Abstract
Enhanced locomotory activity (ELA), such as wandering, is a normal behavior that occurs at the end of the larval stage in lepidopteran (butterflies and moths) insects. Baculovirus infection can also induce ELA in lepidopteran larvae. The belief is that the virus induces this behavior to increase its transmission [Goulson, D. (1997) Oecologia 109, 219–228]. Here we show that a baculovirus-encoded protein tyrosine phosphatase (PTP) gene (ptp) induces ELA that is activated by light. ELA was induced in silkworm Bombyx mori infected with the baculovirus B. mori nucleopolyhedrovirus (BmNPV) beginning at ≈3.75 days postinfection (p.i.) and continued until 4.75 days p.i. The intensity of the ELA was dramatically reduced immediately before death at 5.25 days p.i. Light activated the intensity of the ELA by ≈3-fold, and larvae with ELA showed positive phototropism. ELA was not induced in larvae of B. mori infected with a BmNPV ptp knockout mutant (BmPTPD). However, when a silkworm-derived ptp gene (Bmptp-h) was inserted into BmPTPD, ELA was partially recovered. Bmptp-h was identified from silkworms at 2 days after the start of the natural wandering stage. The deduced amino acid sequence of Bmptp-h showed 48.2% identify (80.7% similarity) to the deduced amino acid sequence of BmNPV ptp. On the basis of the high homology and larval stage at which Bmptp-h was isolated, we postulate that the modern baculovirus may have acquired its ptp gene from an ancestral host and that this gene was selectively maintained because it increases virus transmission.
Keywords: Bombyx mori, enhanced locomotory response, protein tyrosine phosphatase gene, nucleopolyhedrovirus
Enhanced locomotory behaviors, such as wandering, normally occur only during the end of the larval stage of holometabolous insects. Wandering behavior is characterized by enhanced locomotory activity (ELA) that may involve phototaxis, geotaxis, or kinesis (1). This behavior helps the larval insect find a suitable location to metamorphose into an adult and may help to minimize predation and conserve of energy or other resources. Wandering is closely associated with changes in ecdysteroid and juvenile hormone levels, which may trigger this behavior (1). ELA can also be induced in lepidopteran larvae by virus infection. One of the earliest references of this virus-induced behavior is from the late 19th century in Germany, where this behavior was known as Wipfelkrankheit (tree top disease) (2). Baculovirus-induced ELA occurs during a late stage of infection and is characterized by the infected larvae moving to the upper plant foliage, where they are often found dead. The belief is that the virus induces this behavior so that, after death, the decaying cadaver will contaminate a larger surface area of the host plant resulting in increased dispersal and transmission of the virus (3). The molecular mechanisms of this behavior are not understood.
The family Baculoviridae is composed of viruses with circular, double-stranded DNA genomes and rod-shaped, enveloped virions (4). The coding capacity of the baculovirus genome is large, with most baculoviruses encoding well over 100 genes. Several hundred baculovirus species have been identified from Lepidoptera and classified into two genera: nucleopolyhedrovirus (NPV) and granulovirus. Baculoviruses produce two types of progeny during their life cycle, a budded virus for systemic infection of the host and an occluded virus that is spread horizontally in insect populations. The occluded virus, as the name suggests, is occluded within a geometric matrix of virus-encoded protein called polyhedrin for NPVs and granulin for granuloviruses. In this study, the silkworm baculovirus Bombyx mori NPV (BmNPV) and its host, the silkworm B. mori Daizo, were used as a model system to study baculovirus-induced locomotory activity. The B. mori Daizo strain was used because its endogenous dispersal or locomotory activity is well characterized and found to be exceptionally low (5). Initially, the timing and intensity of virus-induced ELA were determined by using the BmNPV–silkworm model system. Subsequently, individual gene knockout mutants of BmNPV were screened for their ability or inability to induce ELA. From these experiments, a BmNPV-encoded phosphatase gene was found to be involved in the induction of ELA. Finally, the ability of a silkworm-derived phosphatase gene to substitute for the BmNPV-encoded phosphate gene was tested. Our study provides the foundation for a better understanding of the molecular mechanisms of baculovirus-induced ELA.
