Abstract
Haemorrhage following injury is associated with significant morbidity and mortality. The role of fibrinogen concentrate in trauma-induced coagulopathy has been the object of intense research in the last 10 years and has been systematically analysed in this review. A systematic search of the literature identified six retrospective studies and one prospective one, involving 1,650 trauma patients. There were no randomised trials. Meta-analysis showed that fibrinogen concentrate has no effect on overall mortality (risk ratio: 1.07, 95% confidence interval: 0.83–1.38). Although the meta-analytic pooling of the current literature evidence suggests no beneficial effect of fibrinogen concentrate in the setting of severe trauma, the quality of data retrieved was poor and the final results of ongoing randomised trials will help to further elucidate the role of fibrinogen concentrate in traumatic bleeding.
Keywords: fibrinogen concentrate, trauma, bleeding, mortality
Introduction
Severe trauma is a major cause of mortality, being responsible for more than five million deaths annually worldwide1. Uncontrolled post-traumatic bleeding is the leading cause of death among these patients and is a major challenge for trauma care providers2. Blood loss that leads to an endogenous derangement of haemostasis, as well as dilutional and consumption coagulopathy complicate the control of haemorrhage in these patients. It has been estimated that about one third of all bleeding trauma patients present with a coagulopathy upon admission to hospital3. This early trauma-induced coagulopathy, which significantly worsens the patients’ prognosis4, is nowadays a well-recognised multifactorial condition resulting from a combination of bleeding-induced shock, tissue injury-related thrombin-thrombomodulin-complex generation and the activation of anticoagulant and fibrinolytic pathways5–7. In this context, it is evident that the immediate correction of the acquired coagulation disorder is one of the primary goals of the treatment protocols of trauma centres8. Among the various haemostatic treatments available, supplementation of fibrinogen has received growing attention in the last years9,10, considering that this is the first coagulation factor to reach a critically low concentration during activation of coagulation and bleeding and its reduction is associated with a worse outcome in injured patients11,12. Fibrinogen is a plasma glycoprotein synthesised by the liver which plays a critical role in haemostasis by acting as an endogenous substrate for fibrin formation and by inducing clot formation and platelet aggregation13,14. Fibrinogen supplementation can be achieved using fresh-frozen plasma, cryoprecipitate or plasma-derived, virally-inactivated, fibrinogen concentrate15–18, with the available therapeutic approaches differing from country to country14. Traditionally, a threshold level of 1.0 g/L was established for fibrinogen supplementation in patients with congenital fibrinogen deficiency14, whereas the threshold for patients with acquired fibrinogen deficiency, including those with trauma-related coagulopathy, is still debated. In fact, with the exception of a few guidelines that indicate trigger levels of fibrinogen less than 1.5–2 g/L19–21, the previous recommendations, including the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI, Società Italiana di Medicina Trasfusionale e Immunoematologia) guidance document17, indicate levels less than 0.8–1 g/L. Current European guidelines recommend supplementation of fibrinogen concentrate in trauma patients if significant bleeding is accompanied by viscoelastic signs of a functional fibrinogen deficit or a plasma fibrinogen level of less than 1.5–2.0 g/L (grade 1C)1, but this recommendation appears to reflect the opinion of the panel of experts rather than being based on evidence22,23. Indeed, although a number of randomised controlled trials investigating the use of fibrinogen concentrate in severe traumatic haemorrhage are currently underway, their results have not yet been published in full24–26. Moreover, the urgently needed, well-conducted, additional prospective clinical trials in bleeding patients with acquired fibrinogen deficiency should not only focus on dosing strategies and thresholds but should also be adequately powered to detect main and secondary selected outcomes27. The aim of this review is to analyse systematically the available literature evaluating the role of fibrinogen concentrate in the management of severe trauma.
Methods
Search methods
A computer-assisted literature search of the MEDLINE, EMBASE and SCOPUS electronic databases was performed to identify reports on clinical experience of the use of fibrinogen concentrate in patients with trauma. We used the search term combination “fibrinogen concentrate” and “trauma” or “bleeding”. In addition, we hand-searched the reference lists of the most relevant items (original studies and reviews) in order to identify further eligible studies not captured in the initial literature search.
