Abstract
Homeodomain interacting protein kinase 2 (HIPK2) is a critical regulator of multiple profibrotic pathways, including that of TGF-β1/Smad3. Genetic ablation of HIPK2 was shown previously to significantly reduce renal fibrosis in the experimental unilateral ureteral obstruction model and Tg26 mice, a model of HIV-associated nephropathy. To develop specific pharmacologic inhibitors of HIPK2 for antifibrotic therapy, we designed and synthesized small molecule inhibitor compounds on the basis of the predicted structure of HIPK2. Among these compounds, we identified one, BT173, that strongly inhibited the ability of HIPK2 to potentiate the downstream transcriptional activity of Smad3 in kidney tubular cells. Notably, binding of BT173 to HIPK2 did not inhibit HIPK2 kinase activity but rather, interfered allosterically with the ability of HIPK2 to associate with Smad3. In vitro, treatment with BT173 inhibited TGF-β1–induced Smad3 phosphorylation and Smad3 target gene expression in human renal tubular epithelial cells. In vivo, administration of BT173 decreased Smad3 phosphorylation and mitigated renal fibrosis and deposition of extracellular matrix in unilateral ureteral obstruction and Tg26 mouse models of renal fibrosis. Our data indicate that BT173 is a novel HIPK2 inhibitor that attenuates renal fibrosis through suppression of the TGF-β1/Smad3 pathway and may be developed as an antifibrotic therapy in patients with kidney disease.
Keywords: HIPK2, renal fibrosis, TGF-beta, Smad3, tubular cells
Fibrosis is characterized by excessive production and accumulation of extracellular matrix proteins, which lead to progressive loss of tissue function and eventual organ failure. CKD, irrespective of primary insults, is usually accompanied by renal interstitial fibrosis.1 Thus, effective therapeutic strategy to halt decline of kidney function in CKD will require not only the removal of the causal factors, such as hyperglycemia, hypertension, and HIV infection, but also, antifibrosis therapy to restore normal kidney structure and function.
TGF-β1 has been identified to be the most important profibrogenic factor for kidney disease.2–4 TGF-β1 binds to type 2 TGF-β receptor, allowing its dimerization with type 1 TGF-β receptor and leading to phosphorylation of Smad2 and Smad3. Phosphorylated Smad3 translocates into nucleus, thereby activating the transcription of the target genes, including profibrotic genes, such as Collagen 1, fibronectin, and α-smooth muscle actin (α-SMA). The critical role of Smad3 in kidney fibrosis is supported by the fact that Smad3 is highly activated in fibrotic kidney5,6 and that knockout of Smad3 attenuates kidney fibrosis in animal models of kidney disease.7,8 Thus, blockade of TGF-β1/Smad3 pathway is considered to be a potential therapeutic strategy for kidney fibrosis.
Using combined systems biology and experimental approaches, we have previously identified homeodomain interacting protein kinase 2 (HIPK2) to be a critical regulator of multiple profibrosis pathways, including the TGF-β1/Smad3 pathway.9 We showed that HIPK2 is essential for the downstream signaling pathway of TGF-β/Smad3 in kidney cells and that HIPK2’s ability to regulate the pathway is achieved by its physical association with Smad3, which modulates its activity. Genetic deletion of HIPK2 resulted in inhibition of TGF-β1/Smad3 activity in vivo and attenuated kidney fibrosis in both HIV1-transgenic (Tg26) mice and mice with unilateral ureteral obstruction (UUO). Therefore, suppression of the TGF-β1/Smad3 pathway through the inhibition of HIPK2 may potentially be a novel approach against fibrosis progression in kidney disease.
HIPK2 inhibitors have not been well developed and are not commercially available. There was a recent study that was reported by Cozza et al.10 describing the identification of a selective HIPK2 inhibitor that competes for the ATP binding in the HIPK2 kinase domain. However, because HIPK2 regulates multiple signaling pathways, including the regulation of p53, there is a concern that broad inhibition of HIPK2 function may not be beneficial in all cellular contexts. Here, we describe a small molecule of HIPK2, BT173, identified using a Smad3 reporter assay that is able to specifically inhibit the TGF-β1/Smad3 pathway through the disruption of HIPK2-Smad3 protein-protein interaction (PPI) without significant inhibition of HIPK2 kinase activity or inhibition of p53 activation. Importantly, administration of BT173 inhibited the profibrosis pathway in vitro in cultured human renal tubular epithelial cells (hRTECs) and halted the course of renal fibrosis in two murine models (Tg26 and UUO mice), suggesting that BT173 may be a novel small molecule compound for attenuation of renal fibrosis in CKD.
Results
Design, Synthesis, and Screening of HIPK2 Inhibitors
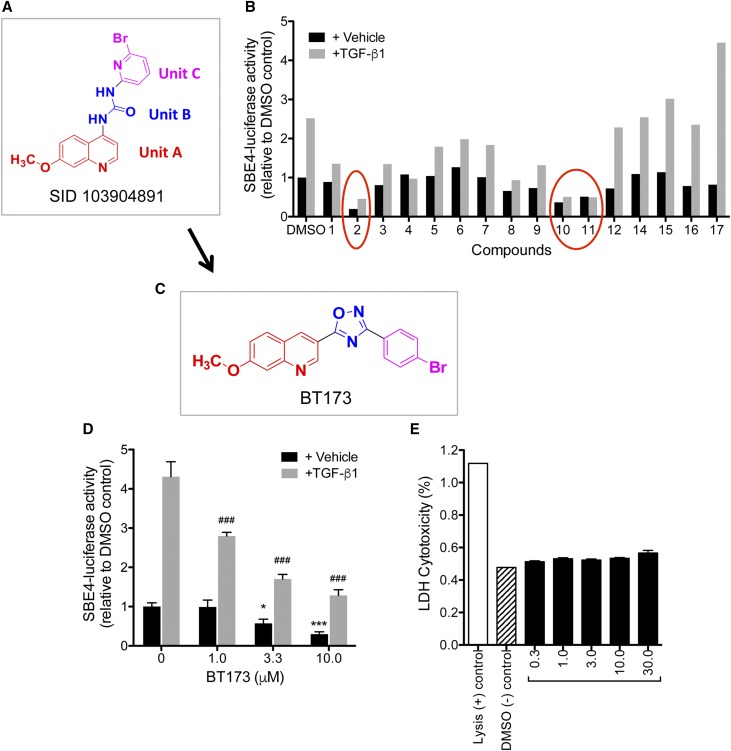
We took two approaches in designing the small molecule inhibitors against HIPK2: (1) Structure Activity Relationship (SAR) study to identify lead molecules (hit to lead) and (2) homology modeling of HIPK2 to develop in silico docking of new pharmacophore groups (target to hit). First, we performed a search for HIPK2 inhibitors and identified several potential compounds from a screening assay previously performed by Abbott Labs,11 which were deposited in the PubChem. We performed the SAR study on one of these compounds (PubChem SID 103904891), which contains three units (Figure 1A): unit A (red in Figure 1A) containing a 7-methoxy-4–substituted quinoline derivative, unit B (blue in Figure 1A) containing a urea derivative, and unit C (pink in Figure 1A) containing a 2-boromo-6–substituted pyridine. We designed and synthesized 17 compounds on the basis of the SAR study. As a readout for the effectiveness of the compound’s ability to inhibit HIPK2, we took advantage of the fact that Smad3, an important mediator of renal fibrosis, is one of the major downstream transcription factors activated by HIPK2 and initially screened the activity of these 17 compounds using a Smad-responsive luciferase reporter (Smad binding element–driven firefly luciferase [SBE4-Luc]) in HEK 293T cells (Figure 1B).
