Abstract
Cognitive decline is a prevalent condition and independent prognostic marker of unfavourable outcomes in patients with heart failure. The highest prevalence, up to 80 %, is reported in patients hospitalised due to acute decompensation. Numerous factors contribute to cognitive dysfunction in heart failure patients, with hypertension, atrial fibrillation, stroke and impaired haemodynamics being the most relevant. Cerebral hypoperfusion, disruption of blood–brain barrier, oxidative damage and brain-derived cytokines are pathogenic links between heart failure and alteration of cognitive functioning. White matter hyperintensities, lacunar infarcts and generalised volume loss are common features revealed by neuroimaging. Typically affected cognitive domains are presented. Assessment of cognitive functioning, even by simple screening tests, should be part of routine clinical examination of heart failure patients.
Keywords: Cognitive impairment, heart failure, adherence, adverse outcomes, cognitive domains, patient self-care
To ensure performance of everyday tasks, adherence to treatment regiments, appointments, and following dietary requirements, cognition is crucial.[1] Cognitive impairment (CI) is a broad and inclusive term used to describe dysfunction of processes in various cognitive domains, such as attention, memory, judgment, reasoning, decision-making and problem solving, comprehension and production of language.
Prevalence and Pathogenic Mechanisms
Evidence is mounting that impaired cardiac function may precipitate early-onset CI. Reported prevalence[2–8] of cognitive dysfunction in the heart failure (HF) population parallels the severity of HF, being the highest in the acute decompensation settings (see Table 1).
Table 1: Reported Prevalence Rate of Cognitive Dysfunction (CD) in Heart Failure Patients.
| Author (year) | Study type | Setting | N | Prevalence of CD (%) | Prevalence of mild CD (%) | CD test used |
|---|---|---|---|---|---|---|
| Zuccala et al.[2] (1997) | Cross-sectional | Mild–moderate CHF | 57 | 53 | NA | MMSE |
| Debette et al.[3] (2007) | Prospective | Hospitalisation for AHF | 83 | 61 | 30 | MMSE |
| Gure et al.[4] (2012) | Cross-sectional | Community (USA) | 6,189 | 39 | 24 | Telephone interview for cognitive status (patterned MMSE) |
| Hajduk et al.[5] (2013) | Prospective | Hospitalisation for AHF | 577 | 79 | NA | Specific protocol* |
| Dodson et al.[6] (2013) | Prospective | Hospitalisation for AHF | 282 | 47 | 25 | MMSE due to AHF |
| Levin et al.[7] (2014) | Prospective | Hospitalisation for AHF | 744 | 80 | 32 | Specific protocol* |
| Hyunh et al.[8] (2016) | Longitudinal | Hospitalisation for AHF | 565 | 45 | NA | MoCA |
*Specific bedside protocol: test of immediate and delayed memory (subscale of MoCA), processing speed (Digit Symbol Substitution Test, DSST) and executive function (Controlled Oral Word Association Test [COWA] for verbal fluency). AHF = acute heart failure; CHF = chronic heart failure; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
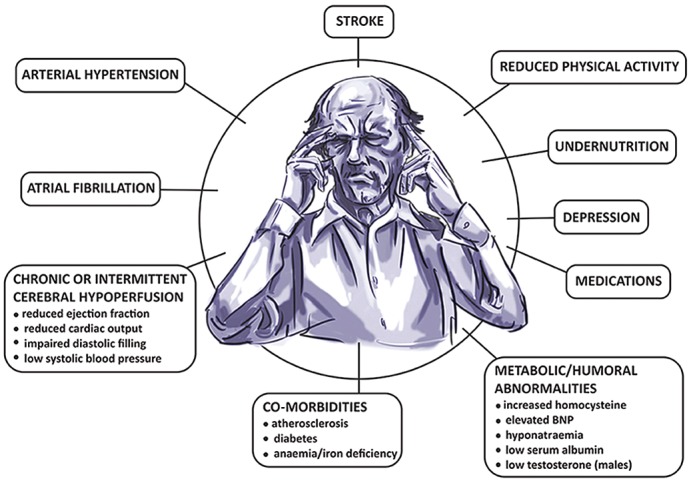
Multiple factors contributing to cognitive decline in HF are presented in Figure 1. Hypertension is found to be independently associated with CI[9,10] through compromise in auto-regulation of cerebral blood flow and cerebral ischaemia (see Figure 2). Neuroimaging has revealed structural brain abnormalities (see Figure 3) correlating with reduced cognition.