Study selection
Studies were selected independently by two reviewers (MF and CM), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (GML). Assessment of potential eligibility was based on the title or abstract and on the full text if required. Articles were eligible if they reported the fibrinogen concentrate used in the management of severe trauma patients. Only studies published in full in English between January 2000 and February 2017 and enrolling at least ten patients were included in this systematic review. For each study included in the systematic review, the following data were extracted by two reviewers (MF and GM) independently: study design, sample size, protocol (fibrinogen plasma level threshold, comparative group) and results (dose of fibrinogen administered, outcomes), adverse events.
Outcomes
According to the study design of the different trials, the following outcomes were considered: mortality (overall in-hospital mortality, mortality at 6 hours, mortality at 24 hours, mortality at 72 hours, mortality at 7 days, mortality at 30 days and time to death), transfusion requirements (red blood cells and platelets), laboratory coagulation parameters and clinical outcomes (sepsis, multi-organ failure, days of ventilation, duration of hospitalisation and thromboembolic events).
Statistical evaluation and meta-analysis
The study designs were reviewed and the quality of the evidence at study level was evaluated. Each study was defined as prospective or retrospective, the presence of a control arm was confirmed, and the randomisation of the treatment allocation was determined. The control group was assessed if assigned by the experimenter, or simply observed a posteriori as exposure, as in cohort/observational studies. Uncontrolled studies were excluded from meta-analytical evaluation, as studies failing to set up an investigational arm treated with fibrinogen concentrate, and a control group treated otherwise. In order to proceed to meta-analytical pooling, all outcomes were reviewed and, if observed in at least three of the included studies, used as a measure of the effect of fibrinogen concentrate in a therapeutic efficacy evaluation. Any relevant binary event (e.g., death) was comparatively pooled as risk incidence (e.g., overall in-hospital mortality) in a cross-sectional way. The protective effect of fibrinogen concentrate was measured as a risk ratio with Mantel-Haenszel weighting. Heterogeneity was reported as the I-squared index.
Results
Literature search
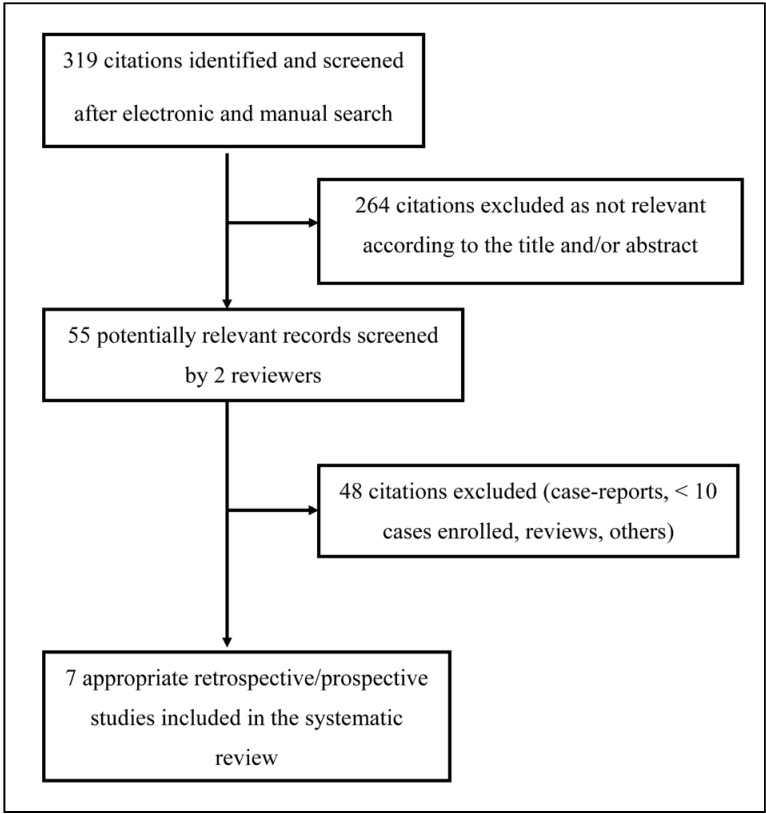
In total, 319 articles were found after the initial electronic and manual search (Figure 1). Of these, 264 were excluded as focusing on other topics. Thus, 55 potentially relevant articles were identified and the next screening led to the exclusion of another 48 studies (case reports, case series with less than ten patients included, reviews, protocols of randomised controlled trials, studies not containing informative data). The remaining seven studies (6 retrospective and 1 prospective)28–34 were included in this systematic review (see Table I for the main characteristics and results of the studies included and the online supplementary content for a more detailed description). Overall, 1,650 trauma patients were enrolled in the seven studies evaluated28–34.
