In silico analysis of iterative coexpression and protein interaction identifies root cell-enriched regulators of the phosphate starvation response in Arabidopsis with impact on phosphate status, transcriptome, and growth.
Abstract
Cellular specialization in abiotic stress responses is an important regulatory feature driving plant acclimation. Our in silico approach of iterative coexpression, interaction, and enrichment analyses predicted root cell-specific regulators of phosphate starvation response networks in Arabidopsis (Arabidopsis thaliana). This included three uncharacterized genes termed Phosphate starvation-induced gene interacting Root Cell Enriched (PRCE1, PRCE2, and PRCE3). Root cell-specific enrichment of 12 candidates was confirmed in promoter-GFP lines. T-DNA insertion lines of 11 genes showed changes in phosphate status and growth responses to phosphate availability compared with the wild type. Some mutants (cbl1, cipk2, prce3, and wdd1) displayed strong biomass gain irrespective of phosphate supply, while others (cipk14, mfs1, prce1, prce2, and s6k2) were able to sustain growth under low phosphate supply better than the wild type. Notably, root or shoot phosphate accumulation did not strictly correlate with organ growth. Mutant response patterns markedly differed from those of master regulators of phosphate homeostasis, PHOSPHATE STARVATION RESPONSE1 (PHR1) and PHOSPHATE2 (PHO2), demonstrating that negative growth responses in the latter can be overcome when cell-specific regulators are targeted. RNA sequencing analysis highlighted the transcriptomic plasticity in these mutants and revealed PHR1-dependent and -independent regulatory circuits with gene coexpression profiles that were highly correlated to the quantified physiological traits. The results demonstrate how in silico prediction of cell-specific, stress-responsive genes uncovers key regulators and how their manipulation can have positive impacts on plant growth under abiotic stress.
Forward and reverse genetics are powerful approaches for the discovery and characterization of gene function. While forward genetics is greatly facilitated by next-generation sequencing-assisted mapping, it is still limited by the design of appropriate screens that involve tens of thousands of plants. Conversely, reverse genetics involves phenotypic characterization of defined mutants, hence attributing the phenotype to the mutation (Winkler et al., 1998; Krysan et al., 1999). The preeminent model plant, Arabidopsis (Arabidopsis thaliana), has an extensive mutant library with substantial genome coverage (Sessions et al., 2002; Rosso et al., 2003), making reverse genetics an attractive strategy for investigating gene function (Wilson-Sánchez et al., 2014). A challenge of reverse genetics is selecting candidates that are biologically relevant to the field of research. One approach is to identify these from large-scale data sets, which are increasingly being generated in the postgenomics era (Ajjawi et al., 2010). Molecular profiling of genotypes on the omics scale has been carried out extensively. However, these studies tend to sample whole plants or plant organs, masking more subtle responses that occur within discrete cell populations. Fluorescence-activated cell sorting (FACS; Birnbaum et al., 2003, 2005) and laser-capture microdissection (Kerk et al., 2003; Nakazono et al., 2003) are techniques that enrich cell populations from organs, allowing profiling of the transcriptome, translatome, proteome, metabolome, or methylome to be carried out (Mustroph et al., 2009; Petricka et al., 2012; Efroni et al., 2015; Petersson et al., 2015). Studies on Arabidopsis primary root cell types have revealed a wealth of information regarding the spatial enrichment of transcriptional programs (Birnbaum et al., 2003; Brady et al., 2007, 2011).
Exogenous nutrient availability is a key determinant of root development that affects nutrient uptake and plant productivity. Utilizing cellular enrichment techniques, root cell type-specific regulatory networks were uncovered in plants subjected to phosphate (Cederholm and Benfey, 2015) or iron deficiency (Dinneny et al., 2008). The former identified signaling peptides ROOT GROWTH FACTOR1 and ROOT GROWTH FACTOR2 to differentially affect phosphate-dependent root elongation and cell division in the root apical meristem. The work by Dinneny and coworkers (2008) not only uncovered new genes of interest but also revealed differential, stress-dependent regulation of biological functions in specific cell types. Examples include the basic helix-loop-helix transcription factors FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR1 (FIT1) and POPEYE, which activate iron-uptake genes under iron deficiency in epidermal cells of the root differentiation zone and in pericycle cells of the mature root, respectively (Long et al., 2010; Wild et al., 2016). Root development also is regulated by microRNA167-dependent control of AUXIN RESPONSE FACTOR8 expression in the root pericycle, mediating lateral root outgrowth in response to nitrogen (Gifford et al., 2008). While these cell-specific studies provide novel biological insight, they are technically challenging. Consequently, it can be efficient to apply data-mining strategies, where existing data sets are reanalyzed to develop new hypotheses. One recent successful example is the nutrient-dependent regulation of root hair development identified through coexpression pipelines (Salazar-Henao and Schmidt, 2016).
Phosphorus (P) is an essential macronutrient for all organisms. Plants take up an inorganic form of P, (dihydrogen) phosphate (H2PO4−; Pi). Pi is indispensable for growth and development as an integral component of primary metabolites, phospholipids, and nucleic acids as well as in a large number of biochemical reactions and signaling pathways (Zhang et al., 2014). However, Pi is one of the most growth-limiting nutrients due to its innate chemical properties, which result in the formation of insoluble mineral complexes in the soil and low bioavailability (Bieleski, 1973). The current agricultural practice of Pi-rich fertilizer use is both ecologically detrimental and financially unsustainable; therefore, identifying components that regulate plant Pi uptake, transport, and metabolism within the plant to sustain plant growth will contribute to developing more sustainable agronomics (Richardson et al., 2011). In response to limited Pi availability in the soil, plants respond with morphological, physiological, and molecular adaptations, which serve to enhance Pi acquisition and distribution within the plant. These include a slowing of primary root growth coupled with lateral root and root hair proliferation (Ma et al., 2001; Williamson et al., 2001) and the translocation of Pi from shoots to the proliferating root systems. Resource mobilization causes an increase in the biomass ratio of roots over shoots (Trull et al., 1997). Examples of biochemical changes are the remobilization of stored Pi (Versaw and Harrison, 2002), the scavenging of Pi from nucleic acids by ribonucleases (Bariola et al., 1994) and from other phosphate esters by purple acid phosphatases (del Pozo et al., 1999), as well as the substitution of membrane phospholipids with sulfolipids (Yu et al., 2002) and galactolipids (Härtel et al., 2000; Gaude et al., 2008). While root architecture remodeling in response to low exogenous Pi is regulated locally at the root tip (Svistoonoff et al., 2007; Ticconi et al., 2009), low endogenous Pi levels trigger a systemic response in Arabidopsis mediated largely by a Myb-like transcription factor of the R2R3-MYB family, PHOSPHATE STARVATION RESPONSE1 (PHR1), and its close homolog, PHR1-LIKE (Rubio et al., 2001; Nilsson et al., 2007; Bustos et al., 2010). High-affinity Pi transporters such as several members of the PHOSPHATE TRANSPORTER1 family (PHT1), including PHT1;1 and PHT1;4 found in the root epidermis (Mitsukawa et al., 1997; Mudge et al., 2002; Ayadi et al., 2015), are responsible for the majority of Pi uptake by roots and are up-regulated by PHR1 under Pi deficiency to increase Pi uptake capacity (Shin et al., 2004; Nilsson et al., 2007). The best characterized signaling cascade in the regulation of Pi metabolism is the transcriptional up-regulation of the microRNA miR399 by PHR1 (Chiou et al., 2006; Pant et al., 2008); miR399, in turn, posttranscriptionally inhibits PHOSPHATE2 (PHO2)/UBIQUITIN-CONJUGATING ENZYME24 expression in roots, suppressing the inhibitory role of PHO2 on high-affinity PHT1 transporters in the root epidermis and PHO1 Pi exporter activity in the stele (Delhaize and Randall, 1995; Birnbaum et al., 2003; Aung et al., 2006; Bari et al., 2006; Liu et al., 2012). Both PHO2 and the E3 ubiquitin ligase NITROGEN LIMITATION ADAPTATION, itself a target of the microRNA miR827, synergistically control PHT1 protein maturation and stability (Lin et al., 2013; Park et al., 2014).
This PHR1-mediated regulatory network has largely been identified by forward genetic screens, followed by the characterization of associated regulators through reverse genetics. Transcriptome studies on Pi-deficient plants show distinct organ-specific responses (Wu et al., 2003; Misson et al., 2005; Woo et al., 2012), and there is mounting evidence of spatial differences in the regulatory networks that mediate Pi transport locally within the root (Thibaud et al., 2010): PHT1;1 and PHT1;4 are involved in the uptake of Pi from the rhizosphere (Shin et al., 2004; Ayadi et al., 2015), PHT1;8 and PHT1;9 facilitate Pi translocation toward the root vasculature (Lapis-Gaza et al., 2014), and PHO1 is responsible for Pi loading into the xylem (Poirier et al., 1991; Hamburger et al., 2002; Arpat et al., 2012).
The aim of this study was to design an in silico approach to predict candidates from large-scale data sets and to confirm these as novel root cell-specific regulators of Pi uptake and translocation in Arabidopsis. This was achieved by integrating landmark P-related transcriptomics data with root cell-specific expression profiles to predict genes involved in root cell-enriched responses to Pi limitation. Combining this with coexpression and protein interaction data sets in an iterative data analysis pipeline resulted in a list of Phosphate starvation-induced (PSI) gene interacting Root Cell Enriched (PRCE) genes that included three genes with unknown function termed PRCE1, PRCE2, and PRCE3. Root cell type-enriched expression of a select number of PRCE genes was confirmed in promoter-GFP transgenic lines. The role of the encoded proteins in the plant’s Pi starvation response was further explored using corresponding T-DNA insertion mutants. Physiological, biochemical, and molecular approaches identified novel mutant phenotypes with respect to organ Pi status and biomass accumulation under Pi limitation. Combining mutant root transcriptome data with physiological responses to Pi availability via a multivariate network model enabled us to link coexpression gene modules with individual physiological mutant traits. These root-specific interaction networks revealed that some of the mutants were less sensitive to Pi limitation or were impaired in intracellular Pi sensing, important attributes for downstream agricultural applications. Future studies of identified hub genes in these modules will offer unprecedented insights into networks controlling important agronomic traits. This work further illustrates that cell-specific regulatory components can alter whole organ and plant responses to changes in Pi availability, which implies that they can generate signals that travel across multiple cell layers, ultimately controlling plant P status.
RESULTS
Prediction of Cell-Specific Regulators of the Pi Starvation Response in Roots
To predict novel genes that are involved in the control of Pi uptake or organ Pi accumulation (Fig. 1), transcript enrichment in six different root cell types (Brady et al., 2007; Supplemental Data Set S1B) was superimposed onto 241 PSI genes in roots subjected to long-term (5–15 d) Pi deprivation (Misson et al., 2005; Supplemental Data Set S1A). This identified 181 genes with a more than 2-fold enrichment (10% or greater total signal across root cell files) in at least one root cell type (Fig. 1). Single cell type enrichments were predicted for 16 PSI genes in epidermal cells, six in cortical cells, five in the endodermis, 26 in the pericycle, 12 in xylem parenchyma cells, and 12 in the phloem (Supplemental Data Set S1C). Eight PSI genes showed enhanced expression in the columella and/or the lateral root cap. The remaining 96 PSI genes showed higher transcript abundance in at least two different root cell types.
Figure 1.
Workflow for iterative cell enrichment and coexpression analysis to identify PRCE genes in Arabidopsis roots. Genes induced under P starvation (Supplemental Data Set S1A; Misson et al., 2005) were overlaid with data sets describing transcriptional root cell type-specific enrichment (top; Supplemental Data Set S1B; Brady et al., 2007), identifying a subset of 181 genes (middle; Supplemental Data Set S1C). At bottom, putative interactors for these genes were identified using the Arabidopsis Interactions Viewer (Supplemental Data Set S1D; Geisler Lee et al., 2007) and were then overlaid with the cell type-specific enrichment data as described above (Supplemental Data Set S1E). Candidate genes (in red) were selected for detailed molecular and physiological characterization (Supplemental Data Set S1F).
Putative interacting partners were identified for 88 of the 181 cell type-enriched PSI genes using the Arabidopsis Interactions Viewer (Geisler-Lee et al., 2007; Brady and Provart, 2009), resulting in a total of 809 putative interactors (Fig. 1; Supplemental Data Set S1D). Out of these predicted interactors, 191 were enriched in the epidermis, 104 in the cortex, and 42 in the endodermis. Another 185 putative interactors were preferentially expressed in the pericycle, 313 in xylem parenchyma cells, and 82 in the phloem (Supplemental Data Set S1E).