Materials and Methods
Insect Cell Line and Larvae. The BmN cell line was maintained at 27°C in TC-100 medium supplemented with 10% FBS as described (6). Larvae of the silkmoth B. mori Daizo (Matsumura) were reared on Silkmate Series M artificial diet (Nosan, Yokohama, Japan) at 27 ± 1°C, at 40 ± 10% relative humidity, and on a 12 h light/12 h dark photoperiod as described (6).
Viruses. The wild-type BmNPV T3 isolate (7), BmNPV protein tyrosine phosphatase (PTP) gene, ptp, knockout mutant (BmPTPD), BmPTPD repair virus (BmPTPD-R), and BmPTPD carrying Bmptp-h (BmPTPD-Bm) were propagated on BmN cells as described in ref. 6. Plaque assays were used for the isolation of the BmPTPD, BmPTPD-R, and BmPTPD-Bm and to determine viral titers as described in ref. 6. BmPTPD was generated by replacing nucleotides 124,424 (PmlI site) to 124,806 (AgeI site) of the BmNPV genome (8) with a lacZ marker cassette [lacZ gene under the heat-shock promoter hsp70 from Drosophila melanogaster (9, 10)]. The deletion encompassed the first 125 of 168 amino acid residues of the deduced amino acid sequence of BmNPV ptp. The other BmNPV knockout mutants were generated in a similar manner as described in Table 1 and references therein. BmPTPD-R is a repair virus of BmPTPD in which the knockout of the ptp gene was repaired with wild type BmNPV-derived sequences. This repair allowed us to confirm that a mutation elsewhere in the genome was not involved in the observed phenotype of BmPTPD. BmPTPD-Bm was generated by replacing the lacZ marker cassette of BmPTPD with a ptp gene homologue, Bmptp-h, that was PCR-amplified from a cDNA (clone wdS20098, www.ab.a.u-tokyo.ac.jp/silkbase) generated from mRNAs isolated from the wing discs of spinning stage, day 2, silkmoths (strain C108) (11). The authenticity of each viral construct was confirmed by restriction endonuclease mapping, Southern hybridization, and/or PCR analysis of the purified genomic DNAs. Polyhedra were produced in fifth-instar larvae and purified as described in ref. 12. The oral infectivity of the viruses was determined by dosage- and time-mortality assays at 27°C with the aid of the polo (13) and vistat (14) computer programs.
Table 1. BmNPV knockout mutants that were screened for the induction of ELA.
| Knockout mutant | Target locus (gene) | Insertion coordinates |
|---|---|---|
| BmORF1D | ORF 1 (polh) | 480 |
| BmEGTZ | ORF 7 (egt) | 7,070-7,695 |
| BmORF13D | ORF 13 | 12,571-13,444 |
| BmBroAD | ORF 22 (bro-a) | 21,287-21,563 |
| BmORF24D | ORF 24 (fgf) | 23,454-23,878 |
| BmORF26D | ORF 26 (ubiquitin) | 25,199 |
| Bm39KD | ORF 27 (39k) | 25,555-26,071 |
| BmORF41D | ORF 41 | 39,246-39,510 |
| BmORF57D | ORF 57 | 54,635-55,031 |
| BmORF71D | ORF 71 (cg30) | 67,093-67,205 |
| BmORF74D | ORF 74 | 70,168 |
| BmBroBD | ORF 80 (bro-b) | 76,871 |
| BmBroCD | ORF 81 (bro-c) | 77,614-78,440 |
| BmORF95D | ORF 95 | 91,697-91,814 |
| BmORF97D | ORF 97 (orl-3) | 92,753-94,011 |
| BmLef7D | ORF 102 (lef-7) | 96,672-96,710 |
| BmORF103D | ORF 103 (chitinase) | 97,229-98,493 |
| BmCysPDu | ORF 104 (cyspro) | 99,090-99,513 |
| BmORF107D | ORF 107 (gp16) | 102,387 |
| BmP35Z | ORF 112 (p35) | 106,000-106,672 |
| Bmhr5D | hr5 | 107,066-107,601 |
| BmORF115D | ORF 115 (p74) | 109,478-110,200 |
| BmORF124D | ORF 124 (odv-e56) | 119,158 |
| Bmle2D | ORF 127 (ie-2) | 120,662-121,103 |
| BmPTPD | ORF 130 (ptp) | 124,424-124,806 |
| BmBroED | ORF 132 (bro-e) | 126,250-126,740 |
| BmORF134D | ORF 134 | 127,467 |
Some of the knockout mutants have been previously described in refs. 8, 35, and 36 and references therein. These 27 knockout mutants were identified after the screening of about half of the 136 putative genes of BmNPV on the basis of their ability to replicate in cultured BmN cells. The position of each ORF within the 128,413-nt genome of BmNPV is given in ref. 8. The coordinates of the point mutation or insertion site(s) of the lacZ marker cassette used to generate the knockout mutants within the 128,413-nt genome of BmNPV are given.