Figure 1.
Flow chart of the inclusion of the studies.
Table I.
Main characteristic of the studies included in the systematic review evaluating fibrinogen concentrate in trauma patients.
| First author, year [ref.] | Study design | N. of patients | Trigger for administering fibrinogen concentrate | Fibrinogen dose | Outcomes | Main results |
|---|---|---|---|---|---|---|
| Danes, 2008 [28] | Retrospective | 11 | Fibrinogen level <1 g/L | Median 4 g | Mortality at 24 hours and 7 days after FC | Association between plasma fibrinogen levels and 7-day survival. No adverse effects. |
| Schochl, 2010 [29] | Retrospective | 128 | ROTEM MCF <10 mm | Median 7 g | Comparison of the observed mortality with the predicted mortality by TRISS and RISC | The observed mortality was 24.4%, lower than the TRISS-predicted mortality of 33.7% (p=0.032) and the RISC score-predicted mortality of 28.7% (p>0.05). |
| Schochl, 2011 [30] | Retrospective | 681 (80 FC ± PCC vs 601 FFP) | ROTEM MCF <10 mm | Median 6 g | Requirements of platelets and RBC units; mortality | RBC: 71% of patients in the FC group vs 97% in the FFP group (p<0.001). Platelets: 9% of patients in the FC group vs 44% in the FFP group (p<0.001). No mortality difference. |
| Weiss, 2011 [31] | Prospective observational | 62 | Fibrinogen level <1.45 g/L, blood loss 2.0 L | 4 g | Hospital mortality | Significant correlation between plasma fibrinogen level at the end of surgery and at 24 h post-FC; 3% thromboembolic complications. |
| Nienaber, 2011 [32] | Retrospective | 36 (18 FC vs 18 FFP) | ROTEM guided | Median 4 g | Morbidity, mortality and transfusion requirements | Fewer RBC transfusions in the FC group (3 U vs 12.5 U, p<0.005). No difference in overall mortality. No FC-related thromboembolic events. |
| Innerhofer, 2013 [33] | Retrospective | 144 (66 FC vs 78 FC + FFP) | ROTEM MCF <7 mm; fibrinogen level <1.5–2 g/L | 2 g FC only; 4 g FC + FFP | Coagulation parameters before and after treatment; blood products for the first 24 hours; clinical outcomes | Fewer blood products transfused in the FC group (RBC: 2 U vs 7 U, p<0.001; platelets: 0 U vs 1 U, p<0.001). No difference in clinical outcomes. |
| Wafaisade, 2013 [34] | Retrospective | 588 (294 with FC and 294 without FC) | NA | NA | 6-hour, 24-hour, 30-day and in-hospital mortality; MOF incidence | 6-hour mortality: 10.5% (FC+) vs 16.7% (p=0.03). No difference in 24-hour, 30-day, and in-hospital mortality. MOF 61.2% in the FC vs 49% in the non-FC group (p=0.003); 6.8% of thromboembolic events in FC vs 3.4% in the non-FC group (p=0.06). |
ROTEM: thromboelastometry; MCF: maximum clot firmness; TRISS: Trauma Injury Severity Score; RISC: Revised Injury Severity Classification; FC: fibrinogen concentrate; FFP: fresh-frozen plasma; PCC: prothrombin complex concentrate; MOF: multi-organ failure; NA: not available; RBC: red blood cell.