In order to select candidates for downstream analyses and to validate our predictive approach, we focused on the following interaction networks (Fig. 1, bottom). (1) Pi transporters PHT1;1, PHT1;2, and PHT1;4 as well as PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 (PHF1) are expressed in root epidermal cells, and high-affinity PHT1 transporters constitute the gateway of Pi into the plant (Shin et al., 2004; Ayadi et al., 2015). Thirty-one candidates were predicted to interact with one of the three PHT1 transporters or PHF1 (Supplemental Data Set S1D). Out of these, WD40 DOMAIN1 (WDD1), AT1G72480, AT3G62770, and AT4G01320 were coexpressed with the respective transporter (Supplemental Data Set S1E). Genes encoding another five putative interactors, SUGAR TRANSPORT PROTEIN10 (STP10), ACYL-COA OXIDASE5 (ACX5), AT1G11530, AT4G23660, and AT5G09390, were expressed in all root cell types. (2) Given emerging evidence that radial Pi transport across cells in the central root cylinder and xylem loading of Pi is critical for P status control (Hamburger et al., 2002; Lapis-Gaza et al., 2014; Zhang et al., 2014; Wege et al., 2016), we chose genes that were enriched in these tissues. Among these candidates were a number of genes encoding Ser/Thr protein kinases, S6 KINASE2 (S6K2), and four members of the CBL-interacting protein kinase (CIPK) family, as well as their calcium-dependent membrane anchor CALCINEURIN B-LIKE PROTEIN1 (CBL1), which showed enhanced expression in the inner stele (Supplemental Data Set S1E). Distinct CBL-CIPK pairs have been associated with the regulation of potassium and nitrate transporter activity (Xu et al., 2006; Ho et al., 2009; Hu et al., 2009), making these gene families attractive candidates for the regulation of radial Pi transport. (3) A number of highly interconnected genes were predicted to interact with a cluster, including PHF1, NITRATE TRANSPORTER1.8, EARLY LIGHT-INDUCABLE PROTEIN1 (ELIP1), and SENESCENCE-ASSOCIATED GENE14 (SAG14), and contained many genes that were enriched in the vasculature. Overall, seven genes (CBL1, CIPK2, CIPK4, CIPK6, S6K2, SAG14, and AT5G41190) selected for downstream analyses were predicted to be expressed in endodermis and/or pericycle, while 14 were transcribed in xylem parenchyma and phloem cells, including CIPK14, MAJOR FACILITATOR SUPERFAMILY1 (MFS1), PRCE1, PRCE2, AT4G30500, and AT5G58190. Six genes (CBL1, CIPK4, CIPK14, S6K2, SAG14, and MFS1) are induced by more than 2-fold in P-limited wild-type roots (Supplemental Data Set S1F; Supplemental Fig. S4).
For detailed further characterization, genes encoding proteins with unknown or regulatory functions were prioritized, because one aim of our study was to identify novel genes involved in Pi metabolism. Genes previously linked to Pi metabolism, such as those encoding Pi transporters of the PHT1 family, Pi sensors of the SYG1/Pho81/XPR1 DOMAIN GENE (SPX) family, mitogen-activated protein kinases, or phospholipases were excluded from the candidate list. In total, 28 PRCE genes were selected for experimental verification of their role in Pi uptake or P status control, which included three genes with yet unknown function that we named PRCE1, PRCE2, and PRCE3 (Supplemental Data Set S1F).
Root Cell-Specific Expression Profiles of Promoter-GFP Lines for PRCE Genes
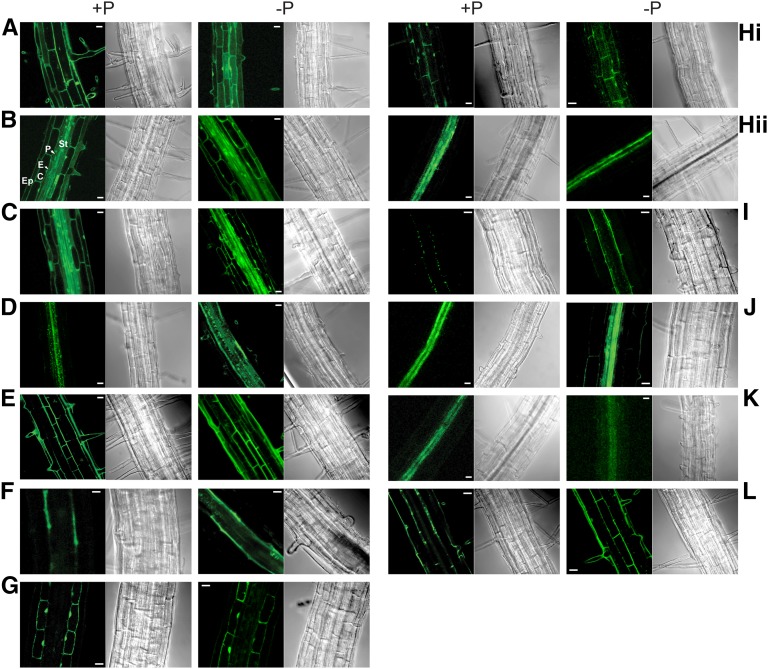
Promoter-GFP reporter lines were generated for 12 PRCE genes listed in Supplemental Data Set S1F to determine if they indeed displayed cell type-specific or cell type-enriched expression (Supplemental Table S1). Overall, 10 of the 12 promoter-GFP constructs displayed a cell-restricted expression, with only CBL1 and CIPK2 displaying ubiquitous promoter activity (Fig. 2). GFP fluorescence imaging confirmed predicted transcript enrichment in the epidermis for WDD1, in the pericycle for S6K2, and in the inner stele for CIPK14 and PARTNER OF SLD FIVE2 (PSF2). Consistent with earlier reports, S6K2 expression in the pericycle was observed only in P-limited roots but was barely detectable in P-replete roots (Misson et al., 2005). SAG14 expression was predicted to be highest in the pericycle (Supplemental Data Set S1C), while GFP fluorescence appeared to be stronger in the inner stele (Fig. 2J; Supplemental Table S1). PRCE2 expression showed the opposite pattern, with FACS-determined transcript abundance peaking in xylem parenchyma cells, while the promoter-GFP lines showed strongest expression in the pericycle. PRCE2 expression in the pericycle matches the enrichment of transcripts encoding ELIP1, one of its putative interactors (Supplemental Data Set S1E). ACX5, PRCE3, and STP10 were determined to be expressed ubiquitously based on FACS and transcript abundance (Brady et al., 2007; Supplemental Data Set S1B), while their respective promoter-GFP lines (Supplemental Table S1) suggest epidermal enrichment for ACX5 (Fig. 2A), cortical enrichment for PRCE3 (Fig. 2G), and high xylem parenchyma expression for STP10 (Fig. 2K). For ACX5 and STP10, our RNA-sequencing (RNA-seq) data (see below) indicated very low overall expression below the read count cutoff in roots. Weak STP10 expression restricted to the root vasculature also was observed in pSTP10:STP10g-GUS reporter lines (Rottmann et al., 2016). The STP10 expression domain observed in this promoter-GUS line and our promoter-GFP line matches that of DUAL-SPECIFICITY PHOSPHATASE1 in the pericycle, encoding one of its putative interactors (Supplemental Data Set S1E). Another weakly expressed gene, MFS1, has two closely related, more strongly expressed isoforms in our root transcriptome data set, AT1G80530 and AT2G16660. MFS1 transcripts were shown to be highly enriched in xylem parenchyma cells (Supplemental Data Set S1, C and E; Brady et al., 2007), while three independent promoter-GFP lines showed strong GFP fluorescence in the epidermis (Fig. 2E; Supplemental Table S1). This might be explained by microarray cross-hybridization to cDNA of the more strongly expressed isoforms, given that AT2G16660 is predicted to be enriched in xylem (Supplemental Data Set S1B).
Figure 2.
Fluorescence imaging of PRCE promoter-GFP lines. Confocal imaging of GFP fluorescence in roots of reporter lines of selected PRCE genes identified their cell type-specific expression patterns. For each line, the left image shows a representative image of observed GFP signals and the right image shows the corresponding bright-field image. Images where taken under P-replete (+P) and P-limited (−P) growth conditions as indicated. Genes and their localizations are as follows: ACX5, epidermis (A); CBL1, ubiquitous (B); CIPK2, ubiquitous (C); CIPK14, stele (D); MFS1, epidermis (E); PRCE2, pericycle (F); PRCE3, cortex (G); PSF2, epidermis (Hi) and inner stele (Hii); S6K2, pericycle (I); SAG14, inner stele (J); STP10, xylem parenchyma (K); and WDD1, epidermis and endodermis (L). For the CBL1 promoter-GFP line with ubiquitous expression throughout the root, the different cell types are marked: C, cortex; E, endodermis; Ep, epidermis; P, pericycle; and St, stele. The details of the number of lines characterized for each gene are outlined in Supplemental Table S1. Bars = 20 µm.
Overall, promoter-GFP lines of PRCE genes showed fluorescence peaks matching transcript profiles in the high-resolution spatiotemporal root map (Brady et al., 2007). Some variation was observed for weakly expressed genes. Differences also may reflect oscillations in dominant expression patterns during development (Brady et al., 2007). Expression domains did not change in response to the stress treatment, validating our initial assumption that informed overlaying stress-responsive with cell type-enriched expression data sets.
Identification of T-DNA Mutant Lines for Selected PRCE Genes
Using the Salk Institute Genomic Analysis Laboratory’s T-DNA Express mapping tool (Alonso et al., 2003), homozygous T-DNA insertion lines were identified for 17 of the 28 candidate genes listed in Supplemental Data Set S1F. For 14 mutants, changes in organ Pi concentrations were detected in an initial screen (Supplemental Data Set S1F). After the determination of left and right border T-DNA insertion sites, significant change of transcript abundance was observed via quantitative reverse transcription-PCR for 10 mutant lines, suggesting disrupted gene function (Supplemental Table S2). T-DNA insertion into the promoter of PSF2 caused significant transcript accumulation (12-fold higher than in Columbia-0 [Col-0]). Therefore, this mutant is considered to be a gain-of-function mutant. The resulting 11 prce mutant lines (Table I) were subjected to an in-depth physiological and molecular characterization of their response to changes in Pi supply.
Table I. PRCE genes selected for detailed characterization.
| Gene Identifier | Arabidopsis Genome Initiative Code | Name | PSR Gene? | Root Cell Typea | Interactor | Root Cell Typea | Mutant Identifier | Reference |
|---|---|---|---|---|---|---|---|---|
| CBL1 | At4g17615 | CALCINEURIN B-LIKE PROTEIN1 | Yes (1.4b) | C, E, P | SALK_110426C (Xu et al., 2006) | Kudla et al. (1999); Shi et al. (1999) | ||
| CIPK2 | At5g07070 | CBL-INTERACTING PROTEIN KINASE2 | No | Ep, P | CBL1 | C, E, P | GK-031A02 (Koprivova, 2014) | Kim et al. (2000) |
| NRT1;8 | ||||||||
| CIPK14 | At5g01820 | CBL-INTERACTING PROTEIN KINASE14 | Yes (1.6b) | X, Ph, Cm | SALK_009699C (Lin et al., 2014) | Nozawa et al. (2001) | ||
| MFS1 | At4g34950 | MAJOR FACILITATOR SUPERFAMILY1 | Yes (1.1b) | X, Cm | SALK_066841C | This work | ||
| PRCE1 | At3g13175 | PSI-INTERACTING ROOT-CELL ENRICHED1 | No | Ph | PHF1 | Ep | SALK_015987C | This work |
| ELIP1 | St | |||||||
| SAG14 | P, Cm, LRC | |||||||
| PRCE2 | At5g03345 | PSI-INTERACTING ROOT-CELL ENRICHED1 | No | Ep, X | ELIP1 | St | SALK_117485C | This work |
| SAG14 | P, Cm, LRC | |||||||
| PRCE3 | At5g52420 | PSI-INTERACTING ROOT-CELL ENRICHED1 | No | ubi | ELIP1 | St | SALK_056788 (McDowell et al., 2013) | This work |
| SAG14 | P, Cm, LRC | |||||||
| PSF2 | At3g12530 | PARTNER OF SLD FIVE2 | No | X | PHT1;2 | Ep, C | SALK_151539C | Shultz et al. (2007) |
| S6K2 | At3g08720 | SER/THR PROTEIN KINASE2 | Yes (1.1b) | P, Ph | SALK_039937C | Turck et al. (1998) | ||
| STP10 | At3g19940 | SUGAR TRANSPORT PROTEIN10 | No | ubi | PHT1;2 | Ep, C | SALK_207063C (Rottmann et al., 2016) | Rottmann et al. (2016) |
| PFA-DSP1 | P | |||||||
| WDD1 | At5g54200 | WD40-DOMAIN1 | No | Ep, Ph | PHT1;2 | Ep, C | SAIL_210_F10 | This work |
Transcript enriched in root cell type (greater than 10% of total signal; Brady et al., 2007). C, Cortex; Cm, columella; E, endodermis; Ep, epidermis; LRC, lateral root cap; P, pericycle; Ph, phloem; St, stele; ubi, ubiquitous; X, xylem parenchyma.
Log2 fold change in transcript abundance in P-limited over P-replete roots (this study).
