Abstract
Anticoagulation by a standard dosage of an inhibitor of thrombin generation presupposes predictable pharmacokinetics and pharmacodynamics of the anticoagulant. We determined the inter-individual variation of the effect on thrombin generation of a fixed concentration of direct and antithrombin-mediated inhibitors of thrombin and factor Xa. Thrombin generation was determined by calibrated automated thrombinography in platelet-poor plasma from 44 apparently healthy subjects which was spiked with fixed concentrations of otamixaban, melagatran, unfractionated heparin, dermatan sulfate and pentasaccharide. The variability of the inhibitory effect of the different anticoagulants within the population was determined using the coefficient of variation, i.e. the standard deviation expressed as a percentage of the mean. The inter-individual coefficients of variation of the endogenous thrombin potential and peak height before inhibition were 18% and 16%, respectively and became 20%-24% and 24%-43% after inhibition. The average inhibition of endogenous thrombin potential and peak height (ETP, peak) brought about by the anticoagulants was respectively: otamixaban (27%, 83%), melagatran (56%, 63%), unfractionated heparin (43%, 58%), dermatan sulfate (68%, 57%) and pentasaccharide (25%, 67%). This study demonstrates that the addition of a fixed concentration of any type of anticoagulant tested causes an inhibition that is highly variable from one individual to another. In this respect there is no difference between direct inhibitors of thrombin and factor Xa and heparin(-like) inhibitors acting on the same factors.
Introduction
Selective inhibitors of factor Xa and thrombin are in clinical development for the prevention and treatment of thrombosis.1 There would be many practical advantages from an antithrombotic that could be taken at a fixed dosage and that would not require its effect to be controlled. Trials showing that direct inhibitors of thrombin or factor Xa (FXa), when given in fixed doses, are not inferior to adjusted dose treatment with vitamin K antagonists are, therefore, hailed with enthusiasm.2-5
Bleeding and thrombosis in patients receiving anticoagulant treatment have a local cause but are influenced by the coagulability of the blood, i.e. by the systematic component that determines the response to the local cause. There is a large body of evidence indicating that the thrombin-generating capacity of plasma is an important element in this systematic component and that it is the function that is diminished by antithrombotics.6,7 The effect of antithrombotic treatment is, therefore, likely due to its effect on the thrombin-generating capacity of blood. This determines the therapeutic results of the treatment, which are measured as the rates of thrombosis and bleeding in the treated group in comparison to a reference group.
The anticoagulant effect itself is a combination of how much of the drug reaches the target organ, i.e. the plasma (pharmacokinetics) and how the function of the target organ, i.e. the coagulability of plasma, is influenced by the drug (pharmacodynamics).
The pharmacokinetics of the new drugs have been reported to be predictable and stable.8-10 For a fixed-dose treatment to be safe and effective one would like the pharmacodynamic response to be predictable and stable within the population as well. We, therefore, measured the response of thrombin generation to a fixed concentration of different anticoagulants in a series of individual normal plasmas (n=44). We tested unfractionated heparin (UFH), known to enhance the anti-thrombin and anti-factor Xa activities of plasma antithrombin;11 dermatan sulfate, which enhances the anti-thrombin activity of heparin cofactor II;12 pentasaccharide, which specifically enhances the anti-factor Xa action of plasma antithrombin;13 otamixaban, a direct and reversible inhibitor of FXa;14 and melagatran, a direct and reversible inhibitor of thrombin.15 Of each of these drugs we used a concentration that inhibits around 50% of either the thrombin generation peak or the endogenous thrombin potential (ETP: area under the thrombin generation curve).
Design and Methods
Preparation of platelet-poor plasma
Blood was acquired from apparently healthy subjects by antecubital venipuncture and was collected into BD vacutainer tubes (1 volume of trisodium citrate 0.105 M to 9 volumes of blood) in the absence of corn trypsin inhibitor (BD Vacutainer System, Roborough, Plymouth, UK). Platelet-poor plasma was obtained by centrifuging the blood at 2,900 g for 10 min at room temperature. Plasma was aspirated and the procedure was repeated. Aliquots of 2 mL were prepared and stored at -80°C until use.
The platelet-poor plasma from the 44 individual donors was pooled (equal volumes from each donor) and is referred to as the ‘pool’ in this article. Normal pooled plasma (NPP) was prepared, previously, as described above, from at least 24 apparently healthy donors, different from those already mentioned and was used as a reference plasma. All enrolled volunteers gave their full informed consent according to the Helsinki Declaration and its amendments; the study fulfilled all institutional ethical requirements and was approved by the Medical Ethical Committee of Maastricht University Medical Center.