Quality assessment and outcome analysis
The quality of evidence of the seven studies evaluated was poor, according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria35. All studies were retrospective, except that by Weiss et al.31. All were cohort studies, in which the treatment allocation was an observed (post-hoc) exposure, instead of a randomised controlled trial or quasi-experimental studies with predetermined eligibility criteria and prior allocation. No study was randomised. Three studies were uncontrolled (Danes et al.28, Schoechl et al.29, Weiss et al.31), and were, therefore, excluded from the meta-analytic approach. The study by Innerhofer et al.33 was also excluded because all patients received fibrinogen concentrate, and the investigational group received fibrinogen concentrate plus fresh-frozen plasma (so, the topic was the effect of fresh-frozen plasma). All the remaining retrospective studies (Schoechl et al. 30, Nienaber et al.32, Wafaisade et al.34) depended on the German Trauma Register of Deutschen Gesellschaft für Unfallchirurgie (TR-DGU) and were evaluated for a possible meta-analytic approach.
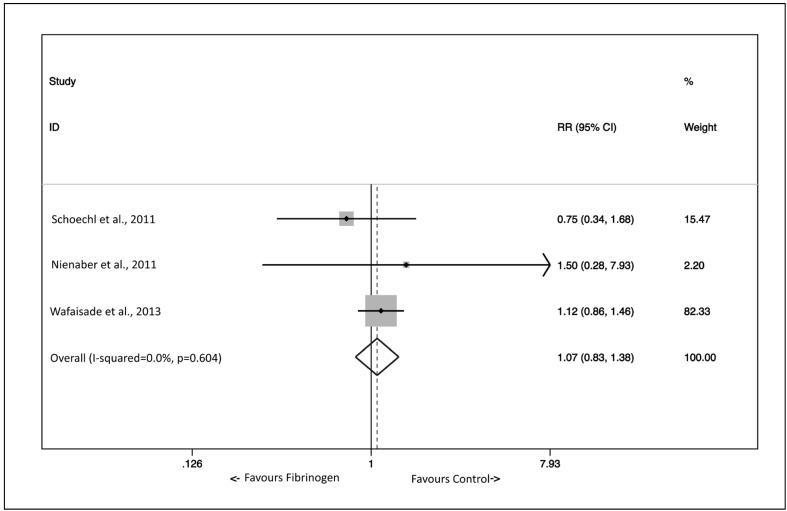
The outcomes were defined in several ways (Table II). However, overall mortality was recorded in all three of these studies30,32,34, and meta-analytic pooling could be performed. The pooled effect of fibrinogen concentrate on overall mortality, measured as a risk ratio (RR) with Mantel-Haenszel weighting and fixed effects method, was not significant (RR 1.07, 95% confidence intervals 0.83–1.38) (Figure 2). No heterogeneity was detected by the I-squared index (0%, p=0.604). However, the range of mortality rates in control groups was broad (9.9% to 25.5%). With regards to the other outcomes of interest, it was not possible to perform meta-analytic pooling of red blood cell transfusion requirements as the three studies with this type of information (Schoechl et al.30, Nienaber et al.32, Innerhofer et al.33) were not comparable since Schoechl et al.30 reported data in a binary form (i.e., the percentage of patients who avoided red blood cell transfusion), Nienaber et al.32 presented their data in a continuous form (i.e., the median number of red blood cell units transfused within the first 6 and 24 hours after admission), and Innerhofer et al.33 evaluated fresh-frozen plasma, and not fibrinogen concentrate, as their end-point. Finally, multi-organ failure was reported by Nienaber et al.32 and Wafaisade et al.34. Whereas the incidence was lower in the fibrinogen concentrate-treated group in the former study (16.7 vs 83.3%), the opposite was observed in the latter one (61.2 vs 49.0%).
Table II.
