Abstract
Cardiovascular imaging is key for the assessment of patients with heart failure. Today, cardiovascular magnetic resonance imaging plays an established role in the assessment of patients with suspected and confirmed heart failure syndromes, in particular identifying aetiology. Its role in informing prognosis and guiding decisions around therapy are evolving. Key strengths include its accuracy; reproducibility; unrestricted field of view; lack of radiation; multiple abilities to characterise myocardial tissue, thrombus and scar; as well as unparalleled assessment of left and right ventricular volumes. T2* has an established role in the assessment and follow-up of iron overload cardiomyopathy and a role for T1 in specific therapies for cardiac amyloid and Anderson–Fabry disease is emerging.
Keywords: Cardiovascular magnetic resonance, heart failure, late gadolinium enhancement, delayed enhancement, T1 mapping, T2*, myocarditis, cardiomyopathy, cardio-oncology, prognosis
Heart failure (HF) can be defined haemodynamically as any abnormality of cardiac structure or function resulting in a failure to deliver oxygen at a rate adequate for tissue requirements, despite normal filling pressures – or only at the expense of increased filling pressures.[1] Around half of patients with HF have reduced left ventricle ejection fraction (LVEF; EF <40 %) at rest (HF-REF).[2]
Diagnosis of suspected HF starts with medical history, physical examination, 12-lead electrocardiogram, chest X-ray and natriuretic peptide measurement. Transthoracic echocardiography is the first-line imaging modality.[3–5] Cardiovascular magnetic resonance (CMR) imaging plays an important complementary role in evaluating the underlying aetiology (or aetiologies) of the suspected HF, informing prognosis and guiding decision making, particularly where echocardiographic windows are inadequate or findings are inconclusive. CMR is not part of the assessment process in acute HF owing to reduced monitoring capability, patient intolerance of lying flat and reduced image quality (arrhythmias and reduced ability to breath hold). For the diagnosis of the ambulatory patient with suspected HF, CMR receives a class IC recommendation in the European Society of Cardiology (ESC) HF Guidelines.[1] CMR is frequently used in the management of HF patients: the European CMR registry reported that the most common indications for CMR include risk stratification in suspected ischaemia, assessment of viability and assessment of suspected myocarditis and cardiomyopathy.[6]
The image signal in MR arises from hydrogen nuclei, which are aligned to the field of the scanner and then ‘excited’ by radiofrequency wave pulses. Energy is released as the excited nuclei or spins relax back to equilibrium magnetisation. Decay of the longitudinal and transverse components of magnetisation are exponential processes named T1 and T2 relaxation, respectively. Whereas T2 relaxation takes into account dephasing due to random proton–proton interaction, T2* relaxation is a faster process, as it also takes into account dephasing accelerated by local inhomogeneities in the global magnetic field. Datasets in patients with HF typically start with T1-weighted black-blood spin-echo sequences for anatomy, gradient-echo (bright-blood steady-state-free precession) cine sequences in three long-axis and three short-axis planes to acquire chamber volumes, and contrast-enhanced inversion-recovery gradient-echo sequences with appropriate nulling of normal myocardium to look for late gadolinium enhancement (LGE). Further tissue characterisation sequences are acquired selectively to answer specific questions.
The advantages of CMR over other non-invasive imaging modalities are accuracy, reproducibility,[7,8] unrestricted field of view, lack of ionizing radiation, and the ability to characterize myocardial tissue. CMR is the gold standard modality for the assessment of LV volumes and EF,[9,10] LV thrombus (see Figure 1),[11–13] left atrium (LA) volumes[14] and the right ventricle (RV).[15] CMR tissue characterisation techniques may include inversion recovery images acquired either early (for thrombus imaging) or late (for scar imaging) after contrast administration, diffuse fibrosis assessment with T1 mapping and extracellular volume (ECV) measurement,[16–18] iron concentration using T2* and non-contrast ‘native’ T1 measurements, oedema evaluation using T2-weighted images, fatty infiltration with fat saturation sequences or T1-weighting, perfusion imaging with first-pass T1-weighted images and metabolism assessment using MR spectroscopy (MRS).[19] Its limitations are its availability, cost, the exclusion of patients with non-MR-compatible devices,[20] cerebrovascular clips or metallic objects in the eye, and the inability to scan patients who are too breathless to lie flat or who have claustrophobia. In patients with HF, electrocardiographic gating may be challenging in those with AF, high ectopic burden or broad QRS width. Furthermore, linear gadolinium chelates are contraindicated in individuals with estimated creatinine clearances <30ml/min, and renal dysfunction is relatively common in HF. Newer macrocyclic chelates have a better safety profile, but should still be used with caution in patients with advanced renal dysfunction.[21]
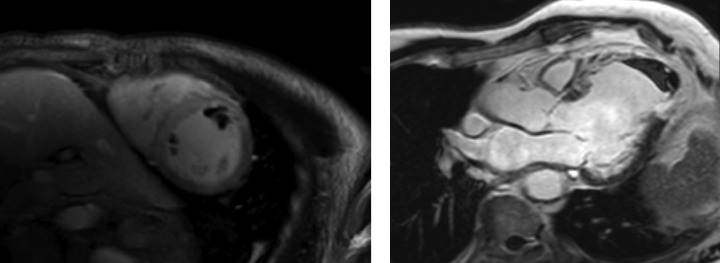
Figure 1: LV Thrombi Identified on Early Gadolinium-enhanced Images.
Left: multiple thrombi in short-axis mid ventricle view; right: apical thrombus with transmural apical enhancement in a three-chamber view.
Ischaemic Cardiomyopathy
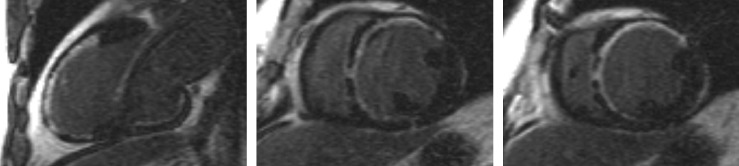
In patients presenting with de novo acute HF and no clinical or electrocardiographic suggestion of ischaemic aetiology, LGE-CMR is sensitive and specific for the presence of underlying significant coronary artery disease (CAD).[22,23] Identifying ischaemic cardiomyopathy (ICM) as the aetiology of HF implies a worse prognosis than non-ICM.[24] Patients with single-vessel disease (<75 % luminal stenosis) not involving the proximal left anterior descending (LAD) or left main arteries and with no history of MI or prior revascularisation have a prognosis similar to patients with non-ischaemic HF.[25] However, the absence of angina and significant stenoses on coronary angiography does not exclude CAD as the cause of HF, as infarction may follow coronary spasm or embolism, or be followed by coronary recanalisation.[26] Around 15 % of patients with unobstructed coronaries are found to have LGE in distributions typical of prior infarction and would be misclassified as having dilated cardiomyopathy (DCM) were LGE-CMR imaging not performed.[27,28] Patterns typical of prior infarction show subendocardial or transmural enhancement respecting one or more coronary territories, reflecting the ‘wavefront phenomenon’ of ischaemic injury (see Figure 2).[29]
Figure 2: Extensive Anterior and Anteroseptal Subendocardial LGE Distribution Typical of Anteroseptal Ischaemic Injury.
LGE = late gadolinium enhancement.
For the detection of suspected stable CAD in patients with intermediate (15–85 %) pre-test probability of disease and preserved and reduced LVEF, vasodilator stress CMR for first-pass perfusion and dobutamine stress CMR for inducible wall-motion abnormalities (WMA) are well established, feasible and safe (see Figure 3).[30–33] Quantitating deformation with strain-encoded CMR improves the accuracy of high-dose dobutamine stress CMR over visual assessment of WMA on cine imaging.[34,35] Three-dimensional stress perfusion techniques acquire datasets covering the whole heart rather than three short-axis slices, allowing quantitation of ischaemic burden with good agreement with stress perfusion single-photon emission computed tomography (SPECT).[36,37] High-resolution stress perfusion CMR is feasible in patients with HF.[38]
Figure 3: Inferior and Inferoseptal Subendocardial Inducible Perfusion Deficit on Vasodilator-stress First-pass Gadolinium Contrast Images.