Accumulation of Free Phosphate in Roots and Shoots of prce Mutants
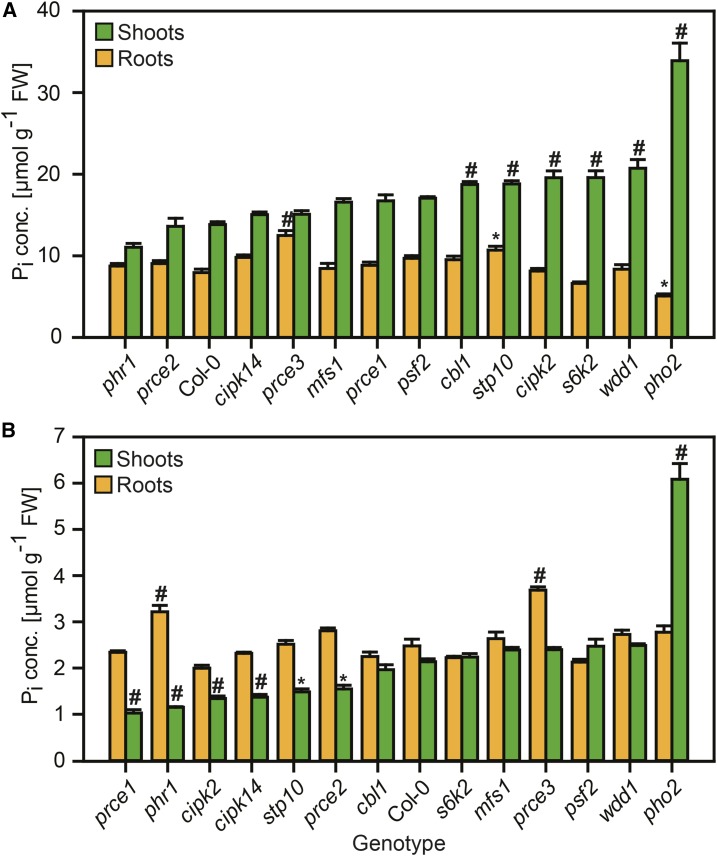
Changes in organ (root and shoot) Pi concentration in response to external Pi availability are indicative of altered P signaling networks that govern Pi transport and resource reallocation from shoots to roots (Williamson et al., 2001; Smith et al., 2011). Organ Pi levels were determined in roots and shoots 5 d after transfer of 9-d-old seedlings to either replete Pi (1 mm) or limiting Pi (12 µm) concentrations in Murashige and Skoog (MS) medium (see “Materials and Methods”). For comparison, prce mutants were grown alongside Col-0 (the wild type), phr1-1 (reduced shoot Pi concentration under limiting Pi supply; Nilsson et al., 2007), and pho2-1 (Pi overaccumulation in shoots; Delhaize and Randall, 1995). Col-0 shoots had higher shoot Pi concentrations than roots when Pi was readily available (Fig. 3A), while the inverse relationship was found under limiting Pi supply (Fig. 3B). pho2-1 showed the typical extreme accumulation of Pi in shoots of P-replete seedlings, which was 2.5-fold higher than in P-replete wild-type shoots and was maintained to some extent when plants were transferred to plates with low available Pi. Pi concentrations in pho2-1 roots were indistinguishable from Col-0 under limiting Pi supply but 36% lower than those of Col-0 roots under replete Pi supply. By contrast, the phr1-1 mutant showed wild-type-like Pi levels in P-replete conditions, while, under limiting Pi supply, roots had significantly higher and shoots had significantly lower Pi concentrations than Col-0 (Fig. 3).
Figure 3.
Pi concentrations in roots and shoots of prce seedlings in response to Pi supply. Organ Pi concentrations (root = orange bars, shoot = green bars) are shown for 14-d-old seedlings 5 d after transfer to P-replete (A) or P-limited (B) medium. Data shown are averages ± se; n = 3 (Supplemental Data Set S2A). Significant differences compared with Col-0 according to two-way ANOVA followed by Tukey’s multiple comparison test are indicated with a hash (#, P < 0.001) or an asterisk (*, P < 0.05). FW, Fresh weight.
While shoot Pi accumulation was less pronounced than in pho2-1, five prce mutants, cbl1, stp10, cipk2, s6k2, and wdd1, showed significant increases of 35% to 50% over Col-0 (Fig. 3; Supplemental Data Set S2A). P-replete stp10 and prce3 roots showed 35% and 57% Pi increases, respectively, compared with the wild type. P-limited prce3 roots were able to maintain higher Pi concentration than those of Col-0. By contrast, P-limited stp10 had wild-type-like Pi levels in roots and 31% lower Pi levels in shoots. This reduction in shoot Pi concentration was similar to that found in phr1-1 (46%) and would suggest that stp10 is overly sensitive to low Pi supply. Similar reductions in shoot Pi concentration also were observed for P-limited prce1 (52%), cipk2 (37%), cipk14 (36%), and prce2 (28%). Unlike phr1-1, all these genotypes had wild-type-like Pi concentrations in P-limited roots.
Physiological Characterization of prce Mutants under P-Replete and P-Limited Conditions
A number of physiological characteristics were chosen to ascertain the role of the candidate genes in Pi acquisition and translocation. A common response to P-limited conditions is to arrest shoot growth (Nielsen et al., 2001; Williamson et al., 2001). The ensuing change in root-to-shoot biomass ratio drives root hair and lateral root growth for top soil exploration. Thus, Pi limitation leads to a change in root system architecture. While lateral root and root hair growth is stimulated, Arabidopsis primary root elongation slows to between 25% and 55% of the final root length of P-replete plants (Williamson et al., 2001; López-Bucio et al., 2002). To determine if the prce mutants were affected in biomass allocation during P-limited growth, 9-d-old seedlings were transferred to vertical MS-agar plates containing either replete Pi (1 mm) or limiting Pi (12 µm) concentrations for 5 d (see “Materials and Methods”). For comparison, the mutants were grown alongside Col-0 (the wild type), phr1-1 (less increase in root-to-shoot ratio under limiting Pi supply; Rubio et al., 2001), and pho2-1 (longer primary roots under replete Pi supply; Williamson et al., 2001).
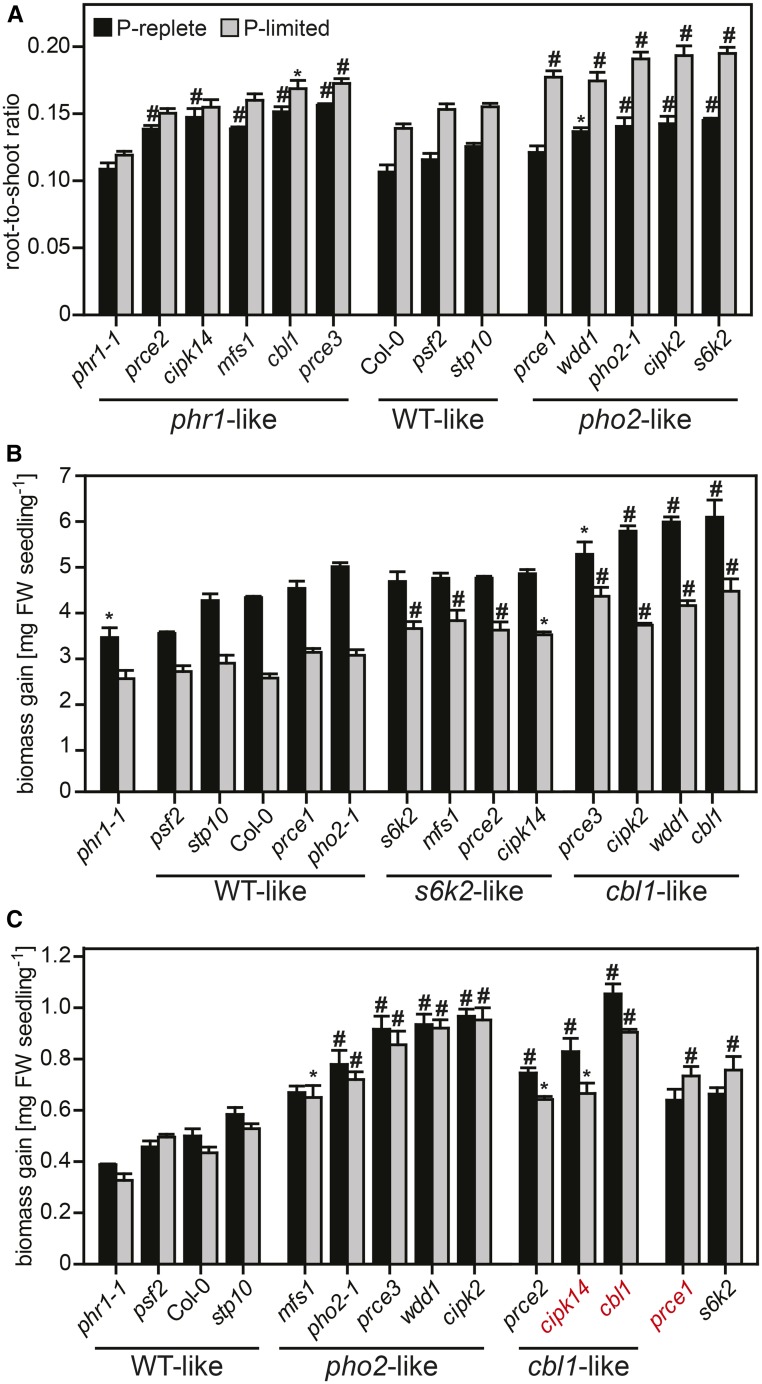
Biomass Allocation under P-Limited Conditions
Col-0 showed a 30% increase in the root-to-shoot ratio in P-limited versus P-replete conditions (Fig. 4A; Supplemental Data Set S2). As reported previously, the phr1-1 mutant lacked this response (Nilsson et al., 2007). By contrast, pho2-1 showed a stronger response to low Pi supply, with a 36% increase in the root-to-shoot ratio. The prce mutants split up into three different groups: stp10 and psf2 showed wild-type-like behavior, while cbl1, cipk14, mfs1, prce2, and prce3 showed little change in the root-to-shoot ratio in response to Pi supply, similar to phr1-1. A stronger, pho2-1-like increase in the root-to-shoot ratio under P-limited growth was displayed by cipk2, prce1, s6k2, and wdd1 (Fig. 4A). This increase is all the more remarkable for cipk2 and wdd1, given that they also feature higher shoot biomass than the wild type, irrespective of Pi supply (Fig. 4B; Supplemental Fig. S1).
Figure 4.
Growth responses of prce seedlings to changes in Pi availability. A, Root-to-shoot ratios of P-replete (black bars) and P-limited (gray bars) seedlings (Supplemental Data Set S2B). Lines with a significant ratio increase under P limitation (P < 0.001) are in the wild-type (WT)-like and pho2-like groups. The phr1-like group showed no P-dependent shift in the root-to-shoot ratio. B and C, Shoot (B) and root (C) biomass gain in seedlings 5 d after transfer to either P-replete (black bars) or P-limited (gray bars) medium. Shoot biomass gains showed significant differences between treatments (P < 0.001) across genotypes. Treatment differences (P < 0.001) in root biomass gain are highlighted in red. B, P-limited shoots of s6k2-like mutants were able to sustain growth, while cbl1-like mutants had higher shoot biomass gain than the wild type irrespective of Pi supply. C, Roots of seedlings in the cbl1-like group showed a hypersensitive response to Pi limitation, while P-limited roots of prce1 and s6k2 accumulated more biomass than P-replete roots. Data shown are averages ± se; n = 3. Significant differences from Col-0 were identified by two-way ANOVA followed by Tukey’s multiple comparison test and are indicated with a hash (#, P < 0.001) or an asterisk (*, P < 0.05). FW, Fresh weight.
Changes in Growth Responses to Limiting Pi Availability
To determine the overall effects of Pi supply on plant growth, shoot and root biomass gains were calculated as the difference in organ biomass of 9-d-old seedlings prior to transfer to that of 14-d-old seedlings that had been subjected to 5 d of either limiting or high Pi supply. While prce mutants were classified into phr1-like and pho2-like biomass allocation patterns, a closer examination of P-limited growth responses of prce roots and shoots revealed more subtle differences: s6k2, and to a lesser extent prce1, were overall more tolerant to low Pi, with higher shoot (Fig. 4B) and in particular root (Fig. 4C) biomass gains (P-limited over P-replete organs) than the wild type. cbl1, cipk14, and prce2 showed a hypersensitive response to low Pi supply in roots (Fig. 4C) while maintaining higher shoot biomass than the P-limited wild type (Fig. 4B). As reported earlier by Williamson et al. (2001), pho2-1 roots had a significantly higher biomass than Col-0 (Fig. 4C), irrespective of Pi supply. Similar to pho2-1, the prce mutants cipk2, mfs1, prce3, and wdd1 all had very prolific root systems irrespective of Pi supply.
A typical response of Arabidopsis to reduced Pi availability is the slowing of primary root elongation that is offset by an increase in lateral root proliferation (Williamson et al., 2001; López-Bucio et al., 2002). Root biomass was reduced by 11% in P-limited compared with P-replete Col-0 plants (Supplemental Data Set S2B). This was accompanied by a 15% reduction in relative root growth rate resulting in 21% shorter primary roots (Supplemental Data Set S2C). P-replete phr1-1 roots overall were shorter than those of Col-0 and showed no significant change in root growth rate when P limited. P-limited pho2-1 roots, on the other hand, showed a strong reduction in relative root growth rate (17% reduction compared with Col-0; Supplemental Data Set S2C). P-limited roots of prce1 and prce3 showed a phr1-like increase in relative growth rates, while P-limited cbl1, cipk2, cipk14, and wdd1 roots showed a pho2-like slowing of relative growth rates.