Locomotion Assay. Neonate (within 12 h of hatching) B. mori were orally inoculated by exposing them to a 380-mm2 area of artificial diet that was surface-contaminated with a LC99 of each virus in H2O as described in ref. 15. The LC99 values of BmNPV, BmPTPD, BmPTPD-R, and BmPTPD-Bm were determined to be 1,860, 7,420, 1,860, and 7,440 polyhedra per mm2, respectively. At least three, and in general four, replicates were performed for each time point. An experimental cohort consisted of 15–25 neonates. Time 0 was defined as the point at which the larvae were placed on the contaminated diet. After an 18-h exposure to the virus, the inoculated larvae were transferred to uncontaminated diet. At the appropriate time after infection, the infected larvae were acclimatized to light (four 20-W fluorescent lamps (Philips F20T12/CW) at a 50-cm average distance) or complete darkness as appropriate for 15 min, then placed in the center of a rectangular grid (200 by 240 mm) with markings at 1-mm intervals. Photographs were taken with a digital camera after release and at 3-min intervals until 15 min after release. This time frame insured that the insects (except in rare instances) stayed within the confines of the grid. The coordinates of the larvae were then determined at each time point after release and the Simplest Diffusion Model (5) of random movement was used to calculate the diffusion coefficient 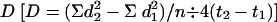 , where d is the distance traveled in millimeters at time t in minutes and n is the number of larvae. This model is based on four assumptions: (i) the environment is constant and homogeneous, (ii) all individuals are identical, (iii) all individuals move randomly, and (iv) all individuals move with constant intensity.
, where d is the distance traveled in millimeters at time t in minutes and n is the number of larvae. This model is based on four assumptions: (i) the environment is constant and homogeneous, (ii) all individuals are identical, (iii) all individuals move randomly, and (iv) all individuals move with constant intensity.
In similar experiments, fourth instar B. mori at 24–48 h after ecdysis (six larvae per cohort) were injected with a 5-μl suspension of TC-100 culture medium containing 6 mg/ml kanamycin and 750, 2,630, 1,550 or 2,670 plaque forming units of BmNPV, BmPTPD, BmPTPD-R, or BmPTPD-Bm, respectively (i.e., a LC99 dose of each virus). Mock-infected insects were injected with TC-100 culture medium containing 6 mg/ml kanamycin. At the appropriate time after infection, the infected larvae were acclimatized to light as described above and then placed in the center of a square grid (60 by 60 cm) with markings at 5-cm intervals. Photographs were taken after release and at 1-min intervals until 5 min after release. This time frame insured that the insects stayed within the confines of the grid. The diffusion coefficients were calculated as described above.
Phototropism Assay. Cohorts of 20 neonate B. mori were orally inoculated as described above. Four days after virus infection or mock infection, the larvae were acclimatized for 10 min to light as described above. The larvae were then placed inside of a light-protected box with a 5- by 28-cm opening at one side. The opening was at the same level as the larvae and 22 cm away from the larvae. The light source (a single 20-W Philips F20T12/CW fluorescent lamp) was placed at 28 cm from the opening of the box. The total distance from the light source to the larvae at the start of the experiment was 50 cm. Phototropic activity was determined by measuring the average distance traveled by each insect either toward or away from the light source. The percentage of insects moving either toward or away from the light source was also determined.
