Abstract
Aim
To evaluate the effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes.
Methods
We conducted systematic searches of PubMed, Embase and Cochrane Central Register of Controlled Trials up to June 2016 and included randomized controlled trials of SGLT2 inhibitors in adult type 2 diabetic patients reporting estimated glomerular filtration rate (eGFR) and/or urine albumin/creatinine ratio (ACR) changes. Data were synthesized using the random-effects model.
Results
Forty-seven studies with 22,843 participants were included. SGLT2 inhibition was not associated with a significant change in eGFR in general (weighted mean difference (WMD), −0.33 ml/min per 1.73 m2, 95% CI [−0.90 to 0.23]) or in patients with chronic kidney disease (CKD) (WMD −0.78 ml/min per 1.73 m2, 95% CI [−2.52 to 0.97]). SGLT2 inhibition was associated with eGFR reduction in short-term trials (WMD −0.98 ml/min per 1.73 m2, 95% CI [−1.42 to −0.54]), and with eGFR preservation in long-term trials (WMD 2.01 ml/min per 1.73 m2, 95% CI [0.86 to 3.16]). Urine ACR reduction after SGLT2 inhibition was not statistically significant in type 2 diabetic patients in general (WMD −7.24 mg/g, 95% CI [−15.54 to 1.06]), but was significant in patients with CKD (WMD −107.35 mg/g, 95% CI [−192.53 to −22.18]).
Conclusions
SGLT2 inhibition was not associated with significant changes in eGFR in patients with type 2 diabetes, likely resulting from a mixture of an initial reduction of eGFR and long-term renal function preservation. SGLT2 inhibition was associated with statistically significant albuminuria reduction in type 2 diabetic patients with CKD.
Keywords: SGLT2 inhibitor, Glomerular filtration rate, Albuminuria, Diabetic nephropathy, Meta-analysis
Introduction
With increasing incidence and prevalence of diabetes mellitus, diabetic nephropathy has become the leading cause of end-stage renal disease (ESRD), accounting for 50% of cases in the developed world (Tuttle et al., 2014; Wild et al., 2004). Current management of diabetic nephropathy includes avoidance of nephrotoxic agents, prevention of infections, glycemic control, and blood pressure control, with emphasis on the use of renin-angiotensin-aldosterone system (RAAS) inhibitors. However, these strategies only provide partial renoprotection against progression of diabetic nephropathy (Bilous et al., 2009; Lewis et al., 1993; Lewis et al., 2001; Mauer et al., 2009). Thus, additional therapeutic interventions for the prevention and treatment of diabetic nephropathy are needed.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors, including canagliflozin, dapagliflozin, empagliflozin, ipragliflozin and tofogliflozin, are a new class of antihyperglycemic drugs that lower blood glucose by blocking glucose reabsorption via SGLT2 at the proximal renal tubule. SGLT2 inhibitors are gaining popularity due to their various beneficial effects. In addition to glycemic control, SGLT2 inhibitors lower blood pressure, control body weight, and reduce cardiovascular mortality in type 2 diabetic patients with high cardiovascular risk (Baker et al., 2014; Matthaei et al., 2015; Tikkanen et al., 2015; Wilding et al., 2015; Zinman et al., 2015b).
SGLT2 inhibition also has profound effects on renal hemodynamics. The tubular hypothesis implicates that impaired tubuloglomerular feedback (TGF) due to upregulation of SGLT2 plays a central role in hyperfiltration in diabetic patients, leading to albuminuria and decline in renal function (Skrtic & Cherney, 2015). While RAAS activation mainly leads to vasoconstriction of efferent arterioles (Sochett et al., 2006), impairment of TGF mediates hyperfiltration via vasodilation of afferent arterioles. SGLT2 inhibitors block glucose and sodium reabsorption at the proximal tubule, increase sodium delivery to the macula densa, and consequently restore impaired TGF. Thus, it is postulated that SGLT2 inhibition alleviates glomerular hyperfiltration in the early stages of diabetic nephropathy, reduces albuminuria, and slows the decline of renal function in the long term. These effects have been observed in micropuncture studies conducted in rats and a proof-of-concept study of type 1 diabetic patients with hyperfiltration (Cherney et al., 2014; Skrtic & Cherney, 2015; Thomson et al., 2012).
A number of clinical trials have reported kidney-related outcomes after SGLT2 inhibitor use (Bailey et al., 2015; Barnett et al., 2014; Wanner et al., 2016; Yale et al., 2013). In the EMPA-REG OUTCOME trial, empagliflozin reduced incident or worsening nephropathy and slowed decline of renal function in type 2 diabetic patients at high cardiovascular risk (Wanner et al., 2016). A previous meta-analysis (Liu et al., 2015) up to December 2014 has concluded that SGLT2 inhibition does not have a significant effect on the estimated glomerular filtration rate (eGFR). However, there has been no up-to-date systematic review examining whether SGLT2 inhibitors attenuate hyperfiltration in acute settings and in the early stages of diabetic nephropathy, whether SGLT2 inhibitors preserve GFR in the long term and for patients with more advanced nephropathy, or whether SGLT2 inhibitors reduce albuminuria. Thus, we conducted this meta-analysis of randomized controlled trials (RCTs) to thoroughly characterize the effects of SGLT2 inhibitors on eGFR and albuminuria compared with placebo or other antidiabetic treatments in patients with type 2 diabetes.
Materials and Methods
This review conforms to the standard guidelines and was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
Search strategy
We conducted a systematic search of PubMed, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) databases through June 19th 2016. The search strategy is provided in Item S3; we used medical subject headings, as well as free-text search terms, including SGLT2 inhibitors, canagliflozin, dapagliflozin, empagliflozin, atigliflozin, ‘bi 44847’, ertugliflozin, ipragliflozin, luseogliflozin, remogliflozin, sergliflozin, sotagliflozin and tofogliflozin. We also conducted a manual search of references of existing reviews in this field to identify additional relevant studies.
Study selection
Two reviewers (LX and YL) independently screened the search results and retrieved relevant studies for further evaluation. The retrieved full-text articles were examined by two reviewers (LX and YL) in parallel for inclusion according to predetermined criteria. We included RCTs conducted on adult type 2 diabetic patients that compared SGLT2 inhibitors with either placebo or other antidiabetic drugs and reported changes in eGFR and/or urine albumin/creatinine ratio (ACR). Only manuscripts published in English were included. For studies reporting renal outcomes in forms other than eGFR and urine ACR (i.e., serum creatinine, urine protein excretion, etc.), an e-mail was sent to the corresponding author requesting further data. For multiple publications from the same study, only the first publication reporting renal outcomes was included. Disagreement was resolved by discussion and/or consultation with a third reviewer (PX).
Data extraction and validity assessment
Two reviewers (LX and JL) independently used a standard data extraction tool to record the following properties of each study: the study characteristics (author, year, study design, method of randomization, duration of follow-up, and number of dropouts), participant characteristics (sample size, age, sex, duration of diabetes, baseline HbA1C level, baseline blood pressure, eGFR and urine ACR), therapeutic intervention (type of SGLT2 inhibitor, dose, frequency and duration of treatment), concomitant therapies (concomitant antidiabetic therapy and RAAS inhibitors), comparison groups (placebo-controlled or active-controlled), outcomes of interest (means and standard deviations (SDs) of changes in eGFR and urine ACR in treatment and control groups), whether outcomes of chronic kidney disease (CKD) subjects (defined as eGFR < 60 ml/min per 1.73 m2 or microalbuminuria or macroalbuminuria) were reported, and the funding source. The program g3data (www.frantz.fi/software/g3data.php) was used to extract relevant data that were reported in figures but not in the text.