In hearts with resting LVEF ≤40 % and established left-main, left-main-equivalent or significant proximal LAD and multivessel disease (with fractional flow reserve <0.80), coronary revascularisation is indicated for the relief of angina and for ‘prognosis’ (ESC/European Association for Cardio-Thoracic Surgery class IA recommendation).[39] CMR strategies for estimating the likelihood of improvement include assessing the response to low-dose dobutamine, extent of LGE transmurality, and myocardial thickness.[40] CMR myocardial feature tracking reduces inter-observer variability compared with visual analysis of the response to low-dose dobutamine.[41] Contractile reserve correlates inversely with infarct transmurality, but cannot be straightforwardly predicted in segments with infarction of intermediate transmural extent.[42] Greater transmurality of infarction as assessed by LGE-CMR has been shown to correlate inversely with the likelihood of segmental and global functional recovery post revascularisation.[43–45] LGE transmurality is often used as a surrogate for viability, the attraction being that no stress or metabolic imaging step is required, and in a meta-analysis of prospective trials was shown to carry the highest sensitivity and negative predictive value for recovery.[46]
Multiple earlier reports and a meta-analysis showed that the presence of viability in patients with ICM, as assessed by thallium nuclear perfusion SPECT, 18-F fluorodeoxyglucose positon emission tomography or dobutamine echocardiography, predicted improved survival after revascularisation.[47–50] However, the viability substudy of the Surgical Treatment for Ischemic Heart Failure (STICH) trial did not find an association between viability and outcomes on multivariate analysis.[51,52] However, this study was criticised for the following reasons: the protocol was amended to make viability testing optional and at the investigators’ choice performed by either SPECT or dobutamine echocardiography, the definition of viability was not prospectively validated and did not require segments to be hypocontractile at rest, and post-revascularisation regional and global changes in LV function were not reported.[53] Further trials investigating the predictive value of CMR viability are warranted.
A meta-analysis of studies of subjects with known or suspected CAD showed that the presence and extent of LGE predict future major adverse cardiovascular events (MACE) and mortality;[54] however, many of these studies did not recruit subjects with HF. In a large cohort with mostly preserved EF, LGE and WMA inducible upon dobutamine stress were independent predictors of MACE; conversely, absence of inducible WMA predicted excellent prognosis over the following 3 years.[55,56] However, in one study in which patients with LVEF <55 % and regional WMA at rest were recruited, dobutamine-induced increases in wall-motion score index provided additive predictive power for MACE beyond resting LVEF when resting LVEF was >40 %.[57] In studies of patients with ICM and reduced EF, greater extents of scar volume as a proportion of total myocardial volume independently predicted MACE,[58,59] and larger peri-infarct ‘intermediate zones’ independently predicted mortality[60,61] and inducibility of monomorphic ventricular tachycardia.[62]
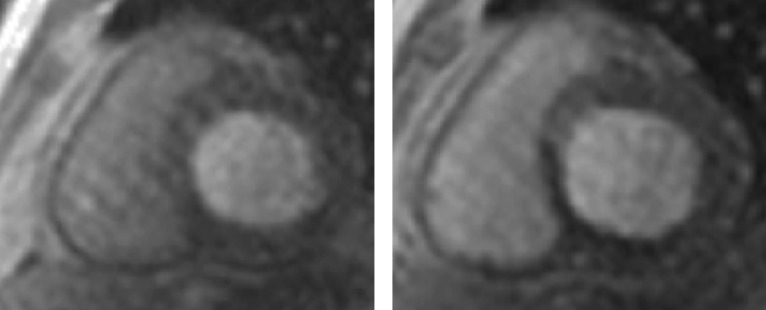
In the setting of recent acute MI, CMR correlates of higher risk include LVEF, infarct and peri-infarct zone sizes, the presence of microvascular obstruction (MVO) (see Figure 4), reduced RVEF,[63] and lower degrees of myocardial salvage, assessed as the difference between the area at risk on T2 weighting and the final infarct size.[64] CMR-detected MVO, defined as a lack of gadolinium retention (dark region) in the core of a segment surrounded by tissue showing gadolinium enhancement, is an independent predictor of MACE[65,66] and adverse LV remodelling.[67] Greater extents of late MVO (assessed 15 minutes after gadolinium administration), rather than early MVO (1 minute after), independently predict MACE after primary percutaneous coronary intervention for ST-segment elevation MI.[68] Hypointense infarct cores on T2 weighting, a marker of intramyocardial haemorrhage (IMH), are associated with larger infarcts and greater extents of late MVO, and predict MACE and adverse LV remodelling independent of the presence of MVO.[69] IMH, alternatively detected by a hypointense infarct core with T2* <20 ms, also independently predicts MACE and adverse LV remodelling.[70]
Figure 4: Microvascular Obstruction at the Core of a Recent Anteroseptal Infarct.
Microvascular obstruction is identified at the core of a recent anteroseptal infarct. There is surrounding subendocardial anteroseptal, inferoseptal and anterior LGE. LGE = late gadolinium enhancement.
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a clinical diagnosis based on dilation and systolic dysfunction of the left or both ventricles that is unexplained by abnormal loading conditions or CAD.[71] CMR studies have shown that increased native T1, LA volumes and RV dysfunction, but not greater degrees of trabeculation, are independent predictors of survival and HF outcomes in patients with DCM.[72–75]
If present in DCM, LGE is typically found in a mid-wall distribution (see Figure 5).[27,76] Co-existent endocardial LGE may indicate concurrent ischaemic contribution to HF aetiology. Mid-wall LGE was found in 10–28 %[28,27] of patients with DCM in adult case series, but may be less common in children.[77] In adults, the presence of LGE in non-ischaemic DCM independently predicts an increased risk of MACE, including hospitalisation for decompensated HF, sudden and non-sudden cardiac death, ventricular arrhythmia[78,79] and all-cause mortality.[80] Mid-wall LGE distribution confers a higher risk of inducible ventricular tachycardia.[81] Larger extents of mid-wall LGE independently predict lower likelihoods of LV reverse remodelling in patients with recent-onset DCM.[82]
Figure 5: Extensive Mid-wall LGE Distribution Seen on Short-axis Imaging in a Patient with DCM.
DCM = dilated cardiomyopathy; LGE = late gadolinium enhancement.