Figure 1: Contributing Factors to Cognitive Dysfunction in Heart Failure.
BNP = brain natriuretic peptide.
Figure 2: Pathogenic Links of Heart Failure and Cognitive Dysfunction.
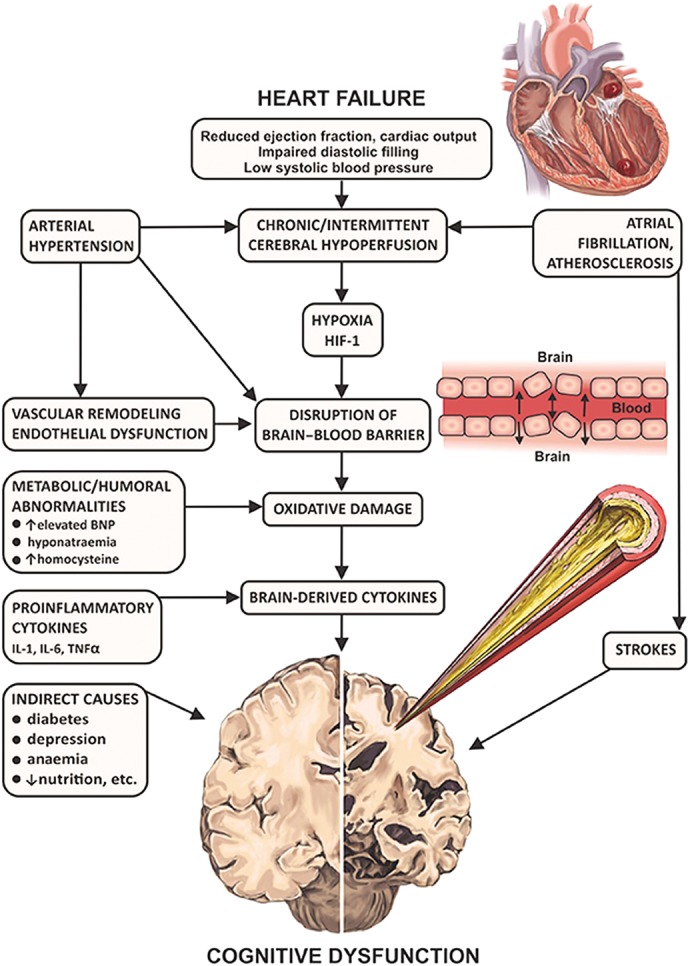
BNP = brain natriuretic peptide; HIF-1 = hypoxia inducible factor-1; IL-1 = interleukin-1; IL-6 = interleukin-6; TNF-alpha = tumour necrosis factor-alpha.
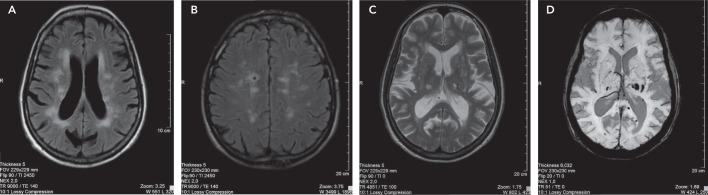
Figure 3: Magnetic Resonance Imaging of Structural Brain Abnormalities in Patients with Cognitive Dysfunction.