Study quality was evaluated by two authors (LX and YL) independently using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1 (2011). The domains of assessment included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.
Statistical analysis
Outcome measures for each trial were mean differences of changes (calculated as (end of trial value for treatment group—baseline value for treatment group)—(end of trial value for control group—baseline value for control group)) in eGFR and urine ACR. For studies in which SD was not directly reported, SD was calculated from SE (standard error) or 95% confidence intervals (CI), or imputed as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1 using a correlation coefficient of 0.8 (with the formula provided in Item S4) (Higgins & Green, 2011). For studies with more than one SGLT2 treatment arm, these groups were combined to create a single treatment arm (with the formulae provided in Item S5) (Higgins & Green, 2011). The inverse variance method was used to estimate the pooled weighted mean differences (WMDs) in eGFR and urine ACR. As clinical and statistical heterogeneity were anticipated, we decided a priori to use the random-effects model in our data synthesis.
Statistical heterogeneity was quantified using the Cochrane Q test and I2 statistic. Subgroup analysis was planned for the type of SGLT2 inhibitor, placebo or active control, concomitant use of RAAS inhibitors, trial duration, mean baseline age, mean duration of diabetes, mean baseline HbA1C level, whether the study population were CKD patients (eGFR < 60 ml/min per 1.73 m2 or microalbuminuria or macroalbuminuria), and whether the study population had hyperfiltration (eGFR ≥ 125 ml/min per 1.73 m2) (Dahlquist, Stattin & Rudberg, 2001). Test for subgroup differences were carried out using RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration).
The possibility of publication bias was assessed using funnel plots and Egger’s test. Sensitivity analysis excluding trials with relatively high risk (defined as ≥1 item with high risk or ≥2 items with unclear risk in the ‘Risk of Bias’ assessment tool) was performed.
Statistical analyses were performed using the Stata 12.0 software package (StataCorp, LP, College Station, TX) and RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration). Statistical significance was set at p < 0.05 for all analyses.
Results
Study selection and characteristics
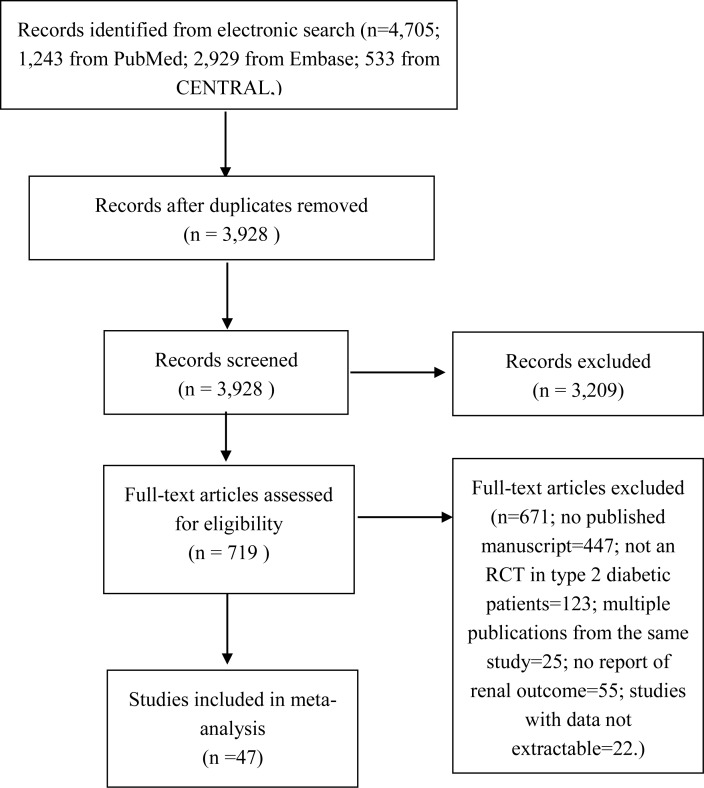
The results of the literature search and study selection are shown in Fig. 1. Details of the study selection process can be found in Item S6. This search process led to inclusion of 47 studies (Bailey et al., 2015; Barnett et al., 2014; Bode et al., 2013; Bolinder et al., 2012; Cefalu et al., 2013; DeFronzo et al., 2015; Fonseca et al., 2013; Forst et al., 2014; Haring et al., 2014; Haring et al., 2013; Inagaki et al., 2014; Ji et al., 2015; Ji et al., 2014; Kadowaki et al., 2014; Kaku et al., 2014; Kashiwagi et al., 2015a; Kashiwagi et al., 2015b; Kashiwagi et al., 2015c; Kashiwagi et al., 2015d; Kohan et al., 2014; Kovacs et al., 2014; Lambers Heerspink et al., 2013; Lavalle-Gonzalez et al., 2013; Lewin et al., 2015; Lu et al., 2016; Nauck et al., 2011; Nishimura et al., 2015; Qiu, Capuano & Meininger, 2014; Ridderstrale et al., 2014; Rodbard et al., 2016; Roden et al., 2013; Rosenstock et al., 2016; Rosenstock et al., 2014; Rosenstock et al., 2015; Ross et al., 2015; Schernthaner et al., 2013; Schumm-Draeger et al., 2015; Sha et al., 2014; Strojek et al., 2011; Tikkanen et al., 2015; Wanner et al., 2016; Weber et al., 2016; Wilding et al., 2013a; Wilding et al., 2013b; Wilding et al., 2009; Wilding et al., 2012; Yale et al., 2013) with 22,843 participants in our meta-analysis.
Figure 1. Identification process for eligible studies.
Abbreviations: CENTRAL, Cochrane Central Register of Controlled Trials; RCT, randomized controlled trial.
The characteristics of the included studies are shown in Table 1. Of the 47 studies, 46 studies with 22,603 participants reported changes in eGFR, and 17 studies with 7,285 participants reported changes in urine ACR. Five SGLT2 inhibitors, including dapagliflozin, canagliflozin, empagliflozin, ipragliflozin and tofogliflozin, were assessed. A total of 38 studies were placebo-controlled, and 9 were controlled by other antidiabetic medications, including metformin, glimepiride, glipizide, linagliptin, and sitagliptin. The trial durations ranged from 4 weeks to 156 weeks. Six studies reported outcomes of CKD subjects. In other studies, the mean baseline eGFR ranged from 76.7 to 149.2 ml/min per 1.73 m2, and the mean baseline urine ACR ranged from 6.7 to 143.7 mg/g. None of the studies reported outcomes in a group of patients with hyperfiltration. Collection of data on concomitant use of RAAS inhibitors was planned a priori; however, this was impeded as only six studies reported the number of patients using RAAS inhibitors (Barnett et al., 2014; Bode et al., 2013; Lambers Heerspink et al., 2013; Sha et al., 2014; Wanner et al., 2016; Weber et al., 2016), among which only one study reported outcomes with stratification according to RAAS inhibitor usage (Weber et al., 2016).