Results and Discussion
To determine the onset, duration, and intensity of virus-induced ELA, neonate B. mori were orally inoculated with a dose of BmNPV polyhedra that resulted in 99% death (i.e., an LC99 dose). This dose insured that the insects were uniformly infected but not overwhelmed with a toxic dose of virus. At this dose, the 90% survival time was 105.9 h (95% confidence limits of 99.3 h and 116.2 h). After inoculation at an LC99 dose, the BmNPV-infected larvae showed early indications of ELA at 3.0 and 3.5 days postinfection (p.i.) (D values of 1.41 and 2.98 mm2/min, respectively) (Fig. 1 A and B). A dramatic increase in ELA was observed at 3.75 days p.i. (D value of 54.4 mm2/min) (Fig. 1B). The intensity of the BmNPV-induced ELA appeared to further increase at 4.0 (Fig. 2A) and 4.25 days p.i. (D values of 86.8 and 80.9 mm2/min, respectively), then appeared to decline at 4.5 and 4.75 days p.i. (D values of 38.9 and 39.9 mm2/min, respectively) (Fig. 1B). At 5 days p.i., the few remaining larvae were near death and showed significantly reduced ELA (D value of 4.75 mm2/min). These findings indicated that the highest intensity of virus-induced ELA occurs ≈12–24 h before death and is significantly reduced during the last several hours before death. In contrast, mock-infected larvae showed essentially no movement at 1, 2, and 3 days after mock infection (Fig. 1 A). By the fourth and fifth days after mock-infection, mock-infected larvae ecdysed to the second instar and showed a minor increase in movement (Fig. 1 A). Larval ecdysis was not observed in Bm-NPV-infected insects putatively because of the inactivation of ecdysteroids (molting hormones) by the virus-encoded ecdysteroid UDP-glucosyl transferase gene product (16).
Fig. 1.
Neonate silkworms show a dramatic increase in locomotion (expressed as diffusion coefficients) after BmNPV infection but not after infection with a ptp gene knockout mutant (BmPTPD) or mock infection. (A) Mean locomotory intensity of BmNPV-, BmPTPD-, or mock-infected larvae at 24-h intervals from 1 to 5 days p.i. (B) Mean locomotory intensity of BmNPV- or BmPTPD-infected larvae at 6-h intervals from 3.5 to 5.0 days p.i. Error bars indicate the SD of three to five independent cohorts.
Fig. 2.
The locomotion of neonate silkworms infected with ptp gene knockout mutant BmPTPD is dramatically lower than larvae infected with BmNPV or BmPTPD-R. The images of the larvae at 4.0 days p.i. were taken 15 min after release from the center position. (A) BmNPV-infected larvae (D = 79.9 mm2/min). (B) BmPTPD-infected larvae (D = 1.27 mm2/min). (C) BmPTPD-R-infected larvae (D = 65.5 mm2/min). Not all of the experimental insects are visible in A and C.
To identify the gene or genes responsible for the BmNPV-induced ELA, gene knockout mutants of BmNPV were generated and screened for their ability to induce ELA. Knockout mutants of nearly half of the 136 putative genes of BmNPV were initially generated; however, many of these were genetically unstable or showed poor replication. In all, 27 knockout mutants (Table 1) were tested by injection into fourth or fifth instar B. mori. With the exception of the knockout mutant BmPTPD of a ptp homolog (ORF 130), all of the other BmNPV knockout mutants tested induced ELA in a manner similar to that of the wild-type virus. The BmPTPD construct lacked ≈76% of the 5′ end of the orf130 gene. The deduced amino acid sequence of the ptp gene of BmNPV contained a signature P-loop motif [(H/V)C(X)5R(S/T); bold indicates invariable amino acid residues] that is found in the catalytic domain of members of the PTP superfamily (17). BmNPV ptp showed 97.0% deduced amino acid sequence identity to ORF 1 of the baculovirus type species Autographa californica multicapsid NPV (18), which encodes a protein termed BVP or BV-PTP that possesses RNA triphosphatase and diphosphatase activities (19, 20). BVP has also been shown to dephosphorylate proteins containing either phosphotyrosine or phosphoserine/phosphothreonine residues (21, 22).
