Abstract
Antibiotic resistance is becoming a pivotal concern for public health that has accelerated the search for new antimicrobial molecules from nature. Numbers of human pathogens have inevitably evolved to become resistant to various currently available drugs causing considerable mortality and morbidity worldwide. It is apparent that novel antibiotics are urgently warranted to combat these life-threatening pathogens. In recent years, there have been an increasing number of studies to discover new bioactive compounds from plant origin with the hope to control antibiotic-resistant bacteria. This review attempts to focus and record the plant-derived compounds and plant extracts against multi-drug-resistant (MDR) pathogens including methicillin-resistant Staphylococcus aureus (MRSA), MDR-Mycobacterium tuberculosis and malarial parasites Plasmodium spp. reported between 2005 and 2015. During this period, a total of 110 purified compounds and 60 plant extracts were obtained from 112 different plants. The plants reviewed in this study belong to 70 different families reported from 36 countries around the world. The present review also discusses the drug resistance in bacteria and emphasizes the urge for new drugs.
Keywords: Plant metabolites, Antibiotic resistance, MRSA, Medicinal plants
Introduction
Approximately, 500,000 species of both identified and unidentified plants have been estimated on Earth. Among them, only 1–10% are being used as foods by animals and humans (Borris 1996; Cowan 1999). Plants are the key source for drugs and an alternative medicine for fighting against diseases since ancient times. Evidential specimens proved that Neanderthals living 60,000 years ago in present-day Iraq used plants such as hollyhock (Thomson 1978; Stockwell 1988; Cowan 1999) and these plants are still widely being used in ethnomedicine across the world. Interestingly, about 50% of all pharmaceutical products distributed in the United States have plant origin. Among which, very few are used as antimicrobials, since the microbial sources are widely relied upon (Cowan 1999). Nevertheless, since the arrival of antibiotics in the 1950s, the use of plant derivatives as antimicrobials has been literally non-existent. Researchers are interested in plant extracts as medicines as they are undisputable substitution for antibiotics prescribed by physicians (Cowan 1999). Besides, the public is becoming increasingly aware of problems with the overuse and misuse of antibiotics. In addition, many people are attracted in having more autonomy over their medical care (Cowan 1999). The self-medication with plant substances is common due to easy availability. The use of plant-derived natural products in medical treatments is attracting more attention due to its potential efficacy and no side effects (Cowan 1999). Indeed, plants are a rich source of valuable secondary metabolites, such as quinones, tannins, terpenoids, alkaloids, flavonoids, and polyphenols that are used by plants as defence mechanisms against predation by microorganisms, insects, and herbivores. Some, such as terpenoids, give plants their odors; quinones and tannins are responsible for plant pigmentation. Many compounds including terpenoids are responsible for plant flavor and some of the herbs and spices, which are being used by humans to season foods, could yield useful medicinal compounds (Cowan 1999; Dixon 2001; Kyaw et al. 2012). The number of bioactive compounds derived from plants has been estimated to be at least 200,000 and it still represents only a fraction of the compounds produced by the plant species growing on Earth (Efferth and Koch 2011). Research interest on medicinal plants is being amplified in recent years, which is seen by the increase in the number of publications on plant-based pharmacological interactions and synergistic principles (van Vuuren and Viljoen 2011). This interest has led to the discovery of new/novel biologically active molecules by the researchers and pharmaceutical industries and the adoption of crude extracts of plants for self-medication by the general public. In this review, an effort is made to summarize one decade (2005–2015) of plant antimicrobials including the purified plant-based bioactive compounds, crude and partially purified plant extracts against MDR human pathogens including MRSA, MDR-M. tuberculosis and malarial parasites Plasmodium spp. This is by no means an exhaustive search of all plant-derived compounds and plant extracts during this past 10-year period. Nevertheless, the list provided in this review is impressive and illustrates the potential of plant antimicrobials against MDR human pathogens.
Drug resistance in bacteria: alarming need of new antibiotics
Antibiotic-resistant bacterial infections are already widespread on the globe (Golkar et al. 2014). In February 2017, World Health Organization (WHO) published its first ever list of antibiotic-resistant ‘priority pathogens’ that pose the greatest threat to human health. The first critical priority pathogens are carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant and extended spectrum beta-lactamase (ESBL) producing Enterobacteriaceae. The second level high priority pathogens are vancomycin-resistant Enterococcus faecium, methicillin-resistant Staphylococcus aureus, clarithromycin-resistant Helicobacter pylori, fluoroquinolone-resistant Campylobacter spp., fluoroquinolone-resistant Salmonellae and cephalosporin and fluoroquinolone-resistant Neisseria gonorrhoeae. These priority pathogens are resistant to multiple antibiotics and have in-built abilities to resist treatment and transfer along genetic material that leads other bacteria to become drug-resistant as well (http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/, accessed on 09/05/2017). Therefore, new/novel antibiotics are desperately needed for battling these rapidly evolving pathogens. The production of new antibiotics has diminished progressively over the past 20 years, entrusting less possibilities to treat these drug-resistant pathogens (Ventola 2015). The treatment of infections caused by MDR pathogens is complicated and limited (Kanj and Kanafani 2011). Meanwhile, clinicians are still prescribing the existing drugs with appropriate dosage and combinations of various drugs for preventing and treating these super bugs (Safavi et al. 2016). Table 1 displays some of the WHO priority drug-resistant pathogens and their current antibiotics of choice for their treatment.
Table 1.
List of some WHO priority drug-resistant pathogens and their current antibiotics of choice for treatment
| WHO priority drug-resistant pathogen | Currently using antibiotics | References |
|---|---|---|
| Carbapenem-resistant A. baumannii | Colistin, carbapenems, sulbactam, rifampin and tigecycline | Viehman et al. (2014) |
| Carbapenem-resistant P. aeruginosa | Ticarcillin-clavulanate, ceftazidime, aztreonam, imipenem, ciprofloxacin and colistin | Kanj and Kanafani (2011) |
| Carbapenem-resistant, ESBL-producing Enterobacteriaceae | Polymyxins, fosfomycin, carbapenems, tigecycline and aminoglycosides | Morrill et al. (2015) |
| Vancomycin-resistant E. faecium | Streptogramin, linezolid, daptomycin, oritavancin and tigecycline | Linden (2002) |
| Methicillin-resistant S. aureus | Vancomycin, trimethoprim-sulfamethoxazole, clindamycin, linezolid, tetracyclines and daptomycin | Kali (2015) |
| Clarithromycin-resistant H. pylori | Amoxicillin, esomeprazole, rabeprazole, omeprazole, metronidazole, levofloxacin and clarithromycin | Safavi et al. (2016) |
| Fluoroquinolone-resistant Campylobacter spp. | Erythromycin, ciprofloxacin and fluoroquinolones | Wieczorek and Osek (2013) |
Multi-drug-resistant (MDR) pathogens: a threat to public health
The underuse, overuse, and misuse of antibiotics by humans are the selective pressure, which eventually lead to the development of antibiotic resistance in microbes (Davies and Davies 2010). Globally, emerging MDR pathogens also called as ‘ESKAPE’ organisms such as Enterococcus spp., S. aureus, Klebsiella spp., A. baumannii, P. aeruginosa and Enterobacter spp. are a serious threat now-a-days to public health (Boucher et al. 2009). MDR microorganisms can survive the treatment with antimicrobial drugs, thereby standard treatments become ineffective and infections persist, increasing the risk of spread to others. In general, MDR microbes are resistant to three or more antibiotics (Styers et al. 2006); however, strains of Mycobacterium tuberculosis are extremely drug-resistant (XDR) that are virtually resistant to all classes of antimicrobials (Gandhi et al. 2006). In 2012, WHO reported a gradual increase in resistance to HIV drugs, although not reaching critical levels (WHO 2012). Since then, further increase in resistance to first-line treatment drugs was reported, which might require using more expensive drugs soon (http://www.who.int/mediacentre/factsheets/fs194/en/, accessed on 18/08/2016). In addition, the Centre for Disease Control (CDC) estimates that each year, nearly 2 million people in the United States acquire an infection while in hospital, resulting in 90,000 deaths. More than 70% of the bacteria that cause these infections are resistant to at least one of the antibiotics commonly used to treat them (http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143568.htm, accessed on 18/08/2016). The globally emerging antibiotic resistance, nevertheless, makes MDR microbes substantially difficult to control or kill and also builds them more stronger. It is clear that the currently available antibiotics are insufficient to control these superbugs and, hence, more research and novel antimicrobial sources are highly demanded.
Methicillin-resistant S. aureus (MRSA)
In this decade, the fastest evolving pathogen is MRSA. There has been a continuous increase in the incidence of MRSA worldwide. In the United States, current MRSA rates exceed 50% of all S. aureus infections and stand close to 90% in some Asian countries (Office for National Statistics 2005). The mortality rates for deaths involving MRSA have increased over 15-fold during the period 1993 to 2002 (Office for National Statistics 2005). MRSA has developed resistance to a number of antibiotics such as oxacillin, penicillin and amoxicillin. In some countries, over 60% of S. aureus cases in hospital intensive care units are now resistant to these first-line antibiotics (Laxminarayan and Malani 2007). MRSA has been categorized into two groups based on their infections such as hospital-acquired and community-acquired MRSA (HA-MRSA and CA-MRSA), which marginally differ in their genetic make-up.
