Abstract
The gastrointestinal tract of vertebrates is inhabited by diverse bacterial communities that induce marked effects on the host physiology and health status. The composition of the gastrointestinal microbiota is characterized by pronounced taxonomic and functional variability among different regions of the vertebrate gastrointestinal tract. Despite the relatively solid knowledge on the among-region variations of the gastrointestinal microbiota in model mammalian species, there are only a few studies concerning among-region variations of the gastrointestinal microbiota in free-living non-mammalian vertebrate taxa. We used Illumina MiSeq sequencing of bacterial 16S rRNA amplicons to compare the diversity as well as taxonomic composition of bacterial communities in proximal vs. distal parts of the gastrointestinal tract (represented by oral swabs and faecal samples, respectively) in a wild passerine bird, the great tit (Parus major). The diversity of the oral microbiota was significantly higher compared to the faecal microbiota, whereas interindividual variation was higher in faecal than in oral samples. We also observed a pronounced difference in taxonomic content between the oral and faecal microbiota. Bacteria belonging to the phyla Proteobacteria, Firmicutes and Actinobacteria typically dominated in both oral and faecal samples. A high abundance of bacteria belonging to Tenericutes was observed only in faecal samples. Surprisingly, we found only a slight correlation between the faecal and oral microbiota at the within-individual level, suggesting that the microbial composition in these body sites is shaped by independent regulatory processes. Given the independence of these two communities at the individual level, we propose that simultaneous sampling of the faecal and oral microbiota will extend our understanding of host vs. microbiota interactions in wild populations.
Introduction
Animal bodies are inhabited by taxonomically and functionally diverse communities of bacteria [1–3] that modulate their host's physiology and health status [4,5]. The modulations mediated by microbial communities are believed to be beneficial in most cases. However, adverse effects can be elicited by obligatory pathogenic bacterial species invading host bodies or facultative pathogens dwelling in immunocompromised hosts [6–8].
The host-associated microbiota exhibits pronounced spatial variation among different parts of the animal body [1,9–11]. Out of this complex system of host-associated microbial consortia, the microbiota of the gastrointestinal tract (hereafter GIT) has attracted considerable research attention during the past two decades. The GIT microbiota provides important benefits to the host, including increased efficiency of food digestion [12–14], stimulation of the immune system [15,16], defence against pathogens [17,18] and beneficial effects on the development and functioning of the gut and central nervous system [19,20]. At the same time, GIT microbiota dysbiosis is associated with metabolic, autoimmune and neurological disorders [21–24]. Last but not least, many bacterial pathogens invade animal bodies through the GIT [25–27], providing a further argument for the importance of the GIT microbiota on animal fitness.
Most current studies focusing on the GIT microbiota, use the microbiota of faecal samples, putatively representing the GIT microbiota of the lower intestine, as a proxy [3,28–30]. However, there is compelling evidence that microbial composition exhibits marked variation between different compartments of the GIT [9,31–33]. Such variation occurs due to spatial changes in biotic and abiotic factors among different GIT regions, including acidity levels, concentrations of oxygen, cholic acids and nutrients as well as changes in the host’s immunity [34–36]. As the relative contributions of these mechanisms to microbial structure may be independent both at the between-GIT region level and the interindividual level, the resulting within-individual correlation of the microbial content among different GIT regions can be of rather low effect size (Kreisinger et al., in prep, [37]). At the same time, the microbiota in different GIT regions can be associated with distinct effects on the hosts' phenotype [33,38]. Consequently, studies relying on samples from single GIT regions, or those based solely on faecal samples, can provide only a limited view on the outcome of interactions between the GIT microbiota and the host.
A few studies have analysed host vs. microbiota interactions using microbiota samples from multiple GIT regions [9,33,37]. However, as acquiring the corresponding samples was typically performed in a destructive way, this approach does not enable assessments of temporal variation of the GIT microbiota based on longitudinal sampling of the same individual. Furthermore, destructive sampling might be ethically questionable in the case of research on protected wild species. Although biopsies taken from different GIT regions offer a non-destructive alternative [39–41], such an approach is methodically challenging, which limits its broader applicability. Therefore, an application of alternative sampling protocols that are non-destructive but allow sampling of multiple GIT regions is desirable.
Moreover, most current knowledge on microbial variation along the GIT relies on data from mammalian captive-bred models and humans [40–42]. Patterns of spatial variation of the microbiota along the GIT in wild populations and in non-mammalian taxa are still understudied, however [9,31,32,43]. This sort of knowledge is crucial for understanding host vs. microbiota interactions, since previous studies, mostly based on samples from single GIT regions, have revealed that the taxonomic and functional content of the GIT microbiota varies considerably between captive-bred and wild populations of the same species [44–47], as well as between mammals vs. other vertebrates [28,30,48,49]. Consequently, approaches based on sampling of multiple GIT regions are essential for understanding host vs. microbiota interactions in wild populations, including resulting effects on the host's fitness.
Here we applied high-throughput amplicon sequencing of bacterial 16S rRNA to study the variation in microbiota among samples of proximal and distal parts of the GIT (represented by oral swabs and faecal samples) noninvasively collected in a free-living passerine bird. According to research on mammals, microbial communities of both the proximal and distal GIT are shaped to a large extent by host-intrinsic regulatory mechanisms, while the effect of environmental bacteria on the composition of these communities is usually limited [43,50]. At the same time, however, host-specific factors affecting microbial populations differ between the proximal and distal GIT in mammals. Diet composition, infection by intestinal parasites and genetic factors are crucial factors leading to lower GIT microbiota variation [51–53]. On the other hand, specific properties of the saliva and gingival crevicular fluid, and to a lesser extent diet, have important effects on the proximal GIT microbiota [54]. Importantly, however, distinct mechanisms seem to drive the variation of host-associated microbiota in mammals vs. non-mammalian vertebrates [28,30,48,55].
In this study, great tit (Parus major) was selected as a model species. The great tit is an eminent model species for the functionally and evolutionary oriented branches of ecological research [56,57]. Despite the current interest in the emerging topic of host vs. GIT microbiota interactions [58–61], there is still rather limited knowledge on these interactions in the great tit [62]. Importantly, no previous studies on the great tit have used culture-independent high-throughput sequencing to characterize the microbial communities associated with this host. In addition, to our knowledge, variation in the microbiota colonizing different parts of body of this species as well as other passerines has not yet been addressed. We compared the diversity, interindividual variation and taxonomic composition of the microbiota from the proximal vs. distal parts of the GIT. Finally, we tested if there was any correlation between the proximal and distal GIT microbiota at the within-individual level.
Materials and methods
Field sampling
Faecal and oral samples used in this study were collected from putatively unrelated adult individuals (n = 29) of the great tit population breeding in artificial nest boxes in the Ďáblický háj forest (50°08'12.4"N, 14°27'57.2"E, Prague, Czech Republic). The sampling locality is covered by secondary deciduous forest with a minor admixture of coniferous trees. All samples were obtained within one week in mid-May 2014.