Figure 1.
HIPK2 inhibitor BT173 effectively inhibits TGF-β–induced Smad3-reporter activity. (A) Synthesis of HIPK2 inhibitor compounds were based on the structure of SID 103904891, which contains 7-methoxy-4–substituted quinoline derivative (unit A), urea derivative (unit B), and 2-bromo-6–substituted pyridine (unit C). (B) 17 potential HIPK2 inhibitor compounds were screened using a Smad3 reporter assay as a readout for HIPK2 activity in HEK 293T cells. Data are expressed as fold change in normalized SBE4-Luc activity to DMSO control. Three compounds (circled) showed the most potent inhibition of Smad3 activity (C2, C10, and C11). (C) Structure of compound 2 (BT173) in relation to SID 103904891. (D) Increasing concentration of BT173 results in significant attenuation of Smad3 activity. Data are expressed as fold change in normalized SBE4-Luc activity to DMSO control. *P<0.05 compared with DMSO control; ***P<0.001 compared with DMSO control; ###P<0.001 compared with TGF-β1–treated control. (E) Cytotoxicity of increasing concentration of BT173 was measured by the release of LDH; 293T cells were treated with various concentrations of C2 for 16 hours. Lysed cells (lysis) served as a positive control, and DMSO-treated cells served as a negative control.
We found that three compounds exhibited significant inhibition, namely compounds 2, 10, and 11 (Figure 1B). Among these, compound 2 (hereafter referred to as BT173) had the most potent inhibition of Smad3 activity in both untreated and TGF-β1–treated cells. Structural modification of BT173 from initial compound SID 103904891 is shown in Figure 1C: unit A was kept intact, but unit B was substituted by the oxadiazole pharmacophore group to increase binding specificity and hydrophilicity, and unit C was substituted with 4-substituted bromo-benzene to increase its hydrophobicity. To further confirm the inhibitory effect of BT173 on Smad3 activity, we determined the dosage effect of BT173 on the SBE4-Luc activity (Figure 1D). In the vehicle-treated cells, BT173 at increasing doses induced a progressive and significant suppression of the baseline Smad reporter activity (approximately 40% and 70% inhibition with 3.3 and 10 μm BT173, respectively, in comparison with DMSO control). In TGF-β–treated cells, BT173 significantly inhibited SBE-Luc activity at all three tested doses in comparison with DMSO control (approximately 40%, 60%, and 75% inhibition with 1.0, 3.3, and 10 μm, respectively). To rule out the possibility of cytotoxic effect of increasing concentration of BT173 in reducing Smad3 activity, we performed a cytotoxicity assay by measuring the level of lactate dehydrogenase (LDH) in cell culture medium, which is released by damaged cells. Even up to 30 μM BT173 for 16 hours of incubation did not induce any significant increase of LDH level in the culture medium (Figure 1E), suggesting that the observed repression of Smad3 activity by BT173 is not due to nonspecific effect of cytotoxicity.
BT173 Inhibits Smad3 Activity in Kidney Cells Independent of HIPK2 Kinase Function
Because BT173 significantly attenuated the HIPK2-mediated Smad3 activation, we next examined the extent of HIPK2 kinase activity inhibition by BT173. Kinase assay was performed by ProQinase GmbH (Freiburg, Germany). Surprisingly, we did not observe any inhibition of HIPK2 kinase activity in the presence of increasing concentrations of BT173, whereas staurosporine as a control showed an IC50 (i.e., half maximal inhibitory concentration) of 4.7×10−6 M (Figure 2A). This suggested that binding of BT173 to HIPK2, resulting in decreased Smad3 activity, is not mediated by the alteration of the kinase function of HIPK2. Therefore, we tested whether HIPK2 activity is nevertheless required for Smad3 activation. We used the kinase-dead mutant of HIPK2 (KD-HIPK2), which serves as a dominant negative mutant of endogenous HIPK2.9 Consistent with our previous report, we found that the overexpression of KD-HIPK2 in HEK 293T cells significantly suppressed the TGF-β1–induced SBE4-Luc reporter activity in comparison with overexpression of wild-type HIPK2 (Figure 2B). In addition, we found that there was an additive inhibitory effect between BT173 and overexpression of KD-HIPK2 on Smad3 activity (Figure 2B). Together with the above results, our data strongly indicated that the effect of BT173 on HIPK2 leading to repression of TGF-β/Smad3–dependent gene transcription is not achieved by the inhibition of its kinase activity. Interestingly, the essential role of HIPK2 in mediating the TGF-β/Smad3–induced gene transcription was also reported in midbrain dopamine neurons,12 and consistent with our result, the kinase activity of HIPK2 was not required for HIPK2’s ability to potentiate Smad3-dependent gene expression.
Figure 2.