HA-MRSA is a deadly pathogen and often infects hospitalized patients particularly those who are immuno-compromised (Sheen 2010). HA-MRSA was first appeared in the United States in 1968 and showed resistance against β-lactam antibiotics and other various types of antibiotics (Chanda et al. 2010). The National Audit Office estimated that HA-MRSA was the primary factor in 5000 deaths per annum (National Audit Office 2000). CA-MRSA emerged in the community setting recovered from a clinical culture from a patient residing in the surveillance area, who had no established risk factors usually correlated with HA-MRSA (Chanda et al. 2010). The established risk factors include the isolation of MRSA two or more days after hospitalization; a history of hospitalization, surgery, dialysis, chronic diseases, or residence in a long-term care facility within 1 year before the MRSA-culture date; the presence of a permanent indwelling catheter or percutaneous medical device at the time of culture; or previous isolation of MRSA (Fridkin et al. 2005; Chanda et al. 2010). CA-MRSA often causes skin infection and severe infection resulting in fatal on certain occasions displaying resistance to β-lactam antibiotics, nevertheless susceptible to trimethoprim/sulfamethoxazole, clindamycin and tetracyclines (Deresinski 2005; Chanda et al. 2010).
Staphylococcus aureus is one of the most critical human pathogens causing wide range of infections from mild skin diseases to life-threatening endocarditis (Chambers 2001). After identifying the evidential occurrence of methicillin resistance among S. aureus strains, vancomycin and quinolones antibiotics have been used as alternative drugs of choice as staphylococcal infections therapy (Tiwari et al. 2009). Nevertheless, over a decade, most of the S. aureus strains including MRSA developed resistance to many commonly used fluoroquinolones by acquiring a rapid mutation in the genes encoding for target enzymes and expression of the efflux pump (Tanaka et al. 2000; Gade and Qazi 2013). Looking at the widespread development of fluoroquinolones resistance in S. aureus (FRSA), potential antibiotics are required and demand more awareness in public health care and community settings.
Vancomycin-resistant Enterococcus (VRE)
Vancomycin-resistant Enterococcus (VRE) is another current threat in emerging drug-resistant pathogens in hospitals worldwide (Johnstone et al. 2017). Approximately, 66,000 healthcare-associated enterococcal infections are reported in the United States every year. Out of them, approx. 20,000 are vancomycin-resistant infections with about 1300 deaths attributes to VRE infections (Centre for Disease Control 2013). Hospital incurred Enterococci infections are resistant to several drugs including daptomycin, linezolid, penicillin and cephalosporins, and their progressive resistance has been discovered across the world posing an alarming concern (Simeon et al. 2006; Johnstone et al. 2017).
Table 2 summarizes 15 plant-derived compounds and 40 plant extracts reported during 2005–2015 against MDR pathogens including MRSA, VRE, Trimethoprim–sulphamethoxazole-resistant uropathogenic Escherichia coli and various MDR Gram-negative and MDR Gram-positive human pathogens. This list clearly demonstrates the substantive and ongoing role of plant-derived compounds against MRSA and other MDR pathogens. A total of 43 plants are reviewed in Table 2; they are belonging to 23 different families. Interestingly, out of the 43 plants, 42 are angiosperms excluding the Brazilian Pinus elliottii (Leandro et al. 2014). Results show that both angiosperms and gymnosperms harboring potential antimicrobials against MDR pathogens; nevertheless, angiosperms are widely studied against MDR pathogens.
Table 2.
Plant-derived compounds and plant extracts reported during 2005–2015 against multi-drug-resistant pathogens
| Compound/extract | Plant | Source | Target | Reported country | References |
|---|---|---|---|---|---|
| Aqueous alkaloid, organic alkaloid and non-alkaloid | Rhazya stricta | Leaves | MRSA | Saudi Arabia | Khan et al. (2016) |
| Aqueous, chloroform, ethanol and hexane | Alkanna tinctoria | Leaves | MRSA, MDR-Acinetobacter baumannii, E. coli and P. aeruginosa | Pakistan | Khan et al. (2015) |
| Dehydroabietic acid | P. elliottii | Resin-oil | MDR-Staphylococcus epidermidis, S. capitis, S. haemolyticus, E. faecium and E. faecalis | Brazil | Leandro et al. (2014) |
| Dichloromethane, methanol, petroleum ether, chloroform, ethyl acetate, acetone, ethanol and water | Lantana camara L. | Leaves | MRSA, VRE, MDR-A. baumannii, P. aeruginosa, Streptococcus pyogenes, Citrobacter freundii, Proteus mirabilis and P. vulgaris | India | Dubey and Padhy (2013) |
| Petroleum ether, acetone, methanol, ethanol and water | Butea monosperma Lam. | Leaves | MRSA, VRSA | India | Sahu and Padhy (2013) |
| Ethanol and water | Anthocephalus cadamba and Pterocarpus santalinus | Leaves and bark | MDR-Acinetobacter sp., P. aeruginosa, C. freundii and Proteus sp. | India | Dubey et al. (2012) |
| Ethanol | Rhus coriaria | Seeds | MDR-P. aeruginosa | Palestine | Adwan et al. (2010) |
| (+)-Lyoniresinol-3 alpha-O-beta-d-glucopyranoside | Lycium chinense Mill. | Roots and bark | MRSA | China | Lee et al. (2005) |
| Baicalin | Scutellaria baicalensis Georgi. | NA | Synergistic effect with β-lactam-resistant strains of S. aureus, synergies between baicalein, tetracycline, β-lactams and ciprofloxacin against MRSA and inhibit MRSA-pyruvate kinase | China | Chan et al. (2011) |
| Sophoraflavanone G, 7,9,2′,4′-tetrahydroxy-8-isopentenyl-5-methoxychalcone | Sophora flavescens | Roots | MRSA, VRE | China | Cha et al. (2009), Lee et al. (2010) |
| Chloroform and chloroform + HCl | Andrographis paniculata | NA | MRSA | India | Roy et al. (2010) |
| Hexane | Sclerocarya birrea | Seeds | MRSA | Malaysia | Mariod et al. (2010) |
| Cold and hot aqueous and ethanol | Terminalia chebula Retz. | Dried seedless ripe fruits | MRSA, trimethoprim-sulphamethoxazole-resistant uropathogenic E. coli | India | Bag et al. (2009) |
| 20-Hydroxyecdysone | Achyranthes japonica | Roots | MRSA | South Korea | Kim et al. (2009) |
| Aqueous and ethanol | Fabiana bryoides, F. densa, F. punensis, Baccharis boliviensis, Chuquiraga atacamensis, Parastrephia lepidophylla, P. lucida, L. phyliciformis, Frankenia triandra, Chiliotrichiopsis keidelii | Aerial parts | MRSA, MSSA, MRSCN, MSSCN and MDR-E. faecalis | Argentina | Zampini et al. (2009) |
| Limonoids | Swietenia mahagoni | Seeds | MRSA, MDR-Group A haemolytic S. aureus, Streptococcus pneumoniae, Haemophilus influenza, E. coli, Klebsiella pneumonia, Salmonella typhi and S. paratyphi | Bangladesh | Rahman et al. (2009) |
| Water | Punica granatum | Pomegranate rind | MRSA, MSSA, PVL-positive-CA-MSSA | London | Gould et al. (2009) |
| Commercial extract (NA) | Olea europaea | Leaves | MRSA | Australia | Sudjana et al. (2009) |
| Ellagic acid, norwogonin, chebulagic acid, chebulinic acid, corilagin and terchebulin | Rosa rugosa, Scutellaria baicalensis and Terminalia chebula | Commercial plant powder | MDR-A. baumannii | USA | Miyasaki et al. (2013) |
| Triterpenes | Planchonia careya | Leaves | MRSA, VRE | Australia | McRae et al. (2008) |
| Butanol | Retama raetam | Flowers | MRSA | Tunisia | Hayet et al. (2008) |
| Ethanol | Atuna racemosa | Seeds | MRSA | USA | Buenz et al. (2007) |
| Methanol | Callistemon rigidus | Leaves | MRSA | India | Gomber and Saxena (2007) |
| Diterpenoids | Croton tonkinensis | Leaves | MRSA | Vietnam | Giang et al. (2006) |
| Aqueous and 80% ethanol | Rosa damascena, Melissa officinalis and Mentha longifolia | Aerial parts and flowers | MRSA | Palestine | Abu-Shanab et al. (2006) |
| Aqueous and ethanol | Terminalia avicennioides, Bridella ferruginea, Ageratum conyzoides, Phyllanthus discoideus, Ocimum gratissimum and Acalypha wilkesiana | Leaves and bark | MRSA | Nigeria | Akinyemi et al. (2005) |
MRSA methicillin-resistant S. aureus, MDR multi-drug-resistant, VRE vancomycin-resistant enterococci, VRSA vancomycin-resistant S. aureus, MSSA methicillin-sensitive S. aureus, MRSCN methicillin-resistant Staphylococcus coagulase negative, MSSCN methicillin-sensitive Staphylococcus coagulase negative, PVL-positive-CA-MSSA Panton-Valentine leukocidin positive community-acquired MSSA, NA not accessed
Multi-drug-resistant tuberculosis (MDR-TB)
Tuberculosis (TB) is an extremely notorious and infectious disease caused by Mycobacterium spp., particularly M. tuberculosis. TB is the second most fatal disease after HIV, accountable for human deaths across the globe according to the World Health Organization (WHO 2014). Around 6.1 million TB patients have been reported in the year 2013, of these, about 5.7 million (93%) cases were new (WHO 2014). About 9.6 million people were reported ill due to TB in 2014, of which approximately 1.5 million died (WHO 2014; Pandit et al. 2015). This disease is highly progressive in Asia and Africa, and more than 80% of all TB cases were reported from these two continents (Zager and McNerney 2008). Evidently, the M. tuberculosis is acquiring resistance against conventional drugs, thus frightening the global health community (Zignol et al. 2006). MDR-M. tuberculosis requires treatment courses that are much longer and less effective than those for non-resistant M. tuberculosis. Extensively drug-resistant TB (XDR-TB, defined as MDR-TB plus resistance to any fluoroquinolone and any second-line injectable drugs) has been identified in 100 countries, in all regions of the world (http://www.who.int/mediacentre/factsheets/fs194/en/, accessed on 18/08/2016). In this review, we have outlined plant-derived compounds and plant derivatives having significant anti-mycobacterial activity against MDR-TB reported during 2005–2015 (Table 3). Table 3 covers a total of 34 plants belonging to 26 different families and all are angiosperms. A total of 36 purified compounds and 20 plant extracts were reported during 2005–2015 against MDR-M. tuberculosis. These data obviously revealed that the plant-derived antimicrobials have a unique competence, as well as alternative and novel solutions to control these deadly MDR- and XDR-TB.
