Abstract
To analyze prioritized outcomes, Buyse [1] and Pocock et al. [2] proposed the win loss approach. In this paper, we first study the relationship between the win loss approach and the traditional survival analysis on the time to the first event. We then propose the weighted win loss statistics to improve the efficiency of the un-weighted methods. A closed-form variance estimator of the weighted win loss statistics is derived to facilitate hypothesis testing and study design. We also calculated the contribution index to better interpret the results of the weighted win loss approach. Simulation studies and real data analysis demonstrated the characteristics of the proposed statistics.
Keywords: clinical trials, composite endpoints, contribution index, prioritized outcomes, weighted win ratio, variance estimation
1. Introduction
In cardiovascular (CV) trials, the term MACE, i.e. Major Adverse Cardiac Events, is a commonly used endpoint. By definition, MACE is a composite of clinical events and usually includes CV death, myocardial infarction, stroke, coronary revascularization, hospitalization for angina, and etc. The use of composite endpoints will increase event rate and effect size therefore reduce sample size and the duration of study. On the other hand, it is often difficult to interpret the findings if the composite is driven by one or two components or some components are going in the opposite direction of the composite, which may weaken the trial’s ability to reach reliable conclusion.
There are two ways to define and compare composite endpoints. The traditional way, which we call “first combine then compare”, is to first combine the multiple outcomes into a single composite endpoint and then compare the composite between the treatment group and the control group. Recently, Buyse [1] and Pocock et al. [2] proposed another approach, which we call “first compare then combine”, that is to first compare each endpoint between the treatment group and the control group and then combine the results from all the endpoints of interest.
This latter approach was proposed to meet the challenges of analyzing prioritized outcomes. In CV trials, it is common that different outcomes may have different clinical importance, and trialists and patients may have different opinions on the order of importance. This phenomenon has been quantified by a recent survey [3], which concludes that equal weights in a composite clinical endpoint do not accurately reflect the preferences of either patients or trialists. If the components of a composite endpoint have different priorities, the traditional time-to-first-event analysis may not be suitable. Only the first event is used in the time-to-first-event analysis and the subsequent, oftentimes more important, events such as CV death are ignored. The “first compare then combine” approach, or more commonly called the win ratio approach, can account for the event hierarchy in a natural way: the most important endpoint is compared first, if tied, then the next important outcome will be compared. This layered comparison procedure will continue until a winner or loser has been determined or the ultimate tie is resulted. Clearly, the order of comparisons aligns with the pre-specified event priorities.
To implement the win loss approach, subjects from the treatment group and the control group are first paired, then a “winner” or “loser” per pair is determined by comparing the endpoints following the hierarchical rule as above. The win ratio and the win difference (also named “proportion in favor of treatment” by Buyse [1]) comparing the total wins and losses per treatment group can then be computed, with large values (win ratio greater than one or win difference greater than zero) indicating the treatment effect. This approach can be applied to all types of endpoints including continuous, categorical and survival [4], even though it was first named by Pocock et al. [2] when analyzing survival endpoints. It can also be coupled with matched [2] and stratified [5] analyses to reduce heterogeneity in the pairwise comparison.
To facilitate hypothesis testing, Luo et al. [6] established a statistical framework for the win loss approach under survival setting. A closed-form variance estimator for the win loss statistics is obtained through an approximation of U-statistics [6]. Later on, Bebu and Lachin [7] generalized this framework to other settings and derived variance estimator based on large sample distribution of multivariate multi-sample U-statistics.
As indicated by Luo et al. [6], the win ratio will depend on the potential follow-up times in the trial. Thus there may be some limitations when applying it to certain trials or populations. To reduce the impact of censoring, Oakes [8] proposed to use a common time horizon for the calculation of the win ratio statistic.
Despite many developments on the win loss approach, there still appears a need of more transparent comparison between the win loss approach and the traditional time-to-first-event analysis. Other than saying that “we first compare the more important endpoint and then the less important endpoint”, it would be more interesting to delineate how the order of importance plays its role in defining the test statistics and how the change of order results in different types of statistics (i.e. win loss vs first-event). We will present this comparison in Section 2 under the survival data setting, in particular, the semi-competing risks data setting where there are a non-terminal event and a terminal event. This setting is very common in CV trials where for instance CV death is the terminal event and the non-fatal stroke or MI is the non-terminal event. We will examine two opposite scenarios with either the terminal event or the non-terminal event as the prioritized outcome. We will show in Section 2 that, when the terminal event has higher priority, the layered comparison procedure will result in the win loss statistics, otherwise, it will end up with the Gehan statistic derived from the first-event analysis. Therefore, different from the common belief, the first-event analysis in fact emphasizes the non-terminal event instead of considering both events of equal importance. Because the non-terminal event always occurs before the terminal event if both events are observed, this analysis assigns the order of importance according to the time course of event occurrence.
In many studies, the log-rank or weighted log-rank statistics are preferred to the Gehan statistic in first-event analysis. From the comparison between the win loss approach and the first-event analysis, it is natural to weigh the win loss statistics similarly to achieve better efficiency and interpretability. The weighted win ratio statistics are proposed in Section 3. In the sequel, weighting is specific to the time that events occur, not the type of events. To facilitate the use of the weighted win loss statistics, we derive a closed-form variance estimator under the null hypothesis and provide some optimality results for weight selection. In order to improve the interpretability of the win loss composite endpoint, the weighted win loss approach is coupled with the contribution index analysis where the proportion of each component event contributing to the overall win/loss is computed. Thus the driving force of the overall win/loss can be identified. The weighted win loss approach is illustrated and compared with the traditional method through real data examples and simulation studies.