Cardiac energy metabolism is deranged in patients with HF, and MRS is the most powerful method for its non-invasive assessment in vivo.[83] Its clinical use has been limited owing to its low temporal and spatial resolution, but this is arguably less important in diffuse myocardial processes such as DCM. In DCM, myocardial phosphocreatine:adenosine triphosphate ratios are reduced and lower ratios independently predict mortality.[84] Forward creatine kinase shuttle flux is reduced in non-ischaemic cardiomyopathy and this also independently predicts mortality.[85,86] The hypothesis that altered energetics play a causal role in HF remains controversial and the modulation of substrate use as a therapeutic target remains under investigation.[87]
Takotsubo Syndrome
Takotsubo syndrome is an acute and usually reversible HF syndrome whose presentation mimics an acute coronary syndrome (ACS).[88,89] Recently proposed diagnostic criteria include transient and reversible regional WMA of the LV or RV frequently preceded by a stressful trigger, circumferential involvement of ventricular segments beyond a single coronary territory, the absence of culprit coronary events and viral myocarditis, new and reversible electrocardiographic changes, significant increases in natriuretic peptide levels, and troponin level increases that are modest for the degree of dysfunction.[90] CMR detects ‘typical’ apical ballooning and ‘atypical’ variants (e.g. biventricular, midventricular, basal and focal ballooning).[91–93] Oedema is detected on T2-weighted CMR in both takotsubo and myocarditis. However, LGE is usually absent acutely in takotsubo,[94,95] unlike in MI (subendocardial) and acute myocarditis (non-ischaemic distribution). Where available, CMR is recommended within 7 days of presentation in suspected takotsubo syndrome to aid diagnosis and detect LV thrombus, and to confirm myocardial recovery on follow-up.[96]
Myocarditis
Myocarditis is an inflammation of myocardial tissue of infectious, immune or toxic aetiology that presents as an ACS, new-onset or worsening HF or life-threatening arrhythmia in the absence of CAD or known causes of HF. It may resolve spontaneously, recur or become chronic, and may predate the development of DCM. Strictly speaking, myocarditis is diagnosed when endomyocardial biopsy (EMB) findings meet certain histological, immunohistochemical and immunological criteria.[97,98] In life-threatening presentations, urgent EMB has a class 1B indication, as only EMB can distinguish aetiologies (e.g. viral from non-viral, lymphocytic from giant-cell). However, as EMB may be limited by sampling error or complicated by tamponade, it is not recommended for all patients.[99] CMR is the primary non-invasive imaging modality for the assessment of suspected myocarditis in clinically stable patients – it supports the diagnosis by identifying abnormalities of cardiac structure, function and tissue characteristics; excludes ischaemic patterns of injury; and acts as a gatekeeper to EMB.[100,101]
The accuracy of CMR diagnosis of myocarditis is variably reported, and depends on the combination of techniques used, the time point in the inflammatory process at which images are taken, the severity of the inflammation in the group studied and whether EMB is the comparison standard. Combining tissue characterisation techniques improves diagnostic performance.[102] The recommended clinical diagnostic algorithm for suspected myocarditis[98] includes the CMR criteria proposed in the 2009 Journal of the American College of Cardiology White Paper for CMR assessment of myocarditis.[103]
These recommendations require two of the following three criteria for diagnosis: T2-weighted images showing increased global or regional myocardial signal intensity relative to skeletal muscle (indicating myocardial oedema), early gadolinium-enhanced T1-weighted images showing increased global myocardial signal intensity relative to skeletal muscle (indicating myocardial hyperaemia/capillary leak) and ≥1 focal lesion on LGE with non-ischaemic distribution (indicating necrosis/fibrosis). The most common LGE distribution is focal and patchy and involves the subepicardial lateral wall.[104,105]
The sensitivity of the 2009 CMR criteria is greatest for patients with infarct-like, rather than HF, presentations.[106] Where conventional techniques do not detect abnormalities or where gadolinium is contraindicated, native (non-contrast) T1 mapping (see Figure 6) can detect oedema in non-ischaemic distributions and thus improve diagnostic confidence when imaging is performed at a median of 3 days from presentation.[107,108] Native T1 values raised >5 standard deviations above the mean of the normal range independently identified acute myocarditis and were more raised in acute compared with convalescent stages of the process.[109] Conversely, in another study of patients with recent-onset HF and clinically suspected myocarditis, T2 mapping revealed higher median global myocardial T2 values in those with biopsy-proven active myocarditis, while there were no significant differences in native or post-contrast global myocardial T1.[110]
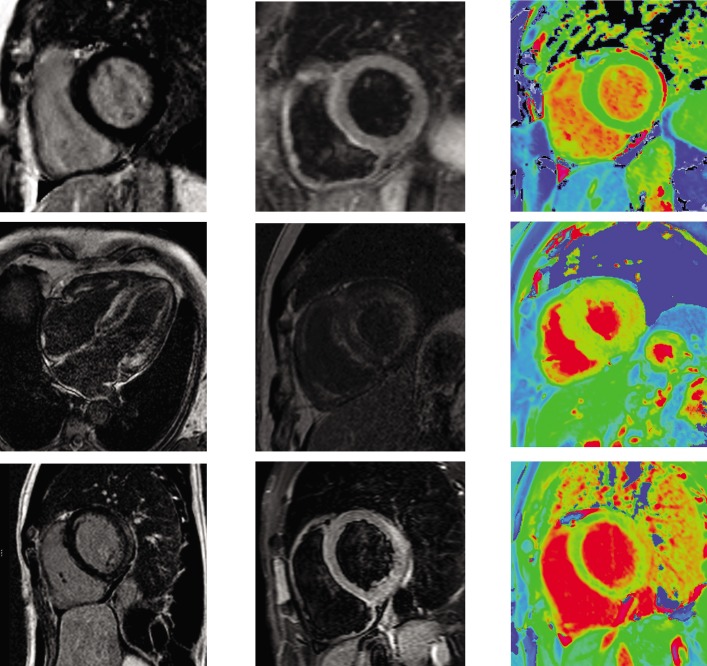
Figure 6: Multi-parametric Assessment of Normal Heart, Cardiac Amyloidosis and Acute Myocarditis.
Top row: normal appearances on LGE (left), T2 STIR imaging (middle), and native T1 mapping (right). Middle row: cardiac amyloidosis on LGE imaging (left, middle) demonstrates abnormal blood-pool appearance with ‘zebra-stripe’ enhancement of and difficulty nulling the myocardium; there is heterogeneous diffuse increase in myocardial T1 on native T1 mapping (right). Bottom row: acute myocarditis demonstrates subepicardial lateral-wall enhancement on LGE imaging (left), diffusely increased signal relative to skeletal muscle on T2 STIR imaging (middle), and increased lateral wall T1 on native T1 mapping (right). LGE = late gadolinium enhancement; STIR = short T1 inversion recovery.
The prognostic value of CMR findings in myocarditis requires further investigation. The presence of LGE on CMR within 5 days of presentation was an independent predictor of all-cause and cardiac mortality in patients with biopsy-proven viral myocarditis,[111] and in an LGE-positive cohort, initial LVEF, but not LGE extent, predicted outcome.[112]
Iron Overload Cardiomyopathy
In patients with HF and suspected cardiac iron overload, and especially in those with transfusion-dependent beta-thalassaemia major, CMR with T2* at 1.5 T field strength should be performed at the earliest opportunity to expedite definitive diagnosis and treatment, and advice from a centre of expertise should be sought.[113] T2* is a magnetic relaxation property of any tissue and is inversely related to intracellular iron stores. Myocardial T2* <20 ms is a reproducible, specific marker of significant cardiac iron content, which does not correlate with liver iron or serum ferritin concentrations (see Figure 7).[114] Myocardial T2* and iron concentration in the septum are excellent predictors of mean total cardiac iron concentration in explanted hearts.[115] T2* declines before LVEF, and is the best predictor of future HF and ventricular arrhythmias, with T2* <10 ms indicating high risk and 10–20 ms indicating intermediate risk.[116] If iron chelation therapy is started early, declines in LVEF are preventable and reversible; T2* imaging has had a major impact on survival in patients with thalassaemia.[117]
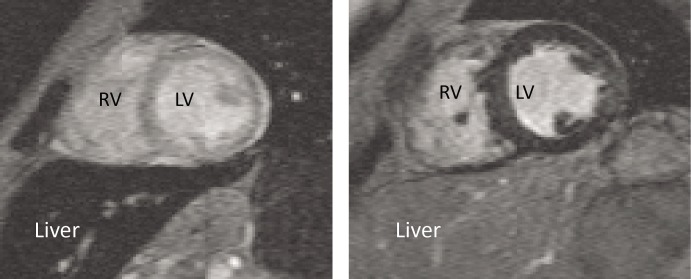
Figure 7: T2* imaging of Two Patients with Thalassaemia.
Left: shows iron loading of the heart sparing the liver; right: shows iron loading of the liver sparing the heart. LV = left ventricle; RV = right ventricle.