Table 1. Characteristics of included studies.
| Study | Dose | Control group | Duration of follow-up (weeks) | Sample size | Mean age (years) | Mean duration of diabetes (years) | Mean baseline HbA1C (%) | Mean baseline blood pressure (mmHg) | Mean baseline eGFR (ml/min/ 1.73 m2) | Mean baseline urine ACR (mg/g) | Reported outcomes of CKD patients | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CANAGLIFLOZIN | ||||||||||||
| Bode et al. (2013) | 100 mg, 300 mg | Placebo | 26 | 584 | 63.6 | 11.7 | 7.7 | 131.0/75.7 | 77.5 | N.R. | No | eGFR |
| Cefalu et al. (2013) | 100 mg, 300 mg | Glimepiride | 52 | 1,038 | 56.2 | 6.6 | 7.8 | 129.8/79.0 | N.R. | 29.1 | No | eGFR, uACR |
| Lavalle-Gonzalez et al. (2013) | 100 mg, 300 mg | Sitagliptin | 52 | 873 | 55.5 | 6.9 | 7.9 | 128.2/77.7 | 89.7 | N.R. | No | eGFR |
| Schernthaner et al. (2013) | 300 mg | Sitagliptin | 52 | 460 | 56.7 | 9.6 | 8.1 | 130.7/78.9 | 88.9 | N.R. | No | eGFR |
| Wilding et al. (2013a) IJCP | 100 mg, 300 mg | Placebo | 52 | 306 | 56.8 | 9.6 | 8.1 | 130.4/78.7 | 90.3 | N.R. | No | eGFR |
| Yale et al. (2013) | 100 mg, 300 mg | Placebo | 26 | 211 | 68.5 | 16.3 | 8.0 | 134.9/74.4 | 39.4 | 30.0 (median) | Yes | eGFR, uACR |
| Forst et al. (2014) | 100 mg, 300 mg | Placebo (26 weeks) + sitagliptin (26 weeks) | 52 | 261 | 57.4 | 10.5 | 7.9 | 127.1/76.4 | 86.4 | N.R. | No | eGFR |
| Inagaki et al. (2014) | 100 mg, 200 mg | Placebo | 24 | 240 | 58.0 | 5.4 | 8.0 | 127.9/77.8 | 84.4 | N.R. | No | uACR |
| Qiu, Capuano & Meininger (2014) | 50 mg bid, 150 mg bid | Placebo | 18 | 239 | 57.4 | 7.0 | 7.6 | 129.3/78.1 | 85.9 | N.R. | No | eGFR |
| Sha et al. (2014) | 300 mg | Placebo | 12 | 35 | 62.8 | 8.5 | 7.7 | 132.9/80.0 | 97.3 | N.R. | No | eGFR |
| Ji et al. (2015) | 100 mg, 300 mg | Placebo | 18 | 636 | 56.2 | 6.7 | 8.0 | 129.5/77.3 | 94.0 | N.R. | No | eGFR |
| Rodbard et al. (2016) | 100 mg or 300 mg (titrated)a | Placebo | 26 | 171 | 57.4 | 9.9 | 8.5 | N.R. | 90.5 | N.R. | No | eGFR |
| Rosenstock et al. (2016) | 100 mg, 300 mg | Placebo | 26 | 618 | 54.9 | 3.2 | 8.8 | 128.6/78.3 | 87.0 | N.R. | No | eGFR |
| DAPAGLIFLOZIN | ||||||||||||
| Wilding et al. (2009) | 10 mg, 20 mg | Placebo | 12 | 68 | 56.7 | 12.3 | 8.4 | 128.8/77.4 | 87.6 | N.R. | No | eGFR |
| Strojek et al. (2011) | 2.5 mg, 5 mg, 10 mg | Placebo | 24 | 596 | 59.8 | 7.4 | 8.1 | N.R. | 76.7 | N.R. | No | eGFR |
| Nauck et al. (2011) | 2.5 mg–10 mg (titrated)b | Glipizide | 52 | 814 | 58.5 | 6.5 | 7.7 | 133.3/80.6 | 90.1 | 58.0 | No | eGFR, uACR |
| Bolinder et al. (2012) | 10 mg | Placebo | 24 | 167 | 60.7 | 5.8 | 7.2 | 134.6/80.5 | 84.3 | 44.3 | No | eGFR, uACR |
| Wilding et al. (2012) | 2.5 mg, 5 mg, 10 mg | Placebo | 48 | 658 | 59.3 | 13.6 | 8.5 | 138.5/80.1 | 78.4 | 75.2 | No | eGFR, uACR |
| Lambers Heerspink et al. (2013) | 10 mg | Placebo | 12 | 48 | 55.9 | 6.5 | 7.6 | 136.41/82.0 | N.R. | N.R. | No | eGFR |
| Ji et al. (2014) | 5 mg, 10 mg | Placebo | 24 | 338 | 51.4 | 1.4 | 8.3 | 123.6/77.8 | 92.5 | N.R. | No | eGFR |
| Kohan et al. (2014) | 5 mg, 10 mg | Placebo | 104 | 132 | 67.0 | 16.9 | 8.4 | 132.1/73.3 | 44.6 | N.R. | Yes | eGFR, uACR |
| Schumm-Draeger et al. (2015) | 2.5 mg bid, 5 mg bid, 10 mg qd | Placebo | 16 | 400 | 57.7 | 5.2 | 7.8 | 132.0/80.7 | 86.3 | N.R. | No | eGFR |
| Bailey et al. (2015) | 2.5 mg, 5 mg, 10 mg | Placebo 24 weeks + 500 mg metformin | 102 | 274 | 52.2 | 1.9 | 7.9 | 125.9/80.5 | 85.1 | 23.5 | No | eGFR, uACR |
| Weber et al. (2016) | 10 mg | Placebo | 12 | 449 | 56.5 | 7.5 | 8.1 | 151.1/91.3 | 85.9 | 143.7 | No | eGFR, uACR |
| EMPAGLIFLOZIN | ||||||||||||
| Haring et al. (2013) | 10 mg, 25 mg | Placebo | 24 | 666 | 57.1 | N.R. | 8.1 | 128.9/78.6 | 87.2 | N.R. | No | eGFR |
| Roden et al. (2013) | 10 mg, 25 mg | Placebo | 24 | 676 | 54.7 | N.R. | 7.9 | 131.1/78.8 | N.R. | N.R. | No | eGFR |
| Barnett et al. (2014) | 10 mg, 25 mg | Placebo | 52 | 637 | 64.1 | N.R. | 8.0 | 135.3/76.9 (CKD2) | 71.6 (CKD2) | 155.0 (CKD2) | Yes | eGFR, uACR |
| 133.7/76.7 (CKD3) | 44.9 (CKD3) | 362.5 (CKD3) | ||||||||||
| 145.6/77.6 (CKD4) | 23.2 (CKD4) | 1387.4 (CKD4) | ||||||||||
| Haring et al. (2014) | 10 mg, 25 mg | Placebo | 24 | 637 | 55.7 | N.R. | 7.9 | 129.4/78.7 | 89.0 | N.R. | No | eGFR |
| Kovacs et al. (2014) | 10 mg, 25 mg | Placebo | 24 | 498 | 54.5 | N.R. | 8.1 | 126.1/76.9 | 85.7 | N.R. | No | eGFR |
| Rosenstock et al. (2014) | 10 mg, 25 mg | Placebo | 52 | 563 | 56.7 | N.R. | 8.3 | 126.2/78.2 | N.R. | N.R. | No | eGFR |