A: Confluent hyperintense changes in the periventricular white matter consistent with small vessel ischaemic changes associated with generalised brain volume loss. B: Multifocal chronic small vessel ischaemic changes, predominantly affecting frontal lobes, and associated with a lacunar infarct on the right side. C: Lacunar infarcts bilaterally in the thalami and small vessel ischaemic changes affecting frontal periventricular white matter and associated generalised brain volume loss. D: Haemosiderin staining bilaterally in the thalami and left occipital lobe due to microbleeds.
Cognitive impairment has been identified as an important clinical issue in numerous recently conducted studies, though no definite consensus has been achieved so far regarding optimal diagnostics and treatment tools in patients suffering both from HF and CI. There is a definite limitation on this issue in the literature as investigators have used plenty of different tools ranging from simple and fast tests to neuropsychological test batteries, and the assessment of cognition has not been standardised. The common approach should be thoroughly investigated and reached using existing data and future clinical studies.
Cognitive Domains and Assessment Tools
The severity of CI may range from mild symptoms to advanced dementia. Mild CI is a commonly used definition for a clinical syndrome in which a patient has subjective complaints as well as objective symptoms of cognitive decline (measured by neuropsychological tests), though daily functioning of the patient is mostly intact.[11] It is established that patients with mild cognitive impairment have an increased risk of progression to dementia.[12,13] Dementia is characterised by a progressive impairment in more than one cognitive domain, and compromised daily functioning is evident.
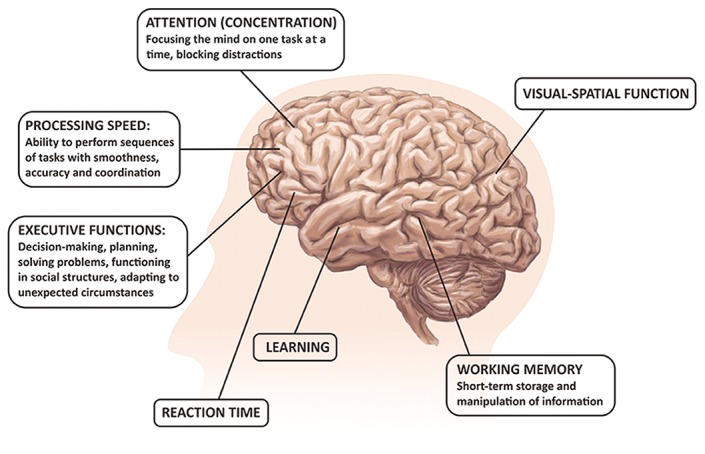
Most of the HF patients suffer from mild impairment in cognition, but some of them may have moderate-to-severe CI.[13] Heart failure adversely affects various cognitive domains, including attention, learning ability and working memory, executive functions, and information processing speed (see Figure 4).[13–15] Cognitive impairment in HF patients usually fulfils the criteria of vascular CI or vascular dementia.[16]
Figure 4: Cognitive Domains Typically Affected in Heart Failure Patients.