Cardiac Amyloidosis
Amyloidosis results from extracellular deposition of abnormal insoluble fibrils derived from a misfolded, normally soluble protein.[118] The three most common types of amyloidosis affecting the heart include systemic amyloid light-chain (AL) amyloidosis, where the fibrils derive from monoclonal immunoglobulin light chains in the setting of B-cell dyscrasias, hereditary systemic (variant) TTR amyloidosis, where the fibrils derive from variant transthyretin, and senile systemic (wild-type) ATTR amyloidosis. The V122I variant is the most common mutation and is found in 3–4 % of African Americans, carriers of which have an increased risk of HF compared with non-carriers over long-term follow-up.[119] ATTR amyloidosis is an underdiagnosed cause of HF. Biopsy remains the gold standard for diagnosis.[120]
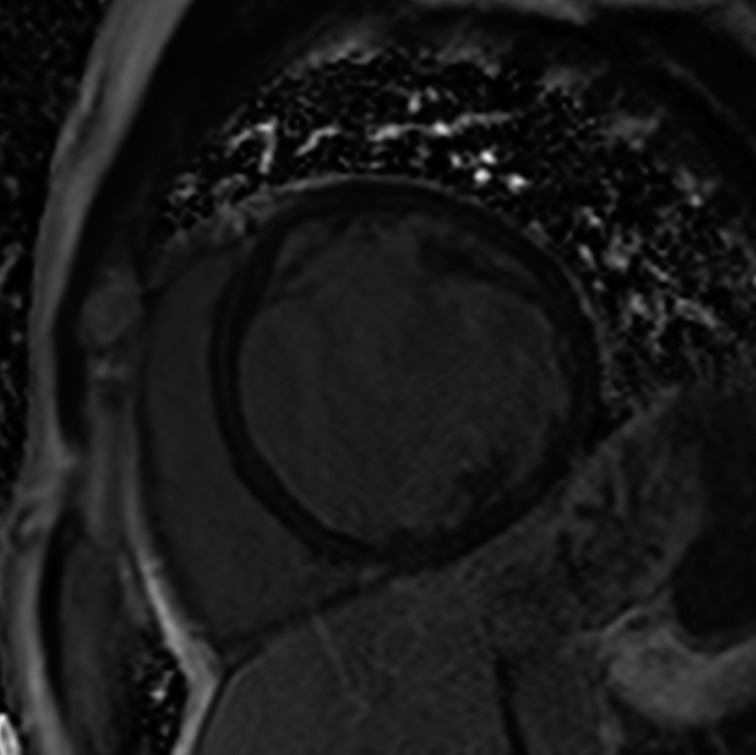
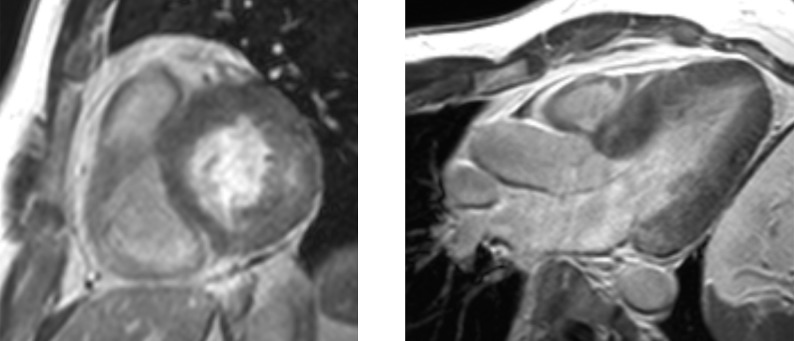
Given the short median survival in cardiac AL amyloidosis (~5 months), CMR is indicated in patients with HF and suspected cardiac amyloidosis to expedite diagnosis and treatment with chemotherapy. CMR findings in cardiac amyloidosis reflect interstitial expansion with high myocardial gadolinium uptake, and typically reveal global subendocardial or transmural LGE, shortening of subendocardial T1, rapid blood pool wash-out and suboptimal myocardial nulling (see Figure 8).[121–124]
Figure 8: LGE in Cardiac Amyloidosis.
Left: widespread transmural distribution in ATTR. Right: global subendocardial distribution in AL with transmurality at the base of the LV. AL = amyloid light chain; ATTR = transthyretin amyloidosis; LGE = late gadolinium enhancement; LV = left ventricle.
Compared with AL, ATTR involvement is characterised by greater LV mass and LGE extent, greater likelihood of transmural and RV LGE, and longer survival.[125] Elevated T1 on native T1 mapping (see Figure 6) has high accuracy for cardiac involvement in AL amyloidosis[126] and together with raised ECV predicts mortality.[127] Raised native T1 also has high accuracy in ATTR cardiac amyloidosis as compared with HCM, ATTR mutation carriers and normal controls, and may represent an early disease marker.[128] The value of native T1 as a marker of disease burden during therapy is under investigation in international trials of TTR-specific therapies.
Anderson–Fabry Disease
Anderson–Fabry disease (AFD) is an X-linked recessive disorder caused by reduced or absent activity of the enzyme alpha-galactosidase A, resulting in lysosomal glycosphingolipid accumulation in several organs. LV hypertrophy (LVH), fibrosis, HF (initially with preserved EF) and sudden arrhythmic death may occur.[129,130] In the presence of renal replacement therapy, cardiac involvement drives mortality. Early enzyme replacement therapy can cause regression of LVH.[131]
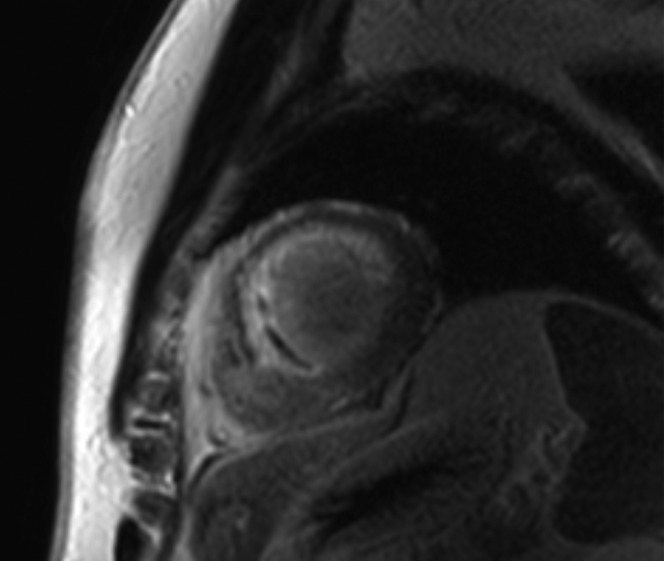
In patients with AFD, LGE may be detected particularly affecting the basal inferolateral wall in the absence of CAD (see Figure 9).[132,133] Native myocardial T1 is reduced in AFD,[134,135] differentiating this condition from HCM, oedema and amyloidosis, where T1 is increased. CMR can identify AFD in unexplained LVH and offers the potential for early detection. In one study, native T1 was lowered in patients with genotype-positive, LVH-negative AFD, although correlation with lipid burden on biopsy was not performed.[136] Current guidelines advocate the CMR measures of LV wall thickness, mass index and LGE to guide enzyme replacement therapy in patients with AFD.[137]
Figure 9: Basal Inferolateral LGE Distribution Seen in a Patient with Anderson–Fabry Disease.
LGE = late gadolinium enhancement.
Heart Failure with Preserved Ejection Fraction
The diagnosis of HF with preserved EF (HF-PEF) requires the following criteria: resting LVEF ≥50 %; a non-dilated LV (indexed volume <97/ml/m[2]); and sufficient biomarker, imaging and/or invasive evidence of diastolic dysfunction.[138] Although outcomes are similar in patients with HF-PEF and HF-REF,[139] no drug therapies have been shown to improve survival in HF-PEF to date.[140,141]
While 2D-echocardiography has superior temporal resolution for assessment of LV filling, CMR may contribute superior assessment of LVEF, LV mass and LA volumes, in addition to correlates of pulmonary hypertension (e.g. pulmonary artery:aorta ratio[142] and RV function[143]). The use of correlations between diastolic dysfunction and diffuse myocardial fibrosis[144] and between post-contrast T1 and outcome[145] is under investigation and CMR indices of diastolic function are not yet routinely measured.[146–148]
Cardio-oncology
Detection and management of the cardiotoxic effects of anticancer treatments is of growing importance.[149,150] Treatments implicated in causing LV dysfunction include anthracyclines, cyclophosphamide, docetaxel, bortezomib, trastuzumab, bevacizumab and sunitinib.[151,152] Most children with cancer will become long-term survivors and be more likely to develop HF than their siblings.[153] Early detection and prompt treatment of anthracycline-related cardiotoxicity can prevent LV dysfunction and promote LV recovery.[154–156]
The Trastuzumab Trials Cardiac Review and Evaluation Committee defined treatment-related cardiac dysfunction as a symptomatic fall in LVEF by >5 % to <55 %, or an asymptomatic fall in LVEF by >10 % to <55 %.[157] Current criteria for discontinuing trastuzumab depend on detection of LVEF 40–49 % and ≥10 % below baseline or LVEF <40 %.[158] Compared with radionuclide cardiac blood pool imaging and echocardiography, the ‘standard’ imaging modality for serial LVEF assessment – CMR – offers a radiation-free, more accurate modality for detecting LVEF <50 %.[159] Expert consensus guidelines recommend CMR in particular when ventricular function nears thresholds for chemotherapy discontinuation or when there is significant regurgitant valve disease.[160]
LGE is an insensitive marker with poor prognostic utility in cancer survivors.[161] The use of ECV, LA volume, oedema, and deformation imaging to detect cardiotoxicity before declines in LVEF is under investigation.[162–166] In a series of childhood cancer survivors who previously received ≥200 mg/m[2] anthracycline and had normal indices of global systolic function by standard CMR parameters, CMR tagging techniques detected significant falls in global and segmental LV peak longitudinal and circumferential strain and detected more widespread regional falls in strain than did speckle-tracking by echocardiography.[167]
Other Cardiomyopathies
HF is an uncommon first presentation for HCM, arrhythmogenic RV cardiomyopathy, and cardiac sarcoidosis, and the role of CMR in the assessment of these conditions has been reviewed extensively elsewhere.[26,168–171]
Conclusion
CMR has established and evolving roles in the assessment of patients with HF, particularly the confirmation of underlying aetiology. The extent of involvement detected on CMR carries prognostic information in patients with ICM, DCM, iron overload, cardiac amyloidosis and AFD. The identification of diffuse interstitial fibrosis in many of the cardiomyopathies is increasing knowledge about the mechanisms of the disease processes involved. The role of T2* in assessment of response to therapy in iron overload is established and a potential role for T1 in specific therapies for cardiac amyloidosis and AFD is emerging. The use of T1, T2 and T2* mapping sequences is increasing for myocardial tissue assessment in the cardiomyopathies.