Reagents
Synthetic procoagulant phospholipids were obtained from Avanti Polar Lipids Inc. (Alabaster, AL, USA) and added in the form of vesicles consisting of phosphatidylserine, phosphatidylethanolamine and phosphatidylcholine (1:1:3, mol:mol). The recombinant tissue factor used was Innovin (Dade-Behring, Marburg, Germany). The fluorogenic substrate Z-Gly-Gly-Arg-aminomethylcoumarine (ZGGR-AMC) was purchased from Bachem (Basel, Switzerland). A calibrator was prepared as described by Hemker et al.16 Hepes buffers containing 5 mg/mL or 60 mg/mL bovine serum albumin (BSA5 and BSA60, respectively) were prepared as described previously.17
The anticoagulants were obtained from different sources: otamixaban (a direct FXa inhibitor) was a gift from Sanofi-Aventis (Frankfurt, Germany), melagatran (a direct thrombin inhibitor) was provided by AstraZeneca (Zoetermeer, the Netherlands), UFH (Liquemin® N) was from Hoffman-La Roche AG (Basel, Switzerland), dermatan sulfate (Mistral) was a gift from Mediolanum Farmaceutici S.p.A. (Milan, Italy) and the synthetic pentasaccharide (Org31540/SR90107A, currently known as fondaparinux) was a gift from Dr. Petitou (Sanofi-Recherche, Toulouse, France).
Calibrated automated thrombinography
Calibrated automated thrombinography (CAT) was performed as described previously by Hemker et al.16 Ten microliters of tissue factor/phospholipid mixture in BSA5 buffer was added to a well with 10 μL of BSA5 buffer with or without the anticoagulant (anticoagulants were not added to calibrator wells). The final concentrations of recombinant tissue factor and phospholipids were 5 pM and 4 μM, respectively. Thrombin generation was initiated by adding 20 mL of ZGGR-AMC (2.5 mM) and CaCl2 (100 mM) in BSA60 buffer. NPP served as a control in each measurement. All experiments were performed in triplicate and the pre-warmed plate was placed in the fluorometer at 37°C for 10 min before initializing the measurement.
Experiments were constructed in such a manner as to measure the effect of all the different anticoagulants on the platelet-poor plasma of one (or more) donor(s) in one run. The experimental error in thrombin generation without any addition, as determined in NPP under these conditions, was 6.5% for the ETP and 4.7% for the peak (n = 37). The experimental error after addition of an anticoagulant to NPP was calculated with data of 14 measurements.
The thrombin generation curves and their parameters were derived from the fluorescence curves using the dedicated software provided with the CAT method (Thrombinoscope BV, Maastricht, the Netherlands).
Anticoagulant concentrations
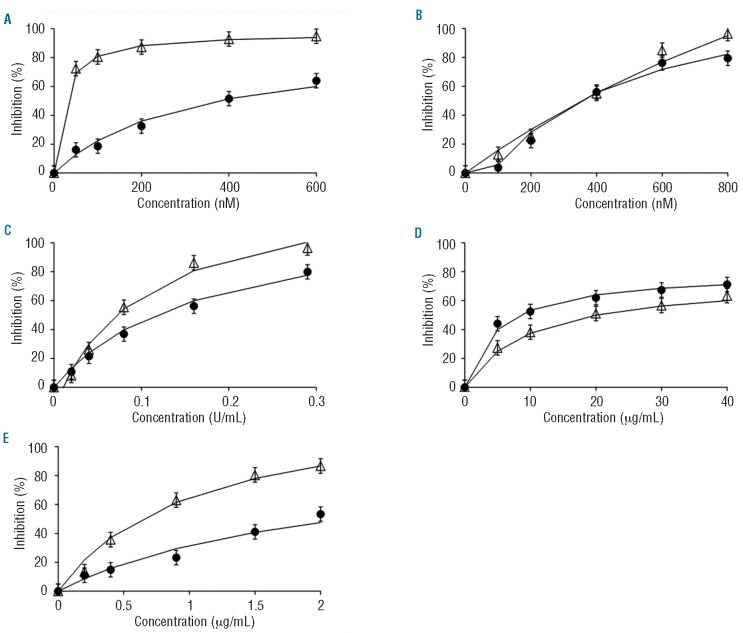
Dose-response curves were constructed in order to select the IC50 concentrations for each anticoagulant. However, the parameters of the CAT assay (ETP, peak height, time to peak and lag time) were affected diversely, which made it impossible to compare the effects of the different agents by considering only one parameter. Since the ETP and peak height were the most informative parameters, we chose a fixed concentration of the anticoagulants at which the sum of the inhibitions of ETP and peak was between 80 and 120%. Consequently, the following concentrations were utilized: otamixaban 200 nM, melagatran 400 nM, dermatan sulfate 20 μg/mL, pentasaccharide 0.9 μg/mL, and UFH 0.08 U/mL (with around 30% high-affinity material18). The concentrations of the new anticoagulants used here are comparable to plasma concentrations effective in-vivo. A study in healthy male subjects used predicted target concentrations of otamixaban of 100 ng/mL; i.e. about 210 nM (200 nM used here).19 For melagatran, the proposed therapeutic plasma concentration is <500 nM, (400 nM used here).15 Therapeutic doses of fondaparinux lead to a concentration of approximately 1.4 μg/mL.20 The concentration of pentasaccharide in the present study is deliberately lower, because the aim was not to measure within the plateau region, which would obscure inter-individual differences.