Table 3.
Plant-derived compounds and plant extracts reported during 2005–2015 against MDR-M. tuberculosis
| Compound/extract | Plant | Source | Reported country | References |
|---|---|---|---|---|
| 20% Ethanol | Prunella vulgaris L. | Whole plant | China | Lu et al. (2011) |
| Dihydro-β-agarofuran sesquiter penes | Celastrus vulcanicola | Dried leaves | Spain | Torres-Romero et al. (2011) |
| n-hexane, ethanol, ethyl acetate, n-butanol, and methanol extracts | Flourensia cernua | Whole plant | Mexico | Molina-Salinas et al. (2011) |
| 70% ethanol | Allium sativum | Cloves | Pakistan | Hannan et al. (2011), Dini et al. (2011) |
| 6α-7-Dehydro-N-formylnornantenine; E/Z-N-formylnornantenine; 7,9-dimethoxytariacuripyrone; 9-methoxytariacuripyrone; aristololactam I; β-sitosterol; stigmasterol; 3-hydroxy-α-terpineol | Aristolochia brevipes | Roots | Mexico | Navarro-Garcia et al. (2011) |
| Bisbenzylisoquinoline alkaloids | Tiliacora triandra | Roots | Thailand | Sureram et al. (2012) |
| Alcohol | Humulus lupulus | Whole plant (stems, leaves and roots) | Iran | Serkani et al. (2012) |
| Essential oil | Citrus sp. | NA | USA | Crandall et al. (2012) |
| Obtusifoliol | Struthanthus marginatus | Aerial parts | Brazil | Leitao et al. (2013) |
| 3-O-n-acil-lup-20(29)-en-3β,7β,1 5α-triol | Struthanthu sconcinnus | Leaves | Brazil | |
| (−) Licarin A | Aristolochia taliscana | Roots | Mexico | Leon-Diaz et al. (2013) |
| Ethanol extract | Hypericum sp. | Aerial parts | Portugal | Nogueira et al. (2013) |
| Ursolic and oleanolic acids | Chamaedorea tepejilote | Aerial parts | Mexico | Jimenez-Arellanes et al. (2013) |
| Maritinone and 3,3′-biplumbagin | Diospyros anisandra | Stem and bark | Mexico | Uc-Cachon et al. (2014) |
| 70% Ethanol and water eluted part of ethanol extract | Ranunculi ternati Radix | Whole plant | China | Zhang et al. (2015) |
| Water, methylene chloride, ethanol, n-hexane and ethyl acetate extracts | Andrographis paniculata, Annona muricata, Centella asiatica, Pluchea indica and Rhoeo spathacea | Whole plant and dried leaves | Indonesia | Radji et al. (2015) |
| Diterpenoids including ent-kaurane, kaurane and grayanane Water extract |
Croton tonkinensis | Whole plants or leaves | South Korea | Jang et al. (2016), Gupta et al. (2010) |
| Acalypha indica L. | Leaves | India | ||
| Adhatoda vasica | Leaves | India | ||
| Allium cepa | Bulbs | India | ||
| Allium sativum L. | Cloves | India | ||
| Pure gel of Aloe vera | Aloe vera L. | Pure gel | India | Gupta et al. (2012) |
| Ethyl p-methoxycinnamate | Kaempferia galanga | Rhizome | India | Lakshmanan et al. (2011) |
| Piperine | Piper nigrum L. | Seeds | India | Birdi et al. (2012) |
| 5,10-Pentadecadiyn-1-ol, a-curcumene, hydroxyjunipene, cycloisosativene, valencine and selino 3,7 (11)-diene | Vetiveria zizanioides | Fresh roots | India | Gupta et al. (2012) |
| Alkaloids, flavonoids | Urtica dioica | Leaves | India | Singh et al. (2013) |
| Plumericin and iso-Plumericin | Plumeria bicolor | Bark | India | Kumar et al. (2013) |
| Emodin | Ventilago madraspatana | Stem and bark | India | Basu et al. (2005) |
| Diospyrin | Diospyros montana | Stem and bark | India | Dey et al. (2014) |
| Andrographolide | Andrographis paniculata | Whole plant | India | Prabu et al. (2015) |
| Aqueous, boiling water and methanol extracts | Punica granatum | Fruit | India | Dey et al. (2015) |
Malaria: a current public health concern
Malaria is a complex deadly blood disease with ravaging effects in the world (Onguéné et al. 2013). Approximately, half of the world’s population is at risk of malaria and that 1–2 million annual deaths can be attributed to malaria alone (Vogel 2010; WHO 2012; Onguéné et al. 2013). There were approximately 245 million cases of malaria in 2006 and 3.3 billion people were at risk of the disease. Among them, about 1 million deaths were mostly of children under the age of five (Oliviera et al. 2009). Currently, there are 109 malarious countries and territories, of which, 45 are within the African region (WHO 2008). A total of four protozoan species of the genus Plasmodium (P. falciparum, P. malariae, P. ovale, and P. vivax) are the causative agents for this infection, although majority of fatal cases are caused by P. falciparum (Nogueira and Lopes 2011). Currently, malaria has been treated with quinine, chloroquine, mefloquine and artemisinin among other drugs (Onguéné et al. 2013). However, the protozoans have developed resistance in many countries of the world over time towards the influential factors: poor hygienic conditions, poorly managed vector control programmes and no approved vaccines so far (White 2004).
Researchers currently put their research efforts on new antimalarial agents, mainly focusing on natural origin and the development of phytomedicines (Onguéné et al. 2013). Table 4 reviews the plant-derived compounds including alkaloids, terpenoids and triterpenoids for antimalarial properties. A total of 59 anti-plasmodial compounds including a potent antimalarial drug artemisinin were reported from different plants documented during 2005–2015 (Table 4). Notably, Table 4 displays a total of 35 plants belonging to 21 families under angiosperms showing antimalarial activity. The data summarized in the Table 4 highlight the rich diversity of plant natural products that manifest to be a promising source for the development of antimalarial agents.
Table 4.