Being one of the most important cognitive functions, episodic memory of specific personal events and experiences was demonstrated to slowly decline in HF patients.[5,13,14,17] Furthermore, deficits in initial learning as well as delayed information recall were reported in the literature.[17] This suggests that CI in HF patients and CI in patients with vascular dementia could share pathophysiological mechanisms.[18] Deficits in executive functioning (problem solving, planning, reasoning and flexibility) have a strong impact on the patient’s everyday life and are common in cases of frontotemporal and vascular dementia, and are also detected in HF patients.[19] Importantly, depression should be considered during the evaluation of patients with HF as a common treatable disorder in elderly persons having chronic diseases. Depression markedly compromises cognitive functioning and treatment adherence in HF patients.[9,19]
A number of neuropsychological tests for measurement of cognitive functioning are available, yet some of them are time-consuming and require special training. There is a need for tests that are informative, yet short and easy to administer in everyday clinical practice. Currently, there is no consensus regarding the optimal battery of neuropsychological tests to assess patients with HF. Brief screening instruments to administer in an outpatient clinical practice, the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment (MoCA), are the most widely used screening tests in the literature. Some studies reported an association between the left ventricular ejection fraction and the MMSE scores.[2] Both tools are 30-point tests to assess basic cognitive domains, the presence of and severity of CI. Cut-off scores of ≤24 are usually used to diagnose mild CI.[20] Though the MMSE and the MoCA are useful tests for screening patients with CI, they could be insufficient in identifying subtle cognitive dysfunction, and more detailed neuropsychological tools may be required.[21]
Importance of Cognitive Decline for Prognosis and Self-care
Several prospective and cross-sectional studies showed a strong independent association of cognitive deterioration and increase in mortality and readmissions in acute heart failure (AHF) patients.[2,6,8,10,22,23] A twofold increase in 30-day death and readmissions,[8] almost fivefold rates of 1-year mortality,[23] as well as increase in hospitalisation and/or death within 5 years were demonstrated.[22] Such association with poor outcomes is observed even in cases of mild CI,[8] which frequently remains undiagnosed. Dodson et al. found that patients with unrecognised cognitive decline exhibited a higher 6-month mortality and hospital readmissions.[6]
Intact memory and executive function are necessary to recognise worsening symptoms, adhere to medication regimens and numerous lifestyle modifications, follow scheduled clinic visits, and comply with dietary recommendations. Even mild cognitive deficits may interfere with adherence to self-care practices.[5,24] Memory and executive function impairments are independently associated with worse instrumental activity, especially with a decreased ability to manage medications.[9,10,25]
Possible Ways to Prevent Cognitive Impairment
Evidence of therapeutic methods for prevention and management of cognitive decline in HF is lacking. The primary approach to the management of the HF patients with CI seems to be optimal pharmacotherapy, outlined in the HF guidelines,[26] restoring central haemodynamics, as well as correction of vascular risks factors. Several studies demonstrated that patients with mild CI can return to normal cognitive functioning.[27] The beneficial influence of HF treatment on cognition processes including angiotensin-converting enzyme inhibitors, diuretics, digoxin, cardiac resynchronisation therapy and heart transplantation were reported.[28–31] Clinical trials (such as the Efficacy and Safety of LCZ696 Compared to Valsartan on Cognitive Function in Patients with Heart Failure and Preserved Ejection Fraction [PERSPECTIVE] trial; NCT02884206) investigating the impact of treatment with angiotensin receptor neprilysin inhibitors and computerised adaptive cognitive training on cognitive function are ongoing.[32] Non-pharmacological measures, such as physical activity, are also important and have been identified to have beneficial effects on cognition as well as protective benefits on brain plasticity in vascular CI and related conditions.[33,34] The main task for physicians is to identify patients with early stages of CI in order to start corrective measures as soon as possible and also manage patients at risk of CI.[35]
There are no particular approved medicines for patients with CI in HF. Given that acetylcholinesterase inhibitors and memantine were found to be effective in vascular dementia, a similar effect might be expected in patients with HF, though no formal regulatory approvals have been received.[36,37]
Conclusion
Cognitive decline is a prevalent condition and an independent prognostic marker of adverse outcomes in patients with HF. Assessment of cognitive functioning, even by simple screening tests, should be part of routine clinical examinations of HF patients. Despite evolving data in clinical practice, CI is still the challenge for clinicians. Future research including cognitive function as a relevant endpoint of HF studies is warranted. Better understanding of pathogenic links between CI and HF including molecular, neuroendocrine, epigenetic and psychosocial factors is needed. It is important to uncover the relationship between cognitive function and HF types and co-morbidities, use of cardiovascular drugs and devices. Furthermore, more randomised and controlled trials should be initiated to provide additional data and implement diagnostic, prevention and management tools for CI in patients with HF.