Data analysis
To quantify the variability of the inhibitory effect of the different antithrombotics within the 44 normal plasma samples we used the coefficient of variation (CV), i.e. the standard deviation expressed as a percentage of the average. The total CV is caused by random experimental error (CVerror) and by inter-individual variation (CVii). The experimental error was derived from the measurements in NPP, which served as a reference plasma during each run.
The coefficient of inter-individual variation was calculated as:
The same formula was used to calculate the variation in susceptibility to inhibition (CVsusc, i.e. how much extra variation is induced by adding an anticoagulant) from the total CV of ETP and peak found in the absolute values of the inhibited (CVinh) plasmas and in the uninhibited plasmas (CVuninh):
The inhibition of thrombin-generating capacity was calculated as follows: 100-[(ETP after addition of an anticoagulant/ETP before addition of an anticoagulant) × 100].
Results
Inhibition of normal pooled plasma
Important qualitative differences in the type of inhibition were observed (Figure 1). The direct inhibitors (otamixaban, melagatran) had a stronger effect on the lag time than the heparin(-like) inhibitors. Inhibition of factor Xa (otamixaban, pentasaccharide) primarily affected the peak and tended to protract thrombin generation so that the ETP was much less affected than the peak. In order to find concentrations that caused around 50% inhibition, we constructed dose-response curves in NPP (Figure 2). Inhibition of factor Xa systematically inhibited the peak more than the ETP, inhibition of thrombin affected both to a similar extent, UFH produced an intermediate position. Because of these differences an unequivocal IC50 could not be defined, as mentioned before, and arbitrarily we chose that concentration at which the sum of the inhibitions of ETP and peak was between 80 and 120%.
Figure 1.
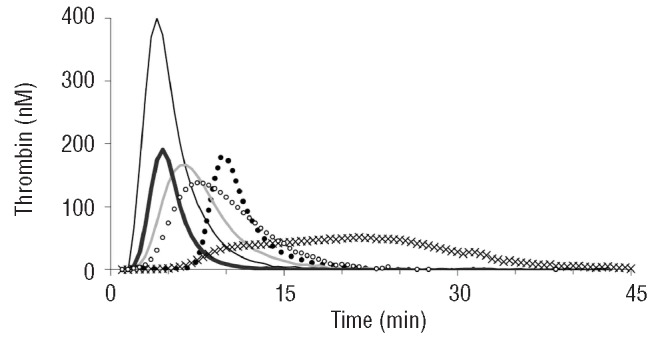
Effect of anticoagulants on thrombin generation in normal pooled plasma. Thin black line: no anticoagulant added; line of Xs: 200 nM otamixaban; closed dots: 400 nM melagatran; thin gray line: 0.08 U/mL unfractionated heparin; bold black line: 20 μg/mL dermatan sulfate; open dots: 0.9 μg/mL pentasaccharide.
Figure 2.
Dose-response curves of ETP and peak height in normal pooled plasma. Open triangles: % inhibition of peak height; closed dots: % inhibition of endogenous thrombin potential (with 5% error bars). (A) otamixaban (B) melagatran, (C) unfractionated heparin, (D) dermatan sulfate, (E) pentasaccharide.
In order to determine the inter-assay CV of thrombin generation, we tested the effect of the above-mentioned concentrations in NPP in 14 fold. The inter-assay CV of the inhibition of the peak was 2-5%, except for UFH (8.5%). The CV of the inhibition of the ETP varied between 2-6%, while otamixaban and pentasaccharide had a higher CV of 16% and 15%, respectively.
Inhibition of individual plasma specimens
The qualitative differences that were observed in NPP were also seen in the individual plasma samples. Table 1 shows that the direct inhibitors had a strong effect on the lag time whereas the effect of the inhibitors requiring plasma cofactors was much less. The variation between individual samples was between 18-30% after correction for experimental errors. Similar effects on time-to-peak were seen, with variations between 13 and 37% in inhibited plasma samples and a CV of 14% in uninhibited plasma after correction for the CV from experimental errors.
Table 1.
Influence of anticoagulants on lag time.