| Ridderstrale et al. (2014) | 25 mg | Glimepiride | 104 | 1500 | 56.0 | N.R. | 7.9 | 133.5/79.5 | 88.0 | 40.2c | Yes | eGFR, uACR |
| Kadowaki et al. (2014) | 5 mg, 10 mg, 25 mg, 50 mg | Placebo | 12 | 547 | 57.5 | N.R. | 8.0 | 129.2/78.7 | 85.7 | N.R. | No | eGFR |
| DeFronzo et al. (2015) | 10 mg, 25 mg | Linagliptin | 52 | 313 | 55.9 | N.R. | 8.0 | 129.8/79.3 | 90.5 | 52.2 | No | eGFR, uACR |
| Lewin et al. (2015) | 10 mg, 25 mg | Linagliptin | 52 | 340 | 54.6 | N.R. | 8.0 | 128.5/78.8 | 88.8 | 36.8 | No | eGFR, uACR |
| Tikkanen et al. (2015) | 10 mg, 25 mg | Placebo | 12 | 723 | 60.2 | N.R. | 7.9 | 142.1/83.9 | 84.0 | N.R. | No | eGFR |
| Nishimura et al. (2015) | 10 mg, 25 mg | Placebo | 4 | 60 | 62.7 | N.R. | 7.9 | 120.9/72.4 | 80.0 | N.R. | No | eGFR |
| Rosenstock et al. (2015) | 10 mg, 25 mg | Placebo | 78 | 364 | 58.8 | N.R. | 8.2 | 133.0/78.3 | 84.0 | N.R. | No | eGFR |
| Ross et al. (2015) | 12.5 mg bid, 25 mg qd, 5 mg bid, 10 mg qd | Placebo | 16 | 965 | 58.2 | N.R. | 7.8 | 131.3/78.6 | 89.2 | N.R. | No | eGFR |
| Wanner et al. (2016) | 10 mg, 25 mg | Placebo | 156(median) | 3064 | 63.1 | N.R. | 8.07 | 135.5/76.7 | 74.1 | N.R. | Yes | eGFR |
| IPRAGLIFLOZIN | ||||||||||||
| Wilding et al. (2013b) DOM | 12.5 mg, 50 mg, 150 mg, 300 mg | Placebo | 12 | 304 | 57.4 | 5.9 | 7.8 | N.R. | N.R. | N.R. | No | eGFR |
| Fonseca et al. (2013) | 12.5 mg, 50 mg, 150 mg, 300 mg | Placebo | 12 | 304 | 53.7 | 4.6 | 7.9 | N.R. | N.R. | N.R. | No | eGFR |
| Kashiwagi et al. (2015a) DI EMIT | 50 mg | Placebo | 24 | 240 | 59.7 | 10.5 | 8.4 | 130.0/76.6 | 84.7 | 50.8 | No | eGFR, uACR |
| Kashiwagi et al. (2015b) DI BRIGHTEN | 50 mg | Placebo | 16 | 129 | 59.4 | 6.7 | 8.3 | 130.0/128.2 | 87.8 | N.R. | No | eGFR |
| Kashiwagi et al. (2015c) DI SPOTLIGHT | 50 mg | Placebo | 24 | 151 | 56.2 | 6.8 | 9.3 | 130.4/77.9 | 91.0 | 39.3 | No | eGFR, uACR |
| Kashiwagi et al. (2015d) DOM LANTERN | 50 mg | Placebo | 24 | 164 | 64.4 | 9.5 | 7.5 | 133.3/77.3 | 60.9 | 148.2 | Yes | eGFR, uACR |
| Lu et al. (2016) | 50 mg | Placebo | 24 | 170 | 53.7 | 6.2 | 7.7 | N.R. | 149.2 | N.R. | No | eGFR, uACR |
| TOFOGLIFLOZIN | ||||||||||||
| Kaku et al. (2014) | 10 mg, 20 mg, 40 mg | Placebo | 24 | 212 | 57.3 | 6.4 | 8.4 | 129.2/78.3 | 85.4 | N.R. | No | eGFR |
Notes.
After six weeks, the canagliflozin dose was increased from 100 to 300 mg (or from placebo to matching placebo) if all of the following criteria were met: baseline eGFR ≥ 70 ml/min/1.73 m2; fasting self-monitored blood glucose ≥ 5.6 mmol/l (100 mg/dl); and no volume depletion-related adverse events within two weeks before dose increase.
From week 0 to week 18 (titration period), patients received an initial dose of dapagliflozin of 2.5 mg, which was up-titrated for patients with fasting blood glucose ≥ 110 mg/dl (6.1 mmol/l) until the maximum dose of 10 mg was reached. From week 19 to week 52 (maintenance period), the dose was no longer up-titrated but could be down-titrated in the event of recurrent hypoglycemia.
Mean baseline urine ACRs: normoalbuminuric group, 9.55 mg/g; microalbuminuric group, 86.3 mg/g; and macroalbuminuric group, 728.9 mg/g.
Abbreviations
- N.R.
- not reported
- uACR
- urine albumin/creatinine ratio
- IJCP
- International Journal of Clinical Practice
- DOM
- Diabetes, Obesity and Metabolism
- DI
- Diabetology International
EMIT, BRIGHTEN, SPOTLIGHT, and LANTERN are names of randomized controlled trials.
Quality assessment (Figs. S1 and S2) revealed that 32 studies described random sequence generation, and that 34 described allocation concealment. Twenty-seven studies described blinding of participants and personnel. All 47 studies had a low risk of detection or reporting bias, and 31 studies had a low risk of attrition bias.
Assessment of publication bias
Visual inspection revealed some asymmetry in the funnel plots for both eGFR (Fig. S3) and urine ACR (Fig. S4). Egger’s regression confirmed statistical significance of publication bias for urine ACR (p = 0.002), but not eGFR (p = 0.057).
Effects of SGLT2 inhibitors on eGFR
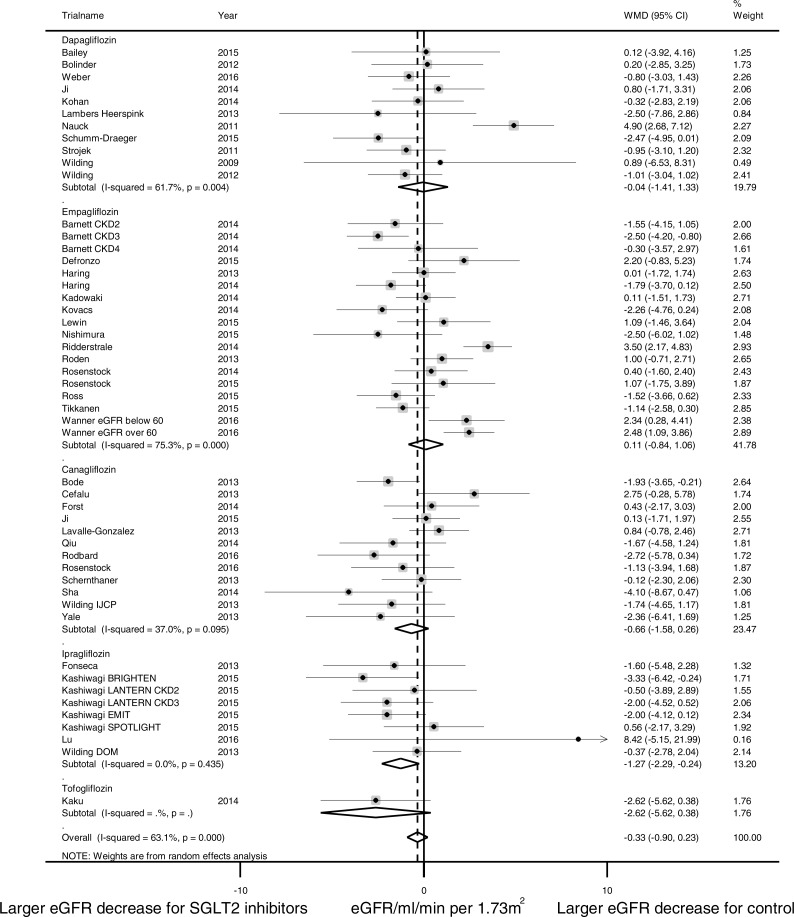
In pooled analysis of 46 studies reporting eGFR changes, no significant difference was observed between the SGLT2 treatment group and control group (calculated as ((end of trial eGFR for SGLT2 inhibition group—baseline eGFR for SGLT2 inhibition group)—(end of trial eGFR for control group—baseline eGFR for control group))) (WMD −0.33 ml/min per 1.73 m2, (95% CI [−0.90 to 0.23]); Fig. 2). Substantial heterogeneity was detected across studies (I2 = 63.1%, p < 0.001).