Collection of microbial samples was performed as follows: adult individuals were captured in mist nets and placed in clean paper bags for approx. 15–20 minutes. Samples of faeces were subsequently collected from the paper surface. The oral microbiota was sampled using sterile microbiological nylon swabs (minitip FLOQSwabs, Copan, Italy) by wiping the oral cavity and upper side of the beak. Both faecal and oral samples were immediately placed in sterile DNA/RNA free cryotubes (Simport, Canada) filled with a self-made DNA/RNA-stabilising buffer on the basis of RNA later (protocol available upon request) and transferred to -80°C within two days. The sex of sampled individuals was determined by external phenotypic traits (e.g. [63]). Data on the body mass and tarsus length were used for calculation of the scaled body mass index following Peig and Green [64]. Birds were then individually marked using aluminium rings following the regulations of the Czech Bird Ringing Centre and released.
All field procedures were approved by the ethical committee of the Czech Academy of Sciences (107/2009).
Microbial genotyping
Metagenomic DNA from faecal and oral samples was extracted in a laminar flow cabinet using the PowerSoil DNA isolation kit (MO BIO Laboratories Inc., USA). To optimise the efficiency of DNA isolation, samples were homogenised using a MagnaLyzer (Roche, Switzerland) for 30s at 6000rpm and extracted DNA was eluted in 50 μl of elution buffer. From 29 sampled individuals, DNA isolated from oral and faecal samples was not of sufficient quantity in 9 and 12 cases, respectively, and therefore these samples were not included in further analyses. The final dataset thus included 17 faecal and 20 oral samples (S1 Table).
Following the recommendations of Klindworth et al., [65], primers covering the V3-V4 variable region of bacterial 16S rRNA (i.e. S-D-Bact-0341-b-S-17 [CCTACGGGNGGCWGCAG] and S-D-Bact-0785-a-A-21 [GACTACHVGGGTATCTAATCC]) were used during the PCR step. Both forward and reverse primers were tagged with 10bp barcodes designed by TagGD software [66]. For the polymerase chain reaction (PCR) we used 8 μl of KAPA HIFI Hot Start Ready Mix (Kapa Biosystems, USA), 0.37 μM of each primer and 7 μl of DNA template. PCR conditions were as follows: initial denaturation at 95°C for 5 min, followed by 35 cycles each of 98°C (20 sec), 61°C (15 sec) and 72°C (40 sec), and a final extension at 72°C (5 min). For individual samples, we prepared technical PCR duplicates. The PCR products, together with negative controls (PCR products of blank DNA isolates), were run on a 1.5% agarose gel and concentration of the PCR product was assessed based on gel band intensity using GenoSoft software (VWR International, Belgium). Samples were subsequently pooled at equimolar concentration. As we did not observe any visible PCR products in negative controls, therefore this type of samples was not included into the final pool. The pooled samples then were run on another 1.5% agarose gel, with bands of appropriate size excised from the gel and purified using the High Pure PCR product Purification Kit (Roche, Switzerland) according to the manufacturer’s instructions. Sequencing adaptors were ligated using TruSeq nano DNA library preparation kits (Illumina, USA) and the resulting amplicon libraries sequenced on a single Miseq run (Illumina, USA) using v3 chemistry and 2 × 300 bp paired-end reads. Raw sequencing data are avialable at http://www.ebi.ac.uk/ena/data/view/PRJEB19204 and sample metadata in S1 Table.
Bioinformatic processing of 16S rRNA data
Paired-end Illumina reads were merged using PEAR [67], and de-mutiplexed using mothur [68] and custom R/Bioconductor scripts (available from the authors on request). We then used the Lotus pipeline [69] for quality filtering of the FASTQ files. Sequences were excluded if the average quality score was lower than 30 or if the average quality score within a 50 bp sliding window decreased below 25. UCHIME (implemented in the Lotus pipeline) [70] was used alongside the gold.fna database (available at http://sourceforge.net/projects/microbiomeutil/files) for the detection and elimination of chimeric sequences. The resulting 16S rRNA sequences were clustered at a 97% similarity threshold using UPARSE [71] in order to define Operational Taxonomic Units (OTU). Taxonomic assignation of representative sequences for each OTU was performed using the RDP classifier [72] and the GreenGenes reference database, (version gg_13_5) [73]. Representative sequences were further aligned using PyNAST [74], the maximum likelihood tree being constructed using FastTree [75]. We observed a considerable excess of chloroplast sequences in our dataset (17.7%). Chloroplast OTUs together with OTUs that were not assigned to any bacterial phylum were considered as diet contaminants or sequencing artefacts, respectively, and we excluded them from all downstream analyses. The resulting OTU tables, sample metadata, OTU tree and taxonomic annotations for individual OTUs were merged into a phyloseq object [76] for statistical analysis in R version 3.2.3 [77].
Statistical analyses
In order to account for uneven sequencing depth among samples, statistical analyses were calculated based on the rarefied OTU table unless otherwise stated. The number of observed OTUs, Shannon diversity and Chao1 based predictions of total microbial diversity for individual samples were calculated using phyloseq [76]. Linear Mixed Effect models (LME, package lme4) [78] were used to test differences in diversity between faecal vs. oral microbiota. To account for statistical nonindependence, the effects of an individual were included as a random intercept. In addition, analysis of variance (ANOVA), running separately on samples from each GIT region, was applied to test differences in microbial alpha diversity between males vs. females and due to scaled body mass index.
We further used Principal Coordinate Analysis (PCoA) based on Bray-Curtis, Jaccard, weighted and unweighted UniFrac [79] distances between samples to visualize the contrast in the composition between faecal and oral microbiota. Adonis (i.e. analysis of variance based on distance matrices) was applied to assess the statistical significance and proportion of variance explained by the contrast in microbial composition between faecal and oral samples. Individual identity was included as a constraint for permutations (i.e. “strata”) in adonis models to account for data nonindependence. Betadisper was further applied to test for the difference in interindividual variation of microbial composition between the two GIT regions. The effects of sex and scaled body mass index were assessed via adonis analyses running separately on faecal and oral samples. For individuals where both oral and faecal microbiota were analysed (n = 8), we used Pearson correlations to assess if there was any interrelationship in microbial alpha diversity between the two GIT regions. Next, within-individual correlations of the microbial composition between oral vs. faecal samples was assessed via Mantel's test. Finally, using Spearman's correlations, we tested if relative abundances of OTUs were correlated between the two GIT regions. This analysis was run on a subset of 216 OTUs that were present both in the oral and faecal microbiota of those individuals for which both these samples were available.