Plant-derived compounds and plant extracts reported during 2005–2015 for anti-plasmodial activity
| Compound | Plant | Source | Reported country | References |
|---|---|---|---|---|
| 17-O-acetyl,10-hydroxycorynantheol | Strychnos usambarensis | Leaves | Belgium | Cao et al. (2011) |
| Alstonine | Picralima nitida | Fruits | USA | Okunji et al. (2005) |
| Methyl uguenenoate, furoquinoline and maculosidine | Vepris uguenensis | Roots | South Africa | Cheplogoi et al. (2008), Kiplimo (2012) |
| Evoxine, arborinine and xanthoxoline | Teclea gerrardii | Roots, bark and fruits | South Africa | Waffo et al. (2007), Tchinda et al. (2009) |
| N-isobutyldeca-2,4-dienamide | Hugonia castaneifolia | Root and bark | Tanzania | Baraza et al. (2008) |
| Pipyahyine | Beilschmiedia zenkeri | Bark | France | Lenta et al. (2009) |
| Cryptoquindoline | Cryptolepis sanguinolenta | Stems | Ghana | Barku et al. (2012) |
| Clerodane and labdane diterpenoids | Nuxia sphaerocephala | Leaves | Sudan | Mambu et al. (2006) |
| 16-Oxolabda-8(17),12(E)-dien-15-oic acid, methyl-14,15-epoxylabda-8(17), 12(E)-Diene-16-oate and Turraeanin A | Turreanthus africanus | Seeds | Cameroon | Ngemenya et al. (2006) |
| 3-Deoxyaulacocarpin A, Zambesiacolactones A and B, aulacocarpin A | Aframomum zambesiacum | Seeds | Cameroon | Kenmogne et al. (2006) |
| Galanal B, galanolactone, (E)-8,17-epoxylabd-12-ene-5,16 dial and (E) Labda-8,12-diene-15,16 dial | Aframomum arundinaceum | Seeds | Cameroon | Wabo et al. (2006) |
| 7α-Obacunylacetate, 7α-acetoxydihydronomilin, 22-hydroxyhopan-3-one and 24-methylene cycloartenol | Entandrophragma angolense | Stem and bark | Cameroon | Bickii et al. (2007) |
| 7-Deacetoxy-7-oxogedunin, Ekeberin C1–C3 and acyclic triterpenes | Ekebergia capensis | Stem and bark | Japan | Murata et al. (2008) |
| Bisnorterpenes | Salacia madagascariensis | Roots | USA | Thiem et al. (2005) |
| Cassane furanoditerpenes | Caesalpinia volkensii | Root and bark | Kenya | Ochieng et al. (2012) |
| Abietane diterpenes | Plectranthus spp. | Leaves | South Africa | Van Zyla et al. (2008) |
| Ferruginol | Fuerstia africana | Aerial parts | USA | Koch et al. (2006) |
| 13-Epi-dioxiabiet-8(14)-en-18-ol | Hyptis suaveolens | Leaves | South Africa | Chukwujekwu et al. (2005) |
| Sesquiterpenes | Acanthospermum hispidum | Flowers, leaves and stems | Belgium | Ganfon et al. (2012) |
| Vernangulides A, B, Vernodalol and Vernodalin | Vernonia angulifolia | Aerial parts | Denmark | Pedersen et al. (2009) |
| Urospermal A-15-O-acetate | Dicoma tomentosa | Whole plant | Belgium | Jansen et al. (2012) |
| Artemisinin | Artemisia annua | Seeds | Italy | Reale et al. (2008) |
| Dehydrobrachylaenolide | Dicoma anomala subsp. gerrardii | Roots stocks | South Africa | Becker et al. (2011) |
| Okundoperoxide | Scleria striatinux | Roots | Cameroon | Efange et al. (2009) |
| Coloratane sesquiterpenes | Warburgia ugandensis | Stem and bark | Austria | Wube et al. (2010) |
| Beilshmiedic acid derivatives | Beilschmiedia cryptocaryoides | Bark | Germany | Talontsi et al. (2013) |
| 3-Hydroxy-20(29)-lupen-28-ol | Schefflera umbellifera | Leaves | South Africa | Mthembu (2007) |
| Pristimerin | Maytenus senegalensis | Root and bark | Sudan | Khalid et al. (2007) |
| Pentacyclic triterpenes | Nuxia sphaerocephala | Leaves | Sudan | Mambu et al. (2006) |
| Lupeol and Lupeyl docosanoate | Hymenocardia acida | Bark and stems | Nigeria | Mahmout et al. (2008), Ajaiyeoba et al. (2008) |
| Betulinic acid | Hypericum lanceolatum | Stem and bark | Cameroon | Zofou et al. (2011) |
| 3-Friedelanone | Psorospermum glaberrimum | Stem and bark | Germany | Lenta et al. (2008) |
| 3-O-betulinic acid p-coumarate | Baillonella toxisperma | Stem and bark | Cameroon | Mbah et al. (2011) |
| 2β,3β,19α-Trihydroxy-urs-12-20-en-28-oic acid | Kigelia africana | Stem and bark | Cameroon | Zofou et al. (2012) |
| Cucurbitacins B, D and 20-epibryonolic acid | Cogniauxia podolaena | Stem and bark | Congo | Banzouzi et al. (2008) |
Drug discovery from plants
The concept of ‘the active principle’ in medicine first reported in the fifteenth century; however, pure compounds isolated from the plant extracts were reported in the late eighteenth and earlier nineteenth century (Houghton 2001). It is important to note that plant-derived pure compounds had the similar effect as the plant extract and thus been promptly substituted in many cases as the important ingredient in medicines (Houghton 2001). Codeine and narcotine were the first natural compounds isolated from Papaver somniferum. Since then, numbers of compounds have been isolated from plants and many of these remain in extensive use in medicine as drugs (Houghton 2001; Lahlou 2013). Some of the prominent plant-derived commercially proven drugs, sources, brand names and their medicinal uses are shown in Table 5. Plant-derived molecules including secondary metabolites that demonstrate medicinal properties may act by similar or different mechanisms. The mode of antimicrobial action is similar between plant-derived quinones (bind to adhesions, inactivate enzymes and complex with cell wall) and flavonoids (bind to adhesions and complex with cell wall). However, polyphenols and tannins (enzyme inhibition, substrate deprivation, membrane disruption and metal ion complexation), terpenoids and essential oils (membrane disruption) and alkaloids (intercalate into cell wall) antimicrobial actions are varied in their mode of action (Pandey and Kumar 2013).
Table 5.
Some of the drugs obtained from plants
| Drug | Brand/trade name | Plant source | Medicinal use |
|---|---|---|---|
| Amitriptylin | Amitrip, Elavil, Levate | Hypericum perforatum L. | Mental illnesses, migraines, fibromyalgia |
| Midazolam | Versed, Dormicum, Hypnovel | Hypericum perforatum L. | Anesthesia, procedural sedation, trouble sleeping |
| Nevirapine | Viramune | Hypericum perforatum L. | Antiretroviral therapy to treat HIV/AIDS |
| Paroxetine | Paxil, Pexeva, Seroxat, Brisdelle, Rexetin | Hypericum perforatum L. | Antidepressant drug |
| Cyclosporine | Neoral, Sandimmune | Hypericum perforatum L. | Rheumatoid arthritis, Psoriasis, Crohn’s disease, Nephrotic syndrome |
| Tacrolimus | Prograf, Advagraf, Protopic | Hypericum perforatum L. | Immunosuppressive drug |
| Simvastatin | Zocor | Hypericum perforatum L. | Lipid-lowering medication |
| Alprazolam | Xanax, Niravam | Piper methysticum G. Forst. | Anxiety disorders |
| Atropine | Atropen | Atropa belladonna, Hyoscyamus spp., Datura spp. | Antispasmodic for gastrointestinal tract, Pupil enlargement in eye |
| Pilocarpine | Isopto Carpine, Salagen | Pilocarpus jaborandi | Glaucoma Treatment, Pupil contraction in eye |
| Indinavir | Crixivan | Hypericum perforatum L. | Antiretroviral therapy to treat HIV/AIDS |
| Sertraline | Zoloft | Hypericum perforatum L. | Antidepressant drug |
| Scopolamine | Transdermscop, Kwells | Atropa belladonna, Hyoscyamus spp., Datura spp. | Preoperative Sedative, Antiemetic in travel sickness |
| Caffeine | Vivarin, Cafcit, Alert | Coffea arabica, Thea sinensis | Psychoactive drug, Central nervous system (CNS) stimulant |
| Digoxin | Lanoxin | Digitalis lanata | Improvement in force of contraction to remedy congestive heart failure |
| Ephedrine | Bronkaid, Primatene | Ephedra spp. | Relief of asthma and hay fever |
| Paclitaxel | Taxol | Taxus brevifolia | Chemotherapy medication used to treat number of types of cancer |
| Quinine | Qualaquin, Quinate, Quinbisul | Cinchona spp. | Antimalarial drug |
| Carbamazepin | Tegretol | Plantago sp. | Epilepsy, Neuropathic pain |
| Clopidogrel | Plavix | Ginkgo biloba | Heart disease, stroke |
| Reserpine | Raudixin, Serpalan, Serpasil | Rauwolfia spp. | Antipsychotic, Antihypertensive drug |
| Phenelzin | Nardil, Nardelzine | Panax ginseng | Antidepressant and anxiolytic drug |
| Vinblastine | Velban | Catharanthus roseus | Chemotherapy medication used to treat number of types of cancer |
| Prednisolon | Orapred, Prelone, PediaPred, Millipred | Glycyrrhiza glabra | Allergies, inflammatory conditions, autoimmune disorders |
| Hydrocortisone | Cortef, Solu-Cortef | Glycyrrhiza glabra | Thyroiditis, rheumatoid arthritis, dermatitis, asthma |
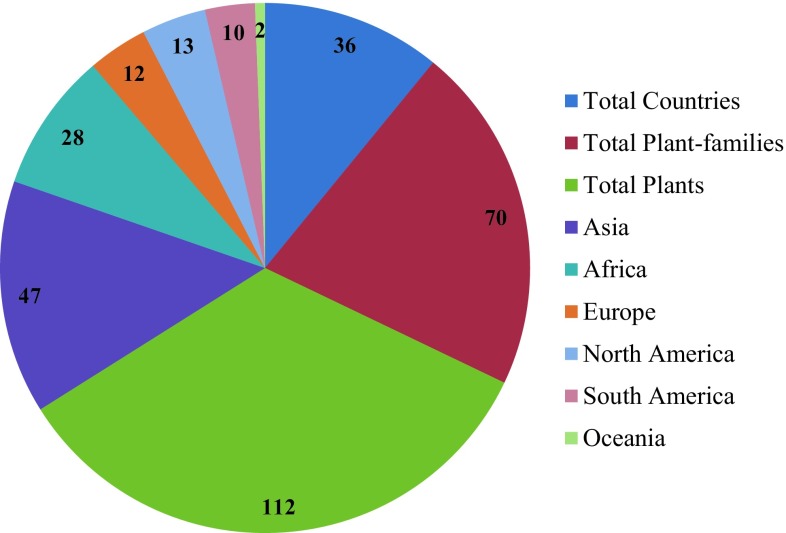
A total of 112 plants were studied during the period 2005–2015 of which, 110 purified bioactive compounds and 60 plant-derived extracts were reported and displayed significant antibacterial, antitubercular, and antimalarial activities (Tables 2, 3, 4). Totally, 40 and 20 plant extracts were reported for antibacterial and antitubercular activities, respectively, during this period (Tables 2, 3, 4). Although these plant extracts showed same therapeutic effects as pure compounds, further investigation of structural elucidation would afford novel chemical structures and new drugs. Plants reviewed in this study are reported from 36 countries around the world. Out of 112 plants, 41.96, 25.00, 10.71, 11.60, 8.90 and 1.78% were reported from Asia, Africa, Europe, North America, South America and Oceania, respectively (Fig. 1). Asia, the highest number (20) of plants, is reported for India for antibacterial and antitubercular activities in the period 2005–2015. Besides, the 112 plants revised in this study belong to 70 different plant families and 99% of them are angiosperms, which is displaying the therapeutic potential. Houghton (2001) reported that 25% of the drugs prescribed by physicians in the developed countries are obtained from flowering plants, angiosperms. Among the 70 families recorded, plants from Apocynaceae, Lamiaceae and Asteraceae showed antibacterial, antitubercular, and antimalarial activities, while plants from Acanthaceae, Lythraceae, and Euphorbiaceae disclosed antibacterial and antitubercular activities. However, plants from Fabaceae and Meliaceae exhibited antibacterial and antimalarial activities, but plants belonging to Celastraceae, Rutaceae, Hypericaceae and Zingiberaceae demonstrated antitubercular and antimalarial activities. The plant-derived extracts mentioned in this review were from various organic solvent extracts including aqueous extracts (Tables 2, 3). Furthermore, the total number of purified compounds given in this review is not an exact number reported in the mentioned period because we also have provided the class of compounds which may contain number of derivatives. Nevertheless, the data collected in this review impose and focus the value of plant antimicrobials against the lethal MDR pathogens including malarial parasites.