Figure 2. Effect of SGLT2 inhibition on eGFR.
The black dots represent mean differences of changes in eGFR, calculated as ((end of trial eGFR for SGLT2 inhibition–baseline eGFR for SGLT2 inhibition)–(end of trial eGFR for control—baseline eGFR for control)). The gray squares represent weights calculated using the random-effects model. The horizontal lines represent 95% confidence intervals (CIs). The hollow diamonds represent pooled mean differences and their 95% CIs. Negative values indicate that SGLT2 inhibitors had larger eGFR decrease than control. eGFR in ml/min per 1.73 m2.
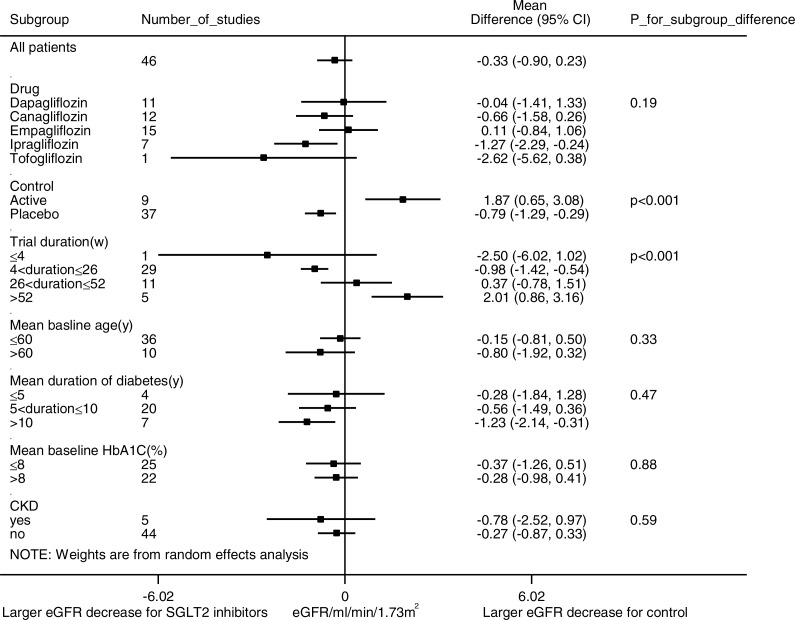
Pre-specified subgroup analyses were conducted to identify possible sources of heterogeneity (Fig. 3). Analysis of trial duration revealed that in the trials with a duration of 4–26 weeks, SGLT2 inhibition was associated with a larger eGFR reduction than control (WMD -0.98 ml/min per 1.73 m2, (95% CI [−1.42 to −0.54]), I2 = 0.9%, 29 studies with 10,946 patients); while in trials that lasted longer than 52 weeks, SGLT2 inhibition was associated with slower eGFR decline than control (WMD 2.01 ml/min per 1.73 m2, (95% CI [0.86 to 3.16]), I2 = 46.0%, 5 studies with 5,334 patients). SGLT2 inhibitors were observed with a larger eGFR reduction than placebo and a smaller eGFR reduction than active control. No significant eGFR difference was observed between the SGLT2 inhibitor group and control group in patients with CKD (WMD −0.78 ml/min per 1.73 m2, (95% CI [−2.52 to 0.97]), I2 = 65.0%, 5 studies with 1,574 patients). No significant subgroup differences were observed in subgroup analysis for the type of SGLT2 inhibitor, the mean duration of diabetes, or the mean baseline HbA1C level. Subgroup analyses for patients with hyperfiltration and RAAS inhibitor use had been planned but were hampered by lack of such stratification in the published data.
Figure 3. Subgroup analysis of the effect of SGLT2 inhibition on eGFR.
Pre-specified subgroup analyses were performed to address sources of heterogeneity. Weighted mean differences for eGFR are represented by small squares. The horizontal lines show 95% confidence intervals. The P values for subgroup differences are listed. Negative values indicate that the eGFR decrease was larger in the SGLT2 inhibition group compared with the control group.
Effects of SGLT2 inhibitors on urine ACR
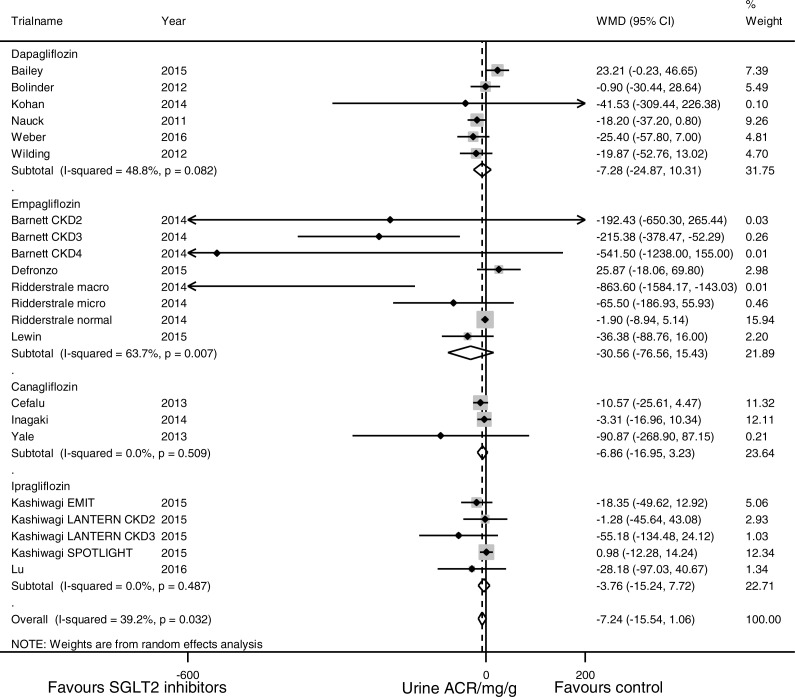
In pooled analysis of 17 studies evaluating the urine ACR, SGLT2 inhibition was not associated with statistically significant albuminuria reduction (WMD −7.24 mg/g, (95% CI [−15.54 to 1.06]), Fig. 4). Substantial heterogeneity was observed across studies (I2 = 39.2%, p = 0.03).