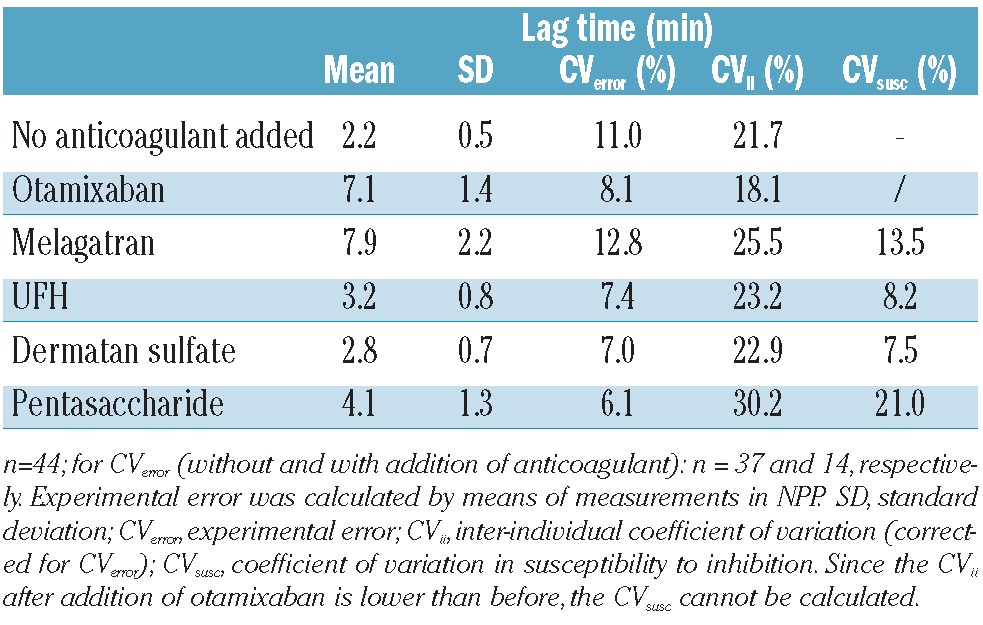
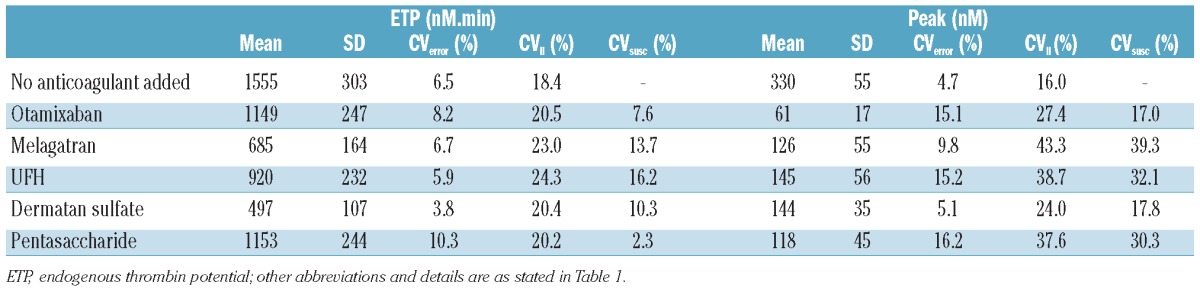
Table 2 summarizes the effect of the antithrombotics on ETP and peak at fixed concentrations in the 44 individual plasma samples. The CV have been corrected for the experimental error. It can be seen that addition of the anticoagulant induced an extra variation superimposed on the interindividual variation of the uninhibited values. The uninhibited peak variation was 16.0% and the CV values became larger after adding antithrombotic agents (24.0 to 43.3%). The variation in ETP without addition of an antithrombotic was 18.4% which increased to between 20.2 and 24.3% after addition of anticoagulants.
Table 2.
Variation in inhibition by anticoagulants in individual plasma samples.
Susceptibility to inhibition
The larger CV after inhibition compared to before suggests that the plasma of some individuals is more susceptible to inhibition than that of others. It is possible that this susceptibility is dependent on the activity of thrombin generation in the uninhibited plasma. We, therefore, investigated whether the degree of inhibition is dependent on the value of uninhibited (“basal”) thrombin generation, i.e. whether individuals with a high basal thrombin generation would be more prone to inhibition than those with a lower one. Plots of the degree of inhibition against the basal ETP or peak did not show any consistent correlation (data not shown). It can, therefore, be assumed that the variation in inhibition superimposes independently upon the variation of the basal values.
The variation in the susceptibility to the anticoagulants (i.e. how much extra variation is induced by the addition of the anticoagulant) was calculated from the variation in the absolute values obtained before and after inhibition. These values varied between 17.0 and 39.3% for peak height and between 2.3% and 16.2% for the ETP, without apparent differences between direct and heparin(-like) inhibitors (Table 2). The variation in susceptibility was also calculated for the lag time, as indicated in Table 1.