In sharp contrast to neonate B. mori infected with an LC99 dose of the wild-type BmNPV, neonate B. mori that were orally inoculated with BmPTPD did not show ELA (Figs. 1 and 2B). Increasing the dose of BmPTPD to twice the LC99 dose had no effect on the induction of ELA (D = 0.75 ± 0.33 mm2/min at 4.0 days p.i.). To confirm that the lack of ELA was due solely to the loss of the ptp gene, BmPTPD-R was generated. Neonate B. mori that were inoculated with an LC99 dose of BmPTPD-R (Fig. 2C) generated diffusion coefficients (e.g., D = 79.6 ± 17.6 mm2/min at 4.0 days p.i.) that were indistinguishable to those generated by BmNPV-infected larvae. These results confirmed that the baculovirus-encoded ptp gene is involved in the induction of ELA.
Light can significantly affect the behavior of larval and adult insects. To assess the effect of light on ELA, the diffusion coefficients generated by virus-infected neonate B. mori were determined (at 4.0 days p.i.) after acclimation of the larvae to either lighted (uniform light from above) or dark conditions. When the same BmNPV-infected cohorts were acclimated to light, then dark, and then light again, D values of 86.8, 22.7, and 78.2 mm2/min, respectively, were generated (Fig. 3A). By measuring the variance of distribution of these cohorts, we found that the intensities of the ELA over time were constant under both the lighted (r2 = 0.944–0.998) and dark (r2 = 0.989) conditions (Fig. 3B). Furthermore, the intensities of the ELA were ≈3-fold higher under the lighted (slope = 293.3–308.5) as opposed to the dark (slope = 97.0) conditions (Fig. 3B). Light had basically no effect on the ELA of larvae infected with BmPTPD. These findings indicated that light activates virus-induced ELA. To determine whether phototropism was associated with the light-activated ELA, an assay in which the light source was placed to one side of the larvae was used. This assay, because of the nonhomogeneous light source, precluded the use of the Simplest Diffusion Model to quantify the intensity of the ELA. Thus, phototropism was quantified by determining the percentage of larvae that moved toward or away from the light source as well as the average distance that each larva moved either toward or away from the light source. This assay indicated that both virus- and mock-infected insects show some positive phototropism (Fig. 4A). However, the intensity of the positive phototropism was more than 2-fold higher in BmNPV- or BmPTPD-R-infected larvae in comparison with larvae infected with BmPTPD (Fig. 4B).
Fig. 3.
Light dramatically increases the locomotion of BmNPV-infected, but not BmPTPD-infected neonate silkworms. (A) Mean locomotory intensity expressed as diffusion coefficients D of BmNPV- or BmPTPD-infected neonate silkworms at 4.0 days p.i. in the presence or absence of light. With light, dark, and light again indicate insects that were acclimated to light, then dark, and then light again, respectively, for 15 min before the determination of D values. (B) Linear regressions of the mean square distance against time after release of the BmNPV- or BmPTPD-infected insects from Fig. 3A. Error bars indicate the SD of four or five independent cohorts.
Fig. 4.
Positive phototropism is enhanced in BmNPV-infected but not BmPTPD-infected neonate silkworms. (A) The percentage of insects showing positive (black bars) or negative (white bars) phototropism at 4.0 days after virus infection or mock infection. (B) The average distance traveled by virus- or mock-infected insects 10 min after release either toward (black bars) or away (white bars) from the light source. Error bars indicate the SD of three independent cohorts.