Fig. 1.
Plants reported from worldwide during 2005–2015 for antibacterial, antitubercular and antimalarial activities
Future perspectives
Plants are the renowned natural laboratories for producing structurally unique, diverse and complex natural products. Besides the plants, extensive efforts are also being undertaken on microorganisms and other organisms from another living world, such as the oceans (Subramani and Aalbersberg 2013; Malve 2016) for pharmaceutically important biomolecules. However, in plants particularly angiosperms, only less than 10% have been screened for natural products discovery (Houghton 2001). Therefore, there is an immense scope for more fascinating bioactive compounds from flowering plants that will yield new/novel drugs. To access this hidden treasure, more integrative approach with various natural product discovery tools will be the key for success in discovery of phytomedicines. The complex and rich chemical diversity in plants pave to the isolation of natural products which is tough and laborious. Therefore, the significant application of tools such as high-performance liquid chromatography coupled to mass spectrometry (HPLC–MS), liquid chromatography–mass spectrometry (LC–MS), liquid chromatography–nuclear magnetic resonance–mass spectrometry (LC–NMR–MS), capillary NMR (cap-NMR) spectroscopy, LC–solid phase extraction (SPE)–NMR along with bioassay-guided fractionation and high-throughput bioassays will accelerate the access of plant-derived natural products. However, the substantial use of medicinal plants for drug discovery programme endangers their existence, so farming of medicinal plants must be instigated for assuring the future accountability (Lahlou 2013).
Conclusion
The use of herbs and herbal products has widely been accepted in our modern way of life and it is estimated that about 80% of the world’s population still rely on traditional medicines for their primary health care. The significance of plant-derived natural products and their extracts used by the lay community has been realized and documented since ancient time. Interestingly, researchers and clinicians pay great attention to plant-derived secondary metabolites because of their antibiotic activity without conferring any antibiotic resistance. Hence, plant-based antimicrobials have widely been used as preventative and curative solutions against multi-drug-resistant pathogens. Globally emerging MDR and XDR pathogens are a serious concern. On the other hand, most of the chemically synthesised antibiotics can cause adverse side effects and are very expensive. Therefore, these days, there is an increasing inclination towards the use of an alternative source of medicines, particularly the medicinal plants. Several plant species have already been widely reported showing potential medicinal properties. However, the emerging new infections, diseases and rapid evolution of pathogens urge the researchers for further exploration into nature for novel natural products. Plants are certainly playing a dynamic role to control antibiotic-resistant bacterial infections. However, these plant-derived active principles should be taken for further research to translate this knowledge into potential therapeutic drugs.
Compliance with ethical standards
Conflict of interest
No conflict of interest was declared.
References
- Abu-Shanab B, Adwan G, Jarrar N, Abu-Hijleh A, Adwan K. Antibacterial activity of four plant extracts used in Palestine in folkloric medicine against methicillin-resistant Staphylococcus aureus. Turk J Biol. 2006;30:195–198. [Google Scholar]
- Adwan G, Abu-Shanab B, Adwan K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug resistant Pseudomonas aeruginosa strains. Asian Pac J Trop Med. 2010;3:266–269. doi: 10.1016/S1995-7645(10)60064-8. [DOI] [Google Scholar]
- Ajaiyeoba EO, Ashidi JS, Okpako LC, Houghton PJ, Wright CW. Antiplasmodial compounds from Cassia siamea stem bark extract. Phytother Res. 2008;22:254–255. doi: 10.1002/ptr.2254. [DOI] [PubMed] [Google Scholar]
- Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005;5:6–12. doi: 10.1186/1472-6882-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag A, Bhattacharyya SK, Bharati P, Pal NK, Chattopadhyay RR. Evaluation of antibacterial properties of Chebulic myrobalan (fruit of Terminalia chebula Retz.) extracts against methicillin resistant Staphylococcus aureus and trimethoprim-sulphamethoxazole resistant uropathogenic Escherichia coli. Afr J Plant Sci. 2009;3:25–29. [Google Scholar]
- Banzouzi JT, Soh PN, Mbatchi B, Cavé A, Ramos S, Retailleau P, Rakotonandrasana O, Berry A, Benoit-Vical F. Cogniauxia podolaena: bioassay-guided fractionation of defoliated stems, isolation of active compounds, antiplasmodial activity and cytotoxicity. Planta Med. 2008;74:1453–1456. doi: 10.1055/s-2008-1081341. [DOI] [PubMed] [Google Scholar]
- Baraza LD, Joseph CC, Munisi JJE, Nkunya MHH, Arnold N, Porzel A, Wessjohann L. Antifungal rosane diterpenes and other constituents of Hugonia castaneifolia. Phytochemistry. 2008;69:200–205. doi: 10.1016/j.phytochem.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Barku VYA, Opoku-Boahen Y, Dzotsi EY. Isolation and pharmacological activities of alkaloids from Cryptolepis sanguinolenta (Lindl) schlt. Int Res J Biochem Bioinform. 2012;2:58–61. [Google Scholar]
- Basu S, Ghosh A, Hazra B. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: isolation of emodin and physcion as active antibacterial agents. Phytother Res. 2005;19:888–894. doi: 10.1002/ptr.1752. [DOI] [PubMed] [Google Scholar]
- Becker JVW, Van der Merwe M, Van Brummelen AC, Pillay P, Crampton BG, Mmutlane EM, Parkinson C, Van Heerden FR, Crouch NR, Smith PJ, Mancama DT, Maharaj VJ. In vitro anti-plasmodial activity of Dicoma anomala subsp. gerrardii (Asteraceae): identification of its main active constituent, structure-activity relationship studies and gene expression profiling. Malar J. 2011;10:295. doi: 10.1186/1475-2875-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickii J, Tchouya GR, Tchouankeu JC, Tsamo E. The antiplasmodial agents of the stem bark of Entandrophragma angolense (Meliaceae) Afr J Tradit Complement Altern Med. 2007;4:135–139. [PMC free article] [PubMed] [Google Scholar]
- Birdi T, D’Souza D, Tolani M, Daswani P, Nair V, Tetali P, Carlos Toro J, Hoffner S. Assessment of the activity of selected Indian medicinal plants against Mycobacterium tuberculosis: a preliminary screening using the microplate alamar blue assay. Eur J Med Plants. 2012;2:308–323. doi: 10.9734/EJMP/2012/1638. [DOI] [Google Scholar]
- Borris RP. Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol. 1996;51:29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Bauer BA, Schnepple DJ, Wahner-Roedler DL, Vandell AG, Howe CL. A randomized phase I study of Atuna racemosa: a potential new anti-MRSA natural product extract. J Ethnopharmacol. 2007;114:371–376. doi: 10.1016/j.jep.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Cao MR, Tits M, Angenot LM, Frédérich M. 17-O-acetyl,10-hydroxycorynantheol, a selective antiplasmodial alkaloid isolated from Strychnos usambarensis leaves. Planta Med. 2011;77:2050–2053. doi: 10.1055/s-0031-1280124. [DOI] [PubMed] [Google Scholar]
- Centre for Disease Control (2013) Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed on 10 May 2017
- Cha JD, Moon SE, Kim JY, Jung EK, Lee YS. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens against methicillin-resistant Staphylococcus aureus. Phytother Res. 2009;23:1326–1331. doi: 10.1002/ptr.2540. [DOI] [PubMed] [Google Scholar]
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BC, Ip M, Lau CB, Lui SL, Jolivalt C, Ganem-Elbaz C, Litaudon M, Reiner NE, Gong H, See RH, Fung KP, Leung PC. Synergistic effects of baicalein with ciprofloxacin against Nor A over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J Ethnopharmacol. 2011;137:767–773. doi: 10.1016/j.jep.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Chanda S, Vyas BRM, Vaghasiya Y, Patel H. Global resistance trends and the potential impact of methicillin resistant Staphylococcus aureus (MRSA) and its solutions, 2nd Series. In: Mendez-Vilas A, editor. Current research, technology, and education topics in applied microbiology and microbial biotechnology. Spain: Formatex; 2010. pp. 529–536. [Google Scholar]