Repression of HIPK2-Smad3 activation by BT173 is independent of kinase function of HIPK2. (A) Kinase activity of HIPK2 in increasing concentrations of BT173 remains unaltered, whereas (B) its activity is decreased in a dose-dependent manner in the presence of staurosporine. (C) Smad3 activity in 293T cells transfected with wild-type (WT) HIPK2 or KD-HIPK2 was compared in response to TGF-β1 and BT173. **P<0.01 compared with respective vehicle control; ***P<0.001 compared with respective vehicle control; #P<0.01 compared with respective TGF-β1–treated control; ###P<0.001 compared with respective TGF-β1–treated control; §§§P<0.001 compared with WT-HIPK2 cells treated with TGF-β1.
Binding of BT173 to HIPK2 Alters the PPI between HIPK2 and Smad3
As kinase activity of HIPK2 was not influenced by BT173, we next sought to determine the mechanism by which BT173 inhibits HIPK2-mediated Smad3 activation. It is known that steric or allosteric binding of compounds to target proteins can change their activity, functioning as either activators or inhibitors. Drug affinity–responsive target stability (DARTS) has recently been developed to identify potential protein targets of small molecules.13,14 It is on the basis of the principle that the binding of a small molecule compound to the target protein changes the protein conformation, leading to increased stability and protection against proteolysis. To test whether BT173 binds directly to HIPK2 and alters its conformation, we performed the DARTS experiment using pronase in absence or presence of BT173. On digestion with a serial dilution of pronase-to-cell lysate concentration ratio, we found that HIPK2 was protected from degradation at a 1:10,000 pronase-to-cell lysate ratio in the presence of BT173 but not in the presence of DMSO control (Figure 3A). To further confirm this result, we examined the dosage effects of BT173 on protection of HIPK2 from pronase digestion. At 1:10,000 pronase-to-cell lysate ratio, we found that a partial protection was observed with 30 μM concentration of BT173 and a complete protection with 100 μM (Figure 3B), confirming that BT173 binds directly to HIPK2 to confer increased resistance to pronase digestion. Because our previous work suggested that HIPK2 interacts with Smad39 and this study showed that the binding of BT173 to HIPK2 represses TGF-β/Smad3-dependent transcription, we wondered whether the binding of BT173 to HIPK2 interferes in the PPI between HIPK2 and Smad3. Because HIPK2 abundance is relatively low in many known cell types, we overexpressed 6xHis-tagged HIPK2 (His6-HIPK2) in 293T cells for testing its interaction with the endogenous Smad3. The expression of His6-HIPK2 and Smad3 in 293T cells was confirmed by Western blot (Figure 3C). We pulled down His6-HIPK2 and checked for the presence of Smad3 in cells treated with different doses of BT173. We found that BT173 indeed reduced the amount of Smad3 that was pulled down with His6-HIPK2 in a dose-dependent manner, confirming that binding of BT173 to HIPK2 interferes with its interaction with Smad3. Furthermore, overexpression of Smad3 alone was insufficient to overcome BT173's inhibition of TGF-β/Smad3 gene expression (Supplemental Figure 1), suggesting that HIPK2-Smad3 interaction is required for full potentiation of TGF-β/Smad3 signaling.
Figure 3.
Binding of BT173 to HIPK2 disrupts its PPI with Smad3. (A) DARTS confirms a direct binding of BT173 to HIPK2. Cleared cell lysate was preincubated with 100 μm BT173 or DMSO vehicle for 1 hour. Lysates were then digested with varying dilutions of pronase for 20 minutes and immunoblotted for HIPK2 and GAPDH loading control. Undigested lysate was used as a positive control. (B) Cell lysates were preincubated with varying concentrations of BT173 and digested with pronase (1:10,000 dilution) as in above experiment. Increasing concentration of BT173 results in greater protection of HIPK2 from pronase digestion. (C) The 293T cells expressing His6-HIPK2 were incubated with DMSO vehicle or BT173 (3.3 or 10 μM) for 16 hours. His6-HIPK2 was pulled down with cobalt beads, and eluted protein complexes were subject to immunoblotting. Total cell lysates were immunoblotted with indicated antibodies as controls.
BT173 Inhibits Phosphorylation of Smad3 and Its Downstream Gene Expression in Primary Human Renal Tubular Epithelial Cells
We next examined the effects of reduced HIPK2-Smad3 interaction by BT173 on TGF-β–induced Smad3 phosphorylation and downstream gene expression. Stimulation of primary hRTECs with TGF-β1 (5 ng/ml) for 20 minutes resulted in robust phosphorylation of Smad3 (Figure 4A). However, this was progressively inhibited when cells were pretreated with increasing doses of BT173 before TGF-β1 treatment (Figure 4A). Interestingly, Smad2 phosphorylation was unaffected by varying doses of BT173, suggesting that BT173 interferes specifically with HIPK2-Smad3 association. We next assessed the expression levels of TGF-β1/Smad downstream target genes (Col I, CTGF, PAI-1, FN, and MMP2) by real-time PCR. Compared with vehicle (DMSO), treatment of cells with TGF-β1 significantly increased the expression of these genes (Figure 4B). Although pretreatment of BT173 did not affect the expression of these genes in the absence of TGF-β1, there was a progressive inhibition of their expression with increasing concentrations of BT173.
Figure 4.
BT173 inhibits Smad3 phosphorylation in human kidney cells in vitro. (A) Early passage primary hRTECs were treated with or without TGF-β1 (10 ng/ml) for 20 minutes after a 16-hour preincubation of BT173. Lysates were immunoblotted for phosphorylated and total Smad2 and Smad3. (B) RNA was prepared from primary hRTECs treated similarly as above with BT173 preincubation and TGF-β1 (5 ng/ml) for 6 hours. Expression level of TGF-β1–responsive genes was determined by real-time PCR. Normalized gene expression is shown as a fold change relative to DMSO control. (C) hRTECs were treated with Wnt1 (10 ng/ml) for 48 hours with or without BT173, and gene expression was analyzed with real-time PCR. (D) hRTECs infected with either control GFP or pNL4/3:Δgag-pol-GFP lentivirus were incubated with BT173 for 24 hours. Lysates were immunoblotted for phosphorylated and total p53 (n=3). ***P<0.001 compared with DMSO control; #P<0.05 compared with agonist-treated control; ##P<0.01 compared with agonist-treated control; ###P<0.001 compared with agonist-treated control.