References
- 1.Borson S. Cognition, aging, and disabilities: conceptual issues. Phys Med Rehabil Clin N Am. 2010;21:375–382. doi: 10.1016/j.pmr.2010.01.001. 10.1016/j.pmr.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuccala G, Cattel C, Manes-Gravina E et al. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. 10.1136/jnnp.63.4.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debette S, Bauters C, Leys D et al. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13:205–208. doi: 10.1111/j.1527-5299.2007.06612.x. 10.1111/j.1527-5299.2007.06612.x [DOI] [PubMed] [Google Scholar]
- 4.Gure TR, Blaum CS, Giordani B et al. The prevalence of cognitive impairment in older adults with heart failure. J Am Geriatr Soc. 2012;60(9):1724–1729. doi: 10.1111/j.1532-5415.2012.04097.x. 10.1111/j.1532-5415.2012.04097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajduk AM, Lemon SC, McManus DD et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol. 2013;5:407–416. doi: 10.2147/CLEP.S44560. 10.2147/CLEP.S44560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodson JA, Truong TT, Towle VR et al. Chaudhry, Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126:120–126. doi: 10.1016/j.amjmed.2012.05.029. 10.1016/j.amjmed.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin SN, Hajduk AM, McManus DD et al. Cognitive status in patients hospitalized with acute decompensated heart failure. Am Heart J. 2014;168:917–923. doi: 10.1016/j.ahj.2014.08.008. 10.1016/j.ahj.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyunh QL, Negishi K, Blizzard L et al. Mild cognitive impairment predicts death and readmission within 30 days of discharge for heart failure. Int J Cardiol. 2016;221:212–217. doi: 10.1016/j.ijcard.2016.07.074. 10.1016/j.ijcard.2016.07.074 [DOI] [PubMed] [Google Scholar]
- 9.Alosco ML, Brickman AM, Spitznagel MB et al. The independent association of hypertension with cognitive function among older adults with heart failure. J Neurol Sci. 2012;323:216–220. doi: 10.1016/j.jns.2012.09.019. 10.1016/j.jns.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. doi: 10.1016/j.ijcard.2014.10.087. 10.1016/j.ijcard.2014.10.087 [DOI] [PubMed] [Google Scholar]
- 11.Lopez OL, Becker JT, Jagust WJ et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. 10.1136/jnnp.2004.045567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleisher AS, Sowell BB, Taylor C et al. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. 10.1212/01.wnl.0000258542.58725.4c [DOI] [PubMed] [Google Scholar]
- 13.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. 10.1016/j.ejheart.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Vogels RL, Oosterman JM, van Harten B et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. 10.1111/j.1532-5415.2007.01395.x [DOI] [PubMed] [Google Scholar]
- 15.Pressler SJ, Subramanian U, Kareken D et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59(2):127–139. doi: 10.1097/NNR.0b013e3181d1a747. 10.1097/NNR.0b013e3181d1a747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelick PB, Scuteri A, Black SE et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mapelli D, Bardi L, Mojoli M et al. Neuropsychological profile in a large group of heart transplant candidates. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028313. 10.1371/journal.pone.0028313 e28313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–255. doi: 10.1016/S1474-4422(08)70040-1. 10.1016/S1474-4422(08)70040-1 [DOI] [PubMed] [Google Scholar]
- 19.Foster ER, Cunnane DF, Edwards DE et al. Executive dysfunction and depressive symptoms associated with reduced participation of people with severe congestive heart failure. Am J Occup Ther. 2011;65:306–313. doi: 10.5014/ajot.2011.000588. 10.5014/ajot.2011.000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron J, Worrall-Carter L, Page K et al. Screening for mild cognitive impairment in patients with heart failure: Montreal Cognitive Assessment versus Mini Mental State Exam. Eur J Cardiovasc Nurs. 2013;12:252–260. doi: 10.1177/1474515111435606. 10.1177/1474515111435606 [DOI] [PubMed] [Google Scholar]