Figure 4. Effect of SGLT2 inhibition on urine albumin/creatinine ratio (ACR).
The black dots represent mean differences of changes in urine ACR, calculated as ((end of trial urine ACR for SGLT2 inhibition–baseline urine ACR for SGLT2 inhibition)–(end of trial urine ACR for control—baseline urine ACR for control)). The gray squares represent weights calculated using the random-effects model. The horizontal lines represent 95% confidence intervals (CIs). The hollow diamonds represent pooled mean differences and their 95% CIs. Negative values indicate that the SGLT2 inhibition group had less albuminuria than control. Urine ACR in mg/g.
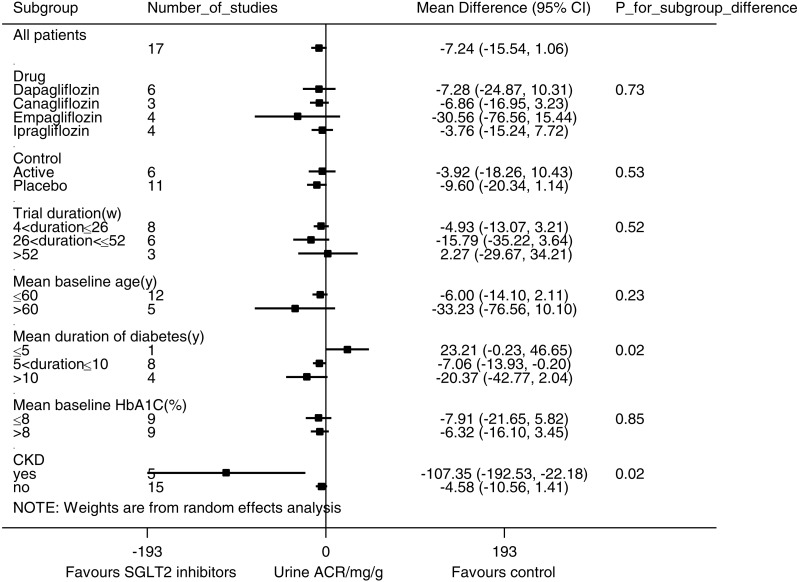
Subgroup analyses (Fig. 5) suggested that SGLT2 inhibition was associated with a significant urine ACR reduction in the participants with CKD (WMD −107.35 mg/g, (95% CI [−192.53 to −22.18]), I2 = 35.7%, 5 studies with 1,063 participants). Stratification according to the duration of diabetes mellitus revealed a trend of enhanced albuminuria reduction in the patients with a longer duration of diabetes mellitus (for the patients with a history of ≤5 years, WMD 23.21 mg/g, (95% CI [−0.23 to 46.65]), for the patients with a history of 5–10 years, WMD −7.06 mg/g, (95% CI [−13.93 to −0.20]), and for the patients with a history of >10 years, WMD −20.37 mg/g, (95% CI [−42.77 to 2.04]), p = 0.02 for subgroup difference). Subgroup analyses for the different SGLT2 inhibitors, use of placebo vs. active control, trial duration, mean baseline age and mean baseline HbA1C level did not reveal any significant subgroup difference.
Figure 5. Subgroup analysis of the effect of SGLT2 inhibition on urine albumin/creatinine ratio (ACR).
Pre-specified subgroup analyses were performed to address sources of heterogeneity. Weighted mean differences for urine ACR are represented by small squares. The horizontal lines show 95% confidence intervals. The P values for subgroup differences are listed. Negative values indicate that the SGLT2 inhibition group had less albuminuria than the control group.
Sensitivity analysis
Similar results were observed when analyses were limited to trials with relatively low risk (defined as no item with high risk and no more than 1 item with unclear risk) for eGFR (WMD −0.06 ml/min per 1.73 m2, (95% CI [−0.90 to 0.78]), I2 = 73.0%, 24 trials with 14,535 participants) and urine ACR (WMD −7.25 mg/g, (95% CI [−17.25 to 2.76]), I2 = 48.0%, 12 trials with 5,308 participants).
Discussion
In this systematic review and meta-analysis, we identified no significant effect of SGLT2 inhibition on eGFR either in type 2 diabetic patients in general or in type 2 diabetic patients with CKD. Subgroup analysis suggested dipping of eGFR in shorter trials and preservation of eGFR in trials of longer duration. Urine ACR reduction after SGLT2 inhibition was not statistically significant in type 2 diabetic patients in general, but was significant in patients with CKD.
SGLT2 inhibitors can exert their effects on the diabetic kidney through several different mechanisms. First, SGLT2 inhibitors can reverse hyperfiltration and attenuate albuminuria by restoring impaired TGF (Cherney et al., 2014; Vallon et al., 2014). In patients with diabetes, upregulation of SGLT2 increases reabsorption of sodium and glucose along the proximal tubules (Rahmoune et al., 2005), attenuates macula densa-mediated vasoconstriction of afferent arterioles, and results in an increased GFR. SGLT2 inhibition is thought to restore impaired TGF and to reverse hyperfiltration (Skrtic & Cherney, 2015). Second, SGLT2 inhibitors have been shown to alleviate inflammation and to protect the kidney by reducing glucose trafficking through proximal tubule cells (Panchapakesan et al., 2013). Third, SGLT2 inhibition can protect the kidney through systematic changes, including enhanced glycemic control, osmotic diuresis, natriuresis (Rajasekeran, Lytvyn & Cherney, 2016), blood pressure lowering (Baker et al., 2014; Weber et al., 2016), and weight loss (Zinman et al., 2015a).
In our meta-analysis, we identified no statistically significant impact of SGLT2 inhibitors on eGFR in type 2 diabetic patients overall, in line with a previous meta-analysis (Liu et al., 2015). However, this might result from a mixture of initial eGFR dipping and long-term eGFR preservation. We noticed a pattern of eGFR reduction in the short-term studies and eGFR preservation in the longer-term studies, as has been reported in several clinical trials (Cefalu et al., 2013; Kohan et al., 2014; Lambers Heerspink et al., 2013; Strojek et al., 2011; Wanner et al., 2016; Yale et al., 2013), including the EMPA-REG OUTCOME study (Wanner et al., 2016), and pooled analyses (Kohan et al., 2016; Yamout et al., 2014). This pattern, as well as the reversibility of eGFR after drug discontinuation (Barnett et al., 2014; Wanner et al., 2016), suggests that initial reduction of eGFR is probably caused by hemodynamic changes, either acute volume contraction or rapid upregulation of TGF, rather than by structural damage. We also noticed that SGLT2 inhibitors had a larger eGFR reduction than placebo and a smaller eGFR reduction than active control. However, confounding by trial duration was likely considering that all 9 trials with active control had a duration of 52 weeks or longer. As none of the included studies had a stratification of hyperfiltrative patients and only one study had a mean baseline eGFR in the hyperfiltrative range (Lu et al., 2016), we were unable to evaluate eGFR changes in the subgroup of patients with hyperfiltration, as has been reported before (Cherney et al., 2014).