In addition to TGF-β/Smad3, our previous work showed that HIPK2 activates other signaling pathways, such as p53 and Wnt.9 Similarly to Smad3, we found that BT173 also inhibited target genes of the Wnt pathway (Figure 4C). HIPK2 has been shown to phosphorylate Ser46 on p53, which is an important site for p53 activation. However, BT173 had no effects on the phosphorylation of p53 on Ser46 in cells infected with HIV (Figure 4D), which is consistent with the previous finding that BT173 does not interfere with HIPK2 kinase activity. As there is a crosstalk between TGF-β and Wnt/β-catenin signaling pathways,15–17 it is not yet clear whether the inhibitory effect of BT173 of HIPK2 on Wnt pathway is mediated directly or indirectly through inhibition of Smad3 pathway.
BT173 Ameliorated Kidney Fibrosis in a Murine UUO Model
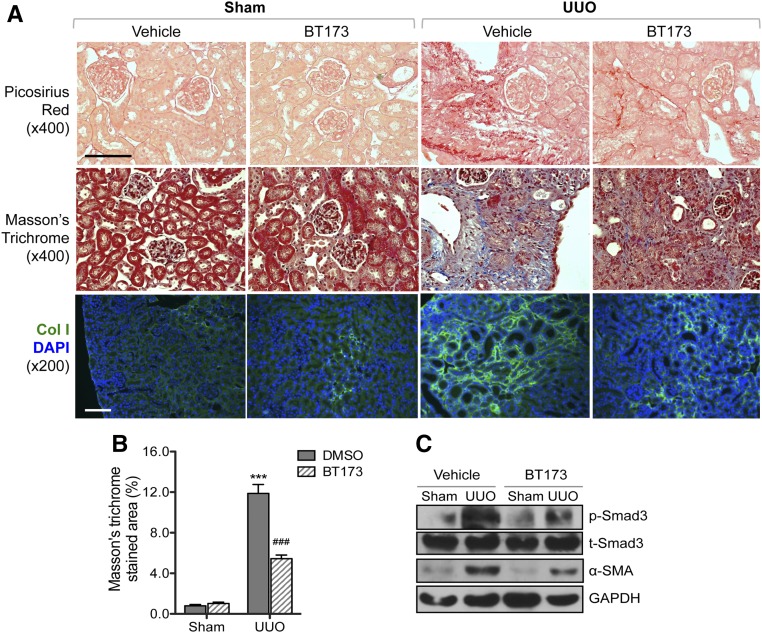
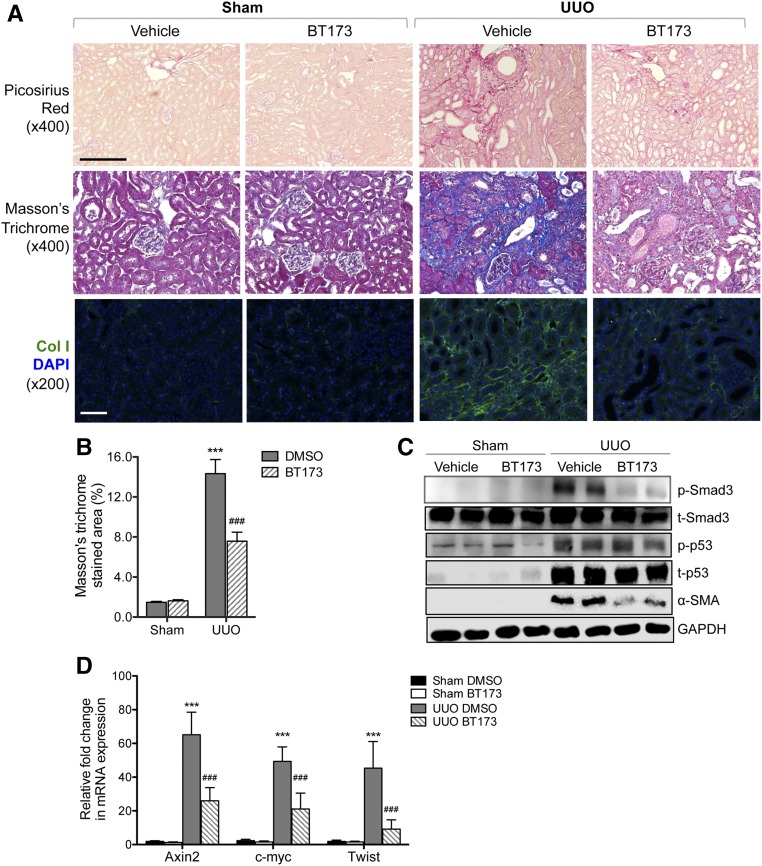
Because TGF-β1/Smad3 pathways are key regulators of profibrosis pathways in kidney disease and our in vitro data above indicated that BT173 inhibited the expression of profibrosis genes, we sought to determine whether BT173 can ameliorate fibrosis in vivo. We used the UUO mouse model, a commonly used model for renal fibrosis using two different treatment time courses. First, we tested the efficacy of BT173 in attenuation of fibrosis development by administration starting on the day of the surgery. Both UUO and sham-operated mice were administered either BT173 (20 mg/kg body wt) or DMSO vehicle by oral gavage daily from the day of the surgery to 7 days postsurgery. We found that treatment of BT173 significantly attenuated renal fibrosis development in the UUO mice compared with vehicle-treated UUO mice at 7 days postsurgery as observed by picrosirius red staining, Masson trichrome staining, and immunofluorescence staining of Collagen 1 (Figure 5, A and B). In addition, Western blot of the kidney cortex lysates showed that the treatment of BT173 significantly decreased Smad3 phosphorylation and α-SMA expression in the UUO kidneys (Figure 5C). Second, we tested the efficacy of BT173 in amelioration of fibrosis progression postfibrosis onset. Both UUO and sham-operated mice were administered either BT173 or vehicle as above but starting at day 7 postsurgery to allow for the onset of fibrosis to commence before BT173 administration. Mice were similarly administered as above for 7 days until day 14 postsurgery. Similar to early treatment, despite starting 7 days postsurgery, BT173 administration led to a significant attenuation of fibrosis at 14 days postsurgery as observed by picosirius red staining, Masson trichrome staining, and immunofluorescence staining of Collagen 1 (Figure 6, A and B). Western blot of kidney cortex showed a marked reduction in p-Smad3 and α-SMA in BT173-treated UUO kidneys in comparison with vehicle-treated UUO kidneys. Although p53 phosphorylation was significantly elevated in UUO kidneys in comparison with sham-operated kidneys, BT173 had no effects on p53 activation, consistent with the above in vitro findings. In addition, real-time PCR analysis revealed a significant upregulation of genes in the Wnt/β-catenin pathway (Axin2, c-myc, and Twist) in UUO kidneys in comparison with sham operated (Figure 6D). Treatment of BT173 also significantly diminished their expressions, further corroborating the above in vitro demonstration of BT173’s effect on HIPK2 to suppress the activation of the Wnt pathway induced in the injured kidney.