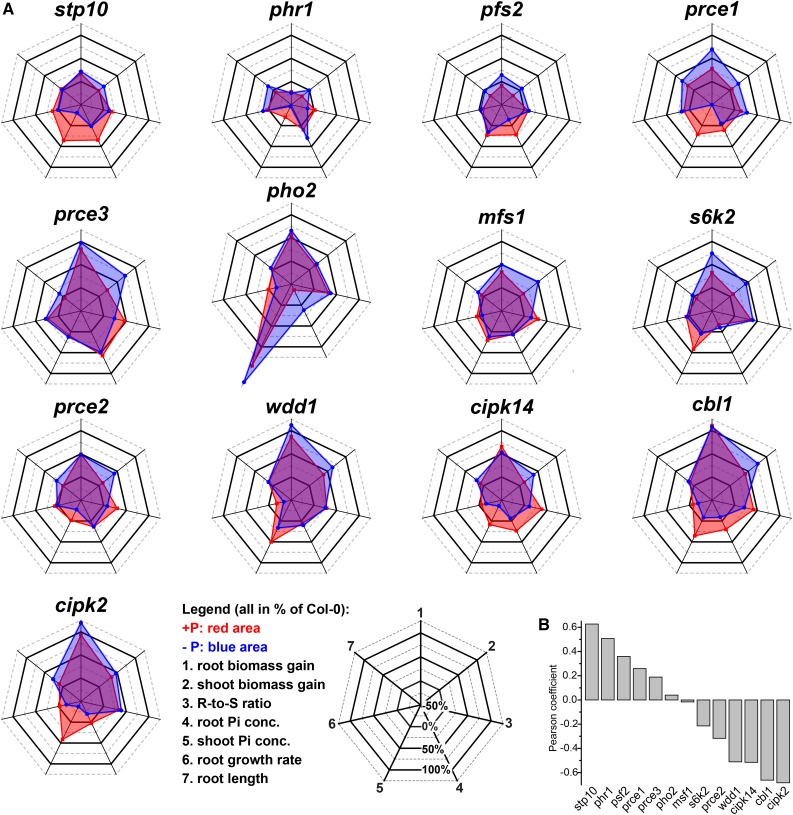
prce Mutants Have Specific Phenotypic Signatures in Response to Pi Supply
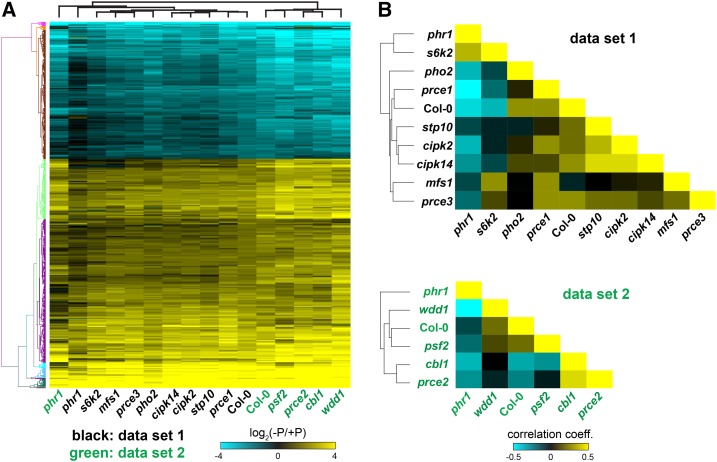
Our prce mutants have distinct signatures to Col-0 across all quantified and derived physiological traits, with the exception of stp10 and pfs2 (Fig. 5). Apart from phr1-1, analyzed traits in the latter two are most similar to the wild type (Pearson’s coefficient > 0.3). cipk2, cbl1, cipk14, and wdd1 showed strong negative trait correlations (Pearson’s coefficient < −0.5). mfs1 and pho2-1 phenotypes have no correlation, while s6k2 and prce2 have weak negative and prce1 and prce3 have weak positive trait correlations to the wild type. A few general trends can be observed that further distinguish overall phenotypic responses of prce mutants to low Pi availability. Mutants with high shoot Pi accumulation under sufficient Pi supply (Fig. 3A) featured strong root growth when P limited (s6k2) or irrespective of Pi supply (cipk2 and wdd1; Fig. 4C). There was no strict correlation with Pi concentration, since pho2-1 showed the strongest Pi accumulation in shoots but cipk2 and wdd1 showed stronger organ biomass gain. Similarly, prce3, which accumulated Pi in roots rather than shoots, showed strong root biomass gains irrespective of Pi supply and was able to maintain higher shoot biomass than the wild type under limiting Pi (Fig. 5). The mfs1 mutant showed wild-type-like organ Pi levels but still showed significantly higher root and, most strikingly, shoot biomass when P limited. As a consequence, both mfs1 and prce3 maintained root-to-shoot ratios irrespective of changes in Pi availability (Fig. 4A). Despite their hypersensitive root response to low Pi supply (Fig. 4C), prce2, as well as cbl1 and cipk14 mutants, were overall larger than the wild type, with significantly higher shoot biomass gains in P-limited conditions (Fig. 5). They showed no P-dependent shift in root-to-shoot ratios either (Fig. 4A). But this was due to substantial biomass reductions in P-limited over P-replete organs and was associated with reduced Pi levels in P-limited roots (Fig. 3B). The only other genotype that showed a similar lack of resource reallocation to these five prce mutants was phr1-1, but these plants were smaller than Col-0, irrespective of Pi supply. Genotypes with increased root-to-shoot ratios under P limitation either featured an increase in root biomass (s6k2 and prce1; Fig. 4C) or a stronger reduction in P-limited over P-replete shoot biomass (cipk2, pho2-1, and wdd1; Fig. 4B). Compared with P-replete organs, s6k2, prce1, cipk2, and wdd1 all featured higher biomass gain in P-limited ones (Fig. 5A). In summary, CBL1, CIPK14, and PRCE2 appear to enhance shoot-to-root resource reallocation under limiting Pi supply, while PRCE1 and S6K2 restrict P-limited root growth, and CIPK2, WDD1, MFS1, and PRCE3 have a greater overall negative impact on organ growth, particularly under low Pi availability. CIPK2 and WDD1 restrict shoot Pi accumulation, while PRCE3 restricts root Pi accumulation. The latter three PRCEs are different from PHO2, which mainly affects shoot Pi accumulation and root biomass but has no (or even a negative) effect on shoot growth. All of these new, negative effectors of P-limited growth, therefore, are interesting targets with which to investigate root cell-specific Pi signaling networks involved in resource reallocation.
Figure 5.
Radar plots summarizing recorded phenotypic data for prce mutants. A, Radar plots of the quantified six physiological traits and root-to-shoot (R-to-S) ratios outline the overall physiological response of prce mutants to P-replete (+P; red areas) and P-limited (−P; blue areas) growth conditions. All values are given in percentage change relative to Col-0 (indicated by the 0% line). The order of plots is according to the trait correlations shown in B. For biomass gain and relative growth rate, data represent values recorded for the period after transfer to +P or −P plates (see “Materials and Methods”), while for all other traits, data were recorded at final harvest (Supplemental Data Set S2). Individual traits are depicted as spokes transecting the concentric rings in each plot and are oriented as indicated by numbers in the key. B, Pearson coefficients for trait correlations of prce mutants with Col-0 were used to determine the order of individual radar plots in A.
Altered and Diverse Transcriptional Responses of P-Responsive Genes in Roots of prce Mutants
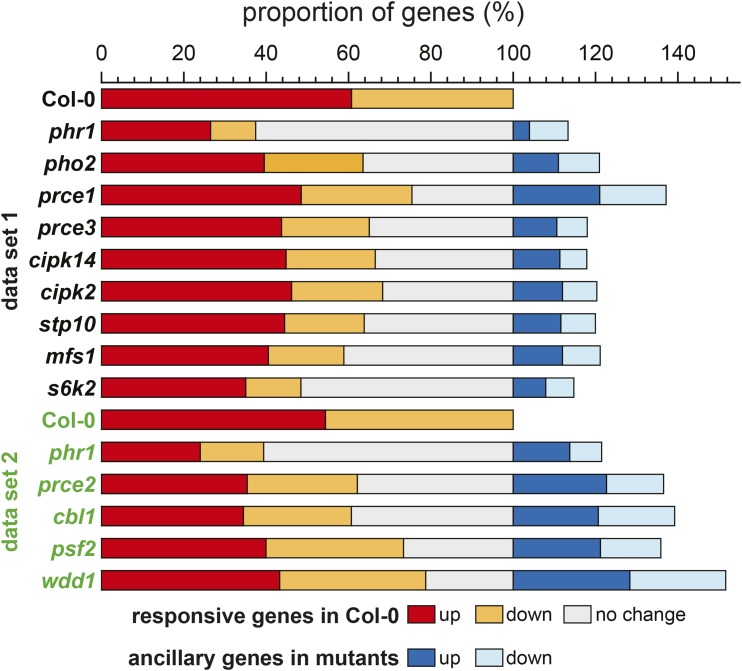
Given that significant biomass gains in P-limited organs were observed in nine out of 11 prce mutants without any strict correlation to organ Pi levels, we investigated the underlying changes in their P-responsive root transcriptomes. Two data sets were generated for subsets of genotypes that were grown in separate experiments, termed data set 1 and data set 2 (Fig. 6; Supplemental Data Set S3). Col-0 and phr1-1 were included as reference genotypes in both sets. As reported earlier by Bustos and coworkers (2010), phr1-1 affects about 47% of Pi starvation-responsive genes in P-limited roots. In our data sets, 63% (data set 1) and 61% (data set 2) of PSR genes in Col-0 showed no response in P-limited phr1-1 roots (gray bars in Fig. 6). The prce mutants showed differences in their transcriptional Pi response across many wild-type PSR genes: the s6k2 mutant showed the strongest disturbance in gene expression (52% nonresponsive genes compared with Col-0), while wdd1 was the least affected prce mutant (21%). The latter also featured the highest number of ancillary genes (51%) that were responding to changes in Pi supply in the wdd1 mutant background but not in the wild type.
Figure 6.
Proportional changes in P-responsive genes compared with Col-0. The proportion of transcripts that are more than 2-fold induced (red) or suppressed (orange) in P-limited over P-replete Col-0 roots is shown for data set 1 (black) and data set 2 (green). The proportion of PSR transcripts not responding in each mutant is shown in gray. Ancillary genes only responsive to changes in Pi supply in mutant but not in Col-0 roots are represented by blue colors.
To analyze the behavior of individual mutants in more detail, we identified an overlapping group of 831 P-responsive core genes that changed their transcript abundance by more than 2-fold in Col-0 in both data sets (Fig. 7A; Supplemental Data Set S3). This group comprised 520 PSI and 311 Pi starvation-suppressed genes (Supplemental Data Set S3, C and D). PSI genes included known genes involved in Pi homeostasis, such as SPX1, SPX3, INDUCED BY PHOSPHATE STARVATION1 (IPS1), PHT1;2, and SULFOQUINOVOSYLDIACYLGLYCEROL2 (SQD2), whose strong transcriptional response to Pi starvation was attenuated in the phr1-1 mutant (Fig. 7A; Supplemental Fig. S3). PHR1-dependent induction also was observed for the PRCE genes CBL1, CIPK14, MFS1, and S6K2 (Table I; Supplemental Fig. S4). Hierarchical clustering of expression profiles of the core PSR genes across genotypes revealed that wdd1 showed an overall stronger induction or repression of P-responsive genes compared with Col-0 (Fig. 7A). This is consistent with the observed higher overlap of PSR genes with Col-0 (Fig. 6) and the strong negative correlation to phr1-1 (Fig. 7B, data set 2). Taken together, these data indicate that the wdd1 mutant is impaired in Pi sensing. It initiates a stronger transcriptional response to P limitation compared with the wild type, while organ Pi levels are similar to or higher than those of the wild type (Fig. 3). Similar to wdd1, prce1 showed a strong negative correlation to phr1-1 and a positive correlation to Col-0 (Fig. 7B, data set 1). Both mutants feature a strong increase in root-to-shoot ratios (Fig. 4A) and similar overall phenotypic response to low Pi supply (Fig. 5A). In contrast to wdd1, however, Pi levels are wild-type like in P-replete, and lower than wild type in P-limited, prce1 shoots (Fig. 3). This suggests that the sensing of P status is not affected in prce1 and that the stronger response in PSR genes in roots is due to lower shoot Pi concentration. The cbl1 and prce2 transcript profiles also suggested enhanced responsiveness to limited Pi supply relative to Col-0 (Fig. 7), with cbl1 sharing higher shoot Pi concentration with wdd1 (Fig. 3A), while prce2 is more similar to prce1, with lower Pi levels than the wild type in P-limited shoots (Fig. 3B). Correlation analysis of P-responsive transcriptomes showed that s6k2 was the only mutant with expression profiles similar to those found in phr1-1 (Fig. 7B). This is consistent with the high percentage of nonresponsive PSR genes observed in both mutants (Fig. 6). Therefore, it is surprising that s6k2 is phenotypically very distinct from phr1-1 (Fig. 5). It features higher biomass gains in P-limited shoots, and in particular roots, than the wild type, while phr1-1 is smaller. It also has higher Pi levels in P-replete shoots, while phr1-1 is impaired in the shoot-to-root translocation of Pi (Fig. 3).
Figure 7.
Comparison of transcriptional responses of prce mutant roots to P starvation. A, Root expression profiles of central P-responsive genes across prce mutants. An overlapping set of 831 P-responsive genes (log2 > 1) in Col-0 was identified in RNA-seq data sets 1 and 2 (Supplemental Data Set S3). The T-DNA mutant s6k2 shows a phr1-1-like repression of the Pi starvation response, while wdd1 shows a stronger Pi starvation response than Col-0. B, Correlation analysis of genes differentially expressed between P-replete and P-limited roots across genotypes in data set 1 (top) and data set 2 (bottom). The color matrix represents the calculated correlation coefficients (Spearman rank correlation) for all pairwise comparisons. For the wild type (Col-0), correlation coefficients indicate statistically significant (P < 0.05) negative correlation with phr1-1 and s6k2 (data set 1) as well as phr1-1, cbl1, and prce2 (data set 2), while correlation coefficients are positive and statistically significant (P < 0.05) for pho2-1 and the remaining prce mutants, except mfs1. Expression profiles in phr1-1 are positively correlated with s6k2 only, with the strongest negative correlation coefficients for prce1 (data set 1) and wdd1 (data set 2).