Overview and outcomes evaluated in the studies included in the systematic review.
| Author | Year | Target | Prospective | Controlled | Randomised | Outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Requirements | Coag. | Clinical | |||||||||||||||||
| 6 h | 24 h | 72 h | 7 d | 30 d | All | TTD | RBC | Plts | Sepsis | MOF | Vent. days | LOS | TE | |||||||
| Danes28 | 2008 | FC | No | No | No | * | * | * | * | |||||||||||
| Schoechl29 | 2010 | FC | No | No | No | * | ||||||||||||||
| Schoechl30 | 2011 | FC | No | Yes | No | * | * | * | ||||||||||||
| Weiss31 | 2011 | FC | Yes | No | No | * | * | * | ||||||||||||
| Nienaber32 | 2011 | FC | No | Yes | No | * | * | * | * | * | * | * | ||||||||
| Innerhofer33 | 2013 | FFP | No | Yes | No | * | * | * | * | * | * | * | * | * | ||||||
| Wafaisade34 | 2013 | FC | No | Yes | No | * | * | * | * | * | ||||||||||
Reported.
FC: fibrinogen concentrate; FFP: fresh-frozen plasma; Mortality: at 6 hours, 24 hours, 72 hours, 7 days, 30 days; All: overall mortality in hospital; TTD: time to death; RBC: red blood cell; Plts: platelets; Coag: laboratory coagulation parameters; MOF: multi-organ failure; Vent. days: ventilation days; LOS: length of stay; Adverse: adverse effects; TE: thromboembolic events.
Figure 2.
Effect of fibrinogen concentrate on overall in-hospital mortality.
The effect was measured comparatively as the risk ratio (RR) in three studies amenable to meta-analytical pooling. Squares denote RR, with size proportional to the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (CI) for each study. The diamond represents the aggregate effect, with the width representing the 95% confidence interval of the total effect.
Discussion
Trauma-related coagulopathy, which results from the rapid depletion of circulating coagulation factors because of consumption and blood loss, is a leading cause of mortality, being responsible for up to 40% of trauma-related deaths36,37. In cases of massive traumatic bleeding, the updated European guidelines recommend first restoration of circulating volume and secondarily haemostatic measures via transfusion of blood products or pharmaceutical agents1. In this context, the role of fibrinogen concentrate administered as immediate treatment in trauma has been investigated recently38,39. In a study conducted by Schochl et al.29 including 128 bleeding trauma patients, goal-directed coagulation management using thromboelastometry-guided administration of fibrinogen concentrate together with prothrombin complex concentrate was evaluated retrospectively. The observed mortality rate was lower in these patients than that predicted by the Trauma Injury Severity Score (TRISS) and the Revised Injury Severity Classification (RISC) score29. In a subsequent retrospective study conducted by the same group, comparing blood product requirements between trauma patients treated with fibrinogen concentrate and/or prothrombin complex concentrate, but no fresh-frozen plasma, and patients receiving only fresh-frozen plasma, it was found that a significantly smaller volume of blood products was transfused in patients receiving coagulation factor concentrates30. Innerhofer et al.33 compared patients who received fibrinogen concentrate without fresh-frozen plasma (n=66) or fibrinogen concentrate with fresh-frozen plasma (n=78), reporting smaller volumes of blood products transfused, including red blood cells and platelets, in patients receiving only the fibrinogen concentrate. There was no difference in clinical outcomes. Propensity score-matching confirmed that additional fresh-frozen plasma administration was associated with more red blood cell and platelet transfusions without additional benefit in restoring haemostasis. A retrospective study of 294 trauma patients conducted by Wafaisade et al.34 further evaluated whether administration of fibrinogen concentrate is associated with improved outcomes. Although 6-hour mortality was significantly reduced in the fibrinogen concentrate group, overall mortality was not significantly different between groups. In contrast to other studies, red blood cell requirement was not reduced in the fibrinogen concentrate group. The only published prospective observational study in this clinical setting is that by Weiss et al.31 in which a total of 223 patients were included, of whom 62 (28%) were trauma patients and received fibrinogen concentrate in association with fresh-frozen plasma. Interestingly, the authors found that plasma fibrinogen levels at the end of surgery and 24 hours after administration of fibrinogen concentrate were significantly higher in the survivors than in the non-survivors.