Figure 5.
Early treatment of BT173 ameliorated kidney fibrosis in UUO mice. BT173 or vehicle control was orally administered in sham- or UUO-operated mice (20 mg/kg body wt) starting on the day of the surgery for 7 days. (A) Representative images showing picosirius red and Masson trichrome staining and Collagen 1 immunostaining in sham- and UUO-operated mouse kidneys 7 days postsurgery. DAPI, 4′,6-diamidino-2-phenyllindole. Scale bar, 50 μM. (B) Quantification of the fibrotic area in Masson trichrome–stained kidneys (n=6). ***P<0.001 compared with sham-operated vehicle (DMSO) control; ###P<0.001 compared with UUO-operated vehicle control. (C) Western blot analysis for phospho-Smad3, total Smad3, α-SMA, and GAPDH in kidney cortices of UUO mice with or without BT173 treatment.
Figure 6.
Treatment of BT173 postonset of fibrosis ameliorated attenuated kidney fibrosis in UUO mice. BT173 or vehicle control was orally administered in sham- or UUO-operated mice (20 mg/kg body wt) starting on day 7 postsurgery for 7 days. (A) Representative images showing picosirius red and Masson trichrome staining and Collagen 1 immunostaining in sham- and UUO-operated mouse kidneys 14 days postsurgery. DAPI, 4′,6-diamidino-2-phenyllindole. Scale bar, 50 μM. (B) Quantification of fibrotic area in Masson trichrome–stained kidneys (n=6). (C) Western blot analysis for phospho-Smad3, total Smad3, p53, α-SMA, and GAPDH in kidney cortices of UUO mice with or without BT173 treatment. (D) Real-time PCR analysis of Wnt pathway target genes in UUO kidneys with or without BT173 treatment. ***P<0.001 compared with sham-operated vehicle (DMSO) control; ###P<0.001 compared with UUO-operated vehicle control.
BT173 Ameliorated Proteinuria and Kidney Fibrosis in Tg26 Mice
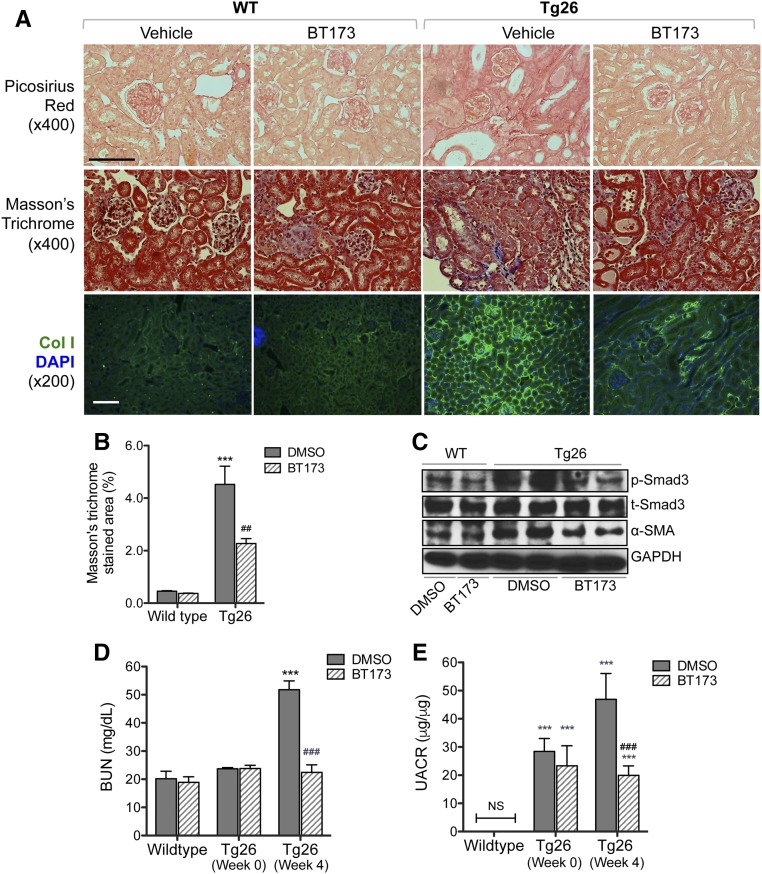
Because the utility of the UUO model is limited to renal fibrosis and cannot allow for examination of the renal function, we used a second mouse model of renal fibrosis. Our initial identification of the pivotal role of HIPK2 was in the setting of renal fibrosis in Tg26 mice.9 Therefore, we also examined whether BT173 can ameliorate the renal disease progression and ensuing fibrosis in Tg26. Tg26 mice are known to develop proteinuria starting from 4 weeks of age, and the proteinuria peaks usually around 8–10 weeks of age.18,19 Tg26 mice also develop significant glomerulosclerosis and renal failure by 8–10 weeks of age.19 To determine whether BT173 could reverse the kidney injury that has already developed, 6-week-old Tg26 mice were treated with either vehicle or BT173 for 4 weeks, and the mice were euthanized at 10 weeks of age. As observed in the UUO model, we found that BT173 significantly attenuated renal fibrosis in Tg26 mice as shown by picrosirius red staining, Masson trichrome staining, and immunofluorescence of Collagen 1 (Figure 7, A and B), which was associated with reduced p-Smad3 and α-SMA levels in kidneys (Figure 7C). Importantly, BT173 significantly improved renal function and reduced proteinuria in Tg26 mice compared with mice treated with vehicle (Figure 7, D and E). Taken together, our data show that BT173, a novel HIPK2 inhibitor, significantly ameliorates renal fibrosis and improves renal function in mouse models of renal fibrosis and CKD.
Figure 7.