In summary, the analyses of root transcriptome changes in response to Pi availability suggest that cbl1 and wdd1 roots are hypersensitive to low Pi supply and that transcriptomes of P-limited prce1 and prce2 roots respond to lower shoot Pi concentration. P-limited s6k2 roots displayed phr1-like impairment of PSR gene expression, while the mutant’s overall phenotypic response more closely resembled that of wdd1.
Identification of Coexpression Networks Associated with prce Mutants
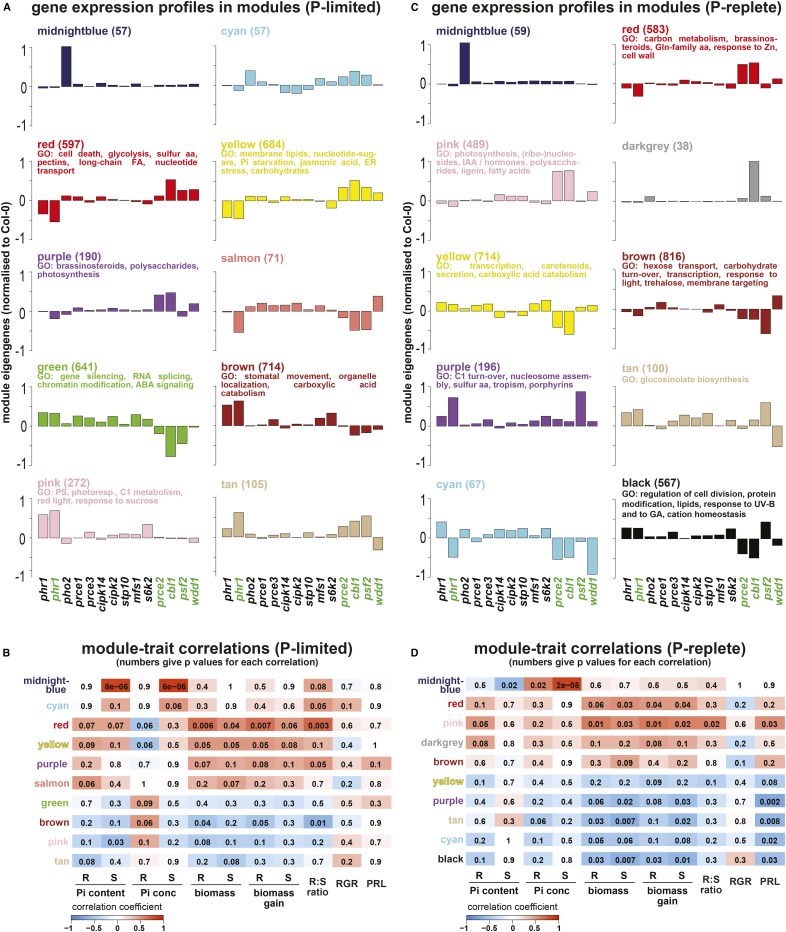
To obtain a better understanding of gene expression patterns and their correlation with physiological traits, a weighted gene coexpression network analysis (WGCNA; Langfelder and Horvath, 2008) for all transcripts (18,782) quantified by RNA-seq was performed across all 32 data sets. For both P-replete and P-limited root expression data, this led to the detection of 18 coexpression modules (Fig. 8, A and C; Supplemental Data Sets S4 and S5). Eigengenes of the obtained modules (i.e. a proxy for the average gene expression profile in each module across genotypes) were used to identify significant correlations between the quantified physiological traits and modules (Fig. 8, B and D; Langfelder and Horvath, 2008). Our analysis focused on identified modules with significant module-trait correlations (10 out of the initial 18 modules for P-limited and P-replete roots, respectively) and contrasting expression patterns between genotypes (Fig. 8, A and C). The numbers of genes in each of those modules varied, with large modules often showing strong correlations for several prce mutants, suggesting a common regulatory pathway, and smaller modules representing more specific interactions for individual mutants. Modules of significance, which explain growth differences between prce mutants (i.e. that show differential gene expression between them and have strong correlation to organ biomass), are the yellow and red modules under P-limited conditions and the black module under P-replete conditions. P-limited yellow and red modules were positively correlated to organ biomass, while the P-replete black module showed strong negative correlation to organ biomass. Other modules with contrasting correlations to biomass in response to Pi supply are the green module in P-limited roots and the pink module in P-replete roots. These five modules are described in detail below.
Figure 8.
WGCNA of RNA-seq and physiological trait data was carried out independently for P-limited (A and B) and P-replete (C and D) plants. This identified groups of genes (color-coded modules) sharing coexpression patterns across genotypes that were subsequently correlated to physiological shoot (S) and root (R) traits (Pi content, Pi concentration, biomass, biomass gain, root-to-shoot ratio, root relative growth rate [RGR], and primary root length [PRL]). For select modules, eigengenes are shown as proxies of expression profiles for genes within each module, with the number of genes given in parentheses and statistically significant (P < 0.05, after Bonferroni correction) enriched Gene Ontology (GO) terms for the top 300 or fewer hub genes listed (A and C). For all mutant lines, the eigengenes were normalized to Col-0. These eigengenes were used to identify gene networks (i.e. modules) with significance for quantified traits by correlation analysis (B and D). This identified several modules with high correlation coefficients to physiological traits, indicated in red color for positive correlations (ranging from 0 to 1) and in blue for negative correlations (ranging from 0 to −1), while the numbers within each colored box give the P values for the statistical significance of each correlation. For example, the midnight blue module for P-limited plants shows very high positive correlation (correlation coefficient of 0.98 and P < 8 × 10−6) with shoot Pi content and concentration (B). aa, Amino acids; ABA, abscisic acid; FA, fatty acids; IAA, indole-3-acetic acid.
In P-limited roots, the yellow module comprised 684 genes (Supplemental Data Set S4) with significant correlations to traits such as organ biomass, Pi content, and root-to-shoot ratio (Fig. 8B). It shares 24% of genes with the core PSI gene set defined earlier in the transcriptome data analysis (Supplemental Data Set S3). Compared with the wild type, module genes are more strongly suppressed in phr1-1 and more strongly induced in the prce mutants cbl1, prce2, psf2, and wdd1. A gene ontology (GO) term enrichment analysis for the 300 genes with the highest module membership (kME) was performed. High kME values identify hub genes with highest connectivity to the genes within each module and, thus, are highly relevant for the module-trait correlations (Langfelder and Horvath, 2008). In the yellow module, GO terms relating to Pi starvation (P < 10−13), phospholipid (P < 0.001) and galactolipid (P < 10−5) metabolism, as well as endoplasmic reticulum (ER) stress (P < 0.001; Supplemental Data Set S4) were highly enriched. Hub genes with kME > 0.9 in the yellow module include genes involved in lipid metabolism (e.g. SQD1/2, GDPD3/5, PLDζ2, and MGD3), glycolytic bypass reactions (e.g. PPCK1 and PEPC1), Pi sensing (SPX1 and SPX3), Pi signaling (IPS1), and Pi transport (PHT1;2, PHT1;5, PHT1;7, PHT1;8, and PHT1;9). The association of the high expression of these yellow module genes, as indicated by a high module eigengene (ME) value in cbl1 (Fig. 8A), may suggest an impact of calcium signaling on Pi transport via CBL1. Indeed, hub genes associated with calcium signaling in the yellow module are CIPK4, a CBL-interacting kinase that is induced in P-limited roots (Supplemental Fig. S4), the calmodulins CAM4 and CAM7, as well as the mitogen-activated protein kinase MAPKKK19. Other PSR genes of note with lower kME values in this module include PHR1 and PHO1;H1 as well as our PRCE gene MFS1. PHO1, which has been implicated previously in Pi signaling between roots and shoots (Wege et al., 2016), is found in the turquoise module, which does not show any significant expression changes across mutants (Supplemental Fig. S3; Supplemental Data Set S4).
The red module showed very similar trait correlations to the yellow module, with a similar pattern of ME values across mutants (Fig. 8A). Unlike the classical Pi starvation genes in the yellow module, hub genes in the red module show very little overlap with core PSI genes (4%) and are associated with programmed cell death, nucleotide metabolism and transport, sulfur metabolism, as well as pectin and very-long fatty acid biosynthesis (Fig. 8A; Supplemental Data Set S4). Interestingly, key hub genes (kME > 0.9) are associated with Ca2+-dependent processes in vesicle trafficking and the ER stress response, such as the ADP-ribosylation factor encoding ARFB1A and ARFA1F (Memon, 2004), the Rab GTPase RAB-5ac/ARA4 (Kirchhelle et al., 2016), and genes encoding chaperones and foldases such as the protein disulfide isomerases PDI5 and PDI11, chaperone HSP90.7, and calreticulins CRT1a and CRT1b (Gupta and Tuteja, 2011). These ER-related processes might be linked to the trafficking of PHTs through this cellular compartment (González et al., 2005), although PHF1 expression does not differ across mutants. Given similar transcriptome responses between cbl1 and prce2 (Fig. 7), it is of note that PRCE2 itself is a hub gene (kME = 0.65) in the red module, which would suggest CBL1-dependent repression.
A role for Ca2+ sensing in Pi starvation responses is further supported by the fact that, in five out of the 10 P-limited root modules (Fig. 8A), ME values for cbl1 were higher than in Col-0, with strongest opposing expression profiles compared with phr1-1 in the key yellow and red modules, pointing to an antagonistic effect of CBL1 and PHR1 on P-dependent growth responses. Together, the observed module-trait correlations suggest PHR1-dependent and -independent regulatory networks that determine growth responses to Pi availability in the prce mutants cbl1, prce2, and wdd1.
Consistent with the transcriptome-wide correlation analysis (Fig. 7B), many modules that are negatively correlated with biomass traits show coexpression of genes in phr1-1 and s6k2 under P-limited conditions. One example is the green module (Fig. 8A). Genes in this module are associated with enriched GO terms relating to DNA methylation, histone modification, RNA splicing, and small interfering RNA-mediated gene silencing (Supplemental Data Set S4). The association with these nuclear processes is notable, given that PHR1 is a transcription factor (Rubio et al., 2001) and S6K2 is a protein kinase that shuttles between the cytosol and nucleus (Mahfouz et al., 2006). Since the suppression of Pi-responsive genes is only indirectly controlled by PHR1 (Bustos et al., 2010), the association with epigenetic modifications in the green module suggests that both PHR1 and S6K2 impact chromatin structure via the suppression of module hub genes such as VERNALIZATION INDEPENDENCE4 (VIP4; kME = 0.99), POLY(A) POLYMERASE4 (PAPS4; kME = 0.97), and GLOBAL TRANSCRIPTION FACTOR GROUP A2 (GTA2; kME = 0.97). This would involve the triple methylation at Lys-4 on histone 3 of target gene loci associated with plant growth, similar to FLOWERING LOCUS C (FLC) regulation by the VIP4 homolog VIP5 and the GTA2 homolog SUPPRESSOR OF TY5 HOMOLOG (Lu et al., 2017), as well as the polyadenylation of target gene transcripts, similar to that of FLC transcripts by PAPS2/4 (Czesnick and Lenhard, 2016).
Given that PHR1 is generally regarded as a positive regulator of PSR, it was surprising that, compared with the wild type, phr1-1 displayed markedly higher gene expression in some modules in P-replete roots such as the black module, with strong negative correlation to root and shoot biomass (Fig. 8, C and D). Consistent with findings in P-limited roots, lower eigengene values in cbl1, prce2, and wdd1 roots in the black module affirm their antagonistic relationship to phr1-1. Hub genes in this module are associated with processes such as cell division, protein modification, lipid metabolism, and response to gibberellic acid (GA) (Supplemental Data Set S5). The top five hub genes up-regulated in phr1-1 and s6k2, but down-regulated in cbl1, prce2, and wdd1, encode a putative target of S6K2-mediated phosphorylation, ribosomal S6 protein (AT2G18110), a Rab5-interacting protein (AT2G29020), the redox-active GA-response regulator GAST1 PROTEIN HOMOLOG4 (Rubinovich and Weiss, 2010), the GRAS transcription factor SCARECROW-LIKE21, and a homeodomain-like transcription factor (AT1G09710). These hub genes suggest a regulatory network that dampens transcriptional and translational activities as well as membrane trafficking and GA-dependent signaling to restrict organ growth.