References
- 1.Ponikowski P, Voors AA, Anker SD ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016. 10.1002/ejhf.592. 2016. 20. [Epub ahead of print] [DOI] [PubMed]
- 2.Gimelli A, Lancellotti P, Badano LP et al. Non-invasive cardiac imaging evaluation of patients with chronic systolic heart failure: a report from the European Association of Cardiovascular Imaging (EACVI). Eur Heart J. 2014;35:3417–3425. doi: 10.1093/eurheartj/ehu433. 10.1093/eurheartj/ehu433 [DOI] [PubMed] [Google Scholar]
- 3.Mebazaa A, Yilmaz MB, Levy P et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur Heart J. 2015;36:1958–1966. doi: 10.1093/eurheartj/ehv066. 10.1093/eurheartj/ehv066 [DOI] [PubMed] [Google Scholar]
- 4.Lancellotti P, Price S, Edvardsen T et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Cardiovasc Imaging. 2015;16:119–146. doi: 10.1093/ehjci/jeu210. 10.1093/ehjci/jeu210 [DOI] [PubMed] [Google Scholar]
- 5.Garbi M, nagh T, Cosyns B et al. Appropriateness criteria for cardiovascular imaging use in heart failure: report of literature review. Eur Heart J Cardiovasc Imaging. 2015;16:147–153. doi: 10.1093/ehjci/jeu299. 10.1093/ehjci/jeu299 [DOI] [PubMed] [Google Scholar]
- 6.Bruder O, Wagner A, Lombardi M et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry–multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15(9) doi: 10.1186/1532-429X-15-9. 10.1186/1532-429X-15-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellenger NG, Davies LC, Francis JM et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 8.Grothues F, Smith GC, Moon JC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 9.Greupner J, Zimmermann E, Grohmann A et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol. 2012;59:1897–1907. doi: 10.1016/j.jacc.2012.01.046. 10.1016/j.jacc.2012.01.046 [DOI] [PubMed] [Google Scholar]
- 10.Bellenger NG, Burgess MI, Ray SG et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. 10.1053/euhj.2000.2011 [DOI] [PubMed] [Google Scholar]
- 11.Srichai MB, Junor C, Rodriguez LL et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75–84. doi: 10.1016/j.ahj.2005.08.021. 10.1016/j.ahj.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 12.Weinsaft JW, Kim HW, Shah DJ et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance: prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–157. doi: 10.1016/j.jacc.2008.03.041. 10.1016/j.jacc.2008.03.041 [DOI] [PubMed] [Google Scholar]
- 13.Weinsaft JW, Kim RJ, Ross M et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009;2:969–979. doi: 10.1016/j.jcmg.2009.03.017. 10.1016/j.jcmg.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habibi M, Chahal H, Opdahl A et al. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014;7:570–579. doi: 10.1016/j.jcmg.2014.01.016. 10.1016/j.jcmg.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawut SM, Barr RG, Lima JA et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: The Multi-Ethnic Study of Atherosclerosis (MESA)-right ventricle study. Circulation. 2012;126:1681–1688. doi: 10.1161/CIRCULATIONAHA.112.095216. 10.1161/CIRCULATIONAHA.112.095216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iles L, Pfluger H, Phrommintikul A et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. 10.1016/j.jacc.2008.06.049 [DOI] [PubMed] [Google Scholar]
- 17.Sado DM, Flett AS, Banypersad SM et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. 10.1136/heartjnl-2012-302346 [DOI] [PubMed] [Google Scholar]
- 18.Maestrini V, Treibel TA, White SK et al. T1 mapping for characterization of intracellular and extracellular myocardial diseases in heart failure. Curr Cardiovasc Imaging Rep. 2014;7:9287. doi: 10.1007/s12410-014-9287-8. 10.1007/s12410-014-9287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudsmith LE, Neubauer S. Magnetic resonance spectroscopy in myocardial disease. JACC Cardiovasc Imaging. 2009;2:87–96. doi: 10.1016/j.jcmg.2008.08.005. 10.1016/j.jcmg.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 20.Naehle CP, Strach K, Thomas D et al. Magnetic resonance imaging at 1.5-T in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2009;54:549–555. doi: 10.1016/j.jacc.2009.04.050. 10.1016/j.jacc.2009.04.050 [DOI] [PubMed] [Google Scholar]
- 21.Knuuti J, Bengel F, Bax JJ et al. Risks and benefits of cardiac imaging: an analysis of risks related to imaging for coronary artery disease . Eur Heart J. 2014;35:633–638. doi: 10.1093/eurheartj/eht512. 10.1093/eurheartj/eht512 [DOI] [PubMed] [Google Scholar]
- 22.Casolo G, Minneci S, Manta R et al. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am Heart J. 2006;151:101–108. doi: 10.1016/j.ahj.2005.03.068. 10.1016/j.ahj.2005.03.068 [DOI] [PubMed] [Google Scholar]
- 23.Valle-Munoz A, Estornell-Erill J, Soriano-Navarro CJ et al. Late gadolinium enhancement-cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure . Eur J Echocardiogr. 2009;10:968–974. doi: 10.1093/ejechocard/jep115. 10.1093/ejechocard/jep115 [DOI] [PubMed] [Google Scholar]
- 24.Adams KF Jr, Dunlap SH, Sueta CA et al. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 25.Felker GM, Shaw LK. O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 26.Karamitsos TD, Francis JM, Myerson S et al. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407–1424. doi: 10.1016/j.jacc.2009.04.094. 10.1016/j.jacc.2009.04.094 [DOI] [PubMed] [Google Scholar]
- 27.McCrohon JA, Moon JC, Prasad SK et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. 10.1161/01.CIR.0000078641.19365.4C [DOI] [PubMed] [Google Scholar]
- 28.Soriano CJ, Ridocci F, Estornell J et al. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–748. doi: 10.1016/j.jacc.2004.11.037. 10.1016/j.jacc.2004.11.037 [DOI] [PubMed] [Google Scholar]
- 29.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 30.Nandalur KR, Dwamena BA, Choudhri AF et al. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis .J Am Coll Cardiol. 2007;50:1343–1353. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Schwitter J, Wacker CM, Wilke N et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775–781. doi: 10.1093/eurheartj/ehs022. 10.1093/eurheartj/ehs022 [DOI] [PubMed] [Google Scholar]
- 32.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. 10.1016/S0140-6736(11)61335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Task Force Members1, Montalescot G, Sechtem U et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 34.Korosoglou G, Lehrke S, Wochele A et al. Strain-encoded CMR for the detection of inducible ischemia during intermediate stress. JACC Cardiovasc Imaging. 2010;3:361–371. doi: 10.1016/j.jcmg.2009.11.015. 10.1016/j.jcmg.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 35.Korosoglou G, Gitsioudis G, Voss A et al. Strain-encoded cardiac magnetic resonance during high-dose dobutamine stress testing for the estimation of cardiac outcomes: comparison to clinical parameters and conventional wall motion readings. J Am Coll Cardiol. 2011;58:1140–1149. doi: 10.1016/j.jacc.2011.03.063. 10.1016/j.jacc.2011.03.063 [DOI] [PubMed] [Google Scholar]
- 36.Jogiya R, Kozerke S, Morton G et al. Validation of dynamic 3-dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol. 2012;60:756–765. doi: 10.1016/j.jacc.2012.02.075. 10.1016/j.jacc.2012.02.075 [DOI] [PubMed] [Google Scholar]
- 37.Jogiya R, Morton G, De Silva K et al. Ischemic burden by 3-dimensional myocardial perfusion cardiovascular magnetic resonance: comparison with myocardial perfusion scintigraphy. Circ Cardiovasc Imaging. 2014;7:647–654. doi: 10.1161/CIRCIMAGING.113.001620. 10.1161/CIRCIMAGING.113.001620 [DOI] [PubMed] [Google Scholar]
- 38.Sammut E, Zarinabad N, Wesolowski R et al. Feasibility of high-resolution quantitative perfusion analysis in patients with heart failure. J Cardiovasc Magn Reson. 2015;17:13. doi: 10.1186/s12968-015-0124-2. 10.1186/s12968-015-0124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Windecker S, Kolh P et al. ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. 10.1093/eurheartj/ehu278 Authors/Task Force members, [DOI] [PubMed] [Google Scholar]
- 40.Knuesel PR, Nanz D, Wyss C et al. Characterization of dysfunctional myocardium by positron emission tomography and magnetic resonance: relation to functional outcome after revascularization. Circulation. 2003;108:1095–1100. doi: 10.1161/01.CIR.0000085993.93936.BA. 10.1161/01.CIR.0000085993.93936.BA [DOI] [PubMed] [Google Scholar]
- 41.Schuster A, Paul M, Bettencourt N et al. Myocardial feature tracking reduces observer-dependence in low-dose dobutamine stress cardiovascular magnetic resonance. PLoS One. 2015;10:e0122858.. doi: 10.1371/journal.pone.0122858. 10.1371/journal.pone.0122858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaandorp TAM, Bax JJ, Schuijf JD et al. Head-to-Head comparison between Contrast-Enhanced magnetic resonance imaging and dobutamine magnetic resonance imaging in men with ischemic cardiomyopathy. Am J Cardiol. 2004;93:1461–1464. doi: 10.1016/j.amjcard.2004.03.003. 10.1016/j.amjcard.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Kim RJ, Wu E, Rafael A et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. 10.1056/NEJM200011163432003 [DOI] [PubMed] [Google Scholar]