- Cheplogoi PK, Mulholland DA, Coombes PH, Randrianarivelojosia M. An azole, an amide and a limonoid from Vepris uguenensis (Rutaceae) Phytochemistry. 2008;69:1384–1388. doi: 10.1016/j.phytochem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Chukwujekwu JC, Smith P, Coombes PH, Mulholland DA, Van Staden J. Antiplasmodial diterpenoid from the leaves of Hyptis suaveolens. J Ethnopharmacol. 2005;102:295–297. doi: 10.1016/j.jep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall PG, Ricke SC, O’Bryan CA, Parrish NM. In vitro effects of citrus oils against Mycobacterium tuberculosis and non-tuberculous Mycobacteria of clinical importance. J Environ Sci Health B. 2012;47:736–741. doi: 10.1080/03601234.2012.669331. [DOI] [PubMed] [Google Scholar]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562–573. doi: 10.1086/427701. [DOI] [PubMed] [Google Scholar]
- Dey D, Ray R, Hazra B. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother Res. 2014;28:1014–1021. doi: 10.1002/ptr.5090. [DOI] [PubMed] [Google Scholar]
- Dey D, Ray R, Hazra B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm Biol. 2015;53:1474–1480. doi: 10.3109/13880209.2014.986687. [DOI] [PubMed] [Google Scholar]
- Dini C, Fabbri A, Geraci A. The potential role of garlic (Allium sativum) against the multi-drug resistant tuberculosis pandemic: a review. Annali dell’Istituto Superiore di Sanita. 2011;47:465–473. doi: 10.4415/ANN_11_04_18. [DOI] [PubMed] [Google Scholar]
- Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Dubey D, Padhy RN. Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J Herb Med. 2013;3:65–75. doi: 10.1016/j.hermed.2012.12.002. [DOI] [Google Scholar]
- Dubey D, Sahu MC, Rath S, Paty BP, Debata NK, Padhy RN. Antimicrobial activity of medicinal plants used by aborigines of Kalahandi, Orissa, India against multidrug resistant bacteria. Asian Pac J Trop Biomed. 2012;2:S846–S854. doi: 10.1016/S2221-1691(12)60322-0. [DOI] [Google Scholar]
- Efange SMN, Brun R, Wittlin S, Connolly JD, Hoye TR, McAkam T, Makolo FL, Mbah JA, Nelson DP, Nyongbela KD, Wirmum CK. Okundoperoxide, a bicyclic cyclofarnesylsesquiterpene endoperoxide from Scleria striatinux with antiplasmodial activity. J Nat Prod. 2009;72:280–283. doi: 10.1021/np800338p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12:122–132. doi: 10.2174/138945011793591626. [DOI] [PubMed] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield RM, Farley M. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- Gade ND, Qazi MS. Fluoroquinolone therapy in Staphylococcus aureus infections: where do we stand? J Lab Physicians. 2013;5:109–112. doi: 10.4103/0974-2727.119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet Infect Dis. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- Ganfon H, Bero J, Tchinda AT, Gbaguidi F, Gbenou J, Moudachirou M, Frédérich M, Quetin-Leclercq J. Antiparasitic activities of two sesquiterpenic lactones isolated from Acanthospermum hispidum DC. J Ethnopharmacol. 2012;141:411–417. doi: 10.1016/j.jep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Giang PM, Son PT, Matsunami K, Otsuka H. Anti-staphylococcal activity of ent-kaurane-type diterpenoids from Croton tonkinensis. J Nat Med. 2006;60:93–95. doi: 10.1007/s11418-005-0011-5. [DOI] [Google Scholar]
- Golkar Z, Bagazra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- Gomber C, Saxena S. Anti-staphylococcal potential of Callistemon rigidus. Central Eur J Med. 2007;2:79–88. [Google Scholar]
- Gould SWJ, Fielder MD, Kelly AF, Naughton DP. Anti-microbial activities of pomegranate rind extracts: enhancement by cupric sulphate against clinical isolates of S. aureus, MRSA and PVL positive CA-MSSA. BMC Complement Altern Med. 2009;9:23–28. doi: 10.1186/1472-6882-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Thakur B, Singh P, Singh HB, Sharma VD, Katoch VM, Chauhan SV. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2010;131:809–813. [PubMed] [Google Scholar]
- Gupta S, Dwivedi GR, Darokar MP, Srivastava SK. Antimycobacterial activity of fractions and isolated compounds from Vetiveria zizanioides. Med Chem Res. 2012;21:1283–1289. doi: 10.1007/s00044-011-9639-8. [DOI] [Google Scholar]
- Hannan A, Ikram Ullah M, Usman M, Hussain S, Absar M, Javed K. Anti-mycobacterial activity of Garlic (Allium sativum) against multi-drug resistant and non-multi-drug resistant Mycobacterium tuberculosis. Pak J Pharm Sci. 2011;24:81–85. [PubMed] [Google Scholar]
- Hayet E, Maha M, Samia A, Mata M, Gros P, Raida H, Ali MM, Mohamed AS, Gutmann L, Mighri Z, Mahjoub A. Antimicrobial, antioxidant, and antiviral activities of Retama raetam (Forssk.) Webb flowers growing in Tunisia. World J Microbiol Biotechnol. 2008;24:2933–2940. doi: 10.1007/s11274-008-9835-y. [DOI] [Google Scholar]
- Houghton PJ. Old yet new—pharmaceuticals from plants. J Chem Educ. 2001;78:175–184. doi: 10.1021/ed078p175. [DOI] [Google Scholar]
- Jang WS, Jyoti MA, Kim S, Nam KW, Ha TK, Oh WK, Song HY. In vitro antituberculosis activity of diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J Nat Med. 2016;70:127–132. doi: 10.1007/s11418-015-0937-1. [DOI] [PubMed] [Google Scholar]
- Jansen O, Tits M, Angenot L, Nicolas JP, De Mol P, Nikiema JB, Frédérich M. Anti-plasmodial activity of Dicoma tomentosa (Asteraceae) and identification of urospermal A-15-O-acetate as the main active compound. Malar J. 2012;11:289. doi: 10.1186/1475-2875-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Arellanes A, Luna-Herrera J, Cornejo-Garrido J, Lopez-Garcia S, Castro-Mussot ME, Meckes-Fischer M, Mata-Espinosa D, Marquina B, Torres J, Hernández-Pando R. Ursolic and Oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement Altern Med. 2013;13:258. doi: 10.1186/1472-6882-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone J, Policarpio ME, Lam F, Adomako K, Prematunge C, Nadolny E, Li Y, Brown K, Kerr E, Garber G. Rates of blood cultures positive for vancomycin-resistant Enterococcus in Ontario: a quasi-experimental study. CMAJ Open. 2017;5:E273–E280. doi: 10.9778/cmajo.20160121. [DOI] [Google Scholar]
- Kali A. Antibiotics and bioactive natural products in treatment of methicillin resistant Staphylococcus aureus: a brief review. Pharmacogn Rev. 2015;9:29–34. doi: 10.4103/0973-7847.156329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant Gram-negative organisms: extended-spectrum β-lactamase-producing enterobacteriaceae, carbapenem-resistant enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250–259. doi: 10.4065/mcp.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenmogne M, Prost E, Harakat D, Jacquier MJ, Frederich M, Sondengam LB, Zeches M, Waffo-Teguo P. Five labdane diterpenoids from the seeds of Aframomum zambesiacum. Phytochemistry. 2006;67:433–438. doi: 10.1016/j.phytochem.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Khalid SA, Friedrichsen GM, Christensen SB, Tahir AE, Sattic GM. Isolation and characterization of pristimerin as the antiplasmodial and antileishmanial agent of Maytenus senegalensis (Lam.) Exell Arkivoc. 2007;9:129–134. [Google Scholar]
- Khan UA, Rahman H, Qasim M, Hussain A, Azizllah A, Murad W, Khan Z, Anees M, Adnan M. Alkanna tinctoria leaves extracts: a prospective remedy against multidrug resistant human pathogenic bacteria. BMC Complement Altern Med. 2015;15:127. doi: 10.1186/s12906-015-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Baeshenb MN, Sainia KS, Boraa RS, Al-Hejin AM, Baeshen NA. Antibacterial activities of Rhazya stricta leaf extracts against multidrug-resistant human pathogens. Biotechnol Biotechnol Equip. 2016;30:1016–1025. doi: 10.1080/13102818.2016.1209087. [DOI] [Google Scholar]
- Kim ES, Jeong SI, Kim JH, Park C, Kim SM, Kim JK, Lee KM, Lee SH, So H, Park R. Synergistic effects of the combination of 20-hydroxyecdysone with ampicillin and gentamicin against methicillin-resistant Staphylococcus aureus. J Microbiol Biotechnol. 2009;19:1576–1581. doi: 10.4014/jmb.0903.03015. [DOI] [PubMed] [Google Scholar]
- Kiplimo JJ (2012) The phytochemical and biological activity of secondary metabolites from Kenyan and Vernonia and Vepris species. PhD thesis. University of Kwazulu-Natal, Department of Chemistry
- Koch TCEFA, Orjala J, Mutiso PC, Soejarto DD. An antimalarial abietane diterpene from Fuerstia africana. Biochem Syst Ecol. 2006;34:270–272. doi: 10.1016/j.bse.2005.08.002. [DOI] [Google Scholar]
- Kumar P, Singh A, Sharma U, Singh D, Dobhal MP, Singh S. Anti-mycobacterial activity of plumericin and isoplumericin against MDR Mycobacterium tuberculosis. Pulm Pharmacol Ther. 2013;26:332–335. doi: 10.1016/j.pupt.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Kyaw BM, Arora S, Lim CS. Bactericidal antibiotic-phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz J Microbiol. 2012;43:938–945. doi: 10.1590/S1517-83822012000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou M. The success of natural products in drug discovery. Pharmacol Pharm. 2013;4:17–31. doi: 10.4236/pp.2013.43A003. [DOI] [Google Scholar]