2. Comparison between the win loss approach and the first-event analysis
Let T1 and T2 be two random variables denoting the time to the non-terminal event and the time to the terminal event, respectively. These two variables are usually correlated. T2 may right-censor T1 but not vice versa. Let Z = 1 denote the treatment group and Z = 0 the control group. In addition, C is the time to censoring, which is assumed to be independent of (T1, T2) given Z. For simplicity, we assume that in both groups the distribution of (T1, T2) is absolutely continuous, whereas the censoring distribution can have jump points.
Due to censoring, we can only observe Y1 = T1 ∧ T2 ∧ C and Y2 = T2 ∧ C and the event indicators δ1 = I(Y1 = T1) and δ2 = I(Y2 = T2). Here and in the sequel a ∧ b = min(a, b) for any real values a and b. The observed data {(Y1i, Y2i, δ1i, δ2i, Zi) : i = 1, . . . , n} are the independently identically distributed samples of (Y1, Y2, δ1, δ2, Z).
For two subjects i and j, the win (i over j) indicators based on the terminal event and the non-terminal event are W2ij = δ2jI(Y2i ≥ Y2j) and W1ij = δ1jI(Y1i ≥ Y1j) respectively. Correspondingly, the loss (i against j) indicators are L2ij = δ2iI(Y2j ≥ Y2i) and L1ij = δ1iI(Y1j ≥ Y1i). The other scenarios are undecidable therefore we define the tie indicators based on the terminal event and the non-terminal event as Ω2ij = (1 − W2ij)(1 − L2ij) and Ω1ij = (1 − W1ij)(1 − L1ij), respectively.
Because the pairwise comparison based on each event results in three categories (win, loss and tie), the pairwise comparison based on two events has nine possible scenarios (see Table 1). Naturally, a win for subject i can be claimed if the three scenarios W2ijW1ij, W2ijΩ1ij and Ω2ijW1ij occur, which indicate subject i wins on one event and at least ties on the other. Similarly, a loss for subject i is claimed if the scenarios L2ijL1ij, L2ijΩ1ij and Ω2ijL1ij occur, meaning subject i losses on one event and does not win on the other. However, rules are needed to classify the two conflicting scenarios W2ijL1ij and L2ijW1ij when subject i wins on one event but loses on the other.
Table 1.
The nine possible outcomes of the pairwise comparison between subjects i and j
| Non-terminal | ||||
|---|---|---|---|---|
| Win | Tie | Loss | ||
|
|
||||
| Terminal | Win | W2ijW1ij | W2ijΩ1ij | W2ijL1ij |
| Tie | Ω2ijW1ij | Ω2ijΩ1ij | Ω2ijL1ij | |
| Loss | L2ijW1ij | L2ijΩ1ij | L2ijL1ij | |
If the terminal event is more important, the scenario W2ijL1ij will be considered as a win for subject i and the scenario L2ijW1ij will be a loss. The difference of total wins and losses in the treatment group is
which is the win difference statistic [2, 6]. This statistic compares the more important terminal event first (W2ij − L2ij), if tied (i.e. Ω2ij = 1), then proceeds to compare the non-terminal event (W1ij − L1ij).
On the contrary, if the non-terminal event ranks higher, scenario W2ijL1ij will be classified as a loss for subject i and L2ijW1ij will be a win. Thus the difference of total wins and total losses in the treatment group is
| (1) |
which is the Gehan statistic based on the first-event time T1 ∧ T2. The equality (1) holds with probability one. This is due to the facts that the first-event indicator δi = δ1i + (1 − δ1i)δ2i, and that, when T2 is continuous, W2ijΩ1ij = (1 − δ1j)δ2jI(Y1i ≥ Y1j) and L2ijΩ1ij = (1 − δ1i)δ2iI(Y1j ≥ Y1i) with probability one. The proof of the latter fact is provided in the Appendix. As compared to the win difference statistic, the Gehan statistic compares the non-terminal event first (W1ij − L1ij), if tied (i.e. Ω1ij = 1), then compares the terminal event (W2ij − L2ij).
Apparently, both the win difference statistic and the Gehan statistic bear a layered comparison structure: to decide a winner or loser, the most important event is compared first, if tied, the second important event will be compared. The difference lies in which event has higher priority. Higher priority in the non-terminal event results in the Gehan statistic and higher order in the terminal event leads to the win difference statistic. The flipping positions in placing W2ijL1ij and L2ijW1ij therefore reflect the fundamental view of the event priorities. The Gehan statistic from the first-event analysis puts more emphasis on the non-terminal event, which is contrary to the common belief that both events are considered equally important. More importantly, we can now delineate the weighting scheme the traditional method entails. This motivates us to explore weighting schemes for the win difference/ratio statistics in next section.
3. Weighted win loss statistics
In practice, weighted log-rank statistics are often preferred to the Gehan statistic because the latter may be less efficient due to equally weighting the wins regardless of when they occurred and heavily depending on the censoring distributions. From equation (1), the weighted log-rank statistics from the first-event analysis can be written as
where G̃(·) is an arbitrary and possibly data-dependent positive function. For example, G̃ = 1 will result in the Gehan statistic, will result in the log-rank statistic, and with ρ ≥ 0 will result in the Gρ family of weighted log-rank statistics proposed by Fleming and Harrington [9], where Ŝ3 is the Kaplan-Meier estimate of the survival function of T1 ∧ T2.
