (2016) Impact of structural polymorphism for the Helicobacter pylori CagA oncoprotein on binding to polarity-regulating kinase PAR1b. Sci. Rep. 6, 30031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Bagnoli F., Buti L., Tompkins L., Covacci A., Amieva M.R. (2005) Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. U.S.A. 102, 16339–16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147).Bessède E., Staede C., Acuña Amador L.A., Nguyen P.H., Chambonnier L., Hatakeyama M., Belleannée G., Mégraud F., Varon C. (2014) Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene 33, 4123–4131. [DOI] [PubMed] [Google Scholar]
- 148).Saito Y., Murata-Kamiya N., Hirayama T., Ohba Y., Hatakeyama M. (2010) Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21. J. Exp. Med. 207, 2157–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Fujii Y., Yoshihashi K., Suzuki H., Tsutsumi S., Mutoh H., Maeda S., Yamagata Y., Seto Y., Aburatani H., Hatakeyama M. (2012) CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. U.S.A. 109, 20584–20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150).Yokoyama K., Higashi H., Ishikawa S., Fujii Y., Kondo S., Kato H., Azuma T., Wada A., Hirayama T., Aburatani H., Hatakeyama M. (2005) Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 102, 9661–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151).Sun M., Liao B., Tao Y., Chen H., Xiao F., Gu J., Gao S., Jin Y. (2016) Calcineurin-NFAT signaling controls somatic cell reprogramming in a stage-dependent manner. J. Cell. Physiol. 231, 1151–1162. [DOI] [PubMed] [Google Scholar]
- 152).Sigal M., Rothenberg M.E., Logan C.Y., Lee J.Y., Honaker R.W., Cooper R.L., Passarelli B., Camorlinga M., Bouley D.M., Alvarez G., Nusse R., Torres J., Amieva M.R. (2015) Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology 148, 1392–1404. [DOI] [PubMed] [Google Scholar]
- 153).Tsugawa H., Suzuki H., Saya H., Hatakeyama M., Hirayama T., Hirata K., Nagano O., Matsuzaki J., Hibi T. (2012) Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12, 764–777. [DOI] [PubMed] [Google Scholar]
- 154).Hatakeyama M. (2014) Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe 15, 306–316. [DOI] [PubMed] [Google Scholar]
- 155).Skinner G.R. (1976) Transformation of primary embryo fibroblasts by type 2 simplex virus: evidence for a “hit and run” mechanism. Br. J. Exp. Pathol. 57, 361–376. [PMC free article] [PubMed] [Google Scholar]
- 156).Umeda M., Murata-Kamiya N., Saito Y., Ohba Y., Takahashi M., Hatakeyama M. (2009) Helicobacter pylori CagA causes mitotic impairment and induces chromosomal instability. J. Biol. Chem. 284, 22166–22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157).Matsumoto Y., Marusawa H., Kinoshita K., Endo Y., Kou T., Morisawa T., Azuma T., Okazaki I.M., Honjo T., Chiba T. (2007) Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 13, 470–476. [DOI] [PubMed] [Google Scholar]
- 158).Chaturvedi R., Asim M., Romero-Gallo J., Barry D.P., Hoge S., de Sablet T., Delgado A.G., Wroblewski L.E., Piazuelo M.B., Yan F., Israel D.A., Casero R.A., Jr., Correa P., Gobert A.P., Polk D.B., Peek R.M., Jr., Wilson K.T. (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141, 1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).Hanada K., Uchida T., Tsukamoto Y., Watada M., Yamaguchi N., Yamamoto K., Shiota S., Moriyama M., Graham D.Y., Yamaoka Y. (2014) Helicobacter pylori infection introduces DNA double-strand breaks in host cells. Infect. Immun. 82, 4182–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Hartung M.L., Gruber D.C., Koch K.N., Grüter L., Rehrauer H., Tegtmeyer N., Backert S., Müller A. (2015) H. pylori-induced DNA strand breaks are introduced by nucleotide excision repair endonucleases and promote NF-κB target gene expression. Cell Reports 13, 70–79. [DOI] [PubMed] [Google Scholar]
- 161).Tanaka H., Katoh H., Negishi M. (2006) Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J. Biol. Chem. 281, 10355–10364. [DOI] [PubMed] [Google Scholar]
- 162).Safari F., Murata-Kamiya N., Saito Y., Hatakeyama M. (2011) Mammalian Pragmin regulates Src family kinases via the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif that is exploited by bacterial effectors. Proc. Natl. Acad. Sci. U.S.A. 108, 14938–14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163).Senda Y., Murata-Kamiya N., Hatakeyama M. (2016) C-terminal Src kinase-mediated EPIYA phosphorylation of Pragmin creates a feed-forward C-terminal Src kinase activation loop that promotes cell motility. Cancer Sci. 107, 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164).Leroy C., Fialin C., Sirvent A., Simon V., Urbach S., Poncet J., Robert B., Jouin P., Roche S. (2009) Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 69, 2279–2286. [DOI] [PubMed] [Google Scholar]
- 165).Tactacan C.M., Phua Y.W., Liu L., Zhang L., Humphrey E.S., Cowley M., Pinese M., Biankin A.V., Daly R.J. (2015) The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol. Cancer 14, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166).Pasceri V., Patti G., Cammarota G., Pristipino C., Richichi G., Di Sciascio G. (2006) Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. Am. Heart J. 151, 1215–1222. [DOI] [PubMed] [Google Scholar]
- 167).Franceschi F., Niccoli G., Ferrante G., Gasbarrini A., Baldi A., Candelli M., Feroce F., Saulnier N., Conte M., Roccarina D., Lanza G.A., Gasbarrini G., Gentiloni S.N., Crea F. (2009) CagA antigen of Helicobacter pylori and coronary instability: insight from a clinico-pathological study and a meta-analysis of 4241 cases. Atherosclerosis 202, 535–542. [DOI] [PubMed] [Google Scholar]
- 168).De Bastiani R., Gabrielli M., Ubaldi E., Benedetto E., Sanna G., Cottone C., Candelli M., Zocco M.A., Saulnier N., Santoliquido A., Papaleo P., Gasbarrini G., Gasbarrini A. (2008) High prevalence of Cag-A positive H. pylori strains in ischemic stroke: a primary care multicenter study. Helicobacter 13, 274–277. [DOI] [PubMed] [Google Scholar]
- 169).Tersigni C., Franceschi F., Todros T., Cardaropoli S., Scambia G., Di Simone N. (2014) Insights into the role of Helicobacter pylori infection in preeclampsia: from the bench to the bedside. Front. Immunol. 5, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170).Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. [DOI] [PubMed] [Google Scholar]
- 171).Azmi A.S., Bao B., Sarkar F.H. (2013) Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 32, 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172).Shimoda A., Ueda K., Nishiumi S., Murata-Kamiya N., Mukai S.A., Sawada S., Azuma T., Hatakeyama M., Akiyoshi K. (2016) Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci. Rep. 6, 18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173).McCracken K.W., Catá E.M., Crawford C.M., Sinagoga K.L., Schumacher M., Rockich B.E., Tsai Y.H., Mayhew C.N., Spence J.R., Zavros Y., Wells J.M. (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174).Noguchi T.K., Ninomiya N., Sekine M., Komazaki S., Wang P.C., Asashima M., Kurisaki A. (2015) Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 17, 984–993. [DOI] [PubMed] [Google Scholar]