- Lakshmanan D, Werngren J, Jose L, Suja K, Nair MS, Varma RL, Mundayoor S, Hoffner S, Kumar RA. Ethyl p-methoxycinnamate isolated from a traditional anti-tuberculosis medicinal herb inhibits drug resistant strains of Mycobacterium tuberculosis in vitro. Fitoterapia. 2011;82:757–761. doi: 10.1016/j.fitote.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R, Malani A. Extending the cure: policy responses to the growing threat of antibiotic resistance. Washington, DC: Resources for the Future; 2007. p. c2007. [Google Scholar]
- Leandro LF, Cardoso MJ, Silva SD, Souza MG, Veneziani RC, Ambrosio SR, Martins CH. Antibacterial activity of Pinus elliottii and its major compound, dehydroabietic acid, against multidrug-resistant strains. J Med Microbiol. 2014;63:1649–1653. doi: 10.1099/jmm.0.081711-0. [DOI] [PubMed] [Google Scholar]
- Lee DG, Jung HJ, Woo ER. Antimicrobial property of (+)-lyoniresinol-3alpha-O-beta-d-glucopyranoside isolated from the root bark of Lycium chinense Miller against human pathogenic microorganisms. Arch Pharm Res. 2005;28:1031–1036. doi: 10.1007/BF02977397. [DOI] [PubMed] [Google Scholar]
- Lee GS, Kim ES, Cho SI, Kim JH, Cho G, Ju YS, Park SH, Jeong SI, Kim HJ. Antibacterial and synergistic activity of prenylated chalcone isolated from the roots of Sophora flavescens. J Korean Soc Appl Biol Chem. 2010;53:290–296. doi: 10.3839/jksabc.2010.045. [DOI] [Google Scholar]
- Leitao F, Leitão SG, de Almeida MZ, Cantos J, Coelho T, da Silva PE. Medicinal plants from open-air markets in the state of Rio de Janeiro, Brazil as a potential source of new antimycobacterial agents. J Ethnopharmacol. 2013;149:513–521. doi: 10.1016/j.jep.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Lenta BN, Devkota PK, Ngouela S, Boyom FF, Naz Q, Choudhary MI, Tsamo E, Rosenthal PJ, Sewald N. Anti-plasmodial and cholinesterase inhibiting activities of some constituents of Psorospermum glaberrimum. Chem Pharm Bull. 2008;56:222–226. doi: 10.1248/cpb.56.222. [DOI] [PubMed] [Google Scholar]
- Lenta BN, Tantangmo F, Devkota KP, Wansi JD, Chouna JR, Soh RCR, Neumann B, Stammler HG, Tsamo E, Sewald N. Bioactive constituents of the stem bark of Beilschmiedia zenkeri. J Nat Prod. 2009;72:2130–2134. doi: 10.1021/np900341f. [DOI] [PubMed] [Google Scholar]
- Leon-Diaz R, Meckes-Fischer M, Valdovinos-Martinez L, Campos MG, Hernandez-Pando R, Jiménez-Arellanes MA. Antitubercular activity and the subacute toxicity of (−)-Licarin A in BALB/c Mice: a neolignan isolated from Aristolochia taliscana. Arch Med Res. 2013;44:99–104. doi: 10.1016/j.arcmed.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Linden PK. Treatment options for vancomycin-resistant enterococcal infections. Drugs. 2002;62:425–441. doi: 10.2165/00003495-200262030-00002. [DOI] [PubMed] [Google Scholar]
- Lu J, Qin R, Ye S, Yang M. Prunella vulgaris L. extract improves cellular immunity in MDR-TB challenged rats. J Med Coll PLA. 2011;26:230–237. doi: 10.1016/S1000-1948(11)60040-3. [DOI] [Google Scholar]
- Mahmout Y, Mianpeurem T, Dolmazon R, Bouchu D, Fenet B. 15ème colloque sur la Pharmacopée et la Médecine Traditionnelles Africaines. Libreville: Conseil Africain et Malgache pour l’Enseignement Supérieur (CAMES) 1; 2008. [Google Scholar]
- Malve H. Exploring the ocean for new drug developments: marine pharmacology. J Pharm Bioallied Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambu L, Grellier P, Florent L, Joyeau R, Ramanitrahasimbola D, Rasoanaivo P, Frappier F. Clerodane and labdane diterpenoids from Nuxia sphaerocephala. Phytochemistry. 2006;67:444–451. doi: 10.1016/j.phytochem.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Mariod AA, Matthaus B, Idris YMA, Abdelwahab SI. Fatty Acids, tocopherols, phenolics and the antimicrobial effect of Sclerocarya birrea kernels with different harvesting dates. J Am Oil Chem Soc. 2010;87:377–384. doi: 10.1007/s11746-009-1510-4. [DOI] [Google Scholar]
- Mbah JA, Ndikum G, Zofou D, Ngemenya MN, Efange SMN. Antiplasmodial triterpenes from the stem bark of Baillonella toxisperma. ISESCO J Sci Technol. 2011;7:84–87. [Google Scholar]
- McRae JM, Yang Q, Crawford RJ, Palombo EA. Antibacterial compounds from Planchonia careya leaf extracts. J Ethnopharmacol. 2008;116:554–560. doi: 10.1016/j.jep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Miyasaki Y, Rabenstein JD, Rhea J, Crouch ML, Mocek UM, Kittell PE, Morgan MA, Nichols WS, Van Benschoten MM, Hardy WD, Liu GY. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS One. 2013;8:e61594. doi: 10.1371/journal.pone.0061594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Salinas GM, Pena-Rodriguez LM, Mata-Cardenas BD, Escalante-Erosa F, Gonzalez-Hernandez S, Torres de la Cruz VM, Martínez-Rodríguez HG, Said-Fernández S. Flourensia cernua: hexane extracts a very active mycobactericidal fraction from an inactive leaf decoction against pansensitive and panresistant Mycobacterium tuberculosis. Evid Based Complement Alternat Med. 2011;2011:782503. doi: 10.1155/2011/782503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mthembu XS. A phytochemical study of Schefflera umbellifera and Elephantorrhiza elephantina, M.Sc. thesis. Pietermaritzburg: School of Chemistry, University of Kwazulu-Natal; 2007. [Google Scholar]
- Murata T, Miyase T, Muregi FW, Naashima-Ishibashi Y, Umehara K, Warashina T, Kanou S, Mkoji GM, Terada M, Ishih A. Antiplasmodial triterpenoids from Ekebergia capensis. J Nat Prod. 2008;71:167–174. doi: 10.1021/np0780093. [DOI] [PubMed] [Google Scholar]
- National Audit Office (2000) The management and control of hospital acquired infections in acute NHS trusts in England. Report by the comptroller and auditor general. HC 230 session 1999-2000. National Audit Office, London [DOI] [PubMed]
- Navarro-Garcia VM, Luna-Herrera J, Rojas-Bribiesca MG, Alvarez-Fitz P, Rios MY. Antibacterial Activity of Aristolochia brevipes against multidrug-resistant Mycobacterium tuberculosis. Molecules. 2011;16:7357–7364. doi: 10.3390/molecules16097357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngemenya MN, Akam TM, Yong JN, Tane P, Fanso- Free SMN, Berzins K, Titanji VPK. Antiplasmodial activities of some products from Turreanthus africanus (Meliaceae) Afr J Health Sci. 2006;13:1–2. [PubMed] [Google Scholar]
- Nogueira CR, Lopes LMX. Antiplasmodial natural products. Molecules. 2011;16:2146–2190. doi: 10.3390/molecules16032146. [DOI] [Google Scholar]
- Nogueira T, Medeiros MA, Marcelo-Curto MJ, García-Pérez B, Luna-Herrera J, Céu Costa M. Profile of antimicrobial potential of fifteen Hypericum species from Portugal. Ind Crops Prod. 2013;47:126–131. doi: 10.1016/j.indcrop.2013.03.005. [DOI] [Google Scholar]
- Ochieng CO, Owuor PO, Manguro LAO, Akala H, Ishola IO. Antinociceptive and antiplasmodial activities of cassane furanoditerpenes from Caesalpinia volkensii H. root bark. Fitoterapia. 2012;83:74–80. doi: 10.1016/j.fitote.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics . Deaths involving MRSA: England and Wales, 1993–2003. Health Statistics Quarterly, Spring 2005, No. 25. London: ONS; 2005. [PubMed] [Google Scholar]
- Okunji CO, Iwu MM, Ito Y, Smith PL. Preparative separation of indole alkaloids from the rind of Picralima nitida (Stapf) T Durand & H Durand by pH zone refining countercurrent chromatography. J Liquid Chromatogr Relat Technol. 2005;28:775–783. doi: 10.1081/JLC-200048915. [DOI] [Google Scholar]
- Oliviera AB, Dolabela MF, Braga FC, Jacome RLRP, Varotti FP, Povoa MM. Plant derived antimalarial agents: new leads and efficient phytomedicines. Part I. Alkaloids. Anias da Academia Brasiliera de Ciencias. 2009;81:715–740. doi: 10.1590/S0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- Onguéné PA, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants. Part I: a pharmacological evaluation of alkaloids and terpenoids. Malar J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kumar S. Perspective on plant products as antimicrobial agents: a review. Pharmacologia. 2013;2013:469–480. doi: 10.5567/pharmacologia.2013.469.480. [DOI] [Google Scholar]
- Pandit R, Kumar PS, Kumar V. Natural remedies against multi-drug resistant Mycobacterium tuberculosis. J Tuberc Res. 2015;3:171–183. doi: 10.4236/jtr.2015.34024. [DOI] [Google Scholar]