It is straightforward to generalize the weighted log-rank statistics to a more general form
| (2) |
where G̃1(·) and G̃2(·, ·) are arbitrary positive univariate and bivariate functions respectively. Clearly, the statistic (2) is a weighted version of the Gehan statistic, which compares the non-terminal event first. The use of a bivariate weight function in the second part is because the strength of win or loss may depend on both observed times Y1 and Y2.
In view of (2), when the terminal event has higher priority, the win ratio and win difference statistics [2, 6] can be generalized as follows. Suppose we have two weight functions G2(·) and G1(·, ·), we can define the total numbers of weighted wins and weighted losses based on the terminal event as
and the total numbers of weighted wins and weighted losses based on the non-terminal event as
The weighted win difference is
and the weighted win ratio is WR(G1, G2) = {W2(G2) + W1(G1)}/{L2(G2) + L1(G1)}. These statistics bear the same idea of the un-weighted ones as they all first compare the terminal event, if tied, non-terminal event is compared. The weighted statistics, however, weight the wins and losses according to when they occur so that for example a win occurring later may be weighted more than a win occurring earlier. To evaluate how much each event will contribute to the final win and loss determination, we calculate the contribution indexes as
where CE = W2(G2), L2(G2), W1(G1) or L1(G1), corresponding to each win or loss situation.
Now we explore some weighting schemes for these win loss statistics. Let for k = 0, 1 and R2(y) = R20(y) + R21(y) with a lower case r being the corresponding expectations, i.e. r20(y) = E{R20(y)}, etc.. If we set the weight function G2(y) = R2(y), then it is easy to show that
which is the log-rank statistic for the terminal event. For a general weight function G2,
which is a weighted log-rank statistic. In addition, if G2 converges to a deterministic function g2 > 0, then n−2{L2(G2) − W2(G2)} will converge to
| (3) |
where, for k = 0, 1, λ2k(·) is the hazard function of T2 in group k. Therefore, for any given weight function G2, the weighted difference L2(G2) − W2(G2) can be used to test the hypothesis
For the non-terminal event, suppose the possibly data-dependent bivariate weight function G1(y1, y2) converges to some deterministic function g1 > 0. Let for k = 0, 1 and R1(y1, y2) = R10(y1, y2) + R11(y1, y2) with a lower case r denoting the corresponding expectations, i.e. r10(y1, y2) = E{R10(y1, y2)}, etc. We show in the Appendix that n−2{L1(G1) − W1(G1)} converges to
| (4) |
where, for k = 0, 1, λ1k(y1 | y2) = pr(T1 = y1 | T1 ≥ y1, T2 ≥ y2, Z = k), Λck is the cumulative hazard function for the censoring time in group k with the differential increment
and Λa(dt) = Λc0(dt) + Λc1(dt) − Λc0(dt) Λc1(dt). Here and in the sequel, for any function f, f (t−) is the left limit at point t. The limit (4) is reasonable because when G1 = 1 and both Λc1 and Λc0 are absolutely continuous, the un-weighted difference does converge to it [6]. Thus, similar to the un-weighted version, the weighted difference L1(G1) − W1(G1) can be used to test the hypothesis
Overall, the weighted win difference and win ratio can be used to test the hypothesis .
4. Properties of the weighted win loss statistics
4.1. Weight selection
To construct weighted log-rank statistics for the terminal event, various weights can be used, for example,
fG2(y) = 1, i.e. Gehan weight;
G2(y) = R2(y), i.e. log-rank weight.
Several choices of G1 are apparent:
G1(y1, y2) = 1, this is the same as the un-weighted win loss endpoints;
G1(y1, y2) = R1(y1, y2), this is similar to the log-rank test for the terminal event;
G1(y1, y2) = R2(y2), this is essentially giving a log-rank weight as the terminal event;
, this is the log-rank weight for the first event analysis.
In general we may choose G1 and G2 to satisfy the following conditions, under which the asymptotic results in next section hold.
Condition 1
G1 converges to a bounded function g1 in the sense that sup0<y1≤y2|G1(y1, y2) − g1(y1, y2)| = o(n−1/4) almost surely and the difference G1 − g1 can be approximated in the sense that almost surely, where V1k(y1, y2) is a bounded random function depending only on the observed data Ok = (Y1k, Y2k, δ1k, δ2k, Zk) from the kth subject such that E{V1k(y1, y2)} = 0 for any 0 < y1 ≤ y2, k = 1, . . . , n;
Condition 2
G2 converges to a bounded function g2 in the sense that supy |G2(y) − g2(y)| = o(n−1/4) almost surely and the difference G2 − g2 can be approximated in the sense that almost surely, where V2k(y) is a bounded random function depending on Ok such that E{V2k(y)} = 0 for any y, k = 1, . . . , n.
These conditions are clearly satisfied by the above choices of the weight functions. In fact, they are applicable to a wide range of selections. For example, one may choose the weight which is the Fleming-Harrington family of weights [9], where Ŝ2 is the Kaplan-Meier estimate of the survival function S2 for T2 and ρ = 0 is a fixed constant. In this case, the convergence rate of supy |G2(y) − g2(y)| will be o(n−1/2+ε) for any ε > 0, which is o(n−1/4) when choosing ε ≤ 1/4. The asymptotic approximation of G2(y) − g2(y) by follows from the Taylor’s series expansion of around the true S2(y) and the approximation of the Kaplan-Meier estimator.