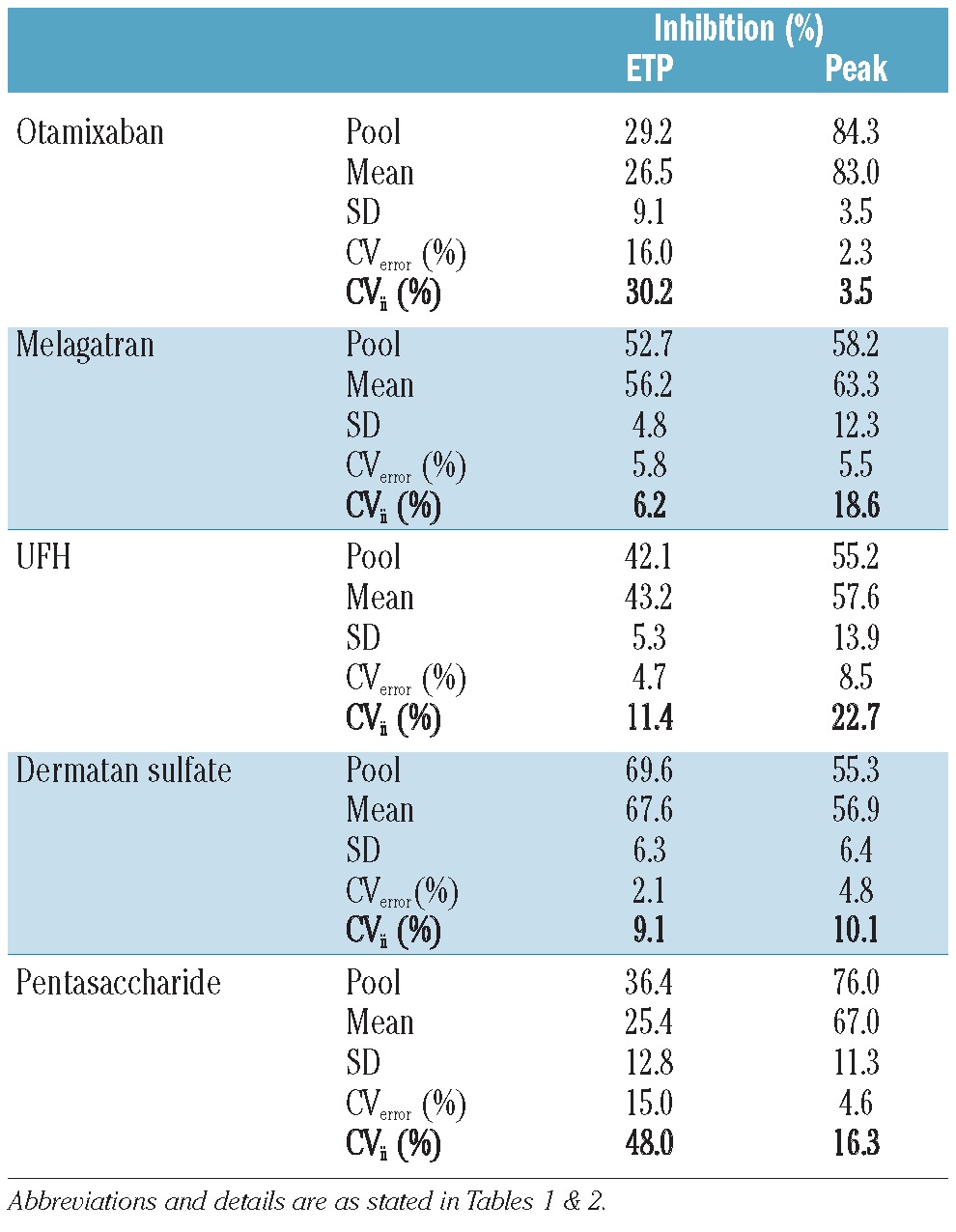
The average inhibition by the anticoagulants was 25-68% for the ETP and 57-83% for peak height (Table 3). The level of inhibition was also determined in the pool prepared from the 44 individual plasmas. It was comparable to the mean inhibition obtained from the 44 individual plasma samples (within a range of 5%) for all anticoagulants, with the exception of pentasaccharide.
Table 3.
Inhibition (%) of the individual plasmas samples (with the variation) and their pool after addition of anticoagulants.
Discussion
In clinical trials, direct inhibitors of thrombin or of FXa are not inferior to prophylaxis with traditional vitamin K antagonists.2-5 It has been surmised, however, that certain patients could benefit from dose adaptation.22,23 This raises the question of whether dose adaptation should be restricted to certain categories or whether it could improve the results with direct inhibitors so that these become better than the – admittedly not ideal - results of prophylaxis with vitamin K antagonists. This article deals with a partial problem pertaining to this question, viz. will the plasma of different individuals react similarly to a fixed dose of antithrombotic?