- Pedersen MM, Chukwujekwu JC, Lategan CA, Van Staden J, Smith PJ, Staerk D. Antimalarial sesquiterpene lactones from Distephanus angulifolius. Phytochemistry. 2009;70:601–607. doi: 10.1016/j.phytochem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Prabu A, Hassan S, Prabuseenivasan Shainaba AS, Hanna LE, Kumar V. Andrographolide: a potent antituberculosis compound that targets aminoglycoside 2′-N-acetyltransferase in Mycobacterium tuberculosis. J Mol Graph Model. 2015;61:133–140. doi: 10.1016/j.jmgm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Radji M, Kurniati M, Kiranasari A. Comparative antimycobacterial activity of some Indonesian medicinal plants against multi-drug resistant Mycobacterium tuberculosis. J Appl Pharm Sci. 2015;5:19–22. [Google Scholar]
- Rahman AKMS, Chowdhury AKA, Ali HA, Raihan SZ, Ali MS, Nahar L, Sarker SD. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J Nat Med. 2009;63:41–45. doi: 10.1007/s11418-008-0287-3. [DOI] [PubMed] [Google Scholar]
- Reale S, Pace L, Monti P, De Angelis F, Marcozzi G. A rapid method for the quantification of artemisinin in Artemisia annua L. plants cultivated for the first time in Burundi. Nat Prod Res. 2008;22:360–364. doi: 10.1080/14786410701855951. [DOI] [PubMed] [Google Scholar]
- Roy S, Rao K, Bhuvaneswari CH, Giri A, Mangamoori LN. Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity. World J Microbiol Biotechnol. 2010;26:85–91. doi: 10.1007/s11274-009-0146-8. [DOI] [Google Scholar]
- Safavi M, Sabourian R, Foroumadi A. Treatment of Helicobacter pylori infection: current and future insights. World J Clin Cases. 2016;4:5–19. doi: 10.12998/wjcc.v4.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu MC, Padhy RN. In vitro antimicrobial potency of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pac J Trop Dis. 2013;3:217–226. doi: 10.1016/S2222-1808(13)60044-4. [DOI] [Google Scholar]
- Serkani JE, Isfahani BN, Safaei HG, Kermanshahi RK, Asghari G. Evaluation of the effect of Humulus lupulus alcoholic extract on rifampin-sensitive and resistant isolates of Mycobacterium tuberculosis. Res Pharm Sci. 2012;7:235–242. [PMC free article] [PubMed] [Google Scholar]
- Sheen B. Diseases and disorders: MRSA. Gale/Farmington: Lucent Books/Cengage Learning; 2010. p. 18. [Google Scholar]
- Simeon M, Maria C, Afroditi T, Alexandros B, Georgia L, Eleni K, Ahilleas G, Stela AD, Pavlos N. Vancomycin-resistant Enterococci, colonizing the intestinal tract of patients in a university hospital in Greece. Braz J Infect Dis. 2006;10:179–184. doi: 10.1590/S1413-86702006000300005. [DOI] [PubMed] [Google Scholar]
- Singh R, Hussain S, Verma R, Sharma P. Anti-mycobacterial screening of five Indian medicinal plants and partial purification of active extracts of Cassia sophera and Urtica dioica. Asian Pac J Trop Med. 2013;6:366–371. doi: 10.1016/S1995-7645(13)60040-1. [DOI] [PubMed] [Google Scholar]
- Stockwell C. Nature’s pharmacy. London: Century Hutchinson Ltd.; 1988. [Google Scholar]
- Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microb Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani R, Aalbersberg W. Culturable rare actinomycetes: diversity, isolation and marine natural product discovery. Appl Microbiol Biotechnol. 2013;97:9291–9321. doi: 10.1007/s00253-013-5229-7. [DOI] [PubMed] [Google Scholar]
- Sudjana AN, D’Orazio C, Ryan V, Rasool N, Ng J, Islam N, Riley TV, Hammer KA. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J Antimicrob Agents. 2009;33:461–463. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Sureram S, Senadeera SP, Hongmanee P, Mahidol C, Ruchirawat S, Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorg Med Chem Lett. 2012;22:2902–2905. doi: 10.1016/j.bmcl.2012.02.053. [DOI] [PubMed] [Google Scholar]
- Talontsi FM, Lamshöft M, Bauer JO, Razakarivony AA, Andriamihaja B, Strohmann C, Spiteller M. Antibacterial and antiplasmodial constituents of Beilschmiedia cryptocaryoides. J Nat Prod. 2013;76:97–102. doi: 10.1021/np300773x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Wang T, Onodera Y, Uchida Y, Sato K. Mechanism of quinolone resistance in Staphylococcus aureus. J Infect Chemother. 2000;6:131–139. doi: 10.1007/s101560070010. [DOI] [PubMed] [Google Scholar]
- Tchinda AT, Fuendjiep V, Sajjad A, Matchawe C, Wafo P, Khan S, Tane P, Choudhary MI. Bioactive compounds from the fruits of Zanthoxylum leprieurii. Pharmacol Online. 2009;1:406–415. [Google Scholar]
- Thiem DA, Sneden AT, Khan SI, Tekwani LB. Bisnortriterpenes from Salacia madagascariensis. J Nat Prod. 2005;68:251–254. doi: 10.1021/np0497088. [DOI] [PubMed] [Google Scholar]
- Thomson WAR. Medicines from the Earth. Maidenhead: McGraw-Hill Book Co.; 1978. [Google Scholar]
- Tiwari HK, Das AK, Sapkota D, Sivarajan K, Pahwa VK. Methicillin resistant Staphylococcus aureus: prevalence and antibiogram in a tertiary care hospital in western Nepal. J Infect Dev Ctries. 2009;3:681–684. doi: 10.3855/jidc.86. [DOI] [PubMed] [Google Scholar]
- Torres-Romero D, Jimenez IA, Rojas R, Gilman RH, Lopez M, Bazzocchi IL. Dihydro-Beta-Agarofuran sesquiterpenes isolated from Celastrus vulcanicola as potential anti-Mycobacterium tuberculosis multidrug-resistant agents. Bioorg Med Chem Lett. 2011;19:2182–2189. doi: 10.1016/j.bmc.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Uc-Cachon AH, Borges-Argaez R, Said-Fernandez S, Vargas-Villarreal J, Gonzalez-Salazar F, Méndez-González M, Cáceres-Farfán M, Molina-Salinas GM. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm Pharmacol Ther. 2014;27:114–120. doi: 10.1016/j.pupt.2013.08.001. [DOI] [PubMed] [Google Scholar]
- van Vuuren S, Viljoen A. Plant-based antimicrobial studies—methods and approaches to study the interaction between natural products. Planta Med. 2011;77:1168–1182. doi: 10.1055/s-0030-1250736. [DOI] [PubMed] [Google Scholar]
- Van Zyla RL, Khanb F, Edwardsc TJ, Drewesc SE. Antiplasmodial activities of some abietane diterpenes from the leaves of five Plectranthus species. S Afr J Sci. 2008;104:62–65. [Google Scholar]
- Ventola CL. The antibiotic resistance crisis. Part 1: causes and threats. P T. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74:1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. Infectious disease—new map illustrates risk from the other malaria. Science. 2010;329:618. doi: 10.1126/science.329.5992.618. [DOI] [PubMed] [Google Scholar]
- Wabo HK, Tane P, Connolly JD. Diterpenoids and sesquiterpenoids from Aframomum arundinaceum. Biochem Syst Ecol. 2006;34:603–605. doi: 10.1016/j.bse.2006.02.001. [DOI] [Google Scholar]
- Waffo AFK, Coombes PH, Crouch NR, Mulholland DA, El Amin SMM, Smith PJ. Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa. Phytochemistry. 2007;68:663–667. doi: 10.1016/j.phytochem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- White NJ. Antimalarial drug resistance. J Clin Investig. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek K, Osek J. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res Int. 2013;2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Malaria Report. Geneva: WHO; 2008. [Google Scholar]
- World Health Organization . World Malaria Report. Geneva: WHO; 2012. [Google Scholar]
- World Health Organization (2014) Organization WH Global Tuberculosis Report, Geneva
- Wube AA, Bucar F, Gibbons S, Asres K, Rattray L, Croft SL. Antiprotozoal activity of drimane and coloratane sesquiterpenes towards Trypanosoma brucei rhodesiense and Plasmodium falciparum in vitro. Phytother Res. 2010;24:1468–1472. doi: 10.1002/ptr.3126. [DOI] [PubMed] [Google Scholar]
- Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini IC, Cuello S, Alberto MR, Ordonez RM, Almeida RD, Solorzano E, Isla MI. Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J Ethnopharmacol. 2009;124:499–505. doi: 10.1016/j.jep.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li R, Li M, Qi Z, Tian J. In vitro and In vivo study of anti-tuberculosis effect of extracts isolated from Ranunculi ternati Radix. Sarcoidosis vasculitis and diffuse lung diseases. Off J WASOG. 2015;31:336–342. [PubMed] [Google Scholar]
- Zignol M, Hosseini MS, Wright A, Lambregts-van Weezenbeek C, Nunn P, Watt CJ, Williams BG, Dye C. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006;194:479–485. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- Zofou D, Kowa TK, Wabo HK, Ngemenya MN, Tane P, Titanji VPK. Hypericum lanceolatum (Hypericaceae) as a potential source of new anti-malarial agents: a bioassay-guided fractionation of the stem bark. Malar J. 2011;10:167. doi: 10.1186/1475-2875-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofou D, Tene M, Tane P, Titanji VPK. Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol Res. 2012;110:539–544. doi: 10.1007/s00436-011-2519-9. [DOI] [PubMed] [Google Scholar]