4.2. Variance estimation
It can be shown that, under and Conditions 1 and 2, WD(G1, G2) = WD(g1, g2) + op(n3/2). This indicates that using the estimated weights G1 and G2 does not change the asymptotic distribution of WD(g1, g2). A detailed proof can be found in the supporting web materials. We therefore need to find the variance of WD(g1, g2) under H0.
By definition, for any i, j = 1, . . . , n and k = 1, 2, Wkji = Lkij and Ω2ij = Ω2ji, therefore Zi(1 − Zj)(W2ij − L2ij) + Zj(1 − Zi)(W2ji − L2ji) = (Zi − Zj)(W2ij − L2ij) and Zi(1 − Zj)Ω2ij(W1ij − L1ij) + Zj(1 − Zi)Ω2ij(W1ji − L1ji) = (Zi − Zj)Ω2ji(W1ij − L1ij). With these, noting that WD(g1, g2) is a U-statistic, we can use the the exponential inequalities for U-statistics [10, 11] to approximate WD(g1, g2) as
| (5) |
almost surely, where Oi = (Y1i, Y2i, δ1i, δ2i, Zi), i = 1, . . . , n. A detailed proof can be found in the supporting web materials. The approximation (5) provides an explicit variance estimator when we substitute (g1, g2) with its empirical counterpart. In particular, under H0, n−3/2WD(G1, G2) converges in distribution to a normal distribution , where the variance can be consistently estimated by with
Furthermore, for the weighted win ratio WR(G1, G2), n1/2{WR(G1, G2) − 1} converges in distribution to , where the variance can be consistently estimated by
Therefore, the 100 × (1 − α)% confidence intervals for the win difference and win ratio are, respectively, n−2WD(G1, G2) ± qαn−1/2σ̂D and exp{WR(G1, G2) ± qαn−1/2σ̂R/WR(G1, G2)}, where qα is the 100 × (1 − α/2)-th percentile of the standard normal distribution. A R package “WWR”, available in CRAN, has been developed to facilitate the above calculations.
In the supporting web materials, we also discuss some optimality results to guide the selection of the best weights for different alternative hypotheses. However, because the variance usually does not yield a simple form and the equation for calculating the optimal weights is very difficult to solve, these results are of less practical value. We will use simulation to evaluate the performance of the weighted win/loss statistics and their variance estimators.
5. Simulation
5.1. Simulation Setup
We evaluated the performance of the proposed weighted win loss statistics via simulation. The simulation scenarios are the same as [6], which cover three different bivariate exponential distributions with the Gumbel-Hougaard copula, the bivariate normal copula and the Marshall-Olkin distribution.
Let λHz = λH exp(−βHz) be the hazard rate for the non-terminal event hospitalization, where z = 1 if a subject is on the new treatment and z = 0 if on the standard treatment. Similarly, let λDz = λD exp(−βDz) be the hazard rate for the terminal event death. The first joint distribution of (TH, TD) is a bivariate exponential distribution with Gumbel-Hougaard copula
where ρ ≥ 1 is the parameter controlling the correlation between TH and TD (Kendall’s concordance τ equals to 1 − 1/ρ). The second distribution is a bivariate exponential distribution with bivariate normal copula
where Φ is the distribution function of the standardized univariate normal distribution with Φ−1 being its inverse and Φ2(u, v; ρ) is the distribution function of the standardized bivariate normal distribution with correlation coefficient ρ. The third distribution is the Marshall-Oklin bivariate distribution
where ρ modulates the correlation between TH and TD.
Independent of (TH, TD) given Z = z, the censoring variable TC has an exponential distribution with rate λCz = λC exp(−βCz).
Throughout the simulation, we fixed parameters λH = 0.1, λD = 0.08, λC = 0.09, βC = 0.1. We then varied βH, βD and ρ in each distribution. We simulated a two-arm parallel trial with 300 subjects per treatment group, so the sample size n = 600. The number of replications was 1, 000 for each setting. The Gumbel-Hougaard bivariate exponential distribution was generated using the R package “Gumbel” [12, 13].
The proposed statistics were evaluated in terms of power in detecting treatment effect and controlling Type I error under the null hypothesis. We also compared the proposed approach with the log-rank test based on the terminal event and the first event. In addition, the impact of weights assigned to the terminal and non-terminal events on the performance of the proposed approach was also investigated. The power and Type I error are calculated as proportions of |Tx|/SE(Tx) greater than qα in the 1,000 simulations, where Tx is the test statistic (win loss, log-rank based on the first event and log-rank based on death) and SE(Tx) is the corresponding estimated standard error and qα is the 100 × (1 − α/2)-th percentile of the standard normal distribution and α = 0.05.