- 44.Selvanayagam JB, Kardos A, Francis JM et al. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535–1541. doi: 10.1161/01.CIR.0000142045.22628.74. 10.1161/01.CIR.0000142045.22628.74 [DOI] [PubMed] [Google Scholar]
- 45.Shah DJ, Kim HW, James O et al. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309:909–918. doi: 10.1001/jama.2013.1381. 10.1001/jama.2013.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494–508. doi: 10.1016/j.jcmg.2012.02.009. 10.1016/j.jcmg.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 47.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 48.Beanlands RSB, Nichol G, Huszti E et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. 10.1016/j.jacc.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 49.Gerber BL, Rousseau MF, Ahn SA et al. Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: impact of revascularization therapy. J Am Coll Cardiol. 2012;59:825–835. doi: 10.1016/j.jacc.2011.09.073. 10.1016/j.jacc.2011.09.073 [DOI] [PubMed] [Google Scholar]
- 50.Ling LF, Marwick TH, Flores DR et al. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. doi: 10.1161/CIRCIMAGING.112.000138. 10.1161/CIRCIMAGING.112.000138 [DOI] [PubMed] [Google Scholar]
- 51.Bonow RO, Maurer G, Lee KL et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. 10.1056/NEJMoa1100358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velazquez EJ, Lee KL, Deja MA et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. 10.1056/NEJMoa1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrone-Filardi P, Pinto FJ. Looking for myocardial viability after a STICH trial: not enough to close the door. J Nucl Med. 2012;53:349–352. doi: 10.2967/jnumed.111.102210. 10.2967/jnumed.111.102210 [DOI] [PubMed] [Google Scholar]
- 54.Zemrak F, Petersen SE. Late gadolinium enhancement CMR predicts adverse cardiovascular outcomes and mortality in patients with coronary artery disease: systematic review and meta-analysis. Prog Cardiovasc Dis. 2011;54:215–229. doi: 10.1016/j.pcad.2011.07.003. 10.1016/j.pcad.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 55.Kelle S, Nagel E, Voss A et al. A bi-center cardiovascular magnetic resonance prognosis study focusing on dobutamine wall motion and late gadolinium enhancement in 3,138 consecutive patients . J Am Coll Cardiol. 2013;61:2310–2312. doi: 10.1016/j.jacc.2013.02.063. 10.1016/j.jacc.2013.02.063 [DOI] [PubMed] [Google Scholar]
- 56.Kelle S, Chiribiri A, Vierecke J et al. Long-term prognostic value of dobutamine stress CMR. JACC Cardiovasc Imaging. 2011;4:161–172. doi: 10.1016/j.jcmg.2010.11.012. 10.1016/j.jcmg.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 57.Dall’Armellina E, Morgan TM, Mandapaka S et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. J Am Coll Cardiol. 2008;52:279–286. doi: 10.1016/j.jacc.2008.04.025. 10.1016/j.jacc.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokota H, Heidary S, Katikireddy CK et al. Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:17. doi: 10.1186/1532-429X-10-17. 10.1186/1532-429X-10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon DH, Halley CM, Carrigan TP et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009;2:34–44. doi: 10.1016/j.jcmg.2008.09.010. 10.1016/j.jcmg.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 60.Kwon DH, Asamoto L, Popovic ZB et al. Infarct characterization and quantification by delayed enhancement cardiac magnetic resonance imaging is a powerful independent and incremental predictor of mortality in patients with advanced ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:796–804. doi: 10.1161/CIRCIMAGING.114.002077. 10.1161/CIRCIMAGING.114.002077 [DOI] [PubMed] [Google Scholar]
- 61.Yan AT, Shayne AJ, Brown KA et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post– myocardial infarction mortality . Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. 10.1161/CIRCULATIONAHA.106.613414 [DOI] [PubMed] [Google Scholar]
- 62.Schmidt A, Azevedo CF, Cheng A et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. 10.1161/CIRCULATIONAHA.106.653568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lockie T, Nagel E, Redwood S, Plein S. Use of cardiovascular magnetic resonance imaging in acute coronary syndromes. Circulation. 2009;119:1671–1681. doi: 10.1161/CIRCULATIONAHA.108.816512. 10.1161/CIRCULATIONAHA.108.816512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eitel I, Desch S, Fuernau G et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–2479. doi: 10.1016/j.jacc.2010.01.049. 10.1016/j.jacc.2010.01.049 [DOI] [PubMed] [Google Scholar]
- 65.Wu KC, Zerhouni EA, Judd RM et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 66.van Kranenburg M, Magro M, Thiele H et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930–939. doi: 10.1016/j.jcmg.2014.05.010. 10.1016/j.jcmg.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 67.Nijveldt R, Beek AM, Hirsch A et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–189. doi: 10.1016/j.jacc.2008.04.006. 10.1016/j.jacc.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 68.de Waha S, Desch S, Eitel I et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–2668. doi: 10.1093/eurheartj/ehq247. 10.1093/eurheartj/ehq247 [DOI] [PubMed] [Google Scholar]
- 69.Eitel I, Kubusch K, Strohm O et al. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354–362. doi: 10.1161/CIRCIMAGING.110.960500. 10.1161/CIRCIMAGING.110.960500 [DOI] [PubMed] [Google Scholar]
- 70.Carrick D, Haig C, Ahmed N et al. Myocardial hemorrhage after acute reperfused ST-segment–elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004148. 10.1161/CIRCIMAGING.115.004148 e004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elliott P, Andersson B, Arbustini E et al. Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. 10.1093/eurheartj/ehm342 [DOI] [PubMed] [Google Scholar]
- 72.Puntmann VO, Carr-White G, Jabbour A et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. 10.1016/j.jcmg.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 73.Gulati A, Ismail TF, Jabbour A et al. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2013;15:660–670. doi: 10.1093/eurjhf/hft019. 10.1093/eurjhf/hft019 [DOI] [PubMed] [Google Scholar]
- 74.Gulati A, Ismail TF, Jabbour A et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013;128:1623–1633. doi: 10.1161/CIRCULATIONAHA.113.002518. 10.1161/CIRCULATIONAHA.113.002518 [DOI] [PubMed] [Google Scholar]
- 75.Amzulescu M-S, Rousseau MF, Ahn SA et al. Prognostic impact of hypertrabeculation and noncompaction phenotype in dilated cardiomyopathy: a CMR study. JACC Cardiovasc Imaging. 2015;8:934–946. doi: 10.1016/j.jcmg.2015.04.015. 10.1016/j.jcmg.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 76.Mahrholdt H, Wagner A, Judd RM et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. 10.1093/eurheartj/ehi258 [DOI] [PubMed] [Google Scholar]
- 77.Latus H, Gummel K, Klingel K et al. Focal myocardial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance in children and adolescents with dilated cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:34. doi: 10.1186/s12968-015-0142-0. 10.1186/s12968-015-0142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assomull RG, Prasad SK, Lyne J et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy . J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. 10.1016/j.jacc.2006.07.049 [DOI] [PubMed] [Google Scholar]
- 79.Wu KC, Weiss RG, Thiemann DR et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. 10.1016/j.jacc.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gulati A, Jabbour A, Ismail TF et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. 10.1001/jama.2013.1363 [DOI] [PubMed] [Google Scholar]
- 81.Nazarian S, Bluemke DA, Lardo AC et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. 10.1161/CIRCULATIONAHA.105.549659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kubanek M, Sramko M, Maluskova J et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. doi: 10.1016/j.jacc.2012.07.072. 10.1016/j.jacc.2012.07.072 [DOI] [PubMed] [Google Scholar]
- 83.Neubauer S. The failing heart — an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. 10.1056/NEJMra063052 [DOI] [PubMed] [Google Scholar]
- 84.Neubauer S, Horn M, Cramer M et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 85.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. doi: 10.1073/pnas.0408962102. 10.1073/pnas.0408962102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bottomley PA, Panjrath GS, Lai S et al. Metabolic rates of ATP transfer through creatine kinase (CK flux) predict clinical heart failure events and death. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3007328. 10.1126/scitranslmed.3007328 215re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beadle RM, Williams LK, Kuehl M et al. Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy . JACC Heart Fail. 2015;3:202–211. doi: 10.1016/j.jchf.2014.09.009. 10.1016/j.jchf.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 88.Hurst RT, Prasad A, Askew JW. et al. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641–649. doi: 10.1016/j.jcmg.2010.01.009. 10.1016/j.jcmg.2010.01.009 3rd, [DOI] [PubMed] [Google Scholar]