The current evidence on the use of fibrinogen concentrate in massive bleeding associated with severe trauma is mostly restricted to retrospective analyses. Indeed, of the seven studies identified in this systematic review (Table I), only one was prospective. In addition, although a wide array of outcomes were assessed by the different studies, rendering a comparative analysis very difficult, we were able to perform a meta-analysis of three retrospective studies that evaluated the main outcome, overall mortality.
In general, although a number of studies have reported on the use of fibrinogen concentrate for the treatment and prevention of acquired bleeding, its beneficial effect is still debated, mainly because of the low quality of the published clinical evidence and the data collected seem weak and insufficient as a basis for therapeutic or prophylactic guidelines40.
The results of our meta-analysis do not support a benefit in terms of survival from the use of fibrinogen concentrate in the setting of severe trauma. We are, however, aware of the methodological flaws of this research, mainly due to the limited amount and poor quality of the data retrieved41. Nevertheless, this is the first attempt to perform meta-analytic pooling of studies focused on the use of fibrinogen concentrate in traumatic haemorrhage. The final results of ongoing randomised controlled trials are required to overcome the biases present in the current literature and fully elucidate the effects of fibrinogen concentrate on allogeneic blood transfusion requirements and on survival in severely injured patients. We would like to highlight, however, that the early use of the relatively inexpensive and widely available haemostatic agent, tranexamic acid, has already shown a survival benefit in this clinical context in the frame of the Clinical Randomization of an Antifibrinolytic in Significant Haemorrhage-2 (CRASH-2) trial42,43.
Footnotes
Disclosure of conflicts of interest
GML is the Editor-in-Chief of Blood Transfusion and this manuscript has undergone additional external review as a result. The other Authors declare no conflicts of interest.
References
- 1.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–11. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 3.Frith D, Goslings JC, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 4.Floccard B, Rugeri L, Faure A, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2012;43:26–32. doi: 10.1016/j.injury.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 6.Noel P, Cashen S, Patel B. Trauma-induced coagulopathy: from biology to therapy. Semin Hematol. 2013;50:259–69. doi: 10.1053/j.seminhematol.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Maegele M, Schöchl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014;41:S21–5. doi: 10.1097/SHK.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 8.Maegele M, Nardi G, Schöchl H. Hemotherapy algorithm for the management of trauma-induced coagulopathy: the German and European perspective. Curr Opin Anaesthesiol. 2017;30:257–64. doi: 10.1097/ACO.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 9.Franchini M, Lippi G. Fibrinogen replacement therapy. A critical review of the literature. Blood Transfus. 2012;10:23–7. doi: 10.2450/2011.0015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlimp CJ, Schöchl H. The role of fibrinogen in trauma-induced coagulopathy. Hamostaseologie. 2014;34:29–39. doi: 10.5482/HAMO-13-07-0038. [DOI] [PubMed] [Google Scholar]
- 11.Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–5. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10:1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosesson MW. Fibrinogen and fibrin structure and function. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 14.Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54:1389–405. doi: 10.1111/trf.12431. [DOI] [PubMed] [Google Scholar]