5.2. Simulation Results
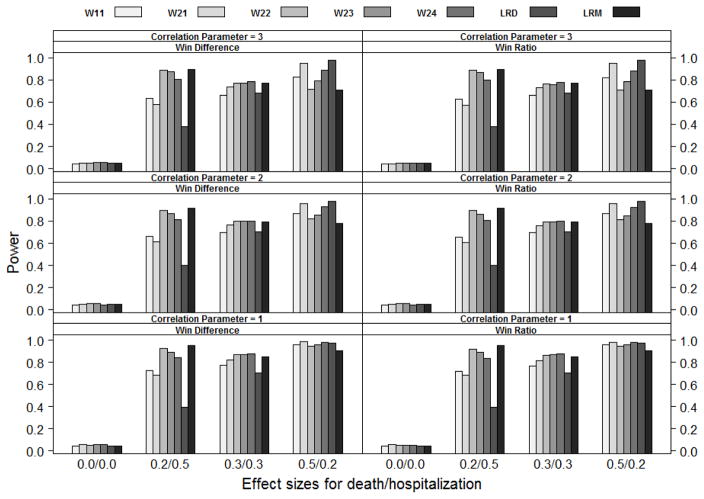
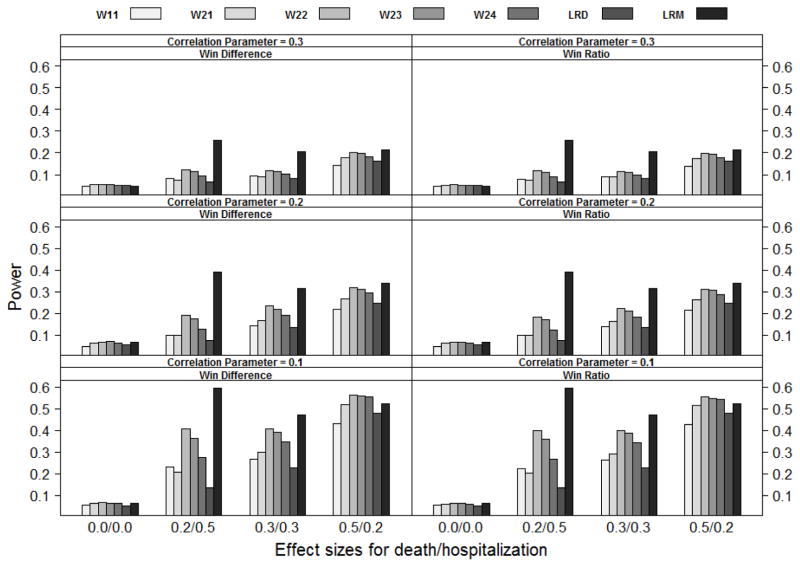
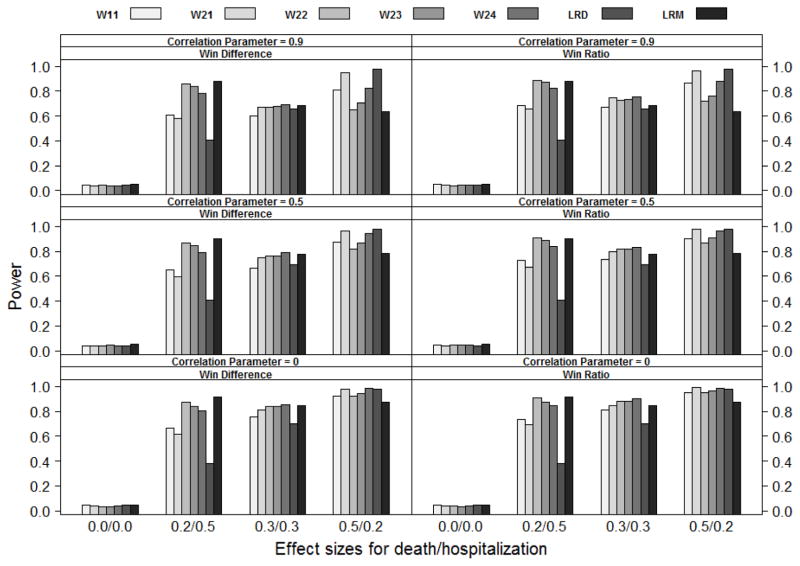
The simulation results are summarized in Figures 1 to 3. As shown in the figures, all the methods listed can control Type I error under different settings. The weighted win loss statistics can improve the efficiency of the un-weighted ones, with the biggest improvements seen when the effect size on hospitalization (βH) is larger than the effect size on death (βD).
Figure 1.
Power comparison of statistics W11, W21, W22, W23, W24, LRD and LRM (from left to right) under the Gumbel-Hougaard bivariate exponential distribution (GH), where Wij is the weighted win loss statistics using weight (i) for the terminal event and weight (j) for the non-terminal event in Section 4.1, LRD are the log-rank statistics based on the terminal event and LRM are the log-rank statistics based on the first event. Effect sizes for death/hospitalization are the log hazard ratios βD and βH respectively. The correlation parameter is ρ. Not all weighted win loss statistics are reported due to the space limit.
Figure 3.
Power comparison of statistics W11, W21, W22, W23, W24, LRD and LRM (from left to right) under the Marshall-Olkin bivariate exponential distribution (MO), where Wij is the weighted win loss statistics using weight (i) for the terminal event and weight (j) for the non-terminal event in Section 4.1, LRD are the log-rank statistics based on the terminal event and LRM are the log-rank statistics based on the first event. Effect sizes for death/hospitalization are the log hazard ratios βD and βH respectively. The correlation parameter is ρ. Not all weighted win loss statistics are reported due to the space limit.
The traditional log-rank test based on the time-to-first-event analysis (LRM) has better power than the other methods when the effect size on hospitalization (βH ) is larger than that on death (βD). The traditional log-rank test based on the time-to-death analysis (LRD) has better power when βD is larger than βH. In terms of power, the win loss approach falls between and lands closer to the better of the traditional methods. Please note that the win loss statistics, LRM and LRD are actually testing completely different null hypotheses albeit we compare them side-by-side here.