- 89.Medeiros K, O’Connor MJ, Baicu CF et al. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129:1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781. 10.1161/CIRCULATIONAHA.113.002781 [DOI] [PubMed] [Google Scholar]
- 90.Lyon AR, Bossone E, Schneider B et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 91.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. 10.1001/jama.2011.992 [DOI] [PubMed] [Google Scholar]
- 92.Haghi D, Fluechter S, Suselbeck T et al. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2007;120:205–211. doi: 10.1016/j.ijcard.2006.09.019. 10.1016/j.ijcard.2006.09.019 [DOI] [PubMed] [Google Scholar]
- 93.Templin C, Ghadri JR, Diekmann J et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 94.Karamitsos TD, Bull S, Spyrou N et al. Tako-tsubo cardiomyopathy presenting with features of left ventricular non-compaction. Int J Cardiol. 2008;128:e34–36. doi: 10.1016/j.ijcard.2007.05.068. 10.1016/j.ijcard.2007.05.068 [DOI] [PubMed] [Google Scholar]
- 95.Syed I, Prasad A, Oh JK et al. Apical ballooning syndrome or aborted acute myocardial infarction? Insights from cardiovascular magnetic resonance imaging. Int J Cardiovasc Imaging. 2008;24:875–882. doi: 10.1007/s10554-008-9320-6. 10.1007/s10554-008-9320-6 [DOI] [PubMed] [Google Scholar]
- 96.Assomull RG, Lyne JC, Keenan N et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. 10.1093/eurheartj/ehm113 [DOI] [PubMed] [Google Scholar]
- 97.Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart. 2015;101:1332–1344. doi: 10.1136/heartjnl-2014-306363. 10.1136/heartjnl-2014-306363 [DOI] [PubMed] [Google Scholar]
- 98.Caforio AL, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 99.Cooper LT, Baughman KL, Feldman AM et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. 10.1016/j.jacc.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 100.Friedrich MG, Marcotte F. Cardiac Magnetic Resonance Assessment of Myocarditis. Circ Cardiovasc Imaging. 2013;6:833–839. doi: 10.1161/CIRCIMAGING.113.000416. 10.1161/CIRCIMAGING.113.000416 [DOI] [PubMed] [Google Scholar]
- 101.Yilmaz A, Ferreira V, Klingel K et al. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev. 2013;18:747–760. doi: 10.1007/s10741-012-9356-5. 10.1007/s10741-012-9356-5 [DOI] [PubMed] [Google Scholar]
- 102.Abdel-Aty H, Boyé P, Zagrosek A et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. 10.1016/j.jacc.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 103.Friedrich MG, Sechtem U, Schulz-Menger J et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahrholdt H, Wagner A, Deluigi CC et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. 10.1161/CIRCULATIONAHA.105.606509 [DOI] [PubMed] [Google Scholar]
- 105.Mahrholdt H, Goedecke C, Wagner A et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. 10.1161/01.CIR.0000118493.13323.81 [DOI] [PubMed] [Google Scholar]
- 106.Francone M, Chimenti C, Galea N et al. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. 2014;7:254–263. doi: 10.1016/j.jcmg.2013.10.011. 10.1016/j.jcmg.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 107.Ferreira VM, Piechnik SK, Dall’Armellina E et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. doi: 10.1186/1532-429X-16-36. 10.1186/1532-429X-16-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira VM, Piechnik SK, Dall’Armellina E et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–1058. doi: 10.1016/j.jcmg.2013.03.008. 10.1016/j.jcmg.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 109.Hinojar R, Foote L, Arroyo Ucar E et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging. 2015;8:37–46. doi: 10.1016/j.jcmg.2014.07.016. 10.1016/j.jcmg.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 110.Bohnen S, Radunski UK, Lund GK et al. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.003073. 10.1161/CIRCIMAGING.114.003073 e003073. [DOI] [PubMed] [Google Scholar]
- 111.Grün S, Schumm J, Greulich S et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. 10.1016/j.jacc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 112.Sanguineti F, Garot P, Mana M et al. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. 2015;17:78. doi: 10.1186/s12968-015-0185-2. 10.1186/s12968-015-0185-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pennell DJ, Udelson JE, Arai AE et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128:281–308. doi: 10.1161/CIR.0b013e31829b2be6. 10.1161/CIR.0b013e31829b2be6 [DOI] [PubMed] [Google Scholar]
- 114.Anderson LJ, Holden S, Davis B et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 115.Carpenter J-P, He T, Kirk P et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123:1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641. 10.1161/CIRCULATIONAHA.110.007641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kirk P, Roughton M, Porter JB et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. 10.1161/CIRCULATIONAHA.109.874487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Modell B, Khan M, Darlison M et al. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. 10.1186/1532-429X-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Selvanayagam JB, Hawkins PN, Paul B et al. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. 10.1016/j.jacc.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 119.Quarta CC, Buxbaum JN, Shah AM et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372:21–29. doi: 10.1056/NEJMoa1404852. 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gertz MA, Benson MD, Dyck PJ et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66:2451–2466. doi: 10.1016/j.jacc.2015.09.075. 10.1016/j.jacc.2015.09.075 [DOI] [PubMed] [Google Scholar]
- 121.Maceira AM, Joshi J, Prasad SK et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. 10.1161/01.CIR.0000152819.97857.9D [DOI] [PubMed] [Google Scholar]
- 122.Perugini E, Rapezzi C, Piva T et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance . Heart. 2006;92:343–349. doi: 10.1136/hrt.2005.061911. 10.1136/hrt.2005.061911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vogelsberg H, Mahrholdt H, Deluigi CC et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. 10.1016/j.jacc.2007.10.049 [DOI] [PubMed] [Google Scholar]
- 124.Syed IS, Glockner JF, Feng D et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3:155–164. doi: 10.1016/j.jcmg.2009.09.023. 10.1016/j.jcmg.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 125.Dungu JN, Valencia O, Pinney JH et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–142. doi: 10.1016/j.jcmg.2013.08.015. 10.1016/j.jcmg.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 126.Karamitsos TD, Piechnik SK, Banypersad SM et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis . JACC Cardiovasc Imaging. 2013;6:488–497. doi: 10.1016/j.jcmg.2012.11.013. 10.1016/j.jcmg.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 127.Banypersad SM, Fontana M, Maestrini V et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244–251. doi: 10.1093/eurheartj/ehu444. 10.1093/eurheartj/ehu444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fontana M, Banypersad SM, Treibel TA et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–165. doi: 10.1016/j.jcmg.2013.10.008. 10.1016/j.jcmg.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 129.Nagueh SF. Anderson-Fabry disease and other lysosomal storage disorders. Circulation. 2014;130:1081–1090. doi: 10.1161/CIRCULATIONAHA.114.009789. 10.1161/CIRCULATIONAHA.114.009789 [DOI] [PubMed] [Google Scholar]
- 130.Putko B, Wen K, Thompson RB et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev. 2015;20:179–191. doi: 10.1007/s10741-014-9452-9. 10.1007/s10741-014-9452-9 [DOI] [PubMed] [Google Scholar]
- 131.Weidemann F, Niemann M, Breunig F et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–529. doi: 10.1161/CIRCULATIONAHA.108.794529. 10.1161/CIRCULATIONAHA.108.794529 [DOI] [PubMed] [Google Scholar]
- 132.Moon JC, Sachdev B, Elkington AG et al. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Eur Heart J. 2003;24:2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 133.Moon JC, Sheppard M, Reed E et al. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson. 2006;8:479–482. doi: 10.1080/10976640600605002. [DOI] [PubMed] [Google Scholar]
- 134.Sado DM, White SK, Piechnik SK et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–398. doi: 10.1161/CIRCIMAGING.112.000070. 10.1161/CIRCIMAGING.112.000070 [DOI] [PubMed] [Google Scholar]
- 135.Thompson RB, Chow K, Khan A et al. T1 mapping with cardiovascular mri is highly sensitive for Fabry disease independent of hypertrophy and sex . Circ Cardiovasc Imaging. 2013;6:637–645. doi: 10.1161/CIRCIMAGING.113.000482. 10.1161/CIRCIMAGING.113.000482 [DOI] [PubMed] [Google Scholar]
- 136.Pica S, Sado DM, Maestrini V et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:99. doi: 10.1186/s12968-014-0099-4. 10.1186/s12968-014-0099-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.West M Canadian Fabry disease treatment guidelines. Ottawa: The Garrod Association, 2012.