Treatment of BT173 ameliorated kidney fibrosis in Tg26 mice. Six-week-old Tg26 mice were treated with either vehicle or BT173 for 4 weeks and euthanized at 10 weeks of age. (A) Representative images showing picosirius red staining, Masson trichrome staining, and Collagen 4 immunostaining in wild-type (WT) and Tg26 kidneys 4 weeks post-treatment. DAPI, 4′,6-diamidino-2-phenyllindole. Scale bar, 50 μM. (B) Quantification of fibrotic area in Masson trichrome–stained kidneys. (C) Western blot analysis for phospho-Smad3, total Smad3, p53, α-SMA, and GAPDH in kidney cortices of WT and Tg26 mice with or without BT173 treatment. (D) BUN levels and (E) urinary albumin-to-creatinine ratio (UCAR) in WT and Tg26 mice at baseline (week 0) and week 4 of BT173 or DMSO treatment (n=6). ***P<0.001 compared with WT control; ##P<0.01 compared with vehicle-treated Tg26; ###P<0.001 compared with vehicle-treated Tg26.
Discussion
Previously, we identified and validated the pivotal role of HIPK2 in renal fibrosis through regulation of multiple profibrosis pathways, including the TGF-β pathway.9 However, specific inhibitors for HIPK2 were not available. Here, we aimed to develop specific inhibitors of HIPK2 as antifibrosis drugs for kidney disease. Using the SAR approach, we designed and synthesized several compounds. We screened them by using a Smad3 reporter assay for its ease of utility to screen large numbers of compounds and because Smad3, a downstream target of HIPK2, is a major regulator of renal fibrosis. Also, this approach would allow for the identification of compounds with inhibitory effects on Smad3 activation, independent of the sites of interaction on HIPK2, such that it may help identify both steric and allosteric inhibitors of HIPK2. Using this approach, we identified BT173, which had almost a complete inhibition on Smad3 activity, while having a minimal inhibition on the kinase activity of HIPK2. Because BT173 did not affect kinase activity of HIPK2, other HIPK2-mediated pathways, such as p53 pathway, were not altered by BT173. This may provide a distinct advantage over a kinase inhibitor, in that BT173 would act as a specific inhibitor in HIPK2-mediated profibrosis pathways without inducing unwanted side effects of a broad inhibition of all HIPK2-regulated pathways, such as tumor growth, a potential side effect of dysregulated p53 pathway.20,21 The inhibitory effect of BT173 on the Wnt/β-catenin pathway, acting directly through inhibition of HIPK2 or indirectly through inhibition of the TGF-β pathway, may further enhance its antifibrosis effect, as Wnt/β-catenin has been show to promote fibrosis in kidneys.22 Also, our data on the HIPK2 kinase mutant suggest that use of BT173 in combination with HIPK2 kinase inhibitors may result in additive renoprotective effects as a combination antifibrosis therapy. Our data further confirmed that BT173 had a strong inhibitory effect of the TGF-β/Smad3 pathway in not only cultured kidney tubular cells but also, two animal models of renal fibrosis: UUO and Tg26. Therefore, BT173 may be developed as a potential drug to treat renal fibrosis.
We also explored the mechanism by which BT173 inhibits Smad3 activity. Our data suggest that BT173 binds to HIPK2 and reduces the interaction between HIPK2 and Smad3, thereby inhibiting the subsequent Smad3 phosphorylation and activation. These results are also supported by a previous observation in dopaminergic neurons in the work by Zhang et al.,12 which showed the necessity of HIPK2 for TGF-β–dependent neuronal survival. They further deduced the region of HIPK2 required for HIPK2-mediated Smad3 gene transcription, which was in the C-terminal regions outside of the HIPK2 kinase domain. However, it is not yet clear how HIPK2-Smad3 interaction results in increased Smad3 phosphorylation. This may be due to enhanced phosphorylation directly by HIPK2 or indirectly by other kinase recruitment and/or suppression of the dephosphorylation of Smad3 through interacting with the phosphatases. Future studies are also required to show the exact mechanisms of and protein domains involved in the interactions between HIPK2-BT173 and HIPK2-Smad3 to elucidate the conformational changes in HIPK2 on BT173 binding and how these changes perturb the HIPK2-Smad3 PPI.
PPIs mediate many crucial biologic activities that govern physical and pathologic processes. It is estimated that 130,000 binary PPIs make up the human interactome, most of which have not yet been identified.23 PPI is druggable, but this remains challenging.24,25 Humanized antibody specific against HIPK2 could be developed to interrupt PPI between HIPK2 and Smad3. However, because HIPK2 is a nuclear protein kinase, it presents added difficulty in targeting by antibodies. Small molecules possess advantages over humanized antibodies, in that they have high cell permeability, low costs of production, and excellent oral bioavailability. BT173, a small molecule that inhibits HIPK2-mediated Smad3 activation, exhibits the above advantages, evidenced by the renoprotective effects observed in our animal studies when administered orally.
One major concern of a small molecule inhibitor against a protein kinase is its nonspecific inhibition of other kinases. We performed kinome scanning and found that BT173 at 10 μm had small inhibitory effects on several protein kinases (<50%; data not shown). Previously reported steric HIPK2 inhibitor10 suppresses ten of 70 kinases with >50% inhibition at a concentration of 10 μm. We cannot exclude the possibility that BT173 affects other protein kinases to inhibit Smad3 activity. However, this is unlikely, because our screening assay did not reveal an effect of BT173 on protein kinases related to the TGF-β pathway.
In summary, we have identified a novel inhibitor of HIPK2, BT173, that blocks TGF-β/Smad3 activation in kidney cells, which is likely mediated through altering PPI between HIPK2 and Smad3. BT173 ameliorated kidney fibrosis in two animal models of kidney disease, largely through the inhibition of Smad3 activation. We propose that BT173 could be developed as a potential drug to treat patients with kidney fibrosis.
Concise Methods
Cell Culture
HEK 293T cells (ATCC) were cultured in DMEM (Invitrogen) containing 10% FBS and 0.5% penicillin and streptomycin at 37°C and 5% CO2 humidified environment. Human primary tubular cells (PromoCell GmbH, Heidelberg, Germany) were cultured in Renal Epithelial Cell Growth Medium-2 (PromoCell GmbH) with supplements according to the manufacturer’s protocol; human primary renal tubular epithelial cells with fewer than five passages were used for all studies. For HIV infection of hRTECs, pNL4–3:ΔG/P-EGFP, a gag/pol-deleted HIV-1 construct that contains EGFP in the gag open reading frame, and pHR-IRES-EGFP, a control EGFP construct, were used to generate the VSV-G–pseudotyped virus.26 Cells were infected with HIV-pseudotyped virus or control virus for 2 days before the treatment of BT173.