We start from the a priori assumption - supported by a wealth of literature16,20,24-30 - that thrombin generation is a sensitive surrogate variable for bleeding or, conversely, thrombotic tendency. All known drugs that diminish thrombin generation have an antithrombotic action, independently of their mode of action. It is, therefore, a reasonable assumption that antithrombotic drugs, such as those tested in this study, act because they diminish thrombin generation and that their action can be quantified by measuring to what degree they diminish it. In contrast, clotting times (activated partial thromboplastin time, prothombin time, lag time of thrombin generation) may or may not be prolonged, depending on the nature of the antithrombotic agent and/or the condition of the assay24,31,32 (Figure 1 and Table 1).
It is known that the capacity to form thrombin varies widely between individuals. In a normal adult population the coefficient of variation is ∼15%.16 Our results show that adding a fixed amount of any anticoagulant causes a variable inhibitory effect with a CV of 6-48% for ETP and 3-23% for peak height, which adds to the variation already present in the population. The variability of effect of the modern antithrombotics, otamixaban, melagatran and pentasaccharide, is just the same as that of UFH or dermatan sulfate. From the literature it is known that low molecular weight heparins do no better either.20
There must be a range of thrombin generation values that minimizes the risk of thrombosis without causing undue bleeding risk. What this “prophylactic window” is, cannot be determined with any accuracy at the moment. It has only been defined for vitamin K antagonist treatment, which however affects the protein C system as well as the procoagulant system33 and is not, therefore, directly comparable to the materials tested here. After a first idiopathic venous thrombosis people with above average thrombin generation have a four times higher risk of recurrence than those with below average thrombin generation.34 This would mean that moderate anticoagulation resulting in below average thrombin generation would already have a beneficial effect.
The evident limit is set by the risk of bleeding. Thrombin generation below ∼20% of normal, i.e. an ETP of below ∼350 nM.min in congenital factor deficiencies, is associated with a definite bleeding risk,35 as is an international normalized ratio >4,36 which corresponds to an ETP of ∼300 nM.min. From this it follows that treatment which keeps a trial population under the average ETP of a normal population, i.e. 1800 nM.min,16 and above the threshold limit for bleeding, i.e. an ETP >300 nM.min, will show a beneficial effect. This is a very large window, which explains the positive outcome of clinical trials despite the large inter-individual variation. An optimal beneficial effect, however, is to be expected within a significantly narrower range. An international normalized ratio of 2-3, which has been proven to be adequate in oral anticoagulation, corresponds roughly to an ETP in the range of 500-700 nM.min.37 Due to the effect of vitamin K antagonists on proteins S and C the optimal range for other antithrombotics may be at higher levels of the ETP, but there is no reason to assume that it would cover a wider range. The large variation of the susceptibility of the target organ to inhibition, together with the variation in individual properties that influence the pharmacokinetics (e.g. weight) will, in all probability, not allow a (trial) population to be kept within narrow limits of optimal prophylaxis unless the dose is tuned to the needs of the individuals.
Previous studies have shown that variations in blood coagulation proteins vary from 50% to 150% of the mean values and can be associated with thrombotic events. However, the most important factors affecting thrombin generation are prothrombin and antithrombin.38-40
Our results are in accordance with previous work by Hacquard et al. on low molecular weight heparins (LMWH) that also showed inter-individual variances of around 20% for the inhibition of ETP.20 In an earlier study by our group on thrombin generation in healthy subjects who received fixed doses of UFH and LMWH, variances in the inhibitory effect were found to be 32% and 13-21%, respectively.41 Freyburger et al. concluded that there is also a high interindividual variability in response to dabigatran and rivaroxaban.42 Pharmacokinetic variation played a role in both these latter two studies.
Some interesting considerations can be made from a more detailed examination of the results.
Inhibition of FXa by otamixaban causes a strong inhibition of the peak but a significant protraction of the thrombin generation process, so that the ETP is much less inhibited than the peak. We surmise that this is due to inhibition of the direct positive feedback action of FXa on FVII,43 which will cause a prolongation of the lag time and a slow start of prothrombinase formation, together with inhibition of the negative feedback on the tissue factor-FVIIa complex by the tissue factor pathway inhibitor-FXa complex which prevents shutting off of the extrinsic pathway.
The inhibition by dermatan sulfate appears to reach a plateau rather than tending to complete inhibition. This may be due to the fact that there is less heparin cofactor II in the plasma (∼ 1 μM) than prothrombin (∼ 2 μM) so that even complete activation of heparin cofactor II by dermatan sulfate would not lead to complete inhibition of thrombin generation. This implies that dermatan sulfate would be an antithrombotic that cannot be overdosed!