- 15.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. I. The pre-operative period. Blood Transfus. 2011;9:19–40. doi: 10.2450/2010.0074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011;9:189–217. doi: 10.2450/2011.0075-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology Working Party. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus. 2011;9:320–35. doi: 10.2450/2011.0076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 20.Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fries D, Innerhofer P, Perger P, et al. [Coagulation management in trauma-related massive bleeding-recommendations of the Task Force for Coagulation (AGPG) of the Austrian Society of Anesthesiology, Resuscitation and Intensive Care Medicine (OGARI)]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2010;45:552–61. doi: 10.1055/s-0030-1265746. [In German.] [DOI] [PubMed] [Google Scholar]
- 22.Schochl H, Schlimp CJ, Voelckel W. Potential value of pharmacological protocols in trauma. Curr Opin Anesthesiol. 2013;26:221–9. doi: 10.1097/ACO.0b013e32835cca92. [DOI] [PubMed] [Google Scholar]
- 23.Meyer MA, Ostrowski SR, Windelov NA, Johansson PI. Fibrinogen concentrate for bleeding trauma patients: what is the evidence? Vox Sang. 2011;101:185–90. doi: 10.1111/j.1423-0410.2011.01478.x. [DOI] [PubMed] [Google Scholar]
- 24.Maegele M, Zinser M, Schlimp C, et al. Injectable hemostatic adjuncts in trauma: fibrinogen and the FIinTIC study. J Trauma Acute Care Surg. 2015;78:S76–82. doi: 10.1097/TA.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento B, Callum J, Tien H, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117:775–82. doi: 10.1093/bja/aew343. [DOI] [PubMed] [Google Scholar]
- 26.Steinmetz J, Sørensen AM, Henriksen HH, et al. Pilot randomized trial of fibrinogen in trauma haemorrhage (PRooF-iTH): study protocol for a randomized controlled trial. Trials. 2016;17:327. doi: 10.1186/s13063-016-1439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liumbruno GM, Vaglio S, Capuzzo E, Franchini M. Fibrinogen concentrate as haemostatic therapy in acquired bleeding disorders: not only a question of dosing strategies and thresholds. Blood Transfus. 2015;13:159–60. doi: 10.2450/2014.0204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danes AF, Cuenca LG, Bueno SR, et al. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94:221–6. doi: 10.1111/j.1423-0410.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 29.Schochl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schochl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss G, Lison S, Glaser M, et al. Observational study of fibrinogen concentrate in massive hemorrhage: evaluation of a multicenter register. Blood Coagul Fibrinolysis. 2011;22:727–34. doi: 10.1097/MBC.0b013e32834cb343. [DOI] [PubMed] [Google Scholar]
- 32.Nienaber U, Innerhofer P, Westermann I, et al. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42:697–701. doi: 10.1016/j.injury.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Innerhofer P, Westermann I, Tauber H, et al. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury. 2013;44:209–16. doi: 10.1016/j.injury.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Wafaisade A, Lefering R, Maegele M, et al. Administration of fibrinogen concentrate in exsanguinating trauma patients is associated with improved survival at 6 hours but not at discharge. J Trauma Acute Care Surg. 2013;74:387–93. doi: 10.1097/TA.0b013e31827e2410. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zentai C, Grottke O, Spahn DR, Rossaint R. Nonsurgical techniques to control massive bleeding. Anesthesiol Clin. 2013;31:41–53. doi: 10.1016/j.anclin.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesth Analg. 2012;114:261–74. doi: 10.1213/ANE.0b013e31822e1853. [DOI] [PubMed] [Google Scholar]
- 38.Winearls J, Campbell D, Hurn C, et al. Fibrinogen in traumatic haemorrhage: a narrative review. Injury. 2017;48:230–42. doi: 10.1016/j.injury.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Lunde J, Stensballe J, Wikkelso A, et al. Fibrinogen concentrate for bleeding: a systematic review. Acta Anaesthesiol Scand. 2014;58:1061–74. doi: 10.1111/aas.12370. [DOI] [PubMed] [Google Scholar]
- 40.Marano G, Mengoli C, Franchini M, et al. Fibrinogen concentrate in surgery. Blood Transfus. 2017;15:215–7. doi: 10.2450/2017.0362-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole D. Coagulopathy and transfusion strategies in trauma. Overwhelmed by literature, supported by weak evidence. Blood Transfus. 2016;14:3–7. doi: 10.2450/2015.0244-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 43.Franchini M, Mannucci PM. Adjunct agents for bleeding. Curr Opin Hematol. 2014;21:503–8. doi: 10.1097/MOH.0000000000000084. [DOI] [PubMed] [Google Scholar]