- 138.Paulus WJ, Tschöpe C, Sanderson JE et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. 10.1093/eurheartj/ehm037 [DOI] [PubMed] [Google Scholar]
- 139.Bhatia RS, Tu JV, Lee DS et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- 140.Garg N, Senthilkumar A, Nusair MB et al. Heart failure with a normal left ventricular ejection fraction: epidemiology, pathophysiology, diagnosis and management. Am J Med Sci. 2013;346:129–136. doi: 10.1097/MAJ.0b013e31828c586e. 10.1097/MAJ.0b013e31828c586e [DOI] [PubMed] [Google Scholar]
- 141.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. 10.1016/j.jacc.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karakus G, Kammerlander AA, Aschauer S et al. Pulmonary artery to aorta ratio for the detection of pulmonary hypertension: cardiovascular magnetic resonance and invasive hemodynamics in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2015;17:79. doi: 10.1186/s12968-015-0184-3. 10.1186/s12968-015-0184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goliasch G, Zotter-Tufaro C, Aschauer S et al. Outcome in heart failure with preserved ejection fraction: the role of myocardial structure and right ventricular performance. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134479. 10.1371/journal.pone.0134479 e0134479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Su M-Y, Lin LY, Tseng YH et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. 10.1016/j.jcmg.2014.04.022 [DOI] [PubMed] [Google Scholar]
- 145.Mascherbauer J, Marzluf BA, Tufaro C et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–1065. doi: 10.1161/CIRCIMAGING.113.000633. 10.1161/CIRCIMAGING.113.000633 [DOI] [PubMed] [Google Scholar]
- 146.Götte MJ, Germans T, Rüssel IK et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002–2011. doi: 10.1016/j.jacc.2006.07.048. 10.1016/j.jacc.2006.07.048 [DOI] [PubMed] [Google Scholar]
- 147.Schuster A, Stahnke VC, Unterberg-Buchwald C et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70:989–998. doi: 10.1016/j.crad.2015.05.006. 10.1016/j.crad.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andre F, Steen H, Matheis P et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. 10.1186/s12968-015-0123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eschenhagen T, Force T, Ewer MS et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. 10.1093/eurjhf/hfq213 [DOI] [PubMed] [Google Scholar]
- 150.Yoon GJ, Telli ML, Kao DP et al. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies: are clinicians responding optimally? J Am Coll Cardiol. 2010;56:1644–1650. doi: 10.1016/j.jacc.2010.07.023. 10.1016/j.jacc.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. 10.1016/j.jacc.2009.02.050 [DOI] [PubMed] [Google Scholar]
- 152.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. 10.1093/eurheartj/ehs181 [DOI] [PubMed] [Google Scholar]
- 153.Oeffinger KC, Mertens AC, Sklar CA et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 154.Cardinale D, Sandri MT, Martinoni A et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 155.Cardinale D, Colombo A, Sandri MT et al. Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. 10.1161/CIRCULATIONAHA.106.635144 [DOI] [PubMed] [Google Scholar]
- 156.Cardinale D, Colombo A, Lamantia G et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 157.Seidman A, Hudis C, Pierri MK et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 158.Curigliano G, Cardinale D, Suter T et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:155–166. doi: 10.1093/annonc/mds293. 10.1093/annonc/mds293 (suppl 7):vii. [DOI] [PubMed] [Google Scholar]
- 159.Armstrong GT, Plana JC, Zhang N et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. 10.1200/JCO.2011.40.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 161.Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6:1080–1091. doi: 10.1161/CIRCIMAGING.113.000899. 10.1161/CIRCIMAGING.113.000899 [DOI] [PubMed] [Google Scholar]
- 162.Neilan TG, Coelho-Filho OR, Shah RV et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111:717–722. doi: 10.1016/j.amjcard.2012.11.022. 10.1016/j.amjcard.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tham EB, Haykowsky MJ, Chow K et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. 10.1186/1532-429X-15-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Ville de Goyet M, Brichard B, Robert A et al. Prospective cardiac MRI for the analysis of biventricular function in children undergoing cancer treatments. Pediatr Blood Cancer. 2015;62:867–874. doi: 10.1002/pbc.25381. 10.1002/pbc.25381 [DOI] [PubMed] [Google Scholar]
- 165.Jordan JH, D’Agostino RB Jr, Hamilton CA et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014;7:872–879. doi: 10.1161/CIRCIMAGING.114.002217. 10.1161/CIRCIMAGING.114.002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fallah-Rad N, Walker JR, Wassef A et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy . J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. 10.1016/j.jacc.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 167.Toro-Salazar OH, Gillan E, O’Loughlin MT et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging. 2013;6:873–880. doi: 10.1161/CIRCIMAGING.113.000798. 10.1161/CIRCIMAGING.113.000798 [DOI] [PubMed] [Google Scholar]
- 168.Karamitsos TD, Neubauer S. Cardiovascular magnetic resonance in heart failure. Curr Cardiol Rep. 2011;13:210–219. doi: 10.1007/s11886-011-0177-2. 10.1007/s11886-011-0177-2 [DOI] [PubMed] [Google Scholar]
- 169.Kim YJ, Kim RJ. The role of cardiac MR in new-onset heart failure. Curr Cardiol Rep. 2011;13:185–193. doi: 10.1007/s11886-011-0179-0. 10.1007/s11886-011-0179-0 [DOI] [PubMed] [Google Scholar]
- 170.Swoboda PP, Plein S. Established and emerging cardiovascular magnetic resonance techniques for prognostication and guiding therapy in heart failure. Expert Rev Cardiovasc Ther. 2014;12:45–55. doi: 10.1586/14779072.2014.870035. 10.1586/14779072.2014.870035 [DOI] [PubMed] [Google Scholar]
- 171.te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson. 2014;16:50. doi: 10.1186/s12968-014-0050-8. 10.1186/s12968-014-0050-8 [DOI] [PMC free article] [PubMed] [Google Scholar]