The pink module for P-replete roots, on the other hand, shows strong positive correlation to root biomass. Eigengene values in this module are increased in cbl1 and prce2 compared with the wild type. The GO term enrichment suggests that, in P-replete roots, CBL1 and PRCE2 attenuate the expression of genes associated with primary and secondary metabolism as well as auxin biosynthesis to restrict root growth. Hub genes of note include FLAVIN-BINDING, KELCH REPEAT, F-BOX1 (FKF1; kME = 0.95), ARABIDOPSIS PSEUDO-RESPONSE REGULATOR5 (APRR5; kME = 0.93), GIGANTEA (GI; kME = 0.89), CONSTANS-LIKE4 (COL4; kME = 0.86), and CYCLING DOF FACTOR3 (CDF3; kME = 0.85). FKF1 and APRR5 have been shown previously to interact with ADAGIO PROTEIN2, which, in turn, interacts with TIMING OF CAB EXPRESSION1 (TOC1; Yasuhara et al., 2004). Both TOC1 and FKF1 promoters responded to changes in iron supply with a lengthening of the free-running period of the circadian clock (Salomé et al., 2013), suggesting that nutrient availability can modulate clock function, most likely through a plastid-derived signal. Both iron and Pi are crucial for maintaining chloroplast function. The presence of the nuclear protein GI, which forms a complex with FKF1 to degrade CDF transcription factors in the CONSTANS promoter (Sawa et al., 2007), as well as COL4 and CDF3 in the list of hub genes further underlines the importance of circadian rhythm and flowering time regulation for Pi stress. The analogy to iron-dependent regulatory networks emphasizes the molecular links that exist between iron and P homeostasis (Bournier et al., 2013).
A number of prce mutants have higher shoot Pi concentrations under P-replete conditions. Therefore, one would expect modules that show shared expression profiles with the strong Pi overaccumulator pho2-1. Surprisingly, there is very little overlap between coexpression modules in P-replete pho2-1 roots and those of cbl1, cipk2, s6k2, and wdd1, indicating that different regulatory circuits determine shoot Pi accumulation in these mutants. Only two modules show significantly altered expression profiles in pho2-1: both the midnight blue modules for P-limited and P-replete roots showed strong positive correlation to shoot Pi concentration and high ME values in pho2-1 (Fig. 8). In P-limited roots, ME values were positively correlated to shoot Pi content and root-to-shoot ratio (Fig. 8B). By contrast, they had a negative correlation with shoot Pi content in P-replete conditions, consistent with growth inhibition due to Pi toxicity (Fig. 8D; Delhaize and Randall, 1995). The midnight blue module for P-replete roots also contained Arabidopsis thaliana4 (At4) and PHT1;8, which were shown previously to be misregulated in pho2-1 seedlings (Bari et al., 2006). For P-limited roots, the hub gene with the highest kME is the transcription factor BASIC HELIX-LOOP-HELIX100 (BHLH100), which has been implicated in FIT-independent responses to iron homeostasis (Sivitz et al., 2012). Our data imply that there are at least two regulatory networks in P-replete roots that adjust root growth (pink module) and shoot Pi accumulation (midnight blue module) to changes in iron and Pi availability (Bustos et al., 2010; Briat et al., 2015).
In summary, the WGCNA identified new networks and corresponding hub genes involved in Pi homeostasis and revealed the phenotypic and transcriptional plasticity of Pi starvation responses. This greatly extends gene interaction networks beyond those associated with the central regulators PHR1 and PHO2. The results also reveal that manipulating genes with restricted expression domains in the root, such as CBL1-associated PRCE2, S6K2, or WDD1, can affect gene regulatory networks in whole roots and, ultimately, the plant’s overall growth response to limiting Pi availability.
DISCUSSION
The aim of this study was to identify new root cell-specific regulators of the plant’s Pi starvation response that influence Pi acquisition and P status. Rather than focusing on individual genes, our successful strategy aimed at identifying groups of genes with similar physiological and molecular responses (Fig. 1). Nine of the 11 tested prce mutants outperformed the reference genotype Col-0 in at least two of the analyzed P response parameters. More strikingly, five of them, cbl1, cipk2, prce3, s6k2, and wdd1, showed highly significant biomass gains under limiting Pi supply in both roots and shoots, with a 50% to 100% increase observed compared with P-limited wild-type organs (Fig. 5A). Therefore, we have identified strong negative regulators of growth under Pi starvation through our iterative data-mining approach. The prce mutant phenotypes differ from those of mutants of known negative regulators of the Pi starvation response, such as PHO2 (Delhaize and Randall, 1995), SPX3 (Duan et al., 2008; Wang et al., 2009), WRKY75 (Devaiah et al., 2007), BHLH32 (Chen et al., 2007), F-BOX PROTEIN2 (Chen et al., 2008), and BOTRYTIS-INDUCED KINASE1 (Zhang et al., 2016): impairing the function of these genes changed PSR gene expression, root architectural traits, anthocyanin and/or organ Pi accumulation, but this had insignificant or detrimental effects on biomass production and, in particular, shoot growth of P-limited plants (Delhaize and Randall, 1995; Aung et al., 2006; Devaiah et al., 2007; Duan et al., 2008; Zhang et al., 2016). Our best-performing five prce mutants (cbl1, cipk2, prce3, s6k2, and wdd1), on the other hand, accumulated moderately higher levels of Pi than the wild type in either root (prce3) or shoot (cbl1, cipk2, s6k2, and wdd1; Fig. 3), with significant growth benefits under both high and, in particular, low Pi supply (Figs. 4 and 5A). This makes the underlying genes excellent targets for improving plant performance in the field (Richardson et al., 2011). Furthermore, our work would suggest that altering the expression of cell type-enriched regulators and associated local cellular functions is beneficial for overall plant performance. This was anticipated, since we aimed at candidates that would alter Pi flux from cell to cell as well as from root to shoot. The altered Pi flux affected both root transcriptome as well as overall organ P status.
The identification of root cell-specific regulators of P-dependent organ growth shows the power of our iterative analysis of cellular enrichment, stress response, as well as coexpression and protein-interaction networks as a means to find new components of complex stress-responsive regulatory networks. The remaining approximately 800 predicted interactors and the 88 P-responsive and cell type-enriched primary targets are a powerful resource for future exploration and will no doubt reveal additional layers of root cell-specific regulatory networks that integrate both local and systemic signals (Thibaud et al., 2010).
With increasingly sophisticated data sets for Arabidopsis root cell-specific transcriptomes (Efroni et al., 2015), translatomes (Mustroph et al., 2009), metabolomes (Petersson et al., 2015), and proteomes (Petricka et al., 2012), the same iterative data-mining approach can be applied to other stress-responsive gene sets. Our proposed in silico approach is perhaps better suited than direct experimental ones, given that there are still methodological challenges. The study of nutrient-related response pathways with FACS is particularly difficult given that metabolically active cells will use up resources during the protoplast generation and cell-sorting phases, which will alter gene expression profiles. This may explain why FACS sorting of P-limited root cells of the pWER:GFP epidermal marker line did not identify PSI genes known to be expressed in the root epidermis, such as PHT1;1 and PHT1;4 (Cederholm and Benfey, 2015). Laser-capture microdissection is a method that can overcome some of the problems associated with protoplasting prior to FACS (Schmid et al., 2012). Efroni and Birnbaum (2016) point out that technical noise and cell-identity issues can be overcome by increasingly sophisticated statistical methods.
Our prce mutant transcriptome analyses gave greater insight into the context in which known regulators of the Pi starvation response, PHR1 and PHO2, operate. They also revealed new regulatory pathways as well as links to other nutrients. RNA-seq and WGCNA showed that PHR1 has additional functions in P-replete roots: the higher expression of genes in phr1-1 across modules with negative correlation to organ biomass (black, purple, and tan modules in Fig. 8C) suggests that associated processes inhibit growth. Interestingly, these processes include aspects of the regulation of cell division, response to GA, but also lipid, sulfur, and glucosinolate metabolism. While PHR1 has already been implicated in increasing shoot-to-root sulfate translocation in P-limited plants (Rouached et al., 2011), the coexpression modules for P-replete roots indicate a greater overall role for PHR1 in sulfate homeostasis. In this context, it is interesting that some glucosinolates accumulated in P-limited plants in a PHR1-dependent fashion (Pant et al., 2015).
The midnight blue coexpression module of P-replete roots (Fig. 8C) shows induced expression of the hub gene BHLH100 in pho2-1, linking Pi and iron regulatory networks (Sivitz et al., 2012). The presence of GI and other clock components in the pink module indicates that sensing of P status and sensing of iron status in chloroplasts (Salomé et al., 2013) both impact the circadian rhythm and, thus, plant development and flowering time. Homologs of components controlling epigenetic modification of the FLC promoter are PHR1- and S6K2-dependent hub genes in the green module in P-limited roots. This provides evidence for a network that governs the transition to flowering in response to Pi stress, similar to the abscisic acid- and GI-dependent drought-escape response (Riboni et al., 2016). Further support for a link between P status and development comes from work in rice (Oryza sativa) showing that GI and PHO2 interact physically (Li et al., 2017).
Some of the candidate genes (CBL1, CIPK14, MFS1, and S6K2) show PHR1-dependent induction in P-limited roots. The cbl1 mutant shows contrasting coexpression profiles to phr1-1, while the s6k2 mutant shows matching coexpression profiles to phr1-1, in modules for both P-limited and P-replete roots. This indicates that the calcium sensor CBL1, through interaction with the ubiquitously expressed CBL-interacting protein kinase CIPK2, the root stele-enriched CIPK14, or other CIPK isoforms, antagonizes PHR1 function. This supports the role of cytosolic calcium levels and their modulation by Pi starvation-induced Ca2+-ATPases (Raghothama, 2000) and vacuolar Ca2+ sequestration (Liu et al., 2011) in P signaling: an intriguing proposition, given that CBL1 has long been established as an abiotic stress (cold, drought, or salt)-responsive Ca2+ sensor (Cheong et al., 2003). Different abiotic stresses have been shown to elicit cell type-specific calcium responses in roots (Kiegle et al., 2000). What is more, cold stress is known to lead to Pi limitation of photosynthesis (Hurry et al., 2000). Drought and salt stress modify the root system response to Pi starvation (Kawa et al., 2016) and affect abscisic acid-dependent regulatory networks through the MYB2-dependent expression of miR399f (Baek et al., 2016). Interestingly, CBL1, and pericycle-enriched PRCE2, appear to act in parallel with PHR1, upstream of PHO2, to adjust transcript levels of the miR399 antagonists At4 and IPS1 (Franco-Zorrilla et al., 2007) and SPX1, encoding a Pi-sensing negative regulator of PHR1 (Puga et al., 2014): IPS1 and SPX1 are hub genes in the yellow module in P-limited roots (Fig. 8A). The lower transcript abundance of At4, IPS1, and SPX1 in P-replete phr1-1 roots contrasts with their higher basal expression levels in P-replete cbl1, prce2, and pho2-1 roots (Supplemental Fig. S3; Bari et al., 2006; Bustos et al., 2010). Our results highlight the role of these regulators and Ca2+ signals in systemic adjustments of plant P status.
While PHR1-dependent coexpression networks are prevalent, some PRCE genes affect P signaling networks more directly: WDD1 expression is not responsive to Pi supply or affected in any of the mutants tested (Supplemental Data Set S3). Gene expression profiles in wdd1 suggest a hypersensitive response to low Pi availability (Fig. 7A), yet Pi levels in P-limited roots and shoots are comparable to those in Col-0 (Fig. 3). Coexpression profiles in wdd1 contrast with those of phr1-1 in P-limited root modules (Fig. 8A), but modules with altered expression profiles in wdd1 are largely PHR1 independent in P-replete roots (Fig. 8C). WDD1 encodes a transducin/WD40 repeat-like protein annotated as a putative β-subunit of a heterotrimeric G-protein complex. Recently, G-protein-mediated signaling was associated with the WRKY75-dependent regulation of PSI genes (Chakraborty et al., 2015). This could explain the high impact of the knockout of WDD1 on yellow and red coexpression modules in P-limited roots, two modules that are largely associated with processes involved directly in Pi signaling and Pi homeostasis. WDD1 is a close homolog of human Rabphilin-11 (van Nocker and Ludwig, 2003), which is a downstream target of the small G-protein Ras-related protein11 (Rab11) and binds preferentially to GTP-Rab11. Both proteins colocalize at perinuclear regions such as the Golgi complex and recycling endosomes in mammals (Mammoto et al., 1999). The WDD1 expression domain in the root epidermis overlaps with that of its predicted interactor, PHT1;2. Our observation that the wdd1 mutant appears to be affected in the sensing of local cellular Pi levels is intriguing given that the WD40 domain has been implicated in the binding of 3-phosphorylated phosphoinositides (Jeffries et al., 2004) and phospho-Ser/Thr peptides (Orlicky et al., 2003; Reinhardt and Yaffe, 2013). Both are of great interest for Pi sensing and signaling, given that SPX proteins also bind phosphoinositides (Wild et al., 2016) and the phosphorylation of PHT1 proteins controls the active, plasma membrane-bound transporter fraction (Bayle et al., 2011). The latter requires PHF1, itself a WD40 domain-containing protein in the endoplasmic reticulum (González et al., 2005).
CONCLUSION
Our iterative data-mining approach is an efficient way of identifying new candidate genes for cell type-specific regulators of stress responses. As proof of concept, we have predicted and validated new regulators of Pi homeostasis. The correlation of quantified traits with transcript expression profiles revealed gene regulatory networks and their underlying hub genes, providing further insight into the biological consequences of altered target gene function. Furthermore, this study provides important resource data sets for future studies of these regulators and networks.