- 21.Hawkins MA, Gathright EC, Gunstad J et al. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: a study with comprehensive neuropsychological testing. Heart Lung. 2014;43:462–468. doi: 10.1016/j.hrtlng.2014.05.011. 10.1016/j.hrtlng.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLennan SN, Pearson SA, Cameron J, Stewart S. Prognostic importance of cognitive impairment in chronic heart failure patients: does specialist management make a difference? Eur J Heart Fail. 2006;8:494–501. doi: 10.1016/j.ejheart.2005.11.013. 10.1016/j.ejheart.2005.11.013 [DOI] [PubMed] [Google Scholar]
- 23.Zuccalà G, Pedone C, Cesari M et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 24.Hughes TF, Snitz BE, Ganguli M. Should mild cognitive impairment be subtyped? Curr Opin Psychiatry. 2011;24:237–242. doi: 10.1097/YCO.0b013e328344696b. 10.1097/YCO.0b013e328344696b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alosco ML, Spitznagel MB, Raz N et al. Executive dysfunction is independently associated with reduced functional independence in heart failure. J Clin Nurs. 2014;23:829–836. doi: 10.1111/jocn.12214. 10.1111/jocn.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponikowski P, Voors AA, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;2016;18:891–975. doi: 10.1002/ejhf.592. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 27.Canevelli M, Grande G, Lacorte E et al. Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta-analysis. J Am Med Dir Assoc. 2016;17:943–948. doi: 10.1016/j.jamda.2016.06.020. 10.1016/j.jamda.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 28.Almeida OP, Tamai S. Clinical treatment reverses attentional deficits in congestive heart failure. BMC Geriatr. 2001;1:2. doi: 10.1186/1471-2318-1-2. 10.1186/1471-2318-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoth KF, Poppas A, Ellison KE et al. Link between change in cognition and left ventricular function following cardiac resynchronization therapy. J Cardiopulm Rehabil Prev. 2010;30:401–408. doi: 10.1097/HCR.0b013e3181e1739a. 10.1097/HCR.0b013e3181e1739a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuccalà G, Onder G, Marzetti E et al. Use of angiotensinconverting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur Heart J. 2005;26:226–233. doi: 10.1093/eurheartj/ehi058. 10.1093/eurheartj/ehi058 [DOI] [PubMed] [Google Scholar]
- 31.Roman DD, Kubo SH, Ormaza SG et al. Memory improvement following cardiac transplantation. J Clin Expl Neuropsychol. 1997;19:692–697. doi: 10.1080/01688639708403754. 10.1080/01688639708403754 [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Zhu Z, Liu Q et al. The efficacy of cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): study protocol for a randomized controlled trial. Trials. 2016;17(1):392. doi: 10.1186/s13063-016-1523-x. 10.1186/s13063-016-1523-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carles S Jr, Curnier D, Pathak A et al. Effects of short-term exercise and exercise training on cognitive function among patients with cardiac disease. J Cardiopulm Rehabil Prev. 2007;27:395–399. doi: 10.1097/01.HCR.0000300268.00140.e6. 10.1097/01.HCR.0000300268.00140.e6 [DOI] [PubMed] [Google Scholar]
- 34.Sofi F, Valecchi D, Bacci D et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- 35.Selnes OA, Royall RM, Grega MA et al. Cognitive changes 5 years after coronary artery bypass grafting: is there evidence of late decline? Arch Neurol. 2001;58:598–604. doi: 10.1001/archneur.58.4.598. [DOI] [PubMed] [Google Scholar]
- 36.Román GC, Salloway S, Black SE et al. Randomized, placebocontrolled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. 10.1161/STROKEAHA.109.570077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 2007;6:782–792. doi: 10.1016/S1474-4422(07)70195-3. 10.1016/S1474-4422(07)70195-3 [DOI] [PubMed] [Google Scholar]