Regarding albuminuria, we found that SGLT2 inhibition was associated with significant urine ACR reduction in type 2 diabetic patients in CKD, but not in type 2 diabetic patients in general. The lack of a substantial urine ACR reduction in patients without CKD may be explained by their low baseline urine ACR, i.e., the urine ACR in normoalbuminuric patients does not decrease by more than 30 mg/g. Although previous pooled analyses reported positive results of SGLT2 inhibitors in albuminuria reduction, they largely included patients with microalbuminuria and macroalbuminuria at baseline (Cherney et al., 2016; Fioretto et al., 2016; Heerspink et al., 2016; Yamout et al., 2014). Our results, in accord with findings of previous clinical trials (Barnett et al., 2014; Wanner et al., 2016) and post hoc analyses (Cherney et al., 2016; Heerspink et al., 2016; Yamout et al., 2014), demonstrate the role of SGLT2 inhibitors in slowing the progression of albuminuria. However, it is still unclear whether SGLT2 inhibitors can prevent incident albuminuria. Empagliflozin was observed to reduce incident albuminuria in the EMPA-REG RENAL trial, but not in the EMPA-REG OUTCOME trial (Barnett et al., 2014; Wanner et al., 2016). Our analysis did not identify significant subgroup difference between different SGLT2 inhibitors either in eGFR or in urine ACR, suggesting drug class rather than molecule specific effects. However, confirmation from long-term trials conducted in different SGLT2 inhibitors, such as the CREDENCE trial (NCT02065791), is still needed.
Given the important yet incomplete renoprotective roles of RAAS inhibitors in diabetic nephropathy (Bilous et al., 2009; Lewis et al., 1993; Lewis et al., 2001; Mauer et al., 2009), another question to consider is whether SGLT2 inhibition has additive renoprotective effects to RAAS inhibition. SGLT2 inhibition reduces intraglomerular pressure by constriction of afferent arterioles through upregulation of TGF, while RAAS blockage mainly dilates efferent arterioles. Besides acting at different intrarenal sites, SGLT2 inhibition activates RAAS systematically, probably due to volume contraction (Cherney et al., 2014). Thus, it is plausible for SGLT2 inhibition and RAAS inhibition to work synergistically and there is accumulating evidence for this synergy. Weber et al. (2016) reported that in diabetic patients on RAAS inhibitors, addition of dapagliflozin was associated with better blood pressure control, no significant difference in eGFR and a trend toward albuminuria reduction relative to placebo. In the EMPA-REG OUTCOME trial, where 80.7% of patients were taking RAAS inhibitors, empagliflozin was associated with slower progression of nephropathy than placebo (Wanner et al., 2016). Future trials focusing on patients with background RAAS inhibition, such as the CREDENCE study, will further shed light on this issue.
Despite rigorous methodology, our study has several limitations. First, the evaluation of eGFR changes in type 2 diabetic patients overall might be obscured by mixing short-term eGFR decrease and long-term eGFR preservation. Second, substantial heterogeneity in analyses of both eGFR and urine ACR may have complicated the interpretation of our data. Third, our study used surrogate endpoints, including eGFR and urine ACR, rather than hard endpoints, such as progression of nephropathy or renal and cardiovascular mortality (Stevens, Greene & Levey, 2006). The EMPA-REG OUTCOME trial provided solid evidence that empagliflozin reduced the risk of progression of albuminuria, doubling of serum creatinine, initiation of renal replacement therapy and cardiovascular death in type 2 diabetic patients with high cardiovascular risk (Wanner et al., 2016), findings to be confirmed by the ongoing CREDENCE trial with primary renal outcomes.
In conclusion, SGLT2 inhibition was not associated with significant changes in eGFR in type 2 diabetic patients, which may result from a mixture of an initial reduction of eGFR and long-term renal function preservation. SGLT2 inhibition was associated with albuminuria reduction in type 2 diabetic patients with CKD. The therapeutic value of SGLT2 inhibitors in the prevention and management of diabetic nephropathy warrants further study.
Supplemental Information
Study quality were evaluated using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1. Green, yellow and red bars represent low, unclear and high risk of bias, respectively.
Study quality were evaluated using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1. Green, yellow and red dots represent low, unclear and high risk of bias, respectively.
There is no statistically significant publication bias.( p = 0.057).
There is substantial publication bias (p = 0.002).
Acknowledgments
This manuscript was edited for English language by American Journal Experts (AJE).
Funding Statement
This work was supported by the Key Project of the Chinese National Program for Fundamental Research and Development (973 Program 2012CB517803 to LC), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-2-004 to LC), the National Natural Science Foundation, China (81170674 and 81470937 to LC), and Xiehe Scholar Fund to LC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Lubin Xu conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Yang Li performed the experiments, analyzed the data, prepared figures and/or tables.
Jiaxin Lang performed the experiments, prepared figures and/or tables.
Peng Xia performed the experiments.
Xinyu Zhao performed the experiments, analyzed the data, contributed reagents/materials/analysis tools.
Li Wang analyzed the data, contributed reagents/materials/analysis tools.
Yang Yu conceived and designed the experiments, reviewed drafts of the paper.
Limeng Chen conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary File.
References
- Bailey et al. (2015).Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabetic Medicine. 2015;32:531–541. doi: 10.1111/dme.12624. [DOI] [PubMed] [Google Scholar]
- Baker et al. (2014).Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. Journal of the American Society of Hypertension. 2014;8:262–275. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Barnett et al. (2014).Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinology. 2014;2:369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- Bilous et al. (2009).Bilous R, Chaturvedi N, Sjolie AK, Fuller J, Klein R, Orchard T, Porta M, Parving HH. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Annals of Internal Medicine. 2009;151:11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. W13–W14. [DOI] [PubMed] [Google Scholar]
- Bode et al. (2013).Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hospital Practice (1995) 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- Bolinder et al. (2012).Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. Journal of Clinical Endocrinology and Metabolism. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- Cefalu et al. (2013).Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- Cherney et al. (2016).Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, Von Eynatten M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860–1870. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- Cherney et al. (2014).Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, Von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- Dahlquist, Stattin & Rudberg (2001).Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrology, Dialysis, Transplantation. 2001;16:1382–1386. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- DeFronzo et al. (2015).DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–393. doi: 10.2337/dc14-2364. [DOI] [PubMed] [Google Scholar]
- Fioretto et al. (2016).Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59(9):2036–2039. doi: 10.1007/s00125-016-4017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca et al. (2013).Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. Journal of Diabetes and its Complications. 2013;27:268–273. doi: 10.1016/j.jdiacomp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Forst et al. (2014).Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes, Obesity and Metabolism. 2014;16:467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring et al. (2014).Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–1659. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- Haring et al. (2013).Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerspink et al. (2016).Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes, Obesity and Metabolism. 2016;18:590–597. doi: 10.1111/dom.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins & Green (2011).Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Wiley-Blackwell; Chichester: 2011. [Google Scholar]
- Inagaki et al. (2014).Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opinion on Pharmacotherapy. 2014;15:1501–1515. doi: 10.1517/14656566.2014.935764. [DOI] [PubMed] [Google Scholar]
- Ji et al. (2015).Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, Qiu R, Meininger G. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes, Obesity and Metabolism. 2015;17:23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- Ji et al. (2014).Ji L, Ma J, Li H, Mansfield TA, T’Joen CL, Iqbal N, Ptaszynska A, List JF. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clinical Therapeutics. 2014;36:84–100. doi: 10.1016/j.clinthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Kadowaki et al. (2014).Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, Rattunde H, Woerle HJ, Broedl UC. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Advances in Therapy. 2014;31:621–638. doi: 10.1007/s12325-014-0126-8. [DOI] [PubMed] [Google Scholar]