Plasmids
The 4× SBE4-Luc plasmid was purchased from Addgene (16495). Renilla luciferase reporter plasmid (pRL) was purchased from Promega. Kinase-dead mutant of HIPK2 was previously described.9 His6-HIPK2 construct was generated by PCR amplification of the coding region using plasmid containing the human HIPK2 gene (GeneCopoeia) as the template.
Transfection and Luciferase Assay
HEK 293T cells seeded in 12-well plates (approximately 60% confluence) were cotransfected with SBE4-Luc (0.5 μg) and pRL plasmids (0.2 μg) using the PolyJet Transfection Kit according to the manufacturer’s instructions (SignaGen Laboratories); 48 hours post-transfection, cells were treated with assigned concentrations of BT173 with or without 10 ng/ml TGF-β1 for 16 hours. Luciferase activities were measured using the Dual-Luciferase Reporter Assay Kit (E1910; Promega). Data are expressed as the ratio of firefly luciferase activity to renilla luciferase activity. For the HIPK2 dominant negative experiment together with SBE4-Luc and pRL (renilla luciferase) plasmids, either pcDNA 3.1 empty vector (0.5 μg) or KD-HIPK2 plasmid (0.5 μg) was cotransfected into 293T cells; 48 hours after transfection, luciferase activities were measured.
LDH Measurement
Medium LDH measurement was performed using the LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
HIPK2 Kinase Assay
HIPK2 kinase assay was performed by ProQinase GmbH.
DARTS
The DARTS method was used to identify protein targets of small molecules as described previously.14 Briefly, 293T lysates were incubated with BT173 at room temperature for 1 hour. After incubation, cell lysates were subjected to proteolysis with various concentrations of pronase (10165921001; Roche) at room temperature for 20 minutes. Proteolysis was stopped by addition of 5× protein loading buffer and heating at 90°C for 5 minutes. Protein samples were subjected to Western blot analysis.
RNA Extraction and cDNA Synthesis
Total RNA was extracted using Trizol (Invitrogen); 400 ng total RNA was reverse transcribed to cDNA using the SuperScript III First Strand Synthesis System (Invitrogen).
Real-Time PCR
Quantitative RT-PCR was performed using the 7500 Real-Time PCR System (Applied Biosystems). Gene level was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and expressed as fold change. The primer sets used were as follows: human Collagen 1 (forward: 5′-CTCCCCAGCTGTCTTATGGC-3′; reverse: 5′-GACCCATGGGACCTGAAGG-3′); CTGF (forward: 5′-CTTCTGTGACTTCGGCTCCC-3′; reverse: 5′-CTGGTACTTGCAGCTGCTCT-3′); fibronectin 1 (forward: 5′-TGCAGTGGCTGAAGACACAA-3′; reverse: 5′-ACGTCCTGCCATTGTAGGTG-3′); MMP2 (forward: 5′-CCTGCAAGTTTCCATTCCGC-3′; reverse: 5′-GTGAAGGGGAAGACACAGGG-3′); PAI1 (forward: 5′-ATCAGCCACTGGAAAGGCAA-3′; reverse: 5′-CTCTAGGGGCTTCCTGAGGT-3′); α-SMA (forward: 5′-GTATGTGGCTATCCAGCCGG-3′; reverse: 5′-AATAGCCACGCTCAGTCAGG-3′); and GAPDH (forward: 5′-AATTGAGCCCGCAGCCTCCC-3′; reverse: 5′-CCAGGCGCCCAATACGACCA-3′).
Immunoprecipitation with His6-HIPK2
The 293T cells were lysed in equilibration/wash buffer (50 mM sodium phosphate, 300 mM sodium chloride, and 10 mM imidazole, pH 7.4) for 48 hours after transfection of His6-HIPK2 expression plasmid. Cleared cell lysate was incubated with HisPur Cobalt Resin (Thermo Fisher Scientific) on an end over end rotator at 4°C for 2 hours. After centrifugation (700×g for 2 minutes at 4°C), the resin was washed three times in a wash buffer. The bound protein complexes were finally eluted with elution buffer (50 mM sodium phosphate, 500 mM sodium chloride, and 150 mM imidazole, pH 7.4) and subjected to Western blot.
Western Blot
Cells were lysed in M-PER Mammalian Protein Extraction Reagent (78501; Thermo Fisher Scientific) containing protease inhibitor cocktail (11836153001; Roche) and phosphatase inhibitors (50 mM NaF, 10 mM β-glycerophosphate, 5 mM sodium pyrophosphate, and 2 mM Na3VO4). Total protein concentration was measured using the Bradford Reagent (500–0006; Bio-Rad). Proteins were separated on SDS-PAGE and transferred to nitrocellulose membrane. Immunoblotting was performed using specific antibodies: phospho-Smad3 (9520; Cell Signaling), total Smad3 (9523; Cell Signaling), 6xHis (ab137839; Abcam), HIPK2, (5091; Cell Signaling), GAPDH (2118; Cell Signaling), and α-SMA (ab5694; Abcam).
Mice
Tg26 mice on FVB/N genetic background bearing a defective HIV-1 provirus lacking gag-pol were described in our previous study27; 6-week-old heterozygous Tg26 mice were used in this study. Wild-type littermates were used as controls. Mice in the treatment group received BT173 dissolved in DMSO and diluted in saline (5% DMSO) by oral gavage at a dose of 20 mg/kg body wt per day (n=6 in each group). Mice in the control group received the same volume of 5% DMSO/saline vehicle. The mice were treated for a total of 4 weeks and euthanized at age of 10 weeks old. The UUO model was established in male C57BL/6 mice (The Jackson Laboratory) by ligation of the left ureter as described previously.28 After the operation, UUO mice were randomly divided into control group (vehicle) and treatment group (BT173). Mice were fed with BT173 by oral gavage at a dose of 20 mg/kg body wt per day for 7 days.