The fact that dermatan sulfate and UFH only slightly prolong the lag time, in contrast to melagatran, indicates that the positive feedback mechanism of factor V activation by thrombin is only accessible to a direct inhibitor, probably because it is membrane-bound and involves meizothrombin rather than thrombin. A similar difference is seen between otamixaban and pentasaccharide. Probably, the feedback activation of FVII by FXa is membrane-bound and therefore inaccessible to inhibition by the pentasaccharide-antithrombin complex.44,45
These two examples together show that the relation between lag time (≈ clotting time) and thrombin generation is mechanism-dependent, so that clotting time measurements cannot be used as a universal indicator of the effect of antithrombotics on thrombin generation. Effects of melagatran on thrombin generation were previously studied by Beilfuss et al. and this group also found a strong effect on the lag time as well as a dose-dependent decrease in ETP.25 Samama et al. who investigated the effect of rivaroxaban and fondaparinux (indirect FXa inhibitor) observed qualitative differences between the two, similar to those reported here.46,47
Our results suggest that the results of direct inhibitors could be improved by tuning the dose to the needs of the individual patient. However, due to the large ‘prophylactic window’, fine tuning is probably not required; differentiation between high, middle and low responders might suffice. However, further in vivo investigation of this facet is warranted.
Supplementary Material
Acknowledgments
The authors would like to thank Bas de Laat for carefully reading the manuscript and providing us with helpful comments. We would also like to thank Leonie Pelkmans for technical assistance.
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bauer KA. New anticoagulants: anti IIa vs anti Xa - is one better? J Thromb Thrombolysis. 2006;21(1):67-72. [DOI] [PubMed] [Google Scholar]
- 2.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med.2009;361(24):2342-52. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-91. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361(12):1139-51. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365(11):981-92. [DOI] [PubMed] [Google Scholar]
- 6.Adams M. Assessment of thrombin generation: useful or hype? Semin Thromb Hemost. 2009;35(1):104-10. [DOI] [PubMed] [Google Scholar]
- 7.Eerenberg ES, van Es J, Sijpkens MK, Büller HR, Kamphuisen PW. New anticoagulants: Moving on from scientific results to clinical implementation. Ann Med. 2011;43(8): 606-16. [DOI] [PubMed] [Google Scholar]
- 8.Ho SJ, Brighton TA. Ximelagatran: direct thrombin inhibitor. Vasc Health Risk Manag. 2006;2(1):49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Outes A, Suarez-Gea ML, Lecumberri R, Rocha E, Pozo-Hernández C, Vargas-Castrillón E. New parenteral anticoagulants in development. Ther Adv Cardiovasc Dis. 2011;5(1):33-59. [DOI] [PubMed] [Google Scholar]
- 10.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285-95. [DOI] [PubMed] [Google Scholar]
- 11.Petitou M, Casu B, Lindahl U. 1976-1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie. 2003;85(1-2):83-9. [DOI] [PubMed] [Google Scholar]
- 12.Tollefsen DM, Pestka CA, Monafo WJ. Activation of heparin cofactor II by dermatan sulfate. J Biol Chem. 1983;258(11): 6713-6. [PubMed] [Google Scholar]
- 13.Boneu B, Necciari J, Cariou R, Sié P, Gabaig AM, Kieffer G, et al. Pharmacokinetics and tolerance of the natural pentasaccharide (SR90107/Org31540) with high affinity to antithrombin III in man. Thromb Haemost. 1995;74(6):1468-73. [PubMed] [Google Scholar]
- 14.Chu V, Brown K, Colussi D, Gao J, Bostwick J, Kasiewski C, et al. Pharmacological characterization of a novel factor Xa inhibitor, FXV673. Thromb Res. 2001;103(4):309-24. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson D, Antonsson T, Bylund R, Eriksson U, Gyzander E, Nilsson I, et al. Effects of melagatran, a new low-molecular-weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb Haemost. 1998;79(1):110-8. [PubMed] [Google Scholar]
- 16.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003; 33(1):4-15. [DOI] [PubMed] [Google Scholar]
- 17.Hemker HC, Hemker PW, Al Dieri R. The technique of measuring thrombin generation with fluorescent substrates: 4. The H-transform, a mathematical procedure to obtain thrombin concentrations without external calibration. Thromb Haemost. 2009;101(1):171-7. [PubMed] [Google Scholar]
- 18.Béguin S, Wielders S, Lormeau JC, Hemker HC. The mode of action of CY216 and CY222 in plasma. Thromb Haemost. 1992; 67(1):33-41. [PubMed] [Google Scholar]
- 19.Paccaly A, Frick A, Rohatagi S, Liu J, Shukla U, Rosenburg R, et al. Pharmacokinetics of otamixaban, a direct factor Xa inhibitor, in healthy male subjects: pharmacokinetic model development for phase 2/3 simulation of exposure. J Clin Pharmacol.2006; 46(1):37-44. [DOI] [PubMed] [Google Scholar]