Recently, an EST database has been generated from various B. mori-derived tissues and cells (ref. 11 and T.S., unpublished data). This database contains >53,000 ESTs of which >13,000 are classified as nonredundant. Of the nonredundant ESTs, 44 different phosphatase sequence-containing ESTs were found. Of these 44 ESTs, the deduced amino acid sequence of only one clone (wdS20098) showed significant homology to ptp genes of baculoviral origin. Clone wdS20098 was generated from the wing discs of silkmoth larvae on the second day of the spinning stage (i.e., 2 days after the start of the natural wandering stage). This clone encoded a deduced protein of 212 amino acid residues that showed 48.2% identity (80.7% similarity) over 166 ungapped amino acid residues to BmNPV PTP (Fig. 5A). To determine whether the ptp coding sequence (i.e., Bmptp-h) of clone wdS20098 could restore the ability of BmPTPD to induce ELA, Bmptp-h was PCR-amplified and cloned into the PmlI and AgeI sites that were used to knock out the authentic ptp gene. This construction generated BmPTPD-Bm, a BmNPV mutant in which the ptp gene of BmNPV was replaced with Bmptp-h in an identical promoter context as the authentic ptp gene. Neonate B. mori that were orally inoculated with BmPTPD-Bm polyhedra did not show ELA (Fig. 4B and data not shown). However, when fourth-instar B. mori were inoculated (by injection) with BmPTPD-Bm, these larvae showed 24.5% of the ELA of wild-type BmNPV-injected larvae (Fig. 5B). The ELA induced by BmPTP-Bm in fourth-instar B. mori was 4.6-fold higher than that induced by BmPTPD (Fig. 5B). Differences in the levels of kinase activity or substrate availability between the neonate and fourth-instar larvae may explain why BmPTPD-Bm was able to only partially restore ELA in the fourth-instar larvae but not in neonates. The partial functional replacement, larval stage at which the Bmptp-h gene was isolated, and high homology between the PTPs of BmNPV and B. mori indicate that BmNPV may have acquired its ptp gene from an ancestral host.
Fig. 5.
Inserting a host-derived ptp gene, Bmptp-h, into the viral ptp gene knockout mutant BmPTPD can partially rescue the inability of BmPTPD to induce ELA. (A) Sequence alignment of the deduced amino acid sequences of BmNPV ptp and Bmptp-h from clustalw (1.82) (34). (B) Mean intensity of ELA expressed as diffusion coefficients of fourth-instar silkworm larvae infected with BmPTPD-Bm, BmNPV, BmPTPD, or BmPTPD-R or mock-infected at 4.0 days p.i. Error bars in B indicate the SD of three to five independent cohorts.
At a very late stage of infection (i.e., 4.75 or 5.0 days p.i.), it appeared that the larvae cuticle was weakened or damaged. This damage often resulted in the release of hemolymph (insect blood) from the anus (e.g., damage to the midgut) and/or prolegs. The weakened cuticle was putatively the result of a virus-encoded or virus-induced cysteine protease and/or chitinase (23, 24). The hemolymph that was lost by the virus-infected larvae was clearly visible as a “trail” in both neonates and fourth instars. Considering that a large number of polyhedra are found in the hemolymph at a late stage of infection, we speculate that contamination of plant surfaces during ELA results in the increased transmission of the virus.
It has long been known that parasitic infection can alter the behavior of an infected host. Our study shows that the baculovirus-encoded protein tyrosine phosphatase gene induces light-activated ELA in lepidopteran larvae and suggests that this gene was obtained from an ancestral host. cGMP-dependent protein kinase genes such as egl-4 (25), Amfor (26, 27), and for (28) have also been shown to be involved in enhanced locomotory behaviors, such as roaming, foraging, and/or positive phototropism, in invertebrates. Consistent with our findings, down-regulation of egl-4 (putatively resulting in lower relative levels of a phosphorylated product) in the nematode results in increased roaming behavior (25). In contrast, up-regulation of Amfor and for result in ELA in the honey bee (26, 27) and fruit fly (28), respectively. Interestingly, orthologs of Amfor and for have recently been identified in the silkworm genome (29). However, the apparent discrepancy in the functional role of Amfor and for in comparison to egl-4 (and our phosphatase) needs further investigation. The availability of sequence data of the silkworm genome (30, 31) as well as microarrays carrying silkworm (32) and baculovirus (33) cDNAs will allow us to identify the specific roles that phosphatase and kinase genes play in the induction of wandering and other ELAs.