The weighted win-ratio approach with optimal weights (optimal within the listed weights) shows comparable power to the best performed traditional method for all scenarios except when the death effect size is not larger than the hospitalization effect size under Marshall-Oklin distribution, see Figure 3 when (βD, βH ) = (0.2, 0.5) and (0.3, 0.3). In these cases, LRM dominates the others. This is understandable as the hazard for TH ∧ TD given Z = z is λHz + λDz + ρ under the Marshall-Oklin distribution, which has the largest effect size as compared to other methods.
In summary, the weighted win loss statistics improve the efficiency of the un-weighted ones. The weighted win loss approach with optimal weights can have a power that is comparable to or better than that of the traditional analyses.
6. Applications
6.1. The PEACE study
In the Prevention of Events with Angiotensin Converting Enzyme (ACE) Inhibition (PEACE) Trial [14], the investigators tested whether ACE inhibitor therapy, when added to modern conventional therapy, reduces cardiovascular death (CVD), myocardial infarction (MI), or coronary revascularization in low-risk, stable coronary artery disease (CAD) in patients with normal or mildly reduced left ventricular function. The trial was a double-blind, placebo-controlled study in which 8290 patients were randomly assigned to receive either trandolapril at a target dose of 4mg per day (4158 patients) or matching placebo (4132 patients). The pre-specified primary endpoint is the composite including MI, CVD, CABG (coronary-artery bypass grafti) and PCTA (percutaneous coronary Coronary Angioplasty). The primary efficacy analysis based on the intent-to-treat principal has shown no benefit among patients who were assigned to trandolapril compared to the patients who were assigned to placebo. In our analysis, the CVD is the prioritized outcome and the minimum of non-terminal MI, CABG and PTCA is the secondary outcome. The analysis results are summarized in Tables 2–3. The weighted win ratio approach with the weight assigned to the non-terminal event results in higher contribution from the non-terminal events (increased from 40.7–42.2% to 47.9–48.7%) and yields the smaller p-values in testing the treatment effect compared to other scenarios. The log-rank test based on time to death analysis has the biggest p-value. Assigning a weight on the prioritized outcome CVD alone does increase the power relative to the traditional approaches, although the improvement is nominal. The log-rank test based on time to first event analysis yields a smaller p-value than the log-rank test based on time to death analysis, but the p-value is far larger than the ones from the weighted win ratio approach with the non-terminal event suitably weighted.
Table 2.
Analysis of PEACE Data using the traditional methods
| Tran | Placebo | HR and 95% CI | log-rank | Gehan | |
|---|---|---|---|---|---|
| No. of patients | 4158 | 4132 | |||
| No. of patients having either MI, CABG, PTCA or CVD | 909 | 929 | 0.96(0.88, 1.06) | ||
| p = 0.43* | p = 0.51 | p = 0.52 | |||
| No. of patients having CVD after MI, CABG or PTCA | 47 | 47 | |||
| No. of CVD | 146 | 152 | 0.95(0.76, 1.19) | ||
| p = 0.67* | p = 0.66 | p = 0.55 |
p-values are based on Wald tests.
Table 3.
Analysis of PEACE Data using the weighted win ratio approach
| W11+ | W21 | W12 | W22 | |
|---|---|---|---|---|
| a: CVD on Tran first | 487,795 (8.1%)# | 602,055.8 (9.6%) | 487,795 (3.3%) | 602,055.8 (4.0%) |
| b: CVD on Placebo first | 524,382 (8.6%) | 633,075.2 (10.1%) | 524,382 (3.6%) | 633,075.2 (4.2%) |
| c: MI, CABG or PTCA on Tran first | 2,492,674 (41.1%) | 2,492,674 (39.6%) | 6,551,555 (44.5%) | 6,551,555 (43.8%) |
| d: MI, CABG or PTCA on Placebo first | 2,561,374 (42.2%) | 2,561,374 (40.7%) | 7,167,545 (48.7%) | 7,167,545 (47.9%) |
| Total: a + b + c + d | 6,067,225 | 6,289,179 | 14,732,277 | 14,954,231 |
| Win ratio: (b + d)/(a + c) | 1.04 | 1.03 | 1.09 | 1.09 |
| Reciprocal of Win ratio | 0.96 | 0.97 | 0.92 | 0.92 |
| 95% CI | (0.88, 1.06) | (0.88, 1.06) | (0.83, 1.01) | (0.83, 1.01) |
| p-value * | 0.46 | 0.50 | 0.083 | 0.088 |
Wij is the weighted win loss statistics using weight (i) for the terminal event and weight (j) for the non-terminal event listed in Section 4.1;
the percentages in the parentheses are the contribution indexes as, for example, a/(a + b + c + d) × 100%;
p-values are from the tests based on the weighted win difference.
6.2. The ATLAS study
ATLAS ACS 2 TIMI 51 was a double-blind, placebo controlled, randomized trial to investigate the effect of Rivaroxaban in preventing cardiovascular outcomes in patients with acute coronary syndrome [15]. For illustration purpose, we reanalyzed the events of MI, Stroke and Death occurred during the first 90 days after randomization among subjects in Rivaroxaban 2.5mg and placebo treatment arms with intention to use Asprin and Thienopyridine at baseline.