- 20.Hacquard M, Perrin J, Lelievre N, Vigneron C, Lecompte T. Inter-individual variability of effect of 7 low molecular weight antithrombin-dependent anticoagulants studied in vitro with calibrated automated thrombography. Thromb Res. 2011;127(1): 29-34. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs G. Mathematik für Mediziner und Biologen. Springer Verlag. 1969; 71. [Google Scholar]
- 22.Mismetti P, Laporte S. New oral antithrombotics: a need for laboratory monitoring. For. J Thromb Haemost. 2010;8(4):621-6. [DOI] [PubMed] [Google Scholar]
- 23.Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost. 2010;8(4):627-30. [DOI] [PubMed] [Google Scholar]
- 24.al Dieri R, Alban S, Béguin S, Hemker HC. Thrombin generation for the control of heparin treatment, comparison with the activated partial thromboplastin time. J Thromb Haemost. 2004;2(8):1395-401. [DOI] [PubMed] [Google Scholar]
- 25.Beilfuss A, Grandoch M, Wenzel F, Hohlfeld T, Schrör K, Weber AA. Differential effects of factor IIa inhibitors on the endogenous thrombin potential. Ther Drug Monit. 2008;30(6):740-3. [DOI] [PubMed] [Google Scholar]
- 26.Castoldi E, Rosing J. Thrombin generation tests. Thromb Res. 2011;127(Suppl 3):S21-5. [DOI] [PubMed] [Google Scholar]
- 27.Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost.2006;96(5):553-61. [PubMed] [Google Scholar]
- 28.Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32(5-6):249-53. [DOI] [PubMed] [Google Scholar]
- 29.van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008; 142(6):889-903. [DOI] [PubMed] [Google Scholar]
- 30.Petros S, Siegemund T, Siegemund A, Engelmann L. The effect of different anticoagulants on thrombin generation. Blood Coagul Fibrinolysis. 2006;17(2):131-7. [DOI] [PubMed] [Google Scholar]
- 31.Tripodi A, van den Besselaar A. Laboratory monitoring of anticoagulation: where do we stand? Semin Thromb Hemost. 2009; 35 (1):34-41. [DOI] [PubMed] [Google Scholar]
- 32.Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2010:9CD001100. [DOI] [PubMed] [Google Scholar]
- 33.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126(3 Suppl):204S-33S. [DOI] [PubMed] [Google Scholar]
- 34.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296(4):397-402. [DOI] [PubMed] [Google Scholar]
- 35.Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002; 88(4):576-82. [PubMed] [Google Scholar]
- 36.Azar AJ, Koudstaal PJ, Wintzen AR, van Bergen PF, Jonker JJ, Deckers JW. Risk of stroke during long-term anticoagulant therapy in patients after myocardial infarction. Ann Neurol. 1996;39(3):301-7. [DOI] [PubMed] [Google Scholar]
- 37.Hemker HC, Béguin S. Phenotyping the clotting system. Thromb Haemost. 2000; 84(5):747-51. [PubMed] [Google Scholar]
- 38.Butenas S, van't Veer C, Mann KG. "Normal" thrombin generation. Blood. 1999;94(7):2169-78. [PubMed] [Google Scholar]
- 39.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80(8):1998-2005. [PubMed] [Google Scholar]
- 40.Woodward M, Lowe GD, Rumley A, Tunstall-Pedoe H, Philippou H, Lane DA, et al. Epidemiology of coagulation factors, inhibitors and activation markers: The Third Glasgow MONICA Survey II. Relationships to cardiovascular risk factors and prevalent cardiovascular disease. Br J Haematol. 1997;97(4):785-97. [DOI] [PubMed] [Google Scholar]
- 41.Al Dieri R, Alban S, Béguin S, Hemker HC. Fixed dosage of low-molecular-weight heparins causes large individual variation in coagulability, only partly correlated to body weight. J Thromb Haemost. 2006; 4(1):83-9. [DOI] [PubMed] [Google Scholar]
- 42.Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127(5): 457-65. [DOI] [PubMed] [Google Scholar]
- 43.Jesty J, Lorenz A, Rodriguez J, Wun TC. Initiation of the tissue factor pathway of coagulation in the presence of heparin: control by antithrombin III and tissue factor pathway inhibitor. Blood. 1996;87(6):2301-7. [PubMed] [Google Scholar]
- 44.Hemker HC, Choay J, Beguin S. Free factor Xa is on the main pathway of thrombin generation in clotting plasma. Biochim Biophys Acta. 1989;992(3):409-11. [DOI] [PubMed] [Google Scholar]
- 45.Herbert JM, Petitou M, Lormeau JC, Cariou R, Necciari J, Magnani HN, et al. SR 90107A/Org 31540, a novel anti-factor Xa antithrombotic agent. Cardiovasc Drug Rev. 1997;15(1):1-26. [Google Scholar]
- 46.Gerotziafas GT, Elalamy I, Depasse F, Perzborn E, Samama MM. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost. 2007;5(4):886-8. [DOI] [PubMed] [Google Scholar]
- 47.Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, et al. Assessment of laboratory assays to measure rivaroxaban- an oral direct factor Xa inhibitor. Thromb Haemost. 2010;103(4): 815-25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.