The LME-based approach was further used to identify OTUs whose abundances differed between oral and faecal samples. These analyses were performed on a subset of 240 OTUs (comprising 90.5% of all high-quality reads) that were detected in at least five samples. For each OTU-specific LME, Box-Cox transformed read counts were used as a response, whereas the effect of GIT regions and individual identity were included as the explanatory variable and random intercept, respectively. In addition, Box-Cox transformed total number of reads per individual samples was included as an offset in LMEs (i.e. assuming its direct relationship with the number of reads per tested OTUs in individual samples). To account for deviance from a Gaussian error distribution, the significance of the GIT region effect was assessed based on permutations. In particular, observed deviance changes due to the elimination of the GIT region effect for the initial model were compared with the null distribution of deviance changes extracted from LMEs, where both the number of total and OTU-specific read counts were randomly resampled (10 000 permutations). The Qvalue method [80] was used to account for false discoveries due to multiple testing. The effect of a given OTU was considered to be significant if the permutation-based p value and associated qvalue were lower than 0.05. The abundance pattern of OTUs that were overrepresented in the oral cavity or faecal samples was visualized using a heatmap (function aheatmap from R package NMF).
Results
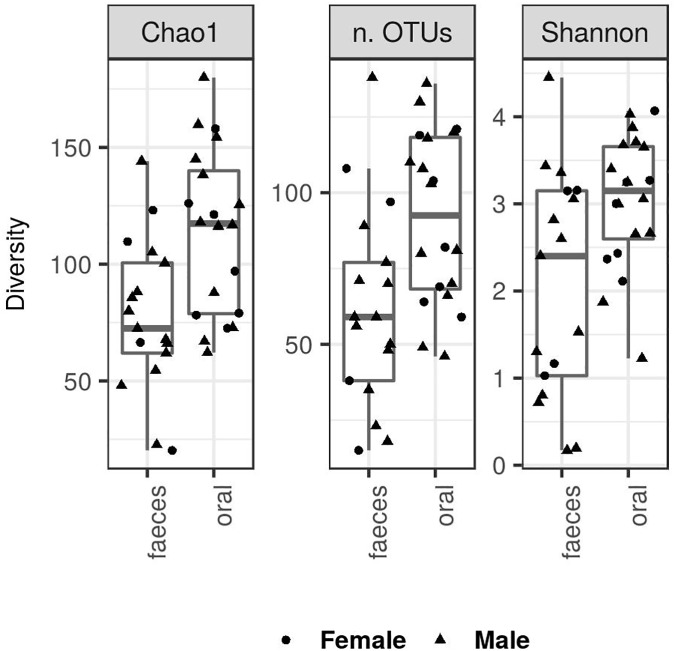
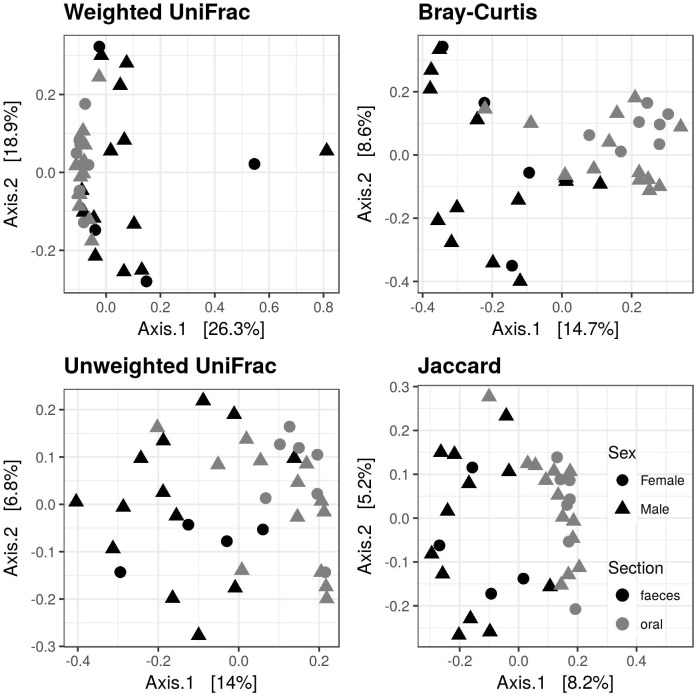
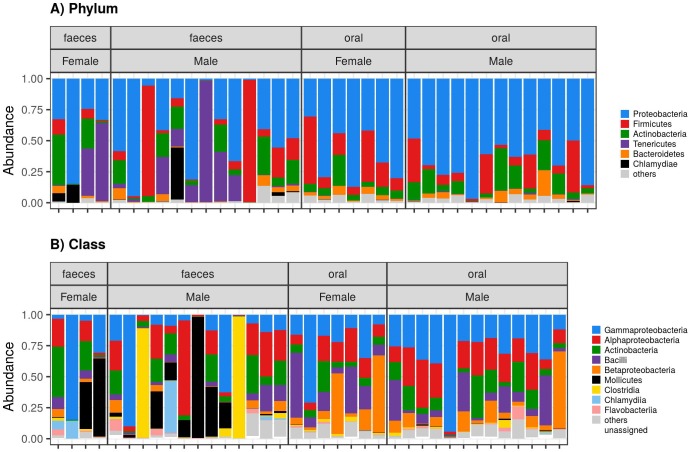
Our dataset included 207 497 high-quality reads that were clustered into 1127 non-chloroplast OTUs. There was a significant decrease of alpha diversity in faecal compared to oral microbiota according to the observed number of OTUs and Shannon index, as well as according to Chao1 (Fig 1, Table 1). Only 384 (34%) OTUs were detected in both the oral and faecal microbiota, whereas 541 (48%) and 202 (18%) OTUs were detected exclusively in oral and faecal samples, respectively. Clear differences in the composition of oral vs. faecal microbiota were also revealed based on PCoA (Fig 2) and the associated adonis analyses (Table 2). In addition, the interindividual variation in the microbial composition, as assessed by betadisper analyses, was lower in oral compared to faecal samples for all types of community dissimilarities, but no significant difference was found in the unweighted UniFrac (Table 2). In line with these results, plots visualising the taxonomic composition on the Phylum and Class levels indicated differentiation in the microbial composition between faecal and oral samples as well as a higher interindividual variation of faecal microbiota (Fig 3, S2 Table). Gammaproteobacteria (genera Diplorickettsia, Pseudomonas, Erwinia, Escherichia/Shigella, Serratia and Acinetobacter), Alphaproteobacteria (genera Methylobacterium, Rickettsia and Sphingomonas) and Actinobacteria (genera Corynebacterium and Pseudonocardia) were the dominating bacterial classes of both the oral and faecal microbiota. However, the abundance of Bacilli (represented by genera Staphylococcus and Lactobacillus) and Betaproteobacteria (represented by genera Methylobacillus, Comamonas and Herbaspirillum) was increased in oral compared to faecal samples. At the same time, several faecal samples exhibited high abundances of Mollicutes (represented by genera Ureaplasma and Mycoplasma), Clostridia and Chlamydia, i.e. bacterial classes that were detected in very low abundances in oral samples.