Acknowledgments
We thank Emeritus Professor Y. Tanada (University of California, Berkeley) for thoughtful discussion of this manuscript, A. I. Samra and E. Hass for assistance with the bioassays, S. Gomi and T. Ohkawa for plasmid constructions, and H. K. Kaya for use of laboratory facilities. Eggs of B. mori Daizo (Matsumura) were provided by the National Institute of Sericultural and Entomological Sciences, Japan (currently known as the National Institute of Agrobiological Sciences). This research was partially funded by United States Department of Agriculture Grants 2001-35302-09919 and 2003-35302-13499; the Bio-oriented Technology Research Advancement Institution; the Ministry of Agriculture, Forestry and Fisheries/National Institute of Agrobiological Sciences (Insect Technology); and Japan Ministry of Education, Culture, Sports, Science, and Technology Grant 15011207.
Author contributions: S.G.K., S.M., and B.D.H. designed research; S.G.K. performed research; J.W.C., T.S., K.M., and M.K. contributed new reagents/analytic tools; S.G.K. and K.N. analyzed data; and S.G.K. and B.D.H. wrote the paper.
Abbreviations: ELA, enhanced locomotory activity; NPV, nucleopolyhedrovirus; BmNPV, Bombyx mori nucleopolyhedrovirus; p.i., postinfection; PTP, protein tyrosine phosphatase; BmPTPD, mutant BmNPV lacking ptp; BmPTPD-R, BmPTPD repair virus; BmPTPD-Bm, BmPTPD carrying Bmptp-h.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB126695).
References
- 1.Nijhout, H. F. (1994) Insect Hormones (Princeton Univ. Press, Princeton).
- 2.Evans, H. F. (1986) in The Biology of Baculoviruses, eds. Granados, R. R. & Federici, B. A. (CRC Press, Boca Raton, FL), Vol. II, pp. 89-132. [Google Scholar]
- 3.Goulson, D. (1997) Oecologia 109, 219-228. [DOI] [PubMed] [Google Scholar]
- 4.Blissard, G., Black, B., Crook, N., Keddie, B. A., Possee, R., Rohrmann, G., Theilmann, D. & Volkman, L. (2000) in Virus Taxonomy: Classification and Nomenclature of Viruses, Seventh Report of the International Committee on Taxonomy of Viruses, eds. van Regenmortel, M. H. V., Fauquet, C. M., Bishop, D. H. L., Carstens, E. B., Estes, M. K., Lemon, S. M., McGeoch, D. J., Maniloff, J., Mayo, M. A., Pringle, C. R. & Wickner, R. B. (Academic, San Diego), pp. 195-202.
- 5.Nagasaka, K., Mase, K., Okada, E. & Yamamoto, T. (1998) J. Seric. Sci. Jpn. 67, 101-107. [Google Scholar]
- 6.Choudary, P. V., Kamita, S. G. & Maeda, S. (1995) in Methods in Molecular Biology: Baculovirus Expression Protocols, ed. Richardson, C. D. (Humana, Totowa, NJ), Vol. 39, pp. 243-264. [DOI] [PubMed] [Google Scholar]
- 7.Maeda, S. & Majima, K. (1990) J. Gen. Virol. 71, 1851-1856. [DOI] [PubMed] [Google Scholar]
- 8.Gomi, S., Majima, K. & Maeda, S. (1999) J. Gen. Virol. 80, 1323-1337. [DOI] [PubMed] [Google Scholar]
- 9.Zuidema, D., Schouten, A., Usmany, M., Maule, A. J., Belsham, G. J., Roosien, J., Klinge-Roode, E. C., Van Lent, J. W. M. & Vlak, J. M. (1990) J. Gen. Virol. 71, 2201-2210. [DOI] [PubMed] [Google Scholar]
- 10.Kamita, S. G., Majima, K. & Maeda, S. (1993) J. Virol. 67, 455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mita, K., Morimyo, M., Okano, K., Koike, Y., Nohata, J., Kawasaki, H., Kadono-Okuda, K., Yamamoto, K., Suzuki, M. G., Shimada, T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14121-14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamita, S. G., Maeda, S. & Hammock, B. D. (2003) J. Virol. 77, 13053-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell, R. M., Robertson, J. L. & Savin, N. E. (1977) Entomol. Soc. Am. Bull. 23, 209-213. [Google Scholar]
- 14.Hughes, P. R. (1990) vistat: Statistical Package for the Analysis of Baculovirus Bioassay Data (Boyce Thompson Institute at Cornell Univ., Ithaca, NY).