Table 4 presents the results using the traditional analyses including Cox proportional hazards model, log-rank test and Gehan test for the time to death and the time to the first occurrence of MI, Stroke or Death. The composite event occurred in 2.77%(132/4765) and 3.57%(170/4760) of Rivaroxaban and placebo subjects respectively. The hazard ratio is 0.78 with 95% confidence interval (0.62, 0.97). The hazard ratio can be interpreted as the hazard of experiencing the composite endpoint for an individual on the Rivaroxaban arm relative to an individual on the placebo arm. Hazard ratio of 0.78 with the upper 95% confidence limit less than 1 demonstrates that Rivaroxaban reduced the risks of experiencing MI, Stroke or Death.
Table 4.
Analysis of ATLAS First 90 Days Data using the traditional methods
| Riva | Placebo | HR and 95% CI | log-rank | Gehan | |
|---|---|---|---|---|---|
| No. of patients | 4765 | 4760 | |||
| No. of patients having either MI, Stroke or Death | 132 | 170 | 0.78(0.62, 0.97) | ||
| p = 0.028* | p = 0.028 | p = 0.026 | |||
| No. of patients having Death after MI or Stroke | 7 | 14 | |||
| No. of Death | 44 | 64 | 0.69(0.47, 1.01) | ||
| p = 0.056* | p = 0.055 | p = 0.061 |
p-values are based on Wald tests.
Table 5 presents the win ratio results where death is considered of higher priority than MI and Stroke. Every subject in Rivaroxaban arm was compared to every subject in placebo arm, which resulted in a total of 22, 681, 400(= 4765 × 4760) patient pairs. Among such pairs, we reported the weighted wins and losses of Rivaroxaban in preventing Death and MI and Stroke respectively. Please note that, due to a slight change in handling the ties, the reported counts in the first column are slightly different from the ones in [6]. Also, we used the variance under the null here as compared to the variance under the alternative in [6], therefore the resulting confidence intervals are slightly different.
Table 5.
Analysis of ATLAS First 90 Days Data using the win ratio approach
| W11+ | W21 | W12 | W22 | |
|---|---|---|---|---|
| a: Death on Riva first | 202,868 (14.8%)# | 209,585.8 (15.1%) | 202,868 (13.8%) | 209,585.8 (14.0%) |
| b: Death on Placebo first | 292,132 (21.4%) | 304,607.4 (22.0%) | 292,132 (19.8%) | 304,607.4 (20.4%) |
| c: MI or Stroke on Riva first | 392,876 (28.7%) | 392,876 (28.3%) | 440,620.4 (29.9%) | 440,620.4 (29.5%) |
| d: MI or Stroke on Placebo first | 480,285 (35.1%) | 480,285 (34.6%) | 536,990.6 (36.5%) | 536,990.6 (36.0%) |
| Total: a + b + c + d | 1,368,161 | 1,387,354 | 1,472,611 | 1,491,804 |
| Win ratio: (b + d)/(a + c) | 1.30 | 1.30 | 1.29 | 1.29 |
| Reciprocal of Win ratio | 0.77 | 0.77 | 0.78 | 0.78 |
| 95% CI | (0.63, 0.94) | (0.63, 0.93) | (0.64, 0.95) | (0.63, 0.94) |
| p-value* | 0.025 | 0.022 | 0.029 | 0.026 |
Wij is the weighted win loss statistics using weight (i) for the terminal event and weight (j) for the non-terminal event listed in Section 4.1;
the percentages in the parentheses are the contribution indexes as, for example, a/(a + b + c + d) × 100%;
p-values are from the tests based on the weighted win difference.
The four methods resulted in that the (weighted) win ratios of Rivaroxaban are around 1.30, which are calculated as the total number of (weighted) wins divided by the total number of (weighted) losses. In order to compare with the traditional methods, we also calculated the reciprocals of the win ratios and their 95% confidence intervals. The reciprocals of the win ratios with values around 0.78 and the upper 95% confidence limits less than 1 show that Rivaroxaban was effective in delaying the occurrence of MI, Stroke or Death. Both the traditional analyses and the (weighted) win loss analysis provide evidence that Rivaroxaban is efficacious in preventing MI, Stroke or Death within first 90 days of randomization. Even though the four weighted win loss analysis produce roughly the same results, weighting applied to the terminal event appears to marginally improve the overall significance. Please note that we reported the p-values in Table 5 based on the weighted win differences as they are more closely related to the log-rank and Gehan test statistics in the traditional analyses.
7. Discussion
Motivated by delineating the relationship between the win ratio approach and the first-event analysis, this paper proposes the weighted win loss statistics to analyze prioritized outcomes in order to improve efficiency of the un-weighted statistics. We derive a closed-form variance estimator under the null hypothesis to facilitate hypothesis testing and study design. The calculated contribution index further compliments the win loss approach to better interpret the results.
As illustrated in Section 2, the choice between the weighted win ratio approach and the weighted log-rank approach based on the first event depends on the relative importance of the two events. If the power is of concern, according to our simulation, when the effect size on non-terminal event is larger than that on the terminal event, the traditional method based on the first event analysis is preferred, vice versa, the win ratio approach is the choice. However, it is important to note that these two approaches are testing completely different null hypotheses.
Within the weighted win loss statistics, one may want to first suitably weight the terminal event (i.e. for example, if the proportional hazards assumption holds, the log-rank test is preferred). It appears that a weight equal to one is good enough for the non-terminal event. More research is needed along this line.