Fig 1. Diversity of the faecal and oral microbiota of the great tit.
Alpha diversity was measured as Chao1, number of observed OTUs and Shannon diversity. To account for uneven sequencing depths, alpha diversities were calculated based on rarefied OTU tables.
Table 1. Diversity of the faecal and oral microbiota of the great tit.
| Oral mean ± SE | Fecal mean ± SE | χ2 | P | |
|---|---|---|---|---|
| Chao1 | 119.0186 ± 10.0396 | 78.4456 ± 9.1118 | 8.21050 | 0.00416 |
| Observed | 92.2500 ± 6.6153 | 62.2353 ± 8.1274 | 7.93768 | 0.00484 |
| Shannon | 3.0168 ± 0.1724 | 2.0839 ± 0.3136 | 7.05950 | 0.00788 |
Alpha diversity was measured as Chao1, number of observed OTUs and Shannon diversity. Significance was assessed based on LME. Mean ± SE for individual sample groups, LME based likelihood-ratio statistic associated probability values are shown.
Fig 2. Differences in the composition between the oral and faecal microbiota of the great tit.
PCoA was performed for four dissimilarity indexes. Sex is indicated by different plotting symbols.
Table 2. Differences in the composition between the oral and faecal microbiota of the great tit.
| composition | interindividual variation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | Mean Sum Sq. | F | R2 | p | Mean Sum Sq. | F | p | ||
| Weighted UniFrac | GIT region | 1 | 0.47575 | 4.09496 | 0.10474 | 0.00781 | 0.24614 | 20.64496 | 0.00006 |
| Residuals | 35 | 0.11618 | 0.01192 | ||||||
| Unweighed UniFrac | GIT region | 1 | 0.78810 | 3.58368 | 0.09288 | 0.00781 | 0.00204 | 0.92960 | 0.34158 |
| Residuals1 | 35 | 0.21991 | 0.00220 | ||||||
| Bray Curtis | GIT region | 1 | 1.51171 | 4.31907 | 0.10985 | 0.00391 | 0.09235 | 14.12805 | 0.00062 |
| Residuals2 | 35 | 0.35001 | 0.00654 | ||||||
| Jaccard | GIT region | 1 | 0.92409 | 2.56352 | 0.06825 | 0.00391 | 0.00884 | 9.14144 | 0.00465 |
| Residuals3 | 35 | 0.36048 | 0.00097 | ||||||
Differences in composition were analysed using adonis, whereas differences in interindividual variation were assessed using betadisper. Both analyses were performed on four types of dissimilarity indexes.
Fig 3. Barplots indicating oral and faecal microbiota composition of the great tit.
Proportions of bacterial (a) phyla and (b) classes are shown.
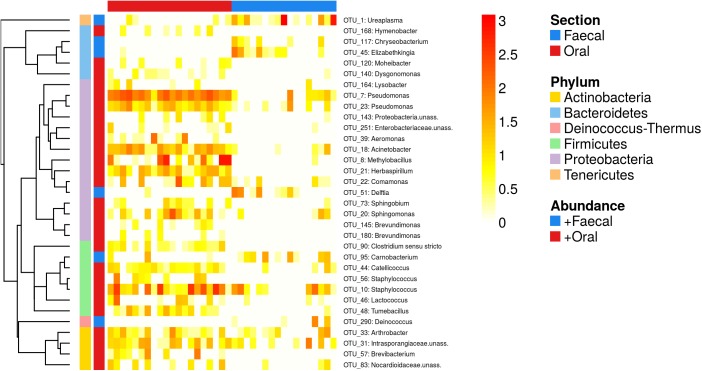
OTU-level analyses identified 33 OTUs (represented by 35.8% reads) whose relative abundances differed between oral and faecal samples (Fig 4). OTUs corresponding to the genera Ureaplasma (phylum Tenericutes), Delftia (phylum Proteobacteria), Carnobacterium (phylum Firmicutes), Deinococcus (phylum Deinococcus-Thermus), Chryseobacterium and Elizabethkingia (both phylum Bacteroidetes) were more abundant in faecal samples. On the other hand, OTUs where the most striking relative abundance increase in oral compared to faecal samples was observed belonged to the genera Pseudomonas, Acinetobacter, Methylobacillus, Herbaspirillum (all from phylum Proteobacteria), Catellicoccus, Staphylococcus, Tumebacillus (phylum Firmicutes) and to Arthrobacter, Brevibacterium and the families Intrasporangiaceae and Nocardioidaceae (phylum Actinobacteria).
Fig 4. Heatmap for OTUs, whose abundance varied between the oral and faecal microbiota of the great tit.
OTUs were identified according to permutations-based LMEs. Cell colours indicate OTU abundances in individual samples (log10 scaled values). Column annotations indicate GIT regions, whereas row annotations show Phylum-level assignations and if the OTU was overrepresented in oral or faecal samples (+oral or +faecal). OTUs are ordered according to their phylogeny (i.e. FastTree-based phylogeny for representative sequences).
ANCOVA analyses did not reveal any effect of sex or scaled body mass index on the diversity of microbial communities associated with these two GIT regions (p < 0.3 in all cases). In addition, according to adonis analyses, there was no effect of these two variables on the composition of oral or faecal microbiota (p > 0.2, R2 < 0.02 in all cases).
Analyses on the subset of individuals with both oral and faecal microbiota sampled did not reveal any correlation of the microbial structure between these two GIT regions at the within-individual level. First, alpha diversity estimates for these two GIT regions were not correlated (range of Pearson's r = -0.22 ~ -0.26, p > 0.5 for all types of diversity indexes). Furthermore, we did not detect any within-individual correlation in microbial composition between faecal and oral samples (Mantel test: p > 0.4, range of cor. coefs. = -0.25 ~ 0.19 for all four community dissimilarity indexes). Finally, the correlation of relative abundances of individual OTUs between oral swabs and faeces was negligible (mean of Spearman correlation coefficient = 0.05185, interquartile range = -0.21600 ~ 0.32870).
Discussion
Many previous studies that focused predominantly on captive bred mammals and humans have found pronounced differences in microbial structure between body sites as well as among different GIT compartments [37,81,82]. Our aim was to extend current knowledge on microbial divergence between GIT regions with data from the free-living population of a passerine bird, i.e. a taxonomic group that, to our knowledge, has not been studied in this context before. Consistent with previous research, we found pronounced differences between the proximal vs. distal GIT microbiota. According to our data, less than 30% of all OTUs were shared between these two GIT compartments in the great tit. Differences in terms of OTU absence vs. presence reflected pronounced variation in the relative abundances of bacterial taxa that were detected in oral vs. faecal microbiota. Compared to faecal microbiota, oral samples were characterized by a higher proportion of the classes Bacilli (phylum Firmicutes) and Betaproteobacteria (phylum Proteobacteria). In addition, bacteria from the phyla Chlamydiae and Tenericutes as well as from the class Clostridia (phylum Firmicutes) were nearly absent in the great tit oral microbiota.