MATERIALS AND METHODS
Meta-Analysis of Transcriptome and Protein Interactome Data
Microarray data describing radial expression gradients across root cell files (Brady et al., 2007) were used to identify the tissue-specific enrichment of transcripts up-regulated under Pi deficiency (Misson et al., 2005). Given the low number of fluorescently labeled, individual cells per root and the associated uncertainty in differential expression calling (Efroni and Birnbaum, 2016), data for the promoter-GFP line tagging cells in the quiescent center were removed from the data set published by Brady et al. (2007) prior to analysis. Due to the overlapping nature of the fluorescent marker used to profile the root stele and those profiling xylem and phloem cells, data from the former marker line were used for this analysis. Relative transcript abundance in individual GFP-expressing root cell populations sorted after fluorescence activation of root samples from the remaining 16 cell- or tissue-specific promoter-GFP lines was converted by setting the combined relative expression value of each individual gene across cell types to 100%. Enrichment in a given root cell type was defined as a fluorescent line with over 9.4% of the overall expression (1.5-fold enriched), with 6.3% denoting even expression in a given GFP line (Supplemental Data Set S1B). Putative interactors of the cell type-enriched genes were data mined using the Arabidopsis Interactions Viewer (Supplemental Data Set S1, C and D; Geisler-Lee et al., 2007). The same process described above was used to determine if these predicted interactors displayed cell type-specific enrichment (Supplemental Data Set S1E).
Plant Material and Growth Conditions
T-DNA insertion lines of Arabidopsis (Arabidopsis thaliana) were chosen to target exons of the genes of interest unless stated otherwise. Insertion mutant information was obtained from the SIGnAL Web site at http://signal.salk.edu. T-DNA lines listed in Table I and wild-type seeds were obtained from the Nottingham Arabidopsis Stock Centre. Col-0 stock number N70000 was used as reference material. Left and right border T-DNA insertion sites were sequenced with primers designed with SIGnAL iSect. The arrangement of T-DNA inserts within the target gene was analyzed by PCR, and the lack of a full-length transcript was confirmed by quantitative reverse transcription-PCR (Supplemental Tables S2 and S3). The fold change in absolute transcript levels between Col-0 and individual mutants also was determined from the RNA-seq data set (Supplemental Table S2). To express altered responses to limiting Pi supply relative to known signaling mutants, phr1-1 (Rubio et al., 2001) and pho2-1 (Delhaize and Randall, 1995) ethyl methanesulfonate mutants were used.
For genotyping and propagation, seeds were surface sterilized with chlorine gas for 1 h. Seeds were sown on soil (0.5 L of coarse vermiculite, 0.33 L of perlite, 33 g of nutricote, 28 g of nitrogen, 25 g of water-holding granules, 15 g of trace elements, and 7 g of garden lime per kg) and stratified at 4°C for 2 d. Trays were then transferred into the growth chamber set to 22°C (day)/19°C (night) with 60% humidity and a 16-h/8-h light/dark cycle with 100 µE m−2 s−1.
For phenotyping and fluorescence imaging of promoter-GFP lines, chlorine-treated seeds were sown on solid MS medium in 10- × 10-cm square petri dishes (Labserv; Thermo Fisher). The MS medium contained 1× MS Modified Basal Salt mixture (M407; Phytotech) adjusted to 20.6 mm NH4NO3, 18.8 mm KNO3, 1 mm KH2PO4, 0.1% (v/v) Gamborg’s B5 vitamins (Sigma-Aldrich), and 0.05% (w/v) MES. The solution was adjusted to pH 5.8, and 0.9% (w/v) Difco Granulated Agar (lot no. 4156518) was used to solidify the medium. A total of 50 mL of medium was used per plate. In agreement with Gruber et al. (2013), the residual Pi concentration of the agar used was 2 µm. Plates were sealed with 3M Micropore tape (Medshop Australia) and placed vertically into racks in the growth chamber (22°C [day]/19°C [night] with 60% humidity and a 16-h/8-h light/dark cycle with 120 µE m−2 s−1) after 2 d of stratification at 4°C in the dark.
Seedlings were established for 10 d (site 1, corresponding to data set 1 for RNA-seq analysis) or 9 d (site 2, corresponding to data set 2 for RNA-seq analysis) on MS medium before being transferred to plates with either replete (1 mm KH2PO4) or limiting (12 µm) Pi supply. For the latter medium, 990 µm KCl was added to adjust osmolarity. One plate with eight seedlings constituted one biological replicate. Root and shoot samples of three independent experiments with one to three replicates each were harvested prior to transfer (9-/10-d-old seedlings) and 5 d after transfer (P-replete/P-limited seedlings). Different seed batches were used for the independent experiments. At harvest, roots were rinsed in MilliQ water for 5 min and patted dry. Sample fresh weights were recorded (Supplemental Data Set S2A). Samples were shock frozen in liquid N2 and stored at −80°C for Pi extraction and RNA isolation.
Promoter-GFP lines were sown directly onto vertically placed MS plates with either replete (1 mm) or limiting (12 µm) Pi and grown for 14 d prior to fluorescence imaging.
Generation of Promoter-GFP Lines for Genes of Interest
The binary vector pCAMBIA1300 was modified using the Gibson Assembly Cloning Kit (New England Biolabs) to include a maximum upstream regulatory sequence of up to 2 kb between the start codon of the gene of interest and its neighboring gene to drive the GFP reporter (Supplemental Table S1). The assembled plasmids were then transformed into Agrobacterium tumefaciens strain GV3130-C58 followed by stable transformation into Arabidopsis Col-0 plants using the floral dipping technique (Clough and Bent, 1998). Primary transformants were selected on 15 µg mL−1 hygromycin-containing MS medium (Harrison et al., 2006). Independent transformants were imaged for GFP fluorescence in roots for each construct, and single-insert lines conferring stable GFP expression by the promoter of interest were propagated and screened for homozygous offspring in the T3 generation. The GFP fluorescence of P-replete and P-limited seedlings, of at least three independent lines for every construct, was analyzed (Supplemental Table S1).
Fluorescence Imaging of Promoter-GFP Lines
Fluorescence imaging was performed using a Zeiss LSM780 confocal microscope with an LD C-Apochromat 40×/1.1 water-immersion objective. GFP fluorescence was excited at 488 nm using an argon ion laser and subsequently detected at 490 to 700 nm.
Root Growth and Biomass
To determine relative root growth rates, the position of the primary root tip on the vertical plates was marked daily at 2 pm, 7 h after the onset of light. At harvest, plates were scanned at 600 dpi resolution (AtrixScan 3200XL; Microtek). Root images were analyzed with the SmartRoot plugin for ImageJ (Lobet et al., 2011). Relative growth rates of P-replete or P-limited seedlings were determined in three independent experiments by integrating incremental primary root length data from day 1 to day 4 after transfer, assuming exponential growth (Supplemental Data Set S2B).
Determination of Free Inorganic Pi Levels
Free inorganic Pi was determined in 1% (v/v) acetic acid extracts of harvested root and shoot samples from three independent experiments as described earlier (Supplemental Data Set S2C; Jost et al., 2015).
RNA Isolation and cDNA Library Preparation
The Spectrum Plant Total RNA kit (Sigma-Aldrich) was used to isolate total RNA from root samples (∼10 mg fresh weight) and shoot samples (∼30 mg fresh weight). DNase I on-column digestion was conducted according to the manufacturer. DNA-free RNA was eluted in 50 µL of RNase-free water. RNA concentrations were determined using the LVis Plate for the SPECTROstar Nano photometer (BMG Labtech). This determination was found to give similar concentration values to the Qubit 3.0 Fluorometer system (Thermo Fisher Scientific; data not shown). RNA integrity was further validated by gel electrophoresis. RNA was extracted from P-replete and P-limited roots of the 14 genotypes collected in three independent experiments.
RNA-seq Analysis
For RNA-seq analysis, libraries were prepared from total RNA using the TruSeq Stranded Total RNA with Ribo-Zero Plant kit according to the manufacturer’s instructions (Illumina), with samples from three independent experiments for each genotype and treatment. Sequencing runs were performed on a HiSeq1500 platform (Illumina) generating 61-bp single-end reads. Mapping of reads to the Arabidopsis genome (TAIR10) and quantification of gene expression were performed using the TopHat (version 2.1.0) and Cufflinks (version 2.1.1) pipelines, respectively (Trapnell et al., 2012). RNA-seq read data have been submitted to the National Center for Biotechnology Information Sequence Read Archive under identifier SRP079906. The average number of mapped reads per sample was 18.4 ± 0.7 million, with a mean quality score of 36. Only genes with an average read count above 10 in at least one genotype per treatment were considered for further analyses. Genes with a log2 fold change > 1 and false discovery rate < 0.05 were considered as differentially expressed. Further data analysis was performed using the Partek Genomics Suite (Partek). Gene ontologies and their enrichment were analyzed with the ClueGO plugin for Cytoscape (Bindea et al., 2009).
WGCNA
For the WGCNA, the corresponding software package for the R environment was used (Langfelder and Horvath, 2008), with a soft thresholding power of 15, a signed-hybrid network type, and a Pearson’s correlation together with an automatic module detection and dynamic tree-cutting algorithm.
Statistical Analysis
Statistical tests for genotype comparisons were performed by ANOVA, followed by Tukey’s pairwise multiple comparison of means (Sigma Plot 12; Systat Software). Unless stated otherwise, one biological replicate consisted of eight seedlings grown on a vertical plate. Each genotype was grown in three independent experiments (n = 3) with one to three replicates each. Changes in question were considered to be significant at P < 0.05 or P < 0.001.
Accession Numbers
Genes encoding proteins of unknown function were named according to TAIR nomenclature and registered as an official gene symbol (www.arabidopsis.org/portals/nomenclature/symbol_main.jsp). Sequence data for the genes characterized in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT4G17615 (CBL1), AT5G07070 (CIPK2), AT5G01820 (CIPK14), AT4G34950 (MFS1), AT4G28610 (PHR1), AT2G33770 (PHO2), AT3G13175 (unknown protein, PRCE1), AT5G03345 (unknown endomembrane protein, PRCE2), AT5G52420 (unknown ER protein, PCRE3), AT3G12530 (PSF2), AT3G08720 (S6K2), AT3G19940 (STP10), and AT5G54200 (WDD1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Shoot phenotypes of prce seedlings in response to Pi availability.
Supplemental Figure S2. Root growth phenotypes of prce seedlings in response to Pi availability.
Supplemental Figure S3. Hierarchical cluster analysis of transcriptional responses of genes with known functions in P homeostasis in prce mutants.
Supplemental Figure S4. P-dependent changes in transcript abundance of PRCE genes in Col-0, phr1-1, and pho2-1.
Supplemental Table S1. Details of promoter-GFP line characterization.
Supplemental Table S2. Overview of genotypic T-DNA mutant characterization.
Supplemental Table S3. Primers for target transcript quantification in the wild type and T-DNA mutants.
Supplemental Data Set S1. Data for candidate selection via iterative coexpression, interaction, and enrichment analyses.
Supplemental Data Set S2. Physiological characterization of T-DNA mutants.
Supplemental Data Set S3. Root transcriptome responses to Pi availability in prce mutants.
Supplemental Data Set S4. Information on highly interconnected genes within each coexpression module for P-limited prce mutant roots.
Supplemental Data Set S5. Information on highly interconnected genes within each coexpression module for P-replete prce mutant roots.
Acknowledgments
We thank Matthew G. Lewsey (La Trobe University) for critical reading of the article, the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants, the LIMS Bioimaging Facility (La Trobe Institute for Molecular Science, La Trobe University) for help with confocal imaging, and the La Trobe University Genomics Platform for next-generation sequencing support.
Glossary
- FACS
fluorescence-activated cell sorting
- Pi
(dihydrogen) phosphate
- RNA-seq
RNA sequencing
- Col-0
Columbia-0
- MS
Murashige and Skoog
- WGCNA
weighted gene coexpression network analysis
- GO
Gene Ontology
- kME
module membership
- ER
endoplasmic reticulum
- ME
module eigengene
Footnotes
This work was supported by the Australian Research Centre (grant no. CE140100008).
Articles can be viewed without a subscription.