The weighted win ratio with the discussed weight functions still seems to rely on the censoring distributions. In viewing of (3) and (4), we may use some suitable weights to get rid of the censoring distributions so that the win ratio will only depend on the hazard functions λ2k(y) and λ1k(y1 | y2), k = 0, 1. However, it may still be hard to interpret such result as the resulting win ratio will be
Note that a suitable weight for the non-terminal event involves density estimate of the overall hazard rate of the censoring time C.
Further improvement may be achieved by differentially weighting the nine scenarios in Table 1 according to the strength of wins, because double wins might be weighted more than one win and one tie. The proposed weighting method and its improvement can be applied to other types of endpoints or recurrent events, with careful adaptation. We shall study this in future papers.
Supplementary Material
Figure 2.
Power comparison of statistics W11, W21, W22, W23, W24, LRD and LRM (from left to right) under the bivariate exponential distribution with bivariate normal copula (BN), where Wij is the weighted win loss statistics using weight (i) for the terminal event and weight (j) for the non-terminal event in Section 4.1, LRD are the log-rank statistics based on the terminal event and LRM are the log-rank statistics based on the first event. Effect sizes for death/hospitalization are the log hazard ratios βD and βH respectively. The correlation parameter is ρ. Not all weighted win loss statistics are reported due to the space limit.
Acknowledgments
The authors thank the PEACE study group for sharing the data through National Institute of Health, USA. Xiaodong Luo was partly supported by National Institute of Health grant P50AG05138.
Appendix
Proof of (1)
Because 1 = I(Y1i ≥ Y1j) + I(Y1j ≥ Y1i) − I(Y1j = Y1i), the tie indicator for the non-terminal event
Because T2 is continuous, the event δ2iI(Y2i = x) will have probability zero for any real number x and i = 1, . . . , n. By definition, Y2i ≥ Y1i and Y2i = Y1i when δ1i = 0, i = 1, . . . , n. With these facts, notice that 1 − δ1iδ1j = (1 − δ1i)δ1j + (1 − δ1j), we have, for i ≠ j,
from which we conclude that Ω1ijW2ij = (1 − δ1j)δ2jI(Y1i ≥ Y1j) with probability one. If we write 1 − δ1iδ1j = (1 − δ1j)δ1i + (1 − δ1i), a similar technique will show Ω1ijW2ij = (1 − δ1i)δ2iI(Y1j ≥ Y1i) with probability one.
Proof of (4)
If T2 has an absolutely distribution, then the tie indicator for the terminal event
with which, we can write
We calculate
Therefore n−2W1(G1) converges to
similarly, n−2L1(G1) converges to
which completes the proof of (4).
Footnotes
DISCLAIMER: This paper reflects the views of the authors and should not be construed to represent FDAs views or policies.
References
- 1.Buyse M. Generalized pairwise comparisons of prioritized outcomes in the two-sample problem. Statistics in Medicine. 2010;29:3245–3257. doi: 10.1002/sim.3923. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. European Heart Journal. 2012;33(2):176–182. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 3.Stolker JM, Spertus JA, Cohen DJ, Jones PG, Jain KK, Bamberger E, Lonergan BB, Chan PS. Re-Thinking Composite Endpoints in Clinical Trials: Insights from Patients and Trialists. Circulation. 2014;134:11–21. doi: 10.1161/CIRCULATIONAHA.113.006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Pocock SJ. A win ratio approach to comparing continuous non-normal outcomes in clinical trials. Pharmaceutical Statistics. 2016;15:238–245. doi: 10.1002/pst.1743. [DOI] [PubMed] [Google Scholar]
- 5.Abdalla S, Montez-Rath M, Parfrey PS, Chertow GM. The win ratio approach to analyzing composite outcomes: An application to the EVOLVE trial. Contemporary Clinical Trials. 2016;48:119–124. doi: 10.1016/j.cct.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Tian H, Mohanty S, Tsai WY. An alternative approach to confidence interval estimation for the win ratio statistic. Biometrics. 2015;71:139–145. doi: 10.1111/biom.12225. [DOI] [PubMed] [Google Scholar]
- 7.Bebu I, Lachin JM. Large sample inference for a win ratio analysis of a composite outcome based on prioritized components. Biostatistics. 2016;17:178–187. doi: 10.1093/biostatistics/kxv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakes D. On the win-ratio statistic in clinical trials with multiple types of event. Biometrika. 2016;103:742–745. [Google Scholar]
- 9.Fleming TR, Harrington DP. A class of hypothesis tests for one and two samples censored survival data. Communications in Statististics. 1981;10:763–794. [Google Scholar]
- 10.Giné E, Latala R, Zinn J. Exponential and moment inequalities for U-statistics. High Dimensional Probability II Progress in Probability. 2000;47:13–38. [Google Scholar]
- 11.Houdré C, Reynaud-Bouret P. Exponential Inequalities, with Constants, for U-statistics of Order Two. Stochastic inequalities and applications Progress in Probability. 2003;56:55–69. [Google Scholar]
- 12.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. http://www.R-project.org/ [Google Scholar]
- 13.Caillat AL, Dutang C, Larrieu V, NGuyen T. R package version 1.01 2008. Gumbel: package for Gumbel copula. [Google Scholar]
- 14.The PEACE Trial Investigators. Angiotensin-Converting-Enzyme Inhibition in Stable Coronary Artery Disease. New England Journal of Medicine. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM ATLAS ACS 2 TIMI 51 Investigators. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. New England Journal of Medicine. 2012;366:9–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.