- Kaku et al. (2014).Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, Tanizawa Y, Araki E, Ueda M, Suganami H, Watanabe D. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovascular Diabetology. 2014;13(1):65. doi: 10.1186/1475-2840-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi et al. (2015a).Kashiwagi A, Akiyama N, Shiga T, Kazuta K, Utsuno A, Yoshida S, Ueyama E. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetology International. 2015a;6:125–138. doi: 10.1007/s13340-014-0184-9. [DOI] [Google Scholar]
- Kashiwagi et al. (2015b).Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study: BRIGHTEN: double-blind randomized study of ipragliflozin to show its efficacy as monotherapy in T2DM patients. Diabetology International. 2015b;6:8–18. doi: 10.1007/s13340-014-0164-0. [DOI] [Google Scholar]
- Kashiwagi et al. (2015c).Kashiwagi A, Shiga T, Akiyama N, Kazuta K, Utsuno A, Yoshida S, Ueyama E. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study) Diabetology International. 2015c;6:104–116. doi: 10.1007/s13340-014-0182-y. [DOI] [Google Scholar]
- Kashiwagi et al. (2015d).Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, Ueyama E. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes, Obesity and Metabolism. 2015d;17:152–160. doi: 10.1111/dom.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan et al. (2016).Kohan DE, Fioretto P, Johnsson K, Parikh S, Ptaszynska A, Ying L. The effect of dapagliflozin on renal function in patients with type 2 diabetes. Journal of Nephrology. 2016;29:391–400. doi: 10.1007/s40620-016-0261-1. [DOI] [PubMed] [Google Scholar]
- Kohan et al. (2014).Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney International. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs et al. (2014).Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2014;16:147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- Lambers Heerspink et al. (2013).Lambers Heerspink HJ, De Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes, Obesity and Metabolism. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalle-Gonzalez et al. (2013).Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin et al. (2015).Lewin A, DeFronzo RA, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38:394–402. doi: 10.2337/dc14-2365. [DOI] [PubMed] [Google Scholar]
- Lewis et al. (1993).Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. New England Journal of Medicine. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- Lewis et al. (2001).Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. Journal of Diabetes and its Complications. 2015;29:1295–1303. doi: 10.1016/j.jdiacomp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. Journal of Diabetes Investigation. 2016;7:366–373. doi: 10.1111/jdi.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei et al. (2015).Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S, Study 05 Group Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial. Diabetes Care. 2015;38:365–372. doi: 10.2337/dc14-0666. [DOI] [PubMed] [Google Scholar]
- Mauer et al. (2009).Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. New England Journal of Medicine. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher et al. (2009).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- Nauck et al. (2011).Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura et al. (2015).Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, Lund SS, Broedl UC. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovascular Diabetology. 2015;14(1):11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchapakesan et al. (2013).Panchapakesan U, Pegg K, Gross S, Komala MG, Mudaliar H, Forbes J, Pollock C, Mather A. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLOS ONE. 2013;8:e54442. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Capuano & Meininger (2014).Qiu R, Capuano G, Meininger G. Efficacy and safety of twice-daily treatment with canagliflozin, a sodium glucose co-transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. Journal of Clinical and Translational Endocrinology. 2014;1:54–60. doi: 10.1016/j.jcte.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmoune et al. (2005).Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- Rajasekeran, Lytvyn & Cherney (2016).Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney International. 2016;89:524–526. doi: 10.1016/j.kint.2015.12.038. [DOI] [PubMed] [Google Scholar]
- Ridderstrale et al. (2014).Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- Rodbard et al. (2016).Rodbard HW, Seufert J, Aggarwal N, Cao A, Fung A, Pfeifer M, Alba M. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes, Obesity and Metabolism. 2016;18(8):812–819. doi: 10.1111/dom.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden et al. (2013).Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- Rosenstock et al. (2016).Rosenstock J, Chuck L, Gonzalez-Ortiz M, Merton K, Craig J, Capuano G, Qiu R. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naive type 2 diabetes. Diabetes Care. 2016;39:353–362. doi: 10.2337/dc15-1736. [DOI] [PubMed] [Google Scholar]
- Rosenstock et al. (2014).Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- Rosenstock et al. (2015).Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2015;17:936–948. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross et al. (2015).Ross S, Thamer C, Cescutti J, Meinicke T, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2015;17:699–702. doi: 10.1111/dom.12469. [DOI] [PubMed] [Google Scholar]
- Schernthaner et al. (2013).Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm-Draeger et al. (2015).Schumm-Draeger PM, Burgess L, Korányi L, Hruba V, Hamer-Maansson JE, Bruin TW. Twice-daily dapagliflozin co-administered with metformin in type 2 diabetes: a 16-week randomized, placebo-controlled clinical trial. Diabetes, Obesity & Metabolism. 2015;17(1):42–51. doi: 10.1111/dom.12387. [DOI] [PubMed] [Google Scholar]
- Sha et al. (2014).Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Morschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2014;16:1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- Skrtic & Cherney (2015).Skrtic M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Current Opinion in Nephrology and Hypertension. 2015;24:96–103. doi: 10.1097/MNH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- Sochett et al. (2006).Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. Journal of the American Society of Nephrology. 2006;17:1703–1709. doi: 10.1681/ASN.2005080872. [DOI] [PubMed] [Google Scholar]
- Stevens, Greene & Levey (2006).Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clinical Journal of the American Society of Nephrology. 2006;1:874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- Strojek et al. (2011).Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2011;13:928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- Thomson et al. (2012).Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen et al. (2015).Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- Tuttle et al. (2014).Tuttle KR, Bakris GL, Bilous RW, Chiang JL, De Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. American Journal of Kidney Diseases. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Vallon et al. (2014).Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. American Journal of Physiology. Renal Physiology. 2014;306:F194–F204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner et al. (2016).Wanner C, Inzucchi SE, Lachin JM, Fitchett D, Von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. New England Journal of Medicine. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- Weber et al. (2016).Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- Wild et al. (2004).Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wilding et al. (2015).Wilding JPH, Blonde L, Leiter LA, Cerdas S, Tong C, Yee J, Meininger G. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. Journal of Diabetes and Its Complications. 2015;29:438–444. doi: 10.1016/j.jdiacomp.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Wilding et al. (2013a).Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. International Journal of Clinical Practice. 2013a;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding et al. (2013b).Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes, Obesity and Metabolism. 2013b;15:403–409. doi: 10.1111/dom.12038. [DOI] [PubMed] [Google Scholar]
- Wilding et al. (2009).Wilding JP, Norwood P, T’Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding et al. (2012).Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Annals of Internal Medicine. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- Yale et al. (2013).Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes, Obesity and Metabolism. 2013;15:463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamout et al. (2014).Yamout H, Perkovic V, Davies M, Woo V, De Zeeuw D, Mayer C, Vijapurkar U, Kline I, Usiskin K, Meininger G, Bakris G. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. American Journal of Nephrology. 2014;40:64–74. doi: 10.1159/000364909. [DOI] [PubMed] [Google Scholar]
- Zinman et al. (2015a).Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015a;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- Zinman et al. (2015b).Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015b;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study quality were evaluated using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1. Green, yellow and red bars represent low, unclear and high risk of bias, respectively.
Study quality were evaluated using the ‘Risk of bias’ assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1. Green, yellow and red dots represent low, unclear and high risk of bias, respectively.
There is no statistically significant publication bias.( p = 0.057).
There is substantial publication bias (p = 0.002).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary File.