Picrosirius Red and Masson Trichrome Staining
Renal fibrosis was evaluated histologically by sirius red staining on paraffin slides of mouse kidney tissues according to the manufacturer’s instructions (ab150681; Abcam). Masson trichrome staining on paraffin slices of mouse kidney tissue was performed according to the manufacturer’s instructions (87019; Thermo Scientific). Fourteen fields were randomly selected in five different sections of the renal cortex area per mouse (n=6). The fibrotic area was quantified by manually tracing the blue-stained area; the total scanned area, excluding the tubular lumen, glomeruli, and vessels, was also quantified using ImageJ 1.38X software (National Institutes of Health, Bethesda, MD). The fibrotic fraction volume ratio was expressed as the interstitial area relative to the total area.
Immunofluorescence Staining
Immunofluorescence staining was conducted on paraffin-embedded kidney sections using standard procedures. Briefly, deparaffinized sections were incubated with primary antibody against Collagen 1 (1:50; NB6–00–4-8; Novus) at 4°C overnight. After being washed, sections were incubated with Alexa Fluor488–labeled second antibody (1:200; A11034; Invitrogen) at room temperature for 1 hour in darkness. After nuclei were stained with 4′,6-diamidino-2-phenyllindole, slides were mounted using Aqua PolyMount (Polysciences, Inc.), and images were acquired using an AxioVision IIe Microscope with a digital camera.
Statistical Analyses
Data are reported as mean±SEM. The ANOVA followed by Bonferroni correction was used for comparison between groups. GraphPad Prism software was used for statistical analyses. All experiments were repeated at least three times, and representative experiments are shown. Data were considered statistically significant when P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
K.L. is supported by National Institutes of Health (NIH) grants 1R01DK098126 and P30DK079307. J.C.H. is supported by NIH grants 1R01DK078897, 1R01DK088541, and 1R01DK109683, and Veterans Affairs Merit award IBX000345C.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016080841/-/DCSupplemental.
References
- 1.Nangaku M: Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern Med 43: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T: TGF-beta signal transduction in chronic kidney disease. Front Biosci (Landmark Ed) 14: 2448–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rüster C, Wolf G: Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22: 1189–1199, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Meng XM, Tang PM, Li J, Lan HY: TGF-β/Smad signaling in renal fibrosis. Front Physiol 6: 82, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, Liu Y, Peng Y, Haruna Y, Kanwar YS: Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest 90: 436–447, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY: Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Mol Ther 21: 388–398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Xia Y, Lin X, Feng XH, Wang Y: Smad3 signaling activates bone marrow-derived fibroblasts in renal fibrosis. Lab Invest 94: 545–556, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, Ross MJ, Chen N, Ma’ayan A, He JC: A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med 18: 580–588, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cozza G, Zanin S, Determann R, Ruzzene M, Kunick C, Pinna LA: Synthesis and properties of a selective inhibitor of homeodomain-interacting protein kinase 2 (HIPK2). PLoS One 9: e89176, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metz JT, Johnson EF, Soni NB, Merta PJ, Kifle L, Hajduk PJ: Navigating the kinome. Nat Chem Biol 7: 200–202, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Pho V, Bonasera SJ, Holtzman J, Tang AT, Hellmuth J, Tang S, Janak PH, Tecott LH, Huang EJ: Essential function of HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. Nat Neurosci 10: 77–86, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, Wang J, Wu RP, Gomez F, Loo JA, Wohlschlegel JA, Vondriska TM, Pelletier J, Herschman HR, Clardy J, Clarke CF, Huang J: Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci USA 106: 21984–21989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai MY, Lomenick B, Hwang H, Schiestl R, McBride W, Loo JA, Huang J: Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol Biol 1263: 287–298, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW: Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature 403: 781–785, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Lee CH, Luo DD, Krupa A, Fraser D, Phillips A: Polarity of response to transforming growth factor-beta1 in proximal tubular epithelial cells is regulated by beta-catenin. J Biol Chem 282: 28639–28647, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D: Smad3 prevents beta-catenin degradation and facilitates beta-catenin nuclear translocation in chondrocytes. J Biol Chem 285: 8703–8710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratnam KK, Feng X, Chuang PY, Verma V, Lu TC, Wang J, Jin Y, Farias EF, Napoli JL, Chen N, Kaufman L, Takano T, D’Agati VD, Klotman PE, He JC: Role of the retinoic acid receptor-α in HIV-associated nephropathy. Kidney Int 79: 624–634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu TC, He JC, Klotman P: Animal models of HIV-associated nephropathy. Curr Opin Nephrol Hypertens 15: 233–237, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Zhang Q, Lu Y, Xue W, Xu Y, Zhu Y, Hu X: Downregulation of HIPK2 increases resistance of bladder cancer cell to cisplatin by regulating Wip1. PLoS One 9: e98418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon MJ, Min SK, Seo J, Kim DH, Sung CO, Lim MS, Cho J, Park HR: HIPK2 expression in progression of cutaneous epithelial neoplasm. Int J Dermatol 54: 347–354, 2015 [DOI] [PubMed] [Google Scholar]
- 22.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesan K, Rual JF, Vazquez A, Stelzl U, Lemmens I, Hirozane-Kishikawa T, Hao T, Zenkner M, Xin X, Goh KI, Yildirim MA, Simonis N, Heinzmann K, Gebreab F, Sahalie JM, Cevik S, Simon C, de Smet AS, Dann E, Smolyar A, Vinayagam A, Yu H, Szeto D, Borick H, Dricot A, Klitgord N, Murray RR, Lin C, Lalowski M, Timm J, Rau K, Boone C, Braun P, Cusick ME, Roth FP, Hill DE, Tavernier J, Wanker EE, Barabási AL, Vidal M: An empirical framework for binary interactome mapping. Nat Methods 6: 83–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arkin MR, Tang Y, Wells JA: Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem Biol 21: 1102–1114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makley LN, Gestwicki JE: Expanding the number of ‘druggable’ targets: Non-enzymes and protein-protein interactions. Chem Biol Drug Des 81: 22–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross MJ, Wosnitzer MS, Ross MD, Granelli B, Gusella GL, Husain M, Kaufman L, Vasievich M, D’Agati VD, Wilson PD, Klotman ME, Klotman PE: Role of ubiquitin-like protein FAT10 in epithelial apoptosis in renal disease. J Am Soc Nephrol 17: 996–1004, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Feng X, Lu TC, Chuang PY, Fang W, Ratnam K, Xiong H, Ouyang X, Shen Y, Levy DE, Hyink D, Klotman M, D’Agati V, Iyengar R, Klotman PE, He JC: Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Liu R, Xie J, Xiong H, He JC, Chen N: Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest 93: 801–811, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.