References
- Ajjawi I, Lu Y, Savage LJ, Bell SM, Last RL (2010) Large-scale reverse genetics in Arabidopsis: case studies from the Chloroplast 2010 Project. Plant Physiol 152: 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Arpat AB, Magliano P, Wege S, Rouached H, Stefanovic A, Poirier Y (2012) Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J 71: 479–491 [DOI] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141: 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayadi A, David P, Arrighi JF, Chiarenza S, Thibaud MC, Nussaume L, Marin E (2015) Reducing the genetic redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 transporters to study phosphate uptake and signaling. Plant Physiol 167: 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Chun HJ, Kang S, Shin G, Park SJ, Hong H, Kim C, Kim DH, Lee SY, Kim MC, et al. (2016) A role for Arabidopsis miR399f in salt, drought, and ABA signaling. Mol Cells 39: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Pant BD, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141: 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ (1994) The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J 6: 673–685 [DOI] [PubMed] [Google Scholar]
- Bayle V, Arrighi JF, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L (2011) Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23: 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski RL. (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol Plant Mol Biol 24: 225–252 [Google Scholar]
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K, Jung JW, Wang JY, Lambert GM, Hirst JA, Galbraith DW, Benfey PN (2005) Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat Methods 2: 615–619 [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Bournier M, Tissot N, Mari S, Boucherez J, Lacombe E, Briat JF, Gaymard F (2013) Arabidopsis ferritin 1 (AtFer1) gene regulation by the phosphate starvation response 1 (AtPHR1) transcription factor reveals a direct molecular link between iron and phosphate homeostasis. J Biol Chem 288: 22670–22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Brady SM, Provart NJ (2009) Web-queryable large-scale data sets for hypothesis generation in plant biology. Plant Cell 21: 1034–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Zhang L, Megraw M, Martinez NJ, Jiang E, Yi CS, Liu W, Zeng A, Taylor-Teeples M, Kim D, et al. (2011) A stele-enriched gene regulatory network in the Arabidopsis root. Mol Syst Biol 7: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Rouached H, Tissot N, Gaymard F, Dubos C (2015) Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front Plant Sci 6: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Pérez-Pérez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6: e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederholm HM, Benfey PN (2015) Distinct sensitivities to phosphate deprivation suggest that RGF peptides play disparate roles in Arabidopsis thaliana root development. New Phytol 207: 683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Sharma P, Kanyuka K, Pathak RR, Choudhury D, Hooley R, Raghuram N (2015) G-protein α-subunit (GPA1) regulates stress, nitrate and phosphate response, flavonoid biosynthesis, fruit/seed development and substantially shares GCR1 regulation in A. thaliana. Plant Mol Biol 89: 559–576 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Jenkins GI, Nimmo HG (2008) Identification of an F-box protein that negatively regulates Pi starvation responses. Plant Cell Physiol 49: 1902–1906 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG (2007) BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem J 405: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S (2003) CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 15: 1833–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Czesnick H, Lenhard M (2016) Antagonistic control of flowering time by functionally specialized poly(A) polymerases in Arabidopsis thaliana. Plant J 88: 570–583 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ (1995) Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol 107: 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Allona I, Rubio V, Leyva A, de la Peña A, Aragoncillo C, Paz-Ares J (1999) A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions. Plant J 19: 579–589 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143: 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Duan K, Yi K, Dang L, Huang H, Wu W, Wu P (2008) Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J 54: 965–975 [DOI] [PubMed] [Google Scholar]
- Efroni I, Birnbaum KD (2016) The potential of single-cell profiling in plants. Genome Biol 17: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efroni I, Ip PL, Nawy T, Mello A, Birnbaum KD (2015) Quantification of cell identity from single-cell gene expression profiles. Genome Biol 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Gaude N, Nakamura Y, Scheible WR, Ohta H, Dörmann P (2008) Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J 56: 28–39 [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, O’Toole N, Ammar R, Provart NJ, Millar AH, Geisler M (2007) A predicted interactome for Arabidopsis. Plant Physiol 145: 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA 105: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BD, Giehl RFH, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163: 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Tuteja N (2011) Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant Signal Behav 6: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Mott EK, Parsley K, Aspinall S, Gray JC, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Hu HC, Wang YY, Tsay YF (2009) AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J 57: 264–278 [DOI] [PubMed] [Google Scholar]
- Hurry V, Strand A, Furbank R, Stitt M (2000) The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant J 24: 383–396 [DOI] [PubMed] [Google Scholar]
- Jeffries TR, Dove SK, Michell RH, Parker PJ (2004) PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol Biol Cell 15: 2652–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost R, Pharmawati M, Lapis-Gaza HR, Rossig C, Berkowitz O, Lambers H, Finnegan PM (2015) Differentiating phosphate-dependent and phosphate-independent systemic phosphate-starvation response networks in Arabidopsis thaliana through the application of phosphite. J Exp Bot 66: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa D, Julkowska MM, Sommerfeld HM, Ter Horst A, Haring MA, Testerink C (2016) Phosphate-dependent root system architecture responses to salt stress. Plant Physiol 172: 690–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk NM, Ceserani T, Tausta SL, Sussex IM, Nelson TM (2003) Laser capture microdissection of cells from plant tissues. Plant Physiol 132: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Kim K-N, Cheong YH, Gupta R, Luan S (2000) Interaction Specificity of Arabidopsis Calcineurin B-Like Calcium Sensors and Their Target Kinases. Plant Physiology 124: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhelle C, Chow CM, Foucart C, Neto H, Stierhof YD, Kalde M, Walton C, Fricker M, Smith RS, Jérusalem A, et al. (2016) The specification of geometric edges by a plant Rab GTPase is an essential cell-patterning principle during organogenesis in Arabidopsis. Dev Cell 36: 386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Harper AL, Trick M, Bancroft I, Kopriva S (2014) Dissection of the Control of Anion Homeostasis by Associative Transcriptomics in Brassica napus. Plant Physiol 166: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Xu Q, Harter K, Gruissem W, Luan S (1999) Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc Natl Acad Sci USA 96: 4718–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapis-Gaza HR, Jost R, Finnegan PM (2014) Arabidopsis PHOSPHATE TRANSPORTER1 genes PHT1;8 and PHT1;9 are involved in root-to-shoot translocation of orthophosphate. BMC Plant Biol 14: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ying Y, Secco D, Wang C, Narsai R, Whelan J, Shou H (2017) Molecular interaction between PHO2 and GIGANTEA reveals a new crosstalk between flowering time and phosphate homeostasis in Oryza sativa. Plant Cell Environ, 10.1111/pce.12945 [DOI] [PubMed] [Google Scholar]
- Lin HX, Du WM, Yang YQ, Schumaker KS, Guo Y (2014) A Calcium-Independent Activation of the Arabidopsis SOS2-Like Protein Kinase24 by Its Interacting SOS3-Like Calcium Binding Protein1. Plant Physiol 164: 2197–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Chiou TJ (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Aung K, Tseng CY, Chang TY, Chen YS, Chiou TJ (2011) Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis. Plant Physiol 156: 1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X (2011) A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol 157: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN (2010) The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22: 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Tian Y, Wang S, Su Y, Mao T, Huang T, Chen Q, Xu Z, Ding Y (2017) Phosphorylation of SPT5 by CDKD;2 is required for VIP5 recruitment and normal flowering in Arabidopsis thaliana. Plant Cell 29: 277–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24: 459–467 [Google Scholar]
- Mahfouz MM, Kim S, Delauney AJ, Verma DPS (2006) Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18: 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Ohtsuka T, Hotta I, Sasaki T, Takai Y (1999) Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein implicated in vesicle recycling. J Biol Chem 274: 25517–25524 [DOI] [PubMed] [Google Scholar]
- McDowell SC, Akmakjian G, Sladek C, Mendoza-Cozatl D, Morrissey JB, Saini N, Mittler R, Baxter I, Salt DE, Ward JM, Schroeder JI, Guerinot ML, Harper JF (2013) Elemental concentrations in the seed of mutants and natural variants of Arabidopsis thaliana grown under varying soil conditions. PLoS One 8: e63014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon AR. (2004) The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants. Biochim Biophys Acta 1664: 9–30 [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al. (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, Harashima S, Shibata D (1997) Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci USA 94: 7098–7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazono M, Qiu F, Borsuk LA, Schnable PS (2003) Laser-capture microdissection, a tool for the global analysis of gene expression in specific plant cell types: identification of genes expressed differentially in epidermal cells or vascular tissues of maize. Plant Cell 15: 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52: 329–339 [PubMed] [Google Scholar]
- Nozawa A, Koizumi N, Sano H (2001) An Arabidopsis SNF1-related protein kinase, AtSR1, interacts with a calcium-binding protein, AtCBL2, of which transcripts respond to light. Plant Cell Physiol 42: 976–981 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Müller R, Nielsen TH (2007) Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ 30: 1499–1512 [DOI] [PubMed] [Google Scholar]
- Orlicky S, Tang X, Willems A, Tyers M, Sicheri F (2003) Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 112: 243–256 [DOI] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant BD, Pant P, Erban A, Huhman D, Kopka J, Scheible WR (2015) Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ 38: 172–187 [DOI] [PubMed] [Google Scholar]
- Park BS, Seo JS, Chua NH (2014) NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26: 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson SV, Lindén P, Moritz T, Ljung K (2015) Cell-type specific metabolic profiling of Arabidopsis thaliana protoplasts as a tool for plant systems biology. Metabolomics 11: 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Schauer MA, Megraw M, Breakfield NW, Thompson JW, Georgiev S, Soderblom EJ, Ohler U, Moseley MA, Grossniklaus U, et al. (2012) The protein expression landscape of the Arabidopsis root. Proc Natl Acad Sci USA 109: 6811–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga MI, Mateos I, Charukesi R, Wang Z, Franco-Zorrilla JM, de Lorenzo L, Irigoyen ML, Masiero S, Bustos R, Rodríguez J, et al. (2014) SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc Natl Acad Sci USA 111: 14947–14952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. (2000) Phosphate transport and signaling. Curr Opin Plant Biol 3: 182–187 [PubMed] [Google Scholar]
- Reinhardt HC, Yaffe MB (2013) Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat Rev Mol Cell Biol 14: 563–580 [DOI] [PubMed] [Google Scholar]
- Riboni M, Robustelli Test A, Galbiati M, Tonelli C, Conti L (2016) ABA-dependent control of GIGANTEA signalling enables drought escape via up-regulation of FLOWERING LOCUS T in Arabidopsis thaliana. J Exp Bot 67: 6309–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, et al. (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349: 121–156 [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Rottmann T, Zierer W, Subert C, Sauer N, Stadler R (2016) STP10 encodes a high-affinity monosaccharide transporter and is induced under low-glucose conditions in pollen tubes of Arabidopsis. J Exp Bot 67: 2387–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Secco D, Arpat B, Poirier Y (2011) The transcription factor PHR1 plays a key role in the regulation of sulfate shoot-to-root flux upon phosphate starvation in Arabidopsis. BMC Plant Biol 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinovich L, Weiss D (2010) The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. Plant J 64: 1018–1027 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Henao JE, Schmidt W (2016) An inventory of nutrient-responsive genes in Arabidopsis root hairs. Front Plant Sci 7: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, Oliva M, Weigel D, Krämer U (2013) Circadian clock adjustment to plant iron status depends on chloroplast and phytochrome function. EMBO J 32: 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MW, Schmidt A, Klostermeier UC, Barann M, Rosenstiel P, Grossniklaus U (2012) A powerful method for transcriptional profiling of specific cell types in eukaryotes: laser-assisted microdissection and RNA sequencing. PLoS ONE 7: e29685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J (1999) Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11: 2393–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Shultz RW, Tatineni VM, Hanley-Bowdoin L, Thompson WF (2007) Genome-wide analysis of the core DNA replication machinery in the higher plants Arabidopsis and rice. Plant Physiol 144: 1697–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz AB, Hermand V, Curie C, Vert G (2012) Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS ONE 7: e44843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Nagarajan VK, Raghothama KG (2011) Arabidopsis Pht1;5 plays an integral role in phosphate homeostasis. Plant Signal Behav 6: 1676–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39: 792–796 [DOI] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 64: 775–789 [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Lucero RD, Sakhonwasee S, Adamson AW, Creff A, Nussaume L, Desnos T, Abel S (2009) ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci USA 106: 14174–14179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull MC, Guiltinan MJ, Lynch JP, Deikman J (1997) The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant Cell Environ 20: 85–92 [Google Scholar]
- Turck F, Kozma SC, Thomas G, Nagy F (1998) A heat-sensitive Arabidopsis thaliana kinase substitutes for human p70s6k function in vivo. Mol Cell Biol 18: 2038–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nocker S, Ludwig P (2003) The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaw WK, Harrison MJ (2002) A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 14: 1751–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hu H, Huang H, Duan K, Wu Z, Wu P (2009) Regulation of OsSPX1 and OsSPX3 on expression of OsSPX domain genes and Pi-starvation signaling in rice. J Integr Plant Biol 51: 663–674 [DOI] [PubMed] [Google Scholar]
- Wege S, Khan GA, Jung JY, Vogiatzaki E, Pradervand S, Aller I, Meyer AJ, Poirier Y (2016) The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol 170: 385–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild R, Gerasimaite R, Jung JY, Truffault V, Pavlovic I, Schmidt A, Saiardi A, Jessen HJ, Poirier Y, Hothorn M, et al. (2016) Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352: 986–990 [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SP, Fitter AH, Leyser HM (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Sánchez D, Rubio-Díaz S, Muñoz-Viana R, Pérez-Pérez JM, Jover-Gil S, Ponce MR, Micol JL (2014) Leaf phenomics: a systematic reverse genetic screen for Arabidopsis leaf mutants. Plant J 79: 878–891 [DOI] [PubMed] [Google Scholar]
- Winkler RG, Frank MR, Galbraith DW, Feyereisen R, Feldmann KA (1998) Systematic reverse genetics of transfer-DNA-tagged lines of Arabidopsis: isolation of mutations in the cytochrome P450 gene superfamily. Plant Physiol 118: 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, MacPherson CR, Liu J, Wang H, Kiba T, Hannah MA, Wang XJ, Bajic VB, Chua NH (2012) The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132: 1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yasuhara M, Mitsui S, Hirano H, Takanabe R, Tokioka Y, Ihara N, Komatsu A, Seki M, Shinozaki K, Kiyosue T (2004) Identification of ASK and clock-associated proteins as molecular partners of LKP2 (LOV Kelch protein 2) in Arabidopsis. J Exp Bot 55: 2015–2027 [DOI] [PubMed] [Google Scholar]
- Yu B, Xu C, Benning C (2002) Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc Natl Acad Sci USA 99: 5732–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Huang L, Hong Y, Song F (2016) BOTRYTIS-INDUCED KINASE1, a plasma membrane-localized receptor-like protein kinase, is a negative regulator of phosphate homeostasis in Arabidopsis thaliana. BMC Plant Biol 16: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao H, Lucas WJ (2014) Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J Integr Plant Biol 56: 192–220 [DOI] [PubMed] [Google Scholar]