- 15.Ignoffo, C. M. (1965) J. Invertebr. Pathol. 7, 315-319. [DOI] [PubMed] [Google Scholar]
- 16.O'Reilly, D. R. & Miller, L. K. (1989) Science 245, 1110-1112. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, Z. Y. (1998) Crit. Rev. Biochem. Mol. Biol. 33, 1-52. [DOI] [PubMed] [Google Scholar]
- 18.Ayres, M. D., Howard, S. C., Kuzio, J., Lopezferber, M. & Possee, R. D. (1994) Virology 202, 586-605. [DOI] [PubMed] [Google Scholar]
- 19.Takagi, T., Taylor, G. S., Kusakabe, T., Charbonneau, H. & Buratowski, S. (1998) Proc. Natl. Acad. Sci. USA 95, 9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross, C. H. & Shuman, S. (1998) J. Virol. 72, 7057-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng, Z. Q. & Charbonneau, H. (1993) J. Biol. Chem. 268, 4728-4733. [PubMed] [Google Scholar]
- 22.Kim, D. & Weaver, R. F. (1993) Virology 195, 587-595. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa, T., Majima, K. & Maeda, S. (1994) J. Virol. 68, 6619-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawtin, R. E., Zarkowska, T., Arnold, K., Thomas, C. J., Gooday, G. W., King, L. A., Kuzio, J. A. & Possee, R. D. (1997) Virology 238, 243-253. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara, M., Sengupta, P. & McIntire, S. L. (2002) Neuron 36, 1091-1102. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Shahar, Y., Robichon, A., Sokolowski, M. B. & Robinson, G. E. (2002) Science 296, 741-744. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Shahar, Y., Leung, H. T., Pak, W. L., Sokolowski, M. B. & Robinson, G. E. (2003) J. Exp. Biol. 206, 2507-2515. [DOI] [PubMed] [Google Scholar]
- 28.Osborne, K. A., Robichon, A., Burgess, E., Butland, S., Shaw, R. A., Coulthard, A., Pereira, H. S., Greenspan, R. J. & Sokolowski, M. B. (1997) Science 277, 834-836. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick, M. J. & Sokolowski, M. B. (2004) Integr. Comp. Biol. 44, 28-36. [DOI] [PubMed] [Google Scholar]
- 30.Mita, K., Kasahara, M., Sasaki, S., Nagayasu, Y., Yamada, T., Kanamori, H., Namiki, N., Kitagawa, M., Yamashita, H., Yasukochi, Y., et al. (2004) DNA Res. 11, 27-35. [DOI] [PubMed] [Google Scholar]
- 31.Xia, Q., Zhou, Z., Lu, C., Cheng, D., Dai, F., Li, B., Zhao, P., Zha, X., Cheng, T., Chai, C., et al. (2004) Science 306, 1937-1940. [DOI] [PubMed] [Google Scholar]
- 32.Ote, M., Mita, K., Kawasaki, H., Seki, M., Nohata, J., Kobayashi, M. & Shimada, T. (2004) Insect Biochem. Mol. Biol. 34, 775-784. [DOI] [PubMed] [Google Scholar]
- 33.Iwanaga, M., Takaya, K., Katsuma, S., Ote, M., Tanaka, S., Kamita, S. G., Kang, W. K., Shimada, T. & Kobayashi, M. (2004) Biochem. Biophys. Res. Commun. 323, 599-614. [DOI] [PubMed] [Google Scholar]
- 34.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22, 4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang, K.-D., Lee, E.-J., Kamita, S. G. & Seong, S.-I. (1998) Korean J. Seric. Sci. 40, 105-110. [Google Scholar]
- 36.Kang, K.-D., Lee, E.-J., Kamita, S. G., Maeda, S. & Seong, S.-I. (2000) J. Biochem. Mol. Biol. 33, 63-68. [Google Scholar]