Altogether, the composition of the great tit faecal microbiota was comparable with previous studies on other passerine birds [29,30,60,83–86], where OTUs from the phyla Proteobacteria, Firmicutes and Actinobacteria represented the dominant components. On the other hand, knowledge on the taxonomic content of the oral microbiota in birds is currently very limited. However, our data indicate that there is pronounced variation between the great tit and other avian hosts. For example, a dominance of Lactobacilli was detected in a recent study on the quail (Coturnix japonica) [87], while this bacterial genus constituted only a low proportion of the oral microbiota in our population. Similarly, abundances of Haemophilus and Streptococcus that dominated the oral microbiota of the kakapo (Strigops habroptilus) [88] were low in the great tit. Compared to mammalian oral microbiota, where bacteria from the phyla Bacteroidetes, Firmicutes and Proteobacteria typically dominate, the oral microbiota in our population was characterized by a low proportion of Bacteroidetes and increased abundances of Actinobacteria [40,43,82,89]. In addition, the diversity of the oral microbiota was significantly increased compared to the faecal microbiota in our population. As studies on other vertebrate species commonly report both higher [1,40,43,90] and lower [82,91] values of alpha diversity in oral vs. faecal microbiota, further research should focus on factors driving this variation.
At the OTU level, the faecal microbiota was characterized by increased abundances of Ureaplasma, Deinococcus, Carnobacterium, Chryseobacterium, Delftia and Elizabethkingia OTUs. Ureaplasma together with another Tenericutes OTU corresponding to the genus Mycoplasma that tended to be increased in the faecal microbiota as well (qvalue ~ 0.08), are common inhabitants of vertebrate gastrointestinal and urogenital tracts. Although these taxa are often asymptomatically present in birds, some of these species are involved in severe pathogenesis [92]. The Deinococcus OTU (phylum Deinococcus-Thermus) has previously been detected in several vertebrate species [85,93]; however, its effect on the host physiology is poorly known. The Carnobacterium OTU (phylum Firmicutes) is a lactic acid bacterium with putative probiotic properties providing protection against various bacterial pathogens [94,95]. On the other hand, Chryseobacterium and Elizabethkingia OTUs (both from the family Flavobacteriaceae) are related to several pathogenic species of human and other vertebrate taxa [96–98].
According to the OTU-level analyses, the oral microbiota was characterized by increased abundance of OTUs from genera that commonly colonize the oral cavity, skin or intestine of various vertebrate taxa (for example Staphylococcus, Acinetobacter, Sphingomonas, Brevundimonas, Dysgonomonas, Hymenobacter, Sphingobium). Many OTUs exhibiting higher abundances in the oral cavity compared to faeces can be involved in interactions with their host's immune system and physiology, or can shape the community composition via interactions with other members of the oral microbiota. This applies, for example, to four Actinobacterial OTUs (from the genus Arthrobacter, Brevibacterium and the family Intrasporangiaceae, Nocardioidaceae). Actinobacteria produce a wide variety of bacteriocines and other compounds suppressing the proliferation of bacterial competitors. Therefore, their presence in the oral cavity could be crucial for the defence against bacterial pathogens as well as for the maintenance of overall microbial structure [99]. In line with this possibility, Arthrobacter abundances in the lower intestine were positively associated with survival rates of passerine species closely related to the great tit [59]. Other bacteria associated with the oral cavity that may be involved in interactions with invading pathogens and other community members included Lysobacter, Pseudomonas and Herbaspirillum. Lysobacter can shape microbial community through the production of bacteriocines and the active predation of other bacteria [100,101]. Pseudomonas and Herbaspirillum produce extracellular siderophores, i.e. iron chelating compounds, which provide them a competitive advantage over other bacteria by reducing the availability of iron in the environment [102]. In addition, Pseudomonas cells secrete exopolysaccharides that make them a difficult target for the host immune system [103], and some Pseudomonas species can be pathogenic for birds [104–106]. It is also worth noting the abundance increase of two lactic acid bacteria in the oral cavity, Catellicoccus and Lactococcus, which can shape the oral community structure by the modulation of abiotic environmental conditions or by direct interactions with host immune system or other community members [107,108].
Even though the sample size was limited, we did not observe any correlation between oral vs. faecal microbiota at the within-individual level. Consequently, we propose that microbial communities associated with the proximal and distal GIT are shaped by independent mechanisms. These can theoretically include (1) host-intrinsic mechanisms such as effects of the immune system and other biotic and abiotic factors operating within the GIT, or (2) extrinsic sources of variation including pools of bacteria present in the diet and other environmental sources.
Relatively low interindividual variation of oral microbiota suggests either that environmental bacterial pools colonizing the oral cavity exhibit high homogeneity in space and time, or that there is low interindividual variation in host-specific mechanisms regulating oral microbiota. As previous research has found only limited effects of environmental bacteria on oral microbiota in non-avian vertebrates [43,50], and as environmental microbial consortia typically exhibit high variation [109–111], we favor the latter explanation. High interindividual variation of faecal microbiota suggests that host-intrinsic factors driving its composition differ markedly among hosts. However, as the passage of food through the passerine gut is extremely fast [112,113] and thus the decomposition of bacteria from the external environment is probably not as effective as in mammals, we cannot exclude the possibility that the variation of faecal microbiota is also driven to certain extent by bacteria that get into the GIT with food. As knowledge of the factors driving within-species variability in the avian GIT microbiota remains limited [59,61,85], further research and specifically designed experiments are required to untangle the relative contribution of transient environmental bacteria to microbial composition in different GIT regions of passerines and other vertebrate taxa.
In conclusion, our study is the first to characterize the oral microbial structure and compared it with the faecal microbiota in a free-living bird population. Our results show that the oral and faecal microbiota of passerines represent two distinct bacterial consortia that exhibit marked differences at all levels of community structure, and that the interindividual variation of these communities is likely to be shaped by independent mechanisms. We propose that aside from the effect of environmental bacteria, the structure of both the faecal and oral microbiota is driven to a large extent by mutual interactions among community members or by the host vs. microbiota interactions including immunity. Consequently, given the putative effects of these two microbial communities on the host's heath status, further research focusing on the microbiota in wild vertebrate populations may benefit from simultaneous sampling of these two communities.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
We are grateful to David Warren Hardekopf and anonymous reviewers for helpful comments on an earlier version of the manuscript. We thank all those who collaborated with fieldwork. Access to computing and storage facilities owned by parties and projects contributing to the National Grid Infrastructure MetaCentrum, provided under the programme "Projects of Large Research, Development, and Innovations Infrastructures" (CESNET LM2015042), is greatly appreciated.
Data Availability
All Raw FASTQ files have been deposited in the European Nucleotide Archive: http://www.ebi.ac.uk/ena/data/view/PRJEB19204.
Funding Statement
Field works were funded by Czech Science foundation project 15-11782S (http://www.gacr.cz/). Wet-lab procedures and data analyses were funded by Czech Science foundation project 14-16596P, and by Grant Agency of Charles University (project 281315, http://www.cuni.cz/UK-33.html). LK, HP, HV and MT were supported by SVV project no. 260 434 / 2017. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326: 1694–1697. doi: 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochman H, Worobey M, Kuo C-H, Ndjango J-BN, Peeters M, Hahn BH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 2010;8: e1000546 doi: 10.1371/journal.pbio.1000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waite DW, Taylor MW. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Microb Symbioses. 2014;5: 223 doi: 10.3389/fmicb.2014.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet. 2003;361: 512–519. doi: 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- 5.Sommer F, Bäckhed F. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 2013;11: 227–238. doi: 10.1038/nrmicro2974 [DOI] [PubMed] [Google Scholar]
- 6.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001;73: 1124S–1130S. [DOI] [PubMed] [Google Scholar]
- 7.Rampelli S, Candela M, Severgnini M, Biagi E, Turroni S, Roselli M, et al. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J Nutr Health Aging. 2013;17: 166–172. doi: 10.1007/s12603-012-0372-x [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Kalyan S, Steck N, Turner LM, Harr B, Künzel S, et al. Analysis of intestinal microbiota in hybrid house mice reveals evolutionary divergence in a vertebrate hologenome. Nat Commun. 2015;6: 6440 doi: 10.1038/ncomms7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Zhang Z, Xu C, Zhao J, Liu H, Fan Z, et al. Bacteria and methanogens differ along the gastrointestinal tract of Chinese roe deer (Capreolus pygargus). PLoS ONE. 2014;9: e114513 doi: 10.1371/journal.pone.0114513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roggenbuck M, Schnell IB, Blom N, Bælum J, Bertelsen MF, Pontén TS, et al. The microbiome of New World vultures. Nat Commun. 2014;5 doi: 10.1038/ncomms6498 [DOI] [PubMed] [Google Scholar]
- 11.Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015;16: 191 doi: 10.1186/s13059-015-0759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kienzle E, Schrag I, Butterwick R, Opitz B. Calculation of gross energy in pet foods: new data on heat combustion and fibre analysis in a selection of foods for dogs and cats. J Anim Physiol Anim Nutr. 2001;85: 148–157. doi: 10.1046/j.1439-0396.2001.00311.x [DOI] [PubMed] [Google Scholar]
- 13.van Schijndel RJMS, Wierdsma NJ, van Heijningen EMB, Weijs PJM, de Groot SDW, Girbes ARJ. Fecal energy losses in enterally fed intensive care patients: an explorative study using bomb calorimetry. Clin Nutr. 2006;25: 758–764. doi: 10.1016/j.clnu.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 14.Wichert B, Müller L, Gebert S, Wenk C, Wanner M. Additional data on energy requirements of young adult cats measured by indirect calorimetry. J Anim Physiol Anim Nutr. 2007;91: 278–281. doi: 10.1111/j.1439-0396.2007.00705.x [DOI] [PubMed] [Google Scholar]
- 15.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31: 677–689. doi: 10.1016/j.immuni.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 16.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4: 478–485. doi: 10.1038/nri1373 [DOI] [PubMed] [Google Scholar]
- 17.Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci. 2011;108: 19288–19292. doi: 10.1073/pnas.1110474108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14: 685–690. doi: 10.1038/ni.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13: 701–712. doi: 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DM, Jenks SM. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav Processes. 2013;96: 27–35. doi: 10.1016/j.beproc.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444: 1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 22.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94: 58–65. doi: 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H-J, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3: 4–14. doi: 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69: 137–143. doi: 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 25.Wells CL, Maddaus MA, Simmons RL. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis. 1988;10: 958–979. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthöft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102: 1815–1823. doi: 10.1172/JCI2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberger M, Andorn N, Agmon V, Cohen D, Shohat T, Pitlik SD. Blood invasiveness of Salmonella enterica as a function of age and serotype. Epidemiol Infect. 2004;132: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley RE, Hamady M, Lozupone C, Turnbaugh P, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320: 1647–1651. doi: 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hird SM, Sánchez C, Carstens BC, Brumfield RT. Comparative gut microbiota of 59 neotropical bird species. Front Microbiol. 2015; 1403 doi: 10.3389/fmicb.2015.01403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kropáčková L, Těšický M, Albrecht T, Kubovčiak J, Čížková D, Tomášek O, et al. Co-diversification of gastrointestinal microbiota and phylogeny in passerines is not explained by ecological divergence. Mol Ecol. 2017;00: 1–1. doi: 10.1111/mec.14144 [DOI] [PubMed] [Google Scholar]
- 31.Godoy-Vitorino F, Goldfarb KC, Karaoz U, Leal S, Garcia-Amado MA, Hugenholtz P, et al. Comparative analyses of foregut and hindgut bacterial communities in hoatzins and cows. ISME J. 2012;6: 531–541. doi: 10.1038/ismej.2011.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colston TJ, Noonan BP, Jackson CR. Phylogenetic analysis of bacterial communities in different regions of the gastrointestinal tract of Agkistrodon piscivorus, the cottonmouth snake. PLoS ONE. 2015;10: e0128793 doi: 10.1371/journal.pone.0128793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han GG, Kim EB, Lee J, Lee J-Y, Jin G, Park J, et al. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. SpringerPlus. 2016;5: 911 doi: 10.1186/s40064-016-2604-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90: 859–904. doi: 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 35.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9: 577–589. doi: 10.1038/nrgastro.2012.156 [DOI] [PubMed] [Google Scholar]
- 36.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14: 667–685. doi: 10.1038/nri3738 [DOI] [PubMed] [Google Scholar]
- 37.Suzuki TA, Nachman MW. Spatial heterogeneity of gut microbial composition along the gastrointestinal tract in natural populations of house mice. PLoS ONE. 2016;11: e0163720 doi: 10.1371/journal.pone.0163720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin F- P, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4: 219 doi: 10.1038/msb.2008.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Ahrné S, Jeppsson B, Molin G. Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol Ecol. 2005;54: 219–231. doi: 10.1016/j.femsec.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 40.Stearns JC, Lynch MDJ, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, et al. Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1: 170 doi: 10.1038/srep00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Geng J, Tang X, Fan H, Xu J, Wen X, et al. Spatial heterogeneity and co-occurrence patterns of human mucosal-associated intestinal microbiota. ISME J. 2014;8: 881–893. doi: 10.1038/ismej.2013.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu S, Chen D, Zhang J- N, Lv X, Wang K, Duan L-P, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE. 2013;8: e74957 doi: 10.1371/journal.pone.0074957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bik EM, Costello EK, Switzer AD, Callahan BJ, Holmes SP, Wells RS, et al. Marine mammals harbor unique microbiotas shaped by and yet distinct from the sea. Nat Commun. 2016;7: 10516 doi: 10.1038/ncomms10516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scupham AJ, Patton TG, Bent E, Bayles DO. Comparison of the cecal microbiota of domestic and wild turkeys. Microb Ecol. 2008;56: 322–331. doi: 10.1007/s00248-007-9349-4 [DOI] [PubMed] [Google Scholar]
- 45.Xenoulis PG, Gray PL, Brightsmith D, Palculict B, Hoppes S, Steiner JM, et al. Molecular characterization of the cloacal microbiota of wild and captive parrots. Vet Microbiol. 2010;146: 320–325. doi: 10.1016/j.vetmic.2010.05.024 [DOI] [PubMed] [Google Scholar]
- 46.Wienemann T, Schmitt-Wagner D, Meuser K, Segelbacher G, Schink B, Brune A, et al. The bacterial microbiota in the ceca of Capercaillie (Tetrao urogallus) differs between wild and captive birds. Syst Appl Microbiol. 2011;34: 542–551. doi: 10.1016/j.syapm.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 47.Kreisinger J, Čížková D, Vohánka J, Piálek J. Gastrointestinal microbiota of wild and inbred individuals of two house mouse subspecies assessed using high-throughput parallel pyrosequencing. Mol Ecol. 2014;23: 5048–5060. doi: 10.1111/mec.12909 [DOI] [PubMed] [Google Scholar]
- 48.Li J, Ni J, Li J, Wang C, Li X, Wu S, et al. Comparative study on gastrointestinal microbiota of eight fish species with different feeding habits. J Appl Microbiol. 2014;117: 1750–1760. doi: 10.1111/jam.12663 [DOI] [PubMed] [Google Scholar]
- 49.Kohl KD, Cary TL, Karasov WH, Dearing MD. Restructuring of the amphibian gut microbiota through metamorphosis. Environ Microbiol Rep. 2013;5: 899–903. doi: 10.1111/1758-2229.12092 [DOI] [PubMed] [Google Scholar]
- 50.Li H, Li T, Yao M, Li J, Zhang S, Wirth S, et al. Pika gut may select for rare but diverse environmental bacteria. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505: 559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreisinger J, Bastien G, Hauffe HC, Marchesi J, Perkins SE. Interactions between multiple helminths and the gut microbiota in wild rodents. Phil Trans R Soc B. 2015;370: 20140295 doi: 10.1098/rstb.2014.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci. 2010;107: 18933–18938. doi: 10.1073/pnas.1007028107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsh PD, Martin MV, Lewis MAO, Williams D. Oral Microbiology 5st ed. Elsevier Health Sciences; 2009. [Google Scholar]
- 55.Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332: 970–974. doi: 10.1126/science.1198719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voegeli B, Saladin V, Wegmann M, Richner H. Parasites as mediators of heterozygosity-fitness correlations in the Great Tit (Parus major). J Evol Biol. 2012;25: 584–590. doi: 10.1111/j.1420-9101.2011.02445.x [DOI] [PubMed] [Google Scholar]
- 57.Ouyang JQ, Sharp P, Quetting M, Hau M. Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J Evol Biol. 2013;26: 1988–1998. doi: 10.1111/jeb.12202 [DOI] [PubMed] [Google Scholar]
- 58.González-Braojos S, Vela AI, Ruiz-de-Castañeda R, Briones V, Moreno J. Age-related changes in abundance of enterococci and Enterobacteriaceae in Pied Flycatcher (Ficedula hypoleuca) nestlings and their association with growth. J Ornithol. 2012;153: 181–188. doi: 10.1007/s10336-011-0725-y [Google Scholar]
- 59.Benskin CMH, Rhodes G, Pickup RW, Mainwaring MC, Wilson K, Hartley IR. Life history correlates of fecal bacterial species richness in a wild population of the blue tit Cyanistes caeruleus. Ecol Evol. 2015;5: 821–835. doi: 10.1002/ece3.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreisinger J, Čížková D, Kropáčková L, Albrecht T. Cloacal microbiome structure in a long-distance migratory bird assessed using deep 16sRNA pyrosequencing. PLoS ONE. 2015;10: e0137401 doi: 10.1371/journal.pone.0137401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escallón C, Becker MH, Walker JB, Jensen RV, Cormier G, Belden LK, et al. Testosterone levels are positively correlated with cloacal bacterial diversity and the relative abundance of Chlamydiae in breeding male rufous-collared sparrows. Funct Ecol. 2017;31: 192–203. [Google Scholar]
- 62.Lucas FS, Heeb P. Environmental factors shape cloacal bacterial assemblages in great tit Parus major and blue tit P. caeruleus nestlings. J Avian Biol. 2005;36: 510–516. doi: 10.1111/j.0908-8857.2005.03479.x [Google Scholar]
- 63.Cramp S, Perrins CM, editors. The Birds of the Western Palearctic Volume VII Oxford; New York: Oxford University Press; 1993. [Google Scholar]
- 64.Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118: 1883–1891. doi: 10.1111/j.1600-0706.2009.17643.x [Google Scholar]
- 65.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41: e1 doi: 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costea PI, Lundeberg J, Akan P. TagGD: fast and accurate software for DNA Tag generation and demultiplexing. PLoS ONE. 2013;8: e57521 doi: 10.1371/journal.pone.0057521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30: 614–620. doi: 10.1093/bioinformatics/btt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75: 7537–7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hildebrand F, Tadeo R, Voigt AY, Bork P, Raes J. LotuS: an efficient and user-friendly OTU processing pipeline. Microbiome. 2014;2: 30 doi: 10.1186/2049-2618-2-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27: 2194–2200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10: 996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 72.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73: 5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72: 5069–5072. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26: 266–267. doi: 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26: 1641–1650. doi: 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8: e61217 doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/. [Internet]. 2016. Available: https://www.R-project.org/
- 78.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67: 1–48. doi: 10.18637/jss.v067.i01 [Google Scholar]
- 79.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71: 8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci. 2003;100: 9440–9445. doi: 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ritchie LE, Steiner JM, Suchodolski JS. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66: 590–598. doi: 10.1111/j.1574-6941.2008.00609.x [DOI] [PubMed] [Google Scholar]
- 82.Alfano N, Courtiol A, Vielgrader H, Timms P, Roca AL, Greenwood AD. Variation in koala microbiomes within and between individuals: effect of body region and captivity status. Sci Rep. 2015;5: 10189 doi: 10.1038/srep10189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hird SM, Carstens BC, Cardiff SW, Dittmann DL, Brumfield RT. Sampling locality is more detectable than taxonomy or ecology in the gut microbiota of the brood-parasitic Brown-headed Cowbird (Molothrus ater). PeerJ. 2014;2: e321 doi: 10.7717/peerj.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirón L, Mira A, Rocha-Ramírez V, Belda-Ferre P, Cabrera-Rubio R, Folch-Mallol J, et al. Gut bacterial diversity of the house sparrow (Passer domesticus) inferred by 16S rRNA sequence analysis. Metagenomics. 2014;3: 1–11. doi: 10.4303/mg/235853 [Google Scholar]
- 85.Kreisinger J, Kropáčková L, Petrželková A, Adámková M, Tomášek O, Martin J-F, et al. Temporal stability and the effect of transgenerational transfer on fecal microbiota structure in a long distance migratory bird. Front Microbiol. 2017;8: 50 doi: 10.3389/fmicb.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis WB, Moore FR, Wang S. Changes in gut microbiota of migratory passerines during stopover after crossing an ecological barrier. The Auk. 2016;134: 137–145. doi: 10.1642/AUK-16-120.1 [Google Scholar]
- 87.Wilkinson N, Hughes RJ, Aspden WJ, Chapman J, Moore RJ, Stanley D. The gastrointestinal tract microbiota of the Japanese quail, Coturnix japonica. Appl Microbiol Biotechnol. 2016;100: 4201–4209. doi: 10.1007/s00253-015-7280-z [DOI] [PubMed] [Google Scholar]
- 88.Waite DW, Deines P, Taylor MW. Gut Microbiome of the Critically Endangered New Zealand Parrot, the Kakapo (Strigops habroptilus). PLoS ONE. 2012;7: e35803 doi: 10.1371/journal.pone.0035803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sturgeon A, Stull JW, Costa MC, Weese JS. Metagenomic analysis of the canine oral cavity as revealed by high-throughput pyrosequencing of the 16S rRNA gene. Vet Microbiol. 2013;162: 891–898. doi: 10.1016/j.vetmic.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 90.Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13: R42 doi: 10.1186/gb-2012-13-6-r42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150: 470–480. doi: 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumithra TG, Chaturvedi VK, Susan C, Siju SJ, Rai AK, Harish C, et al. Mycoplasmosis in wildlife: a review. Eur J Wildl Res. 2013;59: 769–781. doi: 10.1007/s10344-013-0769-9 [Google Scholar]
- 93.Nelson KE. An update on the status of current research on the mammalian microbiome. ILAR J. 2015;56: 163–168. doi: 10.1093/ilar/ilv033 [DOI] [PubMed] [Google Scholar]
- 94.Robertson PAW, O’Dowd C, Burrells C, Williams P, Austin B. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum). Aquaculture. 2000;185: 235–243. doi: 10.1016/S0044-8486(99)00349-X [Google Scholar]
- 95.Nazef L, Belguesmia Y, Tani A, Prévost H, Drider D. Identification of lactic acid bacteria from poultry feces: evidence on anti-campylobacter and anti-listeria activities. Poult Sci. 2008;87: 329–334. doi: 10.3382/ps.2007-00282 [DOI] [PubMed] [Google Scholar]
- 96.Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing healthcare-associated infections. J Hosp Infect. 2014;86: 244–249. doi: 10.1016/j.jhin.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 97.Loch TP, Faisal M. Emerging flavobacterial infections in fish: A review. J Adv Res. 2015;6: 283–300. doi: 10.1016/j.jare.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pereira GH, Garcia D de O, Abboud CS, Barbosa VL de B, da Silva PSL. Nosocomial infections caused by Elizabethkingia meningoseptica: an emergent pathogen. Braz J Infect Dis. 2013;17: 606–609. doi: 10.1016/j.bjid.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012;8: e1002606 doi: 10.1371/journal.pcbi.1002606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boutin S, Sauvage C, Bernatchez L, Audet C, Derome N. Inter individual variations of the fish skin microbiota: host genetics basis of mutualism? PLoS ONE. 2014;9: e102649 doi: 10.1371/journal.pone.0102649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seccareccia I, Kost C, Nett M. Quantitative analysis of Lysobacter predation. Appl Environ Microbiol. 2015;81: 7098–7105. doi: 10.1128/AEM.01781-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer J-M, Geoffroy VA, Baida N, Gardan L, Izard D, Lemanceau P, et al. Siderophore typing, a powerful tool for the identification of fluorescent and nonfluorescent pseudomonads. Appl Environ Microbiol. 2002;68: 2745–2753. doi: 10.1128/AEM.68.6.2745-2753.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol. 2005;175: 7512–7518. [DOI] [PubMed] [Google Scholar]
- 104.Jackson MK, Phillips SN. Necrotizing hepatitis in pet birds associated with Pseudomonas fluorescens. Avian Dis. 1996;40: 473–476. doi: 10.2307/1592248 [PubMed] [Google Scholar]
- 105.Walker SE, Sander JE, Cline JL, Helton JS. Characterization of Pseudomonas aeruginosa isolates associated with mortality in broiler chicks. Avian Dis. 2002;46: 1045–1050. doi: 10.1637/0005-2086(2002)046[1045:COPAIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 106.Benskin CMH, Wilson K, Jones K, Hartley IR. Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol Rev Camb Philos Soc. 2009;84: 349–373. doi: 10.1111/j.1469-185X.2008.00076.x [DOI] [PubMed] [Google Scholar]
- 107.Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, Lemos L, et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014;6: 33 doi: 10.1186/1757-4749-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee N-K, Han KJ, Son S-H, Eom SJ, Lee S-K, Paik H-D. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT—Food Sci Technol. 2015;64: 1036–1041. doi: 10.1016/j.lwt.2015.07.019 [Google Scholar]
- 109.Lear G, Anderson MJ, Smith JP, Boxen K, Lewis GD. Spatial and temporal heterogeneity of the bacterial communities in stream epilithic biofilms. FEMS Microbiol Ecol. 2008;65: 463–473. doi: 10.1111/j.1574-6941.2008.00548.x [DOI] [PubMed] [Google Scholar]
- 110.Carson JK, Campbell L, Rooney D, Clipson N, Gleeson DB. Minerals in soil select distinct bacterial communities in their microhabitats. FEMS Microbiol Ecol. 2009;67: 381–388. doi: 10.1111/j.1574-6941.2008.00645.x [DOI] [PubMed] [Google Scholar]
- 111.Gonzalez A, King A, Robeson MS, Song S, Shade A, Metcalf JL, et al. Characterizing microbial communities through space and time. Curr Opin Biotechnol. 2012;23: 431–436. doi: 10.1016/j.copbio.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caviedes-Vidal E, McWhorter TJ, Lavin SR, Chediack JG, Tracy CR, Karasov WH. The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. Proc Natl Acad Sci. 2007;104: 19132–19137. doi: 10.1073/pnas.0703159104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McWhorter TJ, Caviedes-Vidal E, Karasov WH. The integration of digestion and osmoregulation in the avian gut. Biol Rev Camb Philos Soc. 2009;84: 533–565. doi: 10.1111/j.1469-185X.2009.00086.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All Raw FASTQ files have been deposited in the European Nucleotide Archive: http://www.ebi.ac.uk/ena/data/